Abstract
Dextromethorphan (DM) is a low-affinity, non-competitive NMDA receptor antagonist that has shown promise in pre-clinical and preliminary clinical studies for the reduction of opioid withdrawal symptoms, but when used at higher doses, it is associated with deleterious side effects attributed to its metabolite, dextrorphan. A clinical trial was therefore conducted to test the withdrawal-suppressant effect of a combination of dextromethorphan with quinidine (DM/Q). Quinidine inhibits the metabolism of dextromethorphan, reducing dextrorphan levels. Opioid-dependent patients were admitted to an inpatient unit, stabilized for three days on morphine (25 mg, sc, every six hours), and randomly assigned on day 2 to DM/Q (30 mg/30 mg, twice a day) (n = 22) or matching placebo (n = 9) prior to the discontinuation of morphine on day 4. Withdrawal symptoms, measured with the Modified Himmelsbach Opioid Withdrawal Scale (MHOWS), increased significantly on days 4 and 5 (Z = 3.70, p = .0002), and by day 6, 90% of the sample (28/31) had dropped out of the study. There were no differences between treatment groups on either outcome measure. The combination of dextromethorphan and quinidine appears ineffective as a primary treatment for opioid withdrawal. Future studies should examine dextromethorphan as an adjunct to other anti-withdrawal medications and focus more on the relationship between dextrorphan levels and withdrawal suppression.
INTRODUCTION
Current treatment modalities for opioid withdrawal include opioid medications, such as methadone and buprenorphine; noradrenergic medications, such as clonidine; and benzodiazepines, such as lorazepam and diazepam. Although these medications are effective in treating opioid withdrawal symptoms, they all have certain limitations, including abuse liability, adverse side effects, incomplete relief of withdrawal symptoms, and high rates of relapse.1 This suggests that treatment development efforts should target other neurotransmitter systems that also have been implicated in opioid withdrawal, such as glutamatergic systems. Preclinical and human laboratory data suggest that N-methyl-D-Aspartate receptor (NMDA receptor) antagonists attenuate physical and motivational aspects of opioid withdrawal.2,3 Dextromethorphan (DM) is a low-affinity, non-competitive NMDAR antagonist.4 DM is devoid of opioid effects and favorable side effects profile. DM has been available as an over-the-counter anti-tussive agent for more than forty years5 and has shown some promise at higher doses (>200 mg/day) for the treatment of opioid withdrawal.6 However, DM is rapidly metabolized to dextrorphan in extensive metabolizers, which comprise approximately 90% of the population,7 resulting in low plasma DM levels. A similar impact of extensive metabolism is also seen in methadone patients on antiviral therapy.8 The administration of higher doses of DM (above 500 mg/d) can produce unacceptable levels of adverse effects,9 which can be attributed to the high levels of dextrorphan, a potent NMDA receptor antagonist10 capable of producing severe psychotomimetic effects.11 In order to circumvent this limitation, DM can be combined with quinidine (Q), which inhibits the metabolism of DM at CYP2D6, resulting in higher and sustained plasma concentrations of DM12 without serious side effects. The DM/Q combination has been previously assessed in treatment of neurological disorders.13–15 Here we report the first clinical trial of dextromethorphan/quinidine (DM/Q) for treatment of opioid withdrawal.
METHOD
Participants
Opioid-dependent participants were recruited to the university-based research service through advertisements in local newspapers and postings at the medical center. The internist and psychiatrist (not investigators) at the substance treatment and research service determined medical and psychiatric eligibility. To be included, participants were required to be between the ages of 18 and 60 years, be assessed medically and psychiatrically stable by clinical staff, meet DSM-IV criteria for opioid dependence, and be using opioids for at least 21 of the past 30 days with positive urine toxicology. Participants were excluded from the study for any of the following conditions:
they were pregnant, nursing, or not practicing an effective method of birth control;
they produced urine samples positive for methadone or buprenorphine or had any self-reported use of methadone, buprenorphine, or LAAM in the prior 14 days;
they had used medications containing dextromethorphan within the prior seven days;
they currently met DSM-IV dependence criteria on any psychoactive substance requiring detoxification other than heroin, morphine, hydromorphone, or cocaine;
they were deemed to be clinically inappropriate for opioid detoxification, such as those with a past history of overdose following detoxification;
they had received an investigational drug study within the past three months; or
they were hypersensitive to any of the medications used in the study.
The Institutional Review Board of the New York State Psychiatric Institute approved the study, and all participants gave written informed consent.
PROCEDURES
Participants were admitted to an inpatient research unit for an eight-day trial. During the first three days, participants were stabilized on morphine (25 mg sc every six hours), with the last dose of morphine administered at 8:00 am on day 4. Participants were randomly assigned in a 2-to-1 ratio to receive dextromethorphan/quinidine (30 mg/30 mg) or matching placebo in a single capsule twice daily, beginning on day 2 (see Figure 1). An independent research pharmacist generated the randomization sequence for each participant. All other research and clinical staff and patients were blind to medication assignment. Adjunct over-the-counter medications, such as acetaminophen and antacids, were available on an as-needed basis for withdrawal discomfort, but no prescription medications for withdrawal, such as opioids, clonidine, or benzodiazepines, were available. Participants who had substantial withdrawal symptoms and made a request to withdraw were removed from the study, and were offered additional medications to complete detoxification.
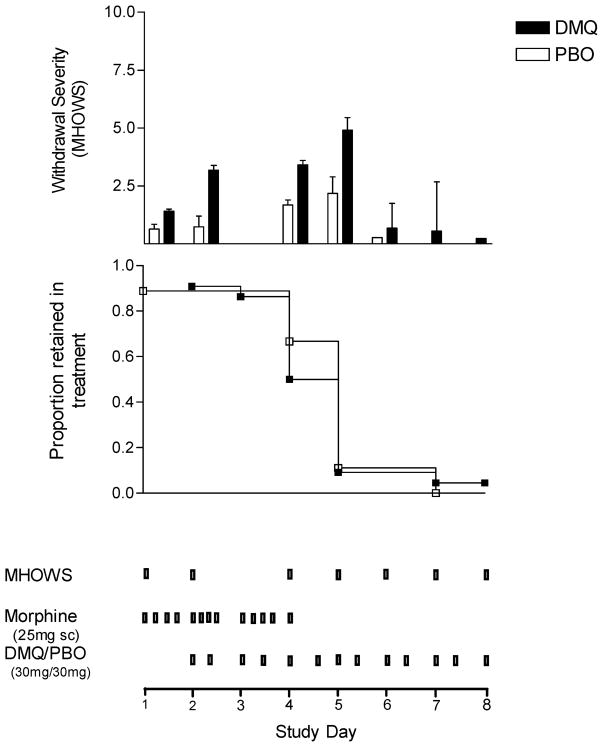
FIGURE 1.
Opiate withdrawal severity and survival. Withdrawal severity (MHOWS, upper panel) and proportion subjects retained (middle panel) in an inpatient, placebo-controlled trial of dextromethorphan/quinidine for the treatment of opioid withdrawal. Lower panel displays design and schedule of morphine dosing, DM/Q dosing, and withdrawal (MHOWS) assessment.
Team members involved in ratings were trained by the principal investigator (EA). Training included repeated ratings of patients in different stages of withdrawal until an acceptable level of inter-rater reliability was achieved. The primary outcome measures were days retained in the study and scores on the Modified Himmelsbach Opioid Withdrawal Scale (MHOWS),16 measured at baseline (day 1), on the first day of DM/Q administration (day 2), and during the withdrawal period (days 4 to 8). We also measured vital signs and participant-rated severity of opioid withdrawal using a Clinical Global Impression Scale (CGI-P).
Data Analysis
Baseline demographic variables were compared between treatment groups using chi-square for categorical variables and t-tests for continuous variables. Retention rates were compared using Kaplan-Meier survival curves and the log-rank test. Daily measures of withdrawal and vital signs were analyzed using linear models with generalized estimating equations (GEE) for parameter estimation, with outcome modeled as a function of time, treatment assignment, time × treatment interaction, and the baseline level of the outcome variable. If the interaction term was not significant, a model without the interaction term was fit. All analyses were performed on the intent-to-treat sample, and all tests were two-sided with alpha=0.05.
RESULTS
Sample Description
Thirty-one patients were randomized, 22 to dextromethorphan/quinidine (DM/Q) and 9 to placebo. Their baseline demographic and clinical features are summarized in Table 1. As can be seen in the table, the patients were predominantly male (68%) and single (58%) with a broad age range. The groups differed in current employment status, with greater unemployment in the DM/Q arm.
TABLE 1.
Demographic and baseline clinical characteristics
| DM/Q (n = 22) | Placebo (n = 9) | χ2 or t | p | DF | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (years) | 40 (9) | 41 (10) | −0.24 | 0.81 | 29 |
| Male | 15 (68%) | 6 (67%) | 0.007 | 0.94 | 1 |
| Race | |||||
| Hispanic | 8 (36%) | 3 (33%) | 4.73 | 0.19 | 4 |
| Black | 6 (27%) | 0 (0%) | |||
| Caucasian | 7 (32%) | 6 (67(%) | |||
| Other | 1 (5%) | 0 (0%) | |||
| Education (high school or higher) | 18 (82%) | 7 (78%) | .07 | .80 | 1 |
| Single | 13 (59%) | 5 (56%) | .03 | .86 | 1 |
| Currently employed | 5 (23%) | 6 (67%) | 5.39 | .02 | 1 |
| Average number of bags of heroin/day | 6.3 (3.0) | 5.8 (4.0) | .41 | .68 | 29 |
Values in the table are N (%) for categorical variables, or mean (SD) for continuous variables.
Retention in Treatment
Figure 1 displays retention in treatment over the 8 days of the study in the upper panel, and opioid withdrawal severity (MHOWS) in the lower panel. Almost the entire sample in both treatment groups (28/31, 90%) dropped out between days 4 and 6, day 4 being the first day of morphine discontinuation, with no difference between DM/Q and placebo (log rank = .16, df = 1, p = .69). Only one subject (randomized to DM/Q) completed all eight days of the study.
Opiate Withdrawal
As can be seen in Figure 1 (upper panel), withdrawal severity (MHOWS) increases on days 4 and 5, day 4 again being the first day when the morphine was discontinued. Low withdrawal scores on days 6 through 8 are not representative, as they reflect only the one or two patients remaining in the study on those days, and days 6 through 8 were not included in the linear models. Withdrawal (MHOWS) scores appear numerically greater for the DM/Q group on days 2 (first day of DM/Q administration) and 4 and 5 (first two withdrawal days), but the linear model, while showing a significant effect of time (Z = 3.70, p = .0002), showed no main effect of treatment (Z = .46, p = .65), baseline (day 1) MHOWS score (Z = .40, p = .69), or treatment × time interaction (Z = −.44, p = 0.66). Similarly, the model for patient rated withdrawal severity (CGI-P) showed increased scores over time (Z = 5.43, p < .0001) but no effect of treatment (Z = .33, p = .74) or treatment × time interaction (Z = −.72, p = .47). Increases over time were also observed in heart rate (Z = 3.15, p = .002) and diastolic blood pressure (Z = 3.63, p = .001). Diastolic blood pressure was greater on DM/Q (81.41 mm Hg, SD = 6.45) compared to placebo (76.05 mm Hg; SD = 4.71; Z = 2.60, p = .01), although such a difference would not be clinically meaningful.
Other Measures
We also explored the effect of treatment on opiate craving. Mean craving severity ranged between 20 and 50 (VAS) and decreased over time for both arms (Z = −2.16, p = .03), but there was no significant main effect of treatment (Z = −1.33, p = .21) or treatment × time interaction (Z = −.17, p = .86). Vital measures such as heart rate and diastolic and systolic blood pressure were also examined. Standing heart rate for the DM/Q arm averaged around 80 bpm (SD 12) and 82 bpm (SD 13) for the PBO arm and increased over time for both arms (Z= 3.15, p = .002) but with no significant treatment effect (Z = .01, p = .99) or treatment × time interaction (Z = .32, p = .75). Systolic blood pressure averaged 124.17 mm Hg (SD 11.40) in the DM/Q arm and 119.43 mm Hg (SD 10.25) in the placebo arm. After adjusting for baseline day 1 SBP, there was no significant main effect of treatment (Z = .99, p = .34) or time (Z = 1.61, p = .11). The groups differed significantly with respect to diastolic blood pressure, with the DM/Q arm averaging around 81.41 mm Hg (SD 6.45) over the course of the study, compared to 76.05 mm Hg (SD 4.71) in the placebo group. There was a significant main effect of treatment (Z= 2.60, p = .01) and time (Z = 3.63, p = .001) after adjusting for baseline day 1 DBP (Z = −.97, p = .33) but no significant treatment × time interaction (Z = −.08, p = .94). Side effects were consistent with opioid withdrawal and did not differ between groups.
DISCUSSION
This small, randomized, placebo-controlled trial suggests that the combination of dextromethorphan with quinidine—at the doses tested—is not effective in facilitating detoxification from opioids. There was no significant difference between the DM/Q and placebo groups in the severity of withdrawal, and dropout was high. This contrasts with several clinical reports,6,17,18 and preclinical studies that showed suppression of opioid withdrawal by dextromethorphan,19–22 although another study in human volunteers failed to show effectiveness of dextromethorphan in blocking naloxone-precipitated opioid withdrawal.23
The present study employed rigorous methodology, including stabilization of patients on a standard dose of morphine prior to discontinuation and absence of ancillary medications with effectiveness against opioid withdrawal. The small sample size was designed to afford sufficient power to detect a large effect size and estimate the effect size for future studies. It was felt that a relatively large effect would be needed for a medication to be clinically useful, given the typical severity of opioid withdrawal. Several of the studies that suggested effectiveness allowed ancillary medications, such as chlorpromazine and benzodiazepines,17,18 suggesting that dextromethorphan might exert a more subtle effect or be more useful as an adjunct rather than an isolated treatment.
The combination of dextromethorphan and quinidine employed here (30 mg plus 30 mg, twice a day) should have resulted in a twenty-fold increase in peak serum DM concentrations,12 matching or exceeding serum levels achieved with higher doses of DM tested in prior studies6,9,24 while producing a six-fold decrease in dextrorphan levels. Thus, it is also possible that dextrorphan, which has a much higher (100-fold) affinity for the NMDA channel site,10 may be necessary to produce a withdrawal suppressing effect.
In summary, we have shown that a combination of dextromethorphan with quinidine does not appear useful as a primary treatment for opioid withdrawal. Future studies should examine dextromethorphan as an adjunct to other anti-withdrawal medications and focus more on the relationship between dextrorphan levels and withdrawal suppression.
References
- 1.Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Expert Opin Pharmacother. 2004;5:713–725. doi: 10.1517/14656566.5.4.713. [DOI] [PubMed] [Google Scholar]
- 2.Bisaga A, Popik P. In search of a new pharmacological treatment for drug and alcohol addiction: N-methyl-D-aspartate (NMDA) antagonists. Drug Alcohol Depend. 2000;59:1–15. doi: 10.1016/s0376-8716(99)00107-6. [DOI] [PubMed] [Google Scholar]
- 3.Bisaga A, Comer SD, Ward AS, Popik P, Kleber HD, Fischman MW. The NMDA antagonist memantine attenuates the expression of opioid physical dependence in humans. Psychopharmacology (Berl) 2001;157:1–10. doi: 10.1007/s002130100739. [DOI] [PubMed] [Google Scholar]
- 4.Netzer R, Pflimlin P, Trube G. Dextromethorphan blocks N-methyl-D-aspartate-induced currents and voltage-operated inward currents in cultured cortical neurons. Eur J Pharmacol. 1993;238:209–216. doi: 10.1016/0014-2999(93)90849-d. [DOI] [PubMed] [Google Scholar]
- 5.Bem JL, Peck R. Dextromethorphan. An overview of safety issues. Drug Saf. 1992;7:190–199. doi: 10.2165/00002018-199207030-00004. [DOI] [PubMed] [Google Scholar]
- 6.Bisaga A, Gianelli P, Popik P. Opiate withdrawal with dextromethorphan. Am J Psychiatry. 1997;154:584. doi: 10.1176/ajp.154.4.584a. [DOI] [PubMed] [Google Scholar]
- 7.Bertilsson L, Dahl ML, Dalen P, Al-Shurbaji A. Molecular genetics of CYP2D6: clinical relevance with focus on psychotropic drugs. Br J Clin Pharmacol. 2002;53:111–122. doi: 10.1046/j.0306-5251.2001.01548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akerele EO, Levin F, Nunes E, Brady R, Kleber H. Effects of HIV triple therapy on methadone levels. Am J Addict. 2002;11:308–314. doi: 10.1080/1055049029008810. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg GK, Bell TE, Yenari MA. Dose escalation safety and tolerance study of the N-methyl-D-aspartate antagonist dextromethorphan in neurosurgery patients. J Neurosurg. 1996;84:860–866. doi: 10.3171/jns.1996.84.5.0860. [DOI] [PubMed] [Google Scholar]
- 10.Wiley JL, Harvey SA, Balster RL, Nicholson KL. Affinity and specificity of N-methyl-D-aspartate channel blockers affect their ability to disrupt prepulse inhibition of acoustic startle in rats. Psychopharmacology (Berl) 2003;165:378–385. doi: 10.1007/s00213-002-1297-6. [DOI] [PubMed] [Google Scholar]
- 11.Albers GW, Atkinson RP, Kelley RE, Rosenbaum DM. Safety, tolerability, and pharmacokinetics of the N-methyl-D-aspartate antagonist dextrorphan in patients with acute stroke. Detrophan Study Group. Stroke. 1995;26:254–258. doi: 10.1161/01.str.26.2.254. [DOI] [PubMed] [Google Scholar]
- 12.Pope LE, Khalil MH, Berg JE, Stiles M, Yakatan GJ, Sellers EM. Pharmacokinetics of dextromethorphan after single or multiple dosing in combination with quinidine in extensive and poor metabolizers. J Clin Pharmacol. 2004;44:1132–1142. doi: 10.1177/0091270004269521. [DOI] [PubMed] [Google Scholar]
- 13.Brooks BR, Thisted RA, Appel SH, et al. Treatment of pseudobulbar affect in ALS with dextromethorphan/quinidine: a randomized trial. Neurology. 2004;63:1364–1370. doi: 10.1212/01.wnl.0000142042.50528.2f. [DOI] [PubMed] [Google Scholar]
- 14.Panitch HS, Thisted RA, Smith RA, et al. Randomized, controlled trial of dextromethorphan/quinidine for pseudobulbar affect in multiple sclerosis. Ann Neurol. 2006;59:780–787. doi: 10.1002/ana.20828. [DOI] [PubMed] [Google Scholar]
- 15.Thisted RA, Klaff L, Schwartz SL, et al. Dextromethorphan and quinidine in adult patients with uncontrolled painful diabetic peripheral neuropathy: a 29-day, multicenter, open-label, dose-escalation study. Clin Ther. 2006;28:1607–1618. doi: 10.1016/j.clinthera.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Himmelsbach C. The morphine abstinence syndrome: its nature and treatment. Ann Intern Med. 1941;15:829–839. [Google Scholar]
- 17.Koyuncuoglu H. The combination of tizanidine markedly improves the treatment with dextromethorphan of heroin addicted outpatients. Int J Clin Pharmacol Ther. 1995;33:13–19. [PubMed] [Google Scholar]
- 18.Koyuncuoglu H, Saydam B. The treatment of heroin addicts with dextromethorphan: a double-blind comparison of dextromethorphan with chlorpromazine. Int J Clin Pharmacol Ther. 1990;28:147–152. [PubMed] [Google Scholar]
- 19.Farzin D. Modification of naloxone-induced withdrawal signs by dextromethorphan in morphine-dependent mice. Eur J Pharmacol. 1999;377:35–42. doi: 10.1016/s0014-2999(99)00396-9. [DOI] [PubMed] [Google Scholar]
- 20.Koyuncuoglu H, Gungor M, Sagduyu H, Aricioglu F. Suppression by ketamine and dextromethorphan of precipitated abstinence syndrome in rats. Pharmacol Biochem Behav. 1990;35:829–832. doi: 10.1016/0091-3057(90)90366-p. [DOI] [PubMed] [Google Scholar]
- 21.Manning BH, Mao J, Frenk H, Price DD, Mayer DJ. Continuous co-administration of dextromethorphan or MK-801 with morphine: attenuation of morphine dependence and naloxone-reversible attenuation of morphine tolerance. Pain. 1996;67:79–88. doi: 10.1016/0304-3959(96)81972-5. [DOI] [PubMed] [Google Scholar]
- 22.Zhu H, Jenab S, Jones KL, Inturrisi CE. The clinically available NMDA receptor antagonist dextromethorphan attenuates acute morphine withdrawal in the neonatal rat. Brain Res Rev. 2003;142:209–213. doi: 10.1016/s0165-3806(03)00059-2. [DOI] [PubMed] [Google Scholar]
- 23.Rosen MI, McMahon TJ, Woods SW, Pearsall HR, Kosten TR. A pilot study of dextromethorphan in naloxone-precipitated opiate withdrawal. Eur J Pharmacol. 1996;307:251–257. doi: 10.1016/0014-2999(96)00249-x. [DOI] [PubMed] [Google Scholar]
- 24.Kazis A, Kimiskidis V, Niopas I. Pharmacokinetics of dextromethorphan and dextrorphan in epileptic patients. Acta Neurol Scand. 1996;93:94–98. doi: 10.1111/j.1600-0404.1996.tb00181.x. [DOI] [PubMed] [Google Scholar]