Abstract
Falling is a serious hazard for older veterans that may lead to severe injury, loss of independence, and death. While the American Geriatric Society (AGS) provides guidelines to screen individuals at-risk for falls, the guidelines may be less successful with specific subgroups of patients. In a veteran sample, we examined whether the Timed Up and Go (TUG) test, including a modified version, the TUG-cognition, effectively detected potential fallers whose risk was associated with cognitive deficits. Specifically, we sought to determine whether TUG tasks and AGS criteria were differentially associated with executive dysfunction, whether the TUG tasks identified potential fallers outside of those recognized by AGS criteria, and whether these tasks distinguished groups of fallers. Participants included 120 mostly male patients referred to the Memory Assessment Clinic due to cognitive impairment. TUG-cognition scores were strongly associated with executive dysfunction and differed systematically between fallers grouped by number of falls. These findings suggest that the TUG-cognition shows promise in identifying fallers whose risk is related to, or compounded by cognitive impairment. Future research should study the predictive validity of these measures by following patients prospectively.
Keywords: American Geriatric Society falls risk guidelines, cognitive impairment, executive function, falls, falls prevention, identification of falls risk, older adults, physical therapy, Timed Up and Go test, veterans
Introduction
For patients, their families, and the health care system, reducing the rate of falls in older adults is a major priority, especially in light of data revealing that falls are the leading cause of injury deaths among older Americans [1]. Addressing falls risk is particularly challenging because falls are multifactorial in origin. According to American Geriatric Society (AGS) guidelines [2], the most common risk factors for falls include muscle weakness, arthritis, history of falls, limited gait, balance or vision problems, use of assistive device, limited activities of daily living, depression, cognitive impairment, and age above 80 years. While these risk factors often occur together, they are unlikely to confer equal risk in all individuals; rather, there may be subgroups of fallers for whom specific risk factors are more salient. That is, even among individuals with multiple falls risks, the strongest risk factor may be hypotension for some; for others, it may be diabetic neuropathy, while for still others, cognitive impairment may represent the most prominent risk factor.
Current guidelines established by the AGS in 2010 [2] suggest that elderly patients should be assessed for falls risk on a yearly basis. According to the recommended screening procedures utilized at the time the study was conducted (based on the 2001 AGS screening criteria), patients were identified as at-risk if they reported a history of two or more falls in the previous year, an acute fall (a fall for which medical attention was sought), or demonstration of gait or balance problems. It was recommended that a single self-reported fall be followed by evaluating the individual’s gait and balance. The 2010 guidelines were modified to probe more specifically into the circumstances of single falls, and to refer for falls evaluation after a self-report of gait or balance problems. Importantly, both versions rely on patient self-report to identify their own falls. Guidelines for 2001 and 2010 are presented in Appendix 2. While the AGS guidelines provide an important benchmark for determining those at-risk for falls, they may not identify all subsets of at-risk patients equally well. Specifically, the guidelines may have limited efficacy in individuals with marked cognitive impairment and relatively well-preserved motor abilities. Yet patients with executive dysfunction, a specific sub-type of cognitive deficit, exhibit increased risk of gait dysfunction and falls [3–7]. Moreover, many older patients may not accurately recall the number of falls they have sustained [8], especially cognitively compromised individuals. Indeed, people with cognitive impairment may not remember that they have fallen, or they may not disclose their falls due to poor insight and judgment, or due to fears of compromising their independence. For example, in one sample of older patients (cognition was not assessed), only 37.2% of all fallers actively disclosed their falls to health care providers [9]. In addition, the AGS guidelines do not identify individuals at-risk for falls who have not yet fallen and who are unaware of (or do not disclose) gait and balance problems. Therefore, current screening guidelines may under-identify individuals at-risk for falls due to cognitive impairment.
We compared two screening methods to detect falls risk: the AGS screening criteria, and the Timed Up and Go (TUG) task, a brief, simple, and established performance-based screening measure of gait and mobility [10]. Specifically, we sought to investigate the relationship of the two falls screening methods (AGS and TUG) with cognition, as well as to examine their identification of at-risk individuals. We hypothesized that, when compared to AGS criteria, TUG performance-based measures would better identify patients at-risk for falls due to executive dysfunction. We also sought to discriminate among fallers using these performance-based criteria. By investigating associations between performance-based measures, executive dysfunction and falls, we are working toward the long-term goal of developing more effective screening methods to identify cognitively impaired older patients at-risk for falls so they may be targeted for appropriate clinical interventions such as physical therapy.
Methods
The study utilized archival data from a quality improvement/quality assurance (QI/QA) demonstration project the goal of which was to improve clinical ability to detect potential fallers. This QI/QA project was reviewed by the University of Wisconsin’s Health Science Institutional Review Board (UW IRB), a subcommittee of the William S. Middleton Memorial Veteran Hospital’s Research and Development (VA R&D) Committee, and determined that data collection for the project was exempt research per federal regulations. UW IRB and VA R&D approval was granted to retrospectively analyze the results.
The sample comprised 120 consecutive patients from the Geriatric Research Education and Clinical Center (GRECC) Memory Assessment Clinic (MAC) at the William S. Middleton Memorial Veterans Hospital. All participants were new MAC patients, referred due to suspected cognitive impairment as reported by the patient, family member, or primary care provider. Inclusion criteria were age 60 years or older, ability to walk 20 feet without pain or risk of injury, with or without an assistive device, ability to comprehend English, and score on the Mini-Mental State Examination [11] of greater than 15. Exclusion criteria included inability to understand task directions.
Procedures
After check-in procedures were completed, a trained clinic nurse administered the three TUG tasks as follows. First the TUG-alone was performed, in which patients were asked to start from a seated position in an arm chair, rise from the chair, walk 10 feet, turn around and walk back to the chair, turn again, and sit down. Patients were asked to perform the task as quickly but as safely as they could. Two additional versions of the TUG were also administered: TUG-manual (performing the TUG while carrying a cup of water) [12] and TUG-cognition (performing the TUG while counting backwards from 50, a variation on that used by Shumway-Cook et al. [13]. After the TUG tasks were explained and demonstrated, patients were provided one opportunity to practice the task. To ensure safety, in cases where balance was unsteady, clinic staff fit patients with a gait belt, and walked behind patients lightly holding the belt for each TUG administration. Following the practice run, all participants were timed on the TUG-alone. Next, the TUG-manual task and the TUG-cognitive task were administered in randomized order, selected using a random number table. Participants who met criteria for being at-risk for falls on any of the 3 performance-based measures were referred to physical therapy for further evaluation.
Classification of falls risk status for the TUG-alone and the TUG-manual was made utilizing cut scores established by Shumway Cook et al. [13]. Risk status on the TUG-cognitive was determined by utilizing the 75th percentile of the interquartile range [13].
Determination of Falls Status
At their clinic visits, all patients were asked how many times they had fallen in the past year. To address the possibility of unreliable self-reporting of falls, self-reported falls were also compared with falls documented in the medical record. Since patients traditionally received their primary care through the VA medical system, records were available to evaluate falls status. Specifically, an additional record review was undertaken for each patient, in which a clinician (BF) reviewed notes from primary care provider visits over a 12 month period, to ascertain whether any mention of falls had been made during the previous year.
Neuropsychological and Mood Measures
After completing the walking tasks, each participant underwent neuropsychological testing, while the participants’ caregivers were interviewed by social work staff. Neuropsychological tests administered are described briefly here. The Mini-Mental State Exam (MMSE) [11] is a 30 item brief screen of global cognition which includes questions on orientation, recall, attention and working memory, language, and construction. The Montreal Cognitive Assessment (MoCA) [14] is another 30 item screening measure of global cognition assessing executive function, visuospatial construction, language, recall, verbal abstract reasoning, and orientation. Serial 7’s and World Backwards are tasks of working memory in which patients are asked to subtract backward from 100 by seven’s, and spell the word “world” backwards, respectively. The Clock Draw Task [15] assesses global cognition, executive function and visual spatial ability on which individuals are asked to draw a clock with hands set to a specific time; it is scored as either pass or fail [16]. Animal Fluency [17] is a measure of executive function and language skills, the score representing the number of animals named in 60 seconds. Trail Making Tests A and B (TMT A &B) [18] measure numeric and alphanumeric sequencing, visuospatial attention and divided attention, and speeded visual processing. On these tasks, individuals are asked to connect numbers in chronological order (TMT A) or by alternating between numbers and letters in order (TMT B). Score on both Trail Making Tests represents time to complete. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) [19] assesses five domains of cognitive function. It includes two tasks each of immediate memory (orally presented word list and story), visuospatial construction ability (line orientation and copying complex figure), language (semantic fluency and confrontation naming), attention (digit span forward, and coding) and delayed memory (list learning, story, and complex figure). The Geriatric Depression Scale (Short Form) [20] is a 15 item self-report questionnaire of mood and depressive symptomatology, on which participants answered yes/no questions about their mood over the past week. One point was assigned for each item answered in the direction suggesting depressed mood.
Development of a Composite Executive Function score
To develop a single combined measure of executive function rather than performing multiple analyses with each individual test score, we first examined Pearson product-moment correlation coefficients between individual test indices. Scores on the Trail Making Tests, representing time to complete the task, were markedly skewed. Thus, log transformed scores were used for analyses. Because significant small to moderate correlations were found between most cognitive tests, 10 cognitive measures (RBANS Coding, logTMT A, logTMT B, MoCA Total Score, Semantic Fluency, Clock Draw, RBANS Figure Copy, RBANS Digit Span, Serial 7’s and World Backward) were subjected to a principal components analysis (PCA). Prior to performing the PCA, the suitability of the data for factor analysis was assessed. Inspection of the component matrix revealed the presence of a number of coefficients of 0.3 and above. The Kaiser-Meyer-Oklin value was established as 0.81, exceeding the recommended value of 0.6, and Bartlett’s Test of Sphericity reached statistical significance, supporting the factorability of the correlation matrix.
The principal components analysis revealed the presence of one component with an eigenvalue exceeding one, explaining 38.17 % of the variance. An inspection of the scree plot revealed a clear break after the first component, with the remaining components each explaining a relatively small amount of the variance. The results of this analysis supported the use of a single component solution, in the form of an Attention/Executive Function Composite Score to represent this factor in further analyses. After inspection of the component matrix, tasks with loadings greater than 0.3 were included in the Composite score. In the two cases where tasks with underlying skills overlapped substantially, the measures with higher loadings in the component matrix were selected. This resulted in final selection of the following tests: RBANS Coding, logTMT A, MoCA Total Score, Semantic Fluency, Clock Draw, RBANS Figure Copy, and World Backwards. Scores on each of these tasks were converted to standard (z) scores to place them on a common metric. Because TMT A is scored in the reverse direction from the other tests (lower time reflects better cognitive functioning), scores on logTMT A were reversed before standard scores were calculated. Then individual patients’ scores on each measure were added to produce a single Composite Executive Function score. This score was utilized in subsequent analyses to represent Attention/Executive Function.
Statistical Analyses
To inform judgments about generalizability of findings, we compared TUG scores for our sample to published means by Bohannon [21] and Shumway-Cook [13]. We investigated relationships between TUG tasks and tasks of attention and executive function (including the Composite Executive Function score), and between TUG-cognition and falls using Pearson product-moment correlation coefficients. