Abstract
Background
PTC299 is a novel, orally-bioavailable small molecule that selectively inhibits vascular endothelial growth factor receptor protein synthesis at the post-transcriptional level. Based on promising preclinical results, we conducted a pediatric phase I study to estimate the maximum tolerated dose (MTD), describe dose-limiting toxicities (DLT) and characterize the pharmacokinetic profile of PTC299 in children with recurrent CNS tumors.
Patients and Methods
PTC299 was administered orally twice or three times daily, depending on the regimen. Four regimens were evaluated using the rolling 6 design, starting with 1.2 mg/kg/dose twice daily and escalating to 2 mg/kg/dose three times daily. Pharmacokinetic studies were performed during the first two courses.
Results
Twenty-seven children (14 male, median age 11.2, range 5.5–21 years) with recurrent brain tumors were treated; 21 were fully evaluable for toxicity assessment. Therapy was well-tolerated, and the only DLT was grade 3 hyponatremia. Grade three and grade four toxicities were uncommon in subsequent cycles. Median AUC0–Tlast values at the 2 mg/kg were similar to those observed in adults. The study was terminated while patients were being treated at the highest planned dose, due to hepatotoxicity encountered in the ongoing adult phase I studies. No complete or partial responses were observed. Two patients with low-grade gliomas were noted to have minor responses, and at the time of the study’s closure, 5 children with low-grade gliomas had been on therapy for 8 or more courses (range 8–16).
Conclusion
PTC299 was well-tolerated at the highest dose level tested (2 mg/kg/dose TID) in children with recurrent brain tumors and prolonged disease stabilization was seen in children with low-grade gliomas.
Keywords: pediatric brain tumors, antiangiogenic agents, low-grade gliomas, biologic therapy, gliomas
Introduction
Tumor growth and metastasis are believed to be dependent, at least in part, on the ability of a tumor to induce vascularization [1, 2]. Bevacizumab, a humanized monoclonal antibody that is highly specific for all vascular endothelial growth factor (VEGF) isoforms, has resulted in a greater than 50% response rate and an increased six-month progression-free survival rate in adults with malignant gliomas [3]. It has been approved for use in recurrent glioblastoma multiforme [6]. Studies utilizing bevacizumab alone or in combination have been performed in children with brain tumors with variable results [4, 7].
The mechanisms involved in tumor-induced angiogenesis are complex and likely involve signaling by other growth factors and cytokines such as basic fibroblast growth factor and platelet-derived growth factor [1, 2, 5, 8]. However, VEGF remains one of the most common angiogenic factors found in tumor-induced angiogenesis [1, 2, 8]. It is known that VEGF production by tumors is controlled post-transcriptionally by sequences both in the 5-prime and 3-prime untranslated regions of its encoding messenger ribonucleic acid [9, 10, 11, 12].
PTC299 is a novel, orally bioavailable small molecule designed to control tumor growth by selectively inhibiting VEGF protein expression at the post-transcriptional level. PTC299 suppresses tumor overproduction of all four VEGF isoforms and inhibits production of other angiogenic cytokines. It also induces a parallel interruption of tumor cell division at the G1/S phase of the cell cycle, offering a potential additional mechanism of action. In vitro studies have demonstrated that PTC299 preferentially inhibits VEGF production in cells stimulated by stressors such as hypoxia. In vivo studies described in the investigator brochure of the drug have also shown that single agent PTC299 reduces tumor and plasma VEGF concentration, decreases tumor microvascular density, induces tumor regression or substantially impedes tumor progression in multiple xenograft models of cancer, including gliomas, and penetrates the blood-brain barrier [13].
The results of a phase I trial of PTC 299 in children with recurrent CNS malignancies are reported. The primary objectives were to estimate the maximum tolerated dosage (MTD) or recommended phase II dose (RP2D) and to describe the dose-limiting toxicities (DLTs) of PTC 299 administered twice or three times daily of a 28 day cycle and to characterize PTC299 plasma pharmacokinetics. The secondary objectives were to obtain preliminary evidence of biologic activity of PTC299 by using MR diffusion as a measure of tumor cellularity.
Patients and Methods
Patient Eligibility
To be eligible for the study, patients had to be ≥3 and ≤21 years of age, with a body weight of 15–100kg and a histologically-verified diagnosis of a recurrent or refractory primary central nervous system malignancy (histology was not required for patients with intrinsic brainstem tumors and optic pathway tumors) and a Lansky or Karnofsky score ≥50%. Patients were required to have recovered from the acute toxic effects of prior therapy. Patients must have been on stable or decreasing doses of steroids for at least 7 days prior to registration and been normotensive for at least 7 days; those on antihypertensives must have been on a stable regimen for at least 7 days. Other requirements included adequate bone marrow, renal, liver function. Patients had to have displayed stable neurological deficits for at least one week, and must be able to swallow pills. Pregnant or lactating females were excluded from the study, as were patients with uncontrolled infections. Patients of childbearing or child fathering potential had to agree to use a medically acceptable form of birth control, including abstinence, while on this study.
Informed consent was obtained from patients, parents or guardians, and assent was obtained as appropriate at the time of protocol enrollment. The initial protocol was approved by the Institutional Review Boards of participating Pediatric Brain Tumor Consortium (PBTC) Institutions.
Drug Administration and Study Design
The planned starting dose for PTC299 was based on the established safety and pharmacokinetic profiles from previous preclinical experience and from prior Phase 1a studies in healthy adult volunteers and ongoing Phase 1b studies in adult patients with tumors. The projected plasma exposures at dose level 1 were to be less than those occurring at the highest doses tested over 7 days in prior Phase 1a studies in healthy adult volunteers, and all dose levels were to have the potential to achieve target plasma trough concentrations associated with PTC299 antitumor activity in preclinical xenograft models. Given the lack of prior dose-limiting toxicity (DLT) observed with PTC299 in adult Phase 1 studies, and an inability to find a maximal tolerated dose in those trials, the protocol was designed to enroll 6 patients in each dose level with a plan to escalate to the highest dose level being used in adult trials, barring excessive toxicity.
PTC299 was supplied by PTC Therapeutics Inc. (South Plainfield, NJ) as 10 and 20 mg. dose capsules. Capsules were to be swallowed whole with water, but if patients had difficulty with swallowing, they could be added to one-half of a cup of yogurt or pudding less than 20 minutes prior to administration. The capsules were to remain intact. Based on preclinical testing and adult clinical experience, dosing was based on mg/kg dose levels. A dosing nomogram based on actual body weight and dosage level (rounded to the nearest 10 mg) was used to minimize interpatient dosing variability. PTC299 was administered twice or three times daily, dependent on the dose level.
Four overall dose levels were planned (see Table 1) starting with 1.2mg/kg/dose twice daily and escalating to a top dose of 2 mg/kg/dose 3 times daily. Dose level 0 (0.6mg/kg/dose twice daily) was provided in case excessive toxicity was observed at dose level 1. No intrapatient dose escalations were permitted.
Table 1.
Dose Levels and Dose Limiting Toxicities
| Dose | Number of patients Enrolled |
Number of Evaluable Patients |
Number of Patients with DLT |
DLT Detail |
|---|---|---|---|---|
| 1.2 mg/kg/dose BID | 6 | 6 | 1 | Gr 3 Hyponatremia |
| 1.2 mg/kg/dose TID | 7 | 6 | 0 | |
| 1.5 mg/kg/dose TID | 8 | 6 | 0 | |
| 2.0 mg/kg/dose TID | 7 | 3 | 0 |
Only DLTs observed during the dose-finding period of therapy were used to guide dose escalation. After completion of enrollment at dose level 2 and at dose level 3, enrollment was suspended until pharmacokinetic concentrations and safety data were fully evaluated. Although dose level 4 was to be the highest dose tested, dependent on pharmacokinetic results, if area under the curve (AUC) levels were found to be lower in the pediatric trial than that obtained at the chosen Phase 2 dose in adults, further dose escalations would be considered.
The dose-finding period was defined as the first course (28 days of treatment). Patients received the drug daily without a break. On Day 1 and Day 28 of the first course of treatment, a dose of PTC299 was to be administered in the clinic with subsequent pharmacokinetic evaluations. Patients were to fill out medication diaries while on treatment to record compliance. Patients could receive up to one year of treatment in the absence of disease progression. Those experiencing clinical benefit could receive up to 2 years of therapy
The MTD was defined as the maximum dose at no more than 1 of 6 patients experience DLT during Cycle 1 of therapy Toxicities were graded according to the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. In the absence of excessive toxicity, 6 patients were to be treated at each dose level. Hematological DLTs were defined as any ≥ grade 3 toxicity other than lymphopenia. Non hematological DLTs were defined as any grade 3, or greater, toxicity at least possibly related to PTC299; any drug-related adverse event that required interruption of the drug for greater than 10 doses or 5 days at dose level 0,1, or > 15 doses or 5 days at dose levels 2,3,4. Patients who experienced DLT during the dose-finding period, or had unacceptable toxicity in later courses, but who demonstrated clinical stability or improvement with evidence of stable disease or objective response on neuroimaging studies, were allowed to continue on treatment at a lower dose after recovering from the toxicity. However, patients were allowed no more than two dose reductions.
If neurosurgical or other surgical procedures were required for reasons other than tumor progression, the procedure was documented but did not necessitate withdrawal of the patient from study. PTC299 was withheld to allow wound healing to occur, and was resumed, subsequently.
Pretreatment Evaluations and Evaluations During Therapy
Pretreatment evaluations included a history, physical examination including a thorough neurological examination, performance status, disease evaluation, complete blood count (CBC), PT, PTT, electrolytes, renal and liver function tests, urinalysis, urine protein/creatinine ratio, pregnancy test for female patients of childbearing age. CBCs were obtained every 2 weeks during course 1, monthly during subsequent courses. History, physical examinations, and serum chemistries, PT, PTT and were obtained every 2 weeks in course 1 and prior to each subsequent course. Patients were clinically re-evaluated on day 14 and day 28 during the first course and monthly, thereafter, until the end of treatment.
Response Criteria and Imaging Assessment
Disease evaluations were obtained at baseline, after course 2 and every other course, thereafter. Tumor response was defined as follows: complete response (CR), disappearance of all measurable lesions on magnetic resonance imaging (MRI); partial response (PR), ≥50% reduction in tumor size by bidimensional measurement on a stable or decreasing dose of corticosteroids, accompanied by a stable or improving neurological exam; progressive disease (PD), worsening neurologic status or >25% increase in the bidimensional measurement, or appearance of new lesions, or increasing corticosteroids doses; and stable disease (SD). For CR or PR, response had to be maintained for at least 8 weeks.
For evaluation of response or progression, gadolinium-enhanced MRIs of the brain with echo planar diffusion were to be obtained within two weeks prior to the start of treatment and every 8 weeks through 12 courses; then, every 12 weeks, thereafter. Using the Vitrea®workstation (Vital Images, Minnetonka, MN) and a perimeter technique, volumetric tumor analyses was performed on axial FLAIR sequences, and post-gadolinium axial T1 images with user-assisted semi-automated software. Diffusion image and region of interest (ROI) analyses were performed using ImageJ (U. S. National Institutes of Health, Bethesda, Maryland, USA). A 2-D ROI encompassing the whole solid portion of the tumor on a representative slice was chosen. All images were electronically transferred to the PBTC Neuroimaging Center for evaluation.
Pharmacokinetic Studies
Pharmacokinetic studies were mandatory. Blood samples for pharmacokinetic studies were collected from patients in heparinized tubes before the morning PTC299 dose, and at 1, 2, 3, 4, 5, 6, and 8 hours after the dose on days 1 and 28 of Course 1. The PTC299 plasma concentrations were determined by use of a validated liquid chromatography tandem mass spectrometry method with a lower limit of quantitation of 5ng/mL [13]. For each patient, the maximum concentration (Cmax) and the time to maximum (tmax) concentration were the observed values. The area under the plasma concentration versus time curve from 0 to tlast (AUC0–Tlast) was estimated using the linear trapezoidal method.
Results
Twenty-eight (28) patients (all eligible) were entered on study between October 2010 and January 2012 (see Table 2). Of the 28 patients, 21 were fully evaluable. Reasons for inevaluability included sudden neurologic deterioration that precluded patient to start therapy (1); incomplete course of treatment during DLT monitoring period due to non-compliance (1), recommendation by the sponsoring company/FDA to take all patients off treatment (1); clinical and/or radiographic tumor progression during the first course of treatment (2); and receiving less than full dose of drug due to dosing errors (2). Thus, in total, 27 patients received drug (see table 1). Although the study was open for all types of recurrent central nervous system tumors, primarily children with gliomas, and ependymomas were entered.
Table 2.
Characteristics of Patients Who Received Treatment (N=27)
| AGE (Years) | at Diagnosis | at Study Entry |
|---|---|---|
| Median | 10.3 | 11.2 |
| Minimum | 2.3 | 5.5 |
| Maximum | 20.2 | 21.1 |
| Number | Percentage | |
| SEX | ||
| Males | 14 | 51.9 |
| Females | 13 | 48.1 |
| ETHNICITY | ||
| Hispanic or Latino | 2 | 7.4 |
| Non-Hispanic | 22 | 81.5 |
| Unknown | 3 | 11.1 |
| RACE | ||
| Black | 2 | 7.4 |
| Unknown | 1 | 3.7 |
| White, Non-Hispanic | 24 | 88.9 |
| DIAGNOSIS | ||
| Astroblastoma | 1 | 3.7 |
| Astrocytoma, Anaplastic | 3 | 11.1 |
| Astrocytoma, Nos | 4 | 14.8 |
| Brain Stem Glioma | 4 | 14.8 |
| Ependymoma, Anaplastic | 4 | 14.8 |
| Ependymoma, Nos | 1 | 3.7 |
| Giant Cell Glioblastoma | 1 | 3.7 |
| Glioblastoma Multiforme | 3 | 11.1 |
| Glioma, Malignant | 1 | 3.7 |
| Gliomatosis Cerebri | 1 | 3.7 |
| Juvenile Astrocytoma | 1 | 3.7 |
| Medulloblastoma, Nos | 1 | 3.7 |
| Pilocytic Astrocytoma | 1 | 3.7 |
| Piloid Astrocytoma | 1 | 3.7 |
Toxicities
Therapy was well tolerated, with only one dose-limiting toxicity during the first course of treatment (see table 1). This occurred in a child who developed hydrocephalus and hyponatremia two weeks into treatment. Although it is conceivable that the hyponatremia was, in part, related to the hydrocephalus, the hyponatremia was considered possibly related to drug. Transient hypokalemia was also noted in the same child.
No child was withdrawn from therapy for toxicity.
Dose Escalation
The dose escalations were performed as planned and 6 evaluable patients were treated at each of the first 3 dose levels. The top level of therapy proposed for the study was at dose level 4 (2mg/kg/d 3 times daily). Seven (7) patients were entered at this dose, as one patient withdrew prior to starting therapy due to a deteriorating neurologic condition. Two of the seven patients were inevaluable due to dosing errors. One additional patient was taken off treatment during the dose finding period because of the decision made by the sponsoring company and the Food and Drug Administration (FDA), that due to toxicities seen in the concurrent adult phase 1b trial, the trial should be halted. These three children were not replaced and dose level 4 was not fully completed. The three evaluable patients treated at this dose were free of DLTs.
Pharmacokinetics
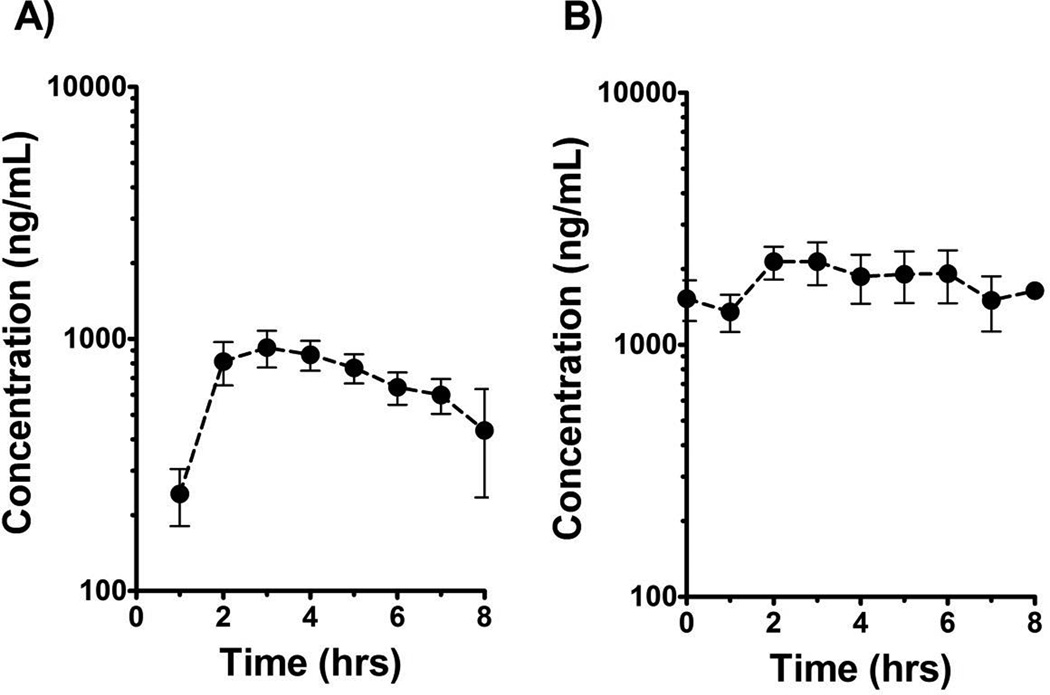
Plasma samples for PTC299 pharmacokinetic studies were collected from all 27 patients on day 1 and from 21 patients on day 28. Concentration-time data from the 1.2 mg/kg dosage cohorts in Figure 1 illustrate the general PTC299 exposure profile for both single dose and steady-state pharmacokinetic studies. In Figure 1A, the average ± SEM PTC299 concentration is plotted versus time for Course 1 Day 1 combined for both the BID and TID groups (since the plot depicts only the concentration-time data for the first dose out to eight hours, for pharmacokinetic purposes, the 1.2 mg/kg BID and TID are the same for Course 1 Day 1). Figure 1B, however, shows the average ± SEM steady-state plasma concentration-time profile for patients treated only on the 1.2 mg/kg TID dosage level. In summary, the Day 1 median (range) Cmax was 1,070 ng/mL (490 to 2,320 ng/mL) for 1.2 mg/kg dosage, 1,715 ng/mL (653 to 2,880 ng/mL) for 1.5 mg/kg dosage, and 2,185 ng/mL (1,150 to 4,300 ng/mL) for 2.0 mg/kg dosage. The overall tmax estimates varied from 2 to 7 hrs with a median value of 3 hrs, independent of both dosage and study day. The median AUC0–Tlast values were proportional to dosage (Kruskall-Wallis; p < 0.05). Similar to the Day 1 values, Day 28 steady-state AUC0–T last values were also proportional to cumulative dosage (Figure 2). The median accumulation factor was 2.2 for 1.2 mg/kg BID, 2.9 for 1.2 mg/kg TID, 2.1 for 1.5 mg/kg TID, and 2.4 for the 2.0 mg/kg TID dosing regimen. Due to inadequate terminal elimination phase sampling, the apparent oral clearance, AUC0-∞, and terminal half-life could not be estimated with these data.
Figure 1.
A) Course 1 Day 1 plasma concentration-time profile for PTC299 at 1.2 mg/kg dosage level. B) Course 1 Day 28 steady-state plasma concentration-time profile for PTC299 for patients on 1.2 mg/kg TID regimen illustrates steady-state accumulation with multiple dosing. Filled circles denote average values with error bars representing ± SEM.
Figure 2.
A) Course 1 Day 1 AUC0–Tlast for PTC299 at three dosage levels studied. B) Course 1 Day 28 steady-state AUC0–Tlast stratified by cumulative dosage. The horizontal bar represents the median value and error bars represent the 90% confidence interval.
Disease Responses
No complete or partial responses were observed. Two (2) patients with low-grade gliomas were noted to have objective shrinkage of tumor (greater than 25%), but less than 50%. At initiation of study, a criterion of minor response was not included, and these patients are considered to have stable disease. The median (range) number of courses on treatment was 2 (1–18). At time of closure of study in February, 2012, three (3) children with low-grade gliomas who had been on study for 8, 13 and 18 courses were withdrawn from treatment. Two additional patients with low grade glioma were on treatment for 8 and 16 courses before progression. One patient with brain stem glioma stayed on treatment for 7 courses before experiencing clinical progression.
While no such association was detected at baseline, we observed a significant negative correlation between tumor enhancement and diffusion at the end of course 2 consistent with increased cellularity in enhancing tumor (Spearman’s correlation=−0.63, p-value=0.0052). No direct relationship was seen between drug dose and change in diffusion.
Discussion
Although PTC299 was well tolerated in this pediatric study, the trial was prematurely concluded at the planned highest dose level due to toxicity seen in the adult studies. Two patients treated on the adult phase I studies with solid tumors developed hepatotoxicity, one requiring liver transplantation and the other resulting in death due to multi-organ dysfunction, including liver failure. Both had been heavily pretreated with multiple chemotherapeutic agents, but had no clear evidence of hepatotoxicity prior to initiation of treatment with PTC299. It was the recommendation of the FDA with agreement by the sponsoring drug company (PTC) to cease the adult trials and also to close the pediatric trial without replacing the last three patients required by the trial design to complete accrual to the fourth dose level.
The reasons for the different toxicity profiles seen in pediatrics compared to adults are unclear, although it could be a function of the number of patients treated, as nearly 300 adult patients were treated with drug on a series of phase I trials compared to 27 treated on this trial. At dose level 4, the PTC299 AUC initially planned for the trial was reached and no dose limiting toxicity was seen. PTC299 plasma concentrations, similar to those seen in the adult phase II study, were also observed.
The pharmacokinetics of PTC299 demonstrated a dose proportional increase in exposure. Steady-state drug exposures suggest accumulation with multiple dosing over time, raising the possibility that adverse events may manifest after multiple dosing due to elevated systemic levels. Furthermore, median AUC0–Tlast values at the 2 mg/kg dosage (7,659 ng/mL × hr) were similar to exposures achieved in the adult trials [13].
As noted, this was a phase I study and no specific statements can be made concerning efficacy. No objective responses, as defined as a 50% reduction in tumor volume, were noted. However, two children with low-grade gliomas did have objective decrease in the size of the tumor. Overall, including the 2 patients with minor responses, five patients with low-grade gliomas had prolonged periods of stability on drug. Interpretation of stability in children with low-grade gliomas is problematic given the somewhat erratic growth course of these tumors and their potential slow rate of growth. Given the evidence now in two clinical trials an anti-angiogenesis drug (bevacizumab), was effective in halting growth and in some cases decreasing tumor size of low-grade gliomas with associated functional improvement, the results seen in this trial in children with low-grade gliomas is of interest [7, 15]. PTC299 is oral and easy to deliver and has a unique mechanism of action. Pre-clinical data had demonstrated potential synergy between PTC299 and bevacizumab and between PTC299 and mTOR-inhibitors. The toxicity profile in pediatrics raised the possibility of utilizing the drug in combination with other chemotherapies or molecular targeted therapies until the adult toxicity issues became apparent. Although further studies are not planned with this drug, other oral drugs with a similar mechanism of action and pediatric toxicity profiles could be candidates for combination therapy in the future.
Table 3.
Type and Grade of Toxicities among Patients (N=27, Number of Patient Courses=109)
Cells Contain the Number of Events (Number of Patients)
| ADVERSE EVENTS (AE Version=CTCAEVer4) |
GRADE | TOTAL | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| TOX | TOX | TOX | TOX | |
| Fatigue | 11(9) | 3(2) | 1(1) | 15(9) |
| Nausea | 8(7) | 1(1) | 9(8) | |
| Lymphocyte count decreased (Lymphocytes (%)) | 5(5) | 1(1) | 1(1) | 7(6) |
| Anemia (HGB (g/dL)) | 6(6) | 1(1) | 7(7) | |
| Anorexia | 3(2) | 2(2) | 1(1) | 6(3) |
| Proteinuria | 4(3) | 2(2) | 6(3) | |
| Hyponatremia (Sodium (mmol/L)) | 3(2) | 2(1) | 5(2) | |
| Personality change | 3(1) | 2(1) | 5(1) | |
| Dehydration | 1(1) | 1(1) | ||
| Weight loss | 1(1) | 1(1) | 2(2) | |
| Movements involuntary | 1(1) | 1(1) | ||
Acknowledgements
Supported in part by National Institutes of Health (NIH) Grant No. U01 CA81457 for the Pediatric Brain Tumor Consortium, and the American Lebanese Syrian Associated Charities.
Footnotes
Conflict of Interest Statement: Dr. Packer and the co-authors have no direct conflicts of interest.
References
- 1.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6(4):243–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 2.Pietsch T, et al. Expression and distribution of vascular endothelial growth factor protein in human brain tumors. Acta Neuropathol (Berl) 1997;93(2):109–117. doi: 10.1007/s004010050591. [DOI] [PubMed] [Google Scholar]
- 3.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1259–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 4.Gururamgam S, Chi SN, Young Poussaint T, et al. Lack of efficacy of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma: a Pediatric Brain Tumor Consortium study. J Clin Onocol. 2010;28(18):3069–3075. doi: 10.1200/JCO.2009.26.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziche M, Donnini S, Morbidelli L. Development of new drugs in angiogenesis. Current Drug Targets. 2004;5:485–493. doi: 10.2174/1389450043345371. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MH, Shen YL, Leegan P, Pazdur R. FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist. 2009;14(11):1131–1138. doi: 10.1634/theoncologist.2009-0121. [DOI] [PubMed] [Google Scholar]
- 7.Packer RJ, Jakacki R, Horn M, et al. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatr Blood Cancer. 2009;52(7):791–795. doi: 10.1002/pbc.21935. [DOI] [PubMed] [Google Scholar]
- 8.Machein MR, Plate KH. VEGF in brain tumors. J Neurooncol. 2000;50(1–2):109–120. doi: 10.1023/a:1006416003964. [DOI] [PubMed] [Google Scholar]
- 9.Levy AP, Levy NS, Goldberg MA. Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem. 1996 Feb 2;271(5):2746–2753. doi: 10.1074/jbc.271.5.2746. [DOI] [PubMed] [Google Scholar]
- 10.Levy AP. Hypoxic Regulation of VEGF mRNA Stability by RNA-binding Proteins. Trens Cardiovasc Med. 1998 Aug;8(6):246–250. doi: 10.1016/s1050-1738(98)00020-6. [DOI] [PubMed] [Google Scholar]
- 11.Akri G, Nahari D, Finkelstein Y, Le SY, Elroy-Stein, Levi BZ. Regulation of vascular endothelial growth factor (VEGF) expression is mediated by internal initiation of translation and alternative initiation of transcription. Oncogene. 1998 Jul 16;17(2):227–236. doi: 10.1038/sj.onc.1202019. [DOI] [PubMed] [Google Scholar]
- 12.Huez I, Creancier L, Audiger S, Gensac MC, Prats AC, Prats H. Two independent internal ribosome entry sites are involved in translation initiation of vascular endothelial growth factor mRNA. Mol Cell Biol. 1998 Nov;18(11):6178–6190. doi: 10.1128/mcb.18.11.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.PTC 299 Investiagor Brochure. PTC Therapeutics, Inc.; Ver 6, 12, 2 July 2011. [Google Scholar]
- 14.Packer RJ, Ater J, Allen J, et al. Carboplatin and vincristine chemotherapy for children with newly diagnosed progressive low-grade gliomas. J Neurosurg. 1997;86(5):747–754. doi: 10.3171/jns.1997.86.5.0747. [DOI] [PubMed] [Google Scholar]
- 15.Gururangan S, Fangusaro J, Young-Poussaint T, Lesh S, Onar A, Gilbertson R, Packer R, McLendon R, Friedman HS, Boyett H, Kun LE. Efficacy of bevacizumab +CPT-11 in children with recurrent low-grade glioma (LGG)- a pediatric brain tumor consortium study. Neuro-Oncology. 2011;13:iii95–iii101. [Google Scholar]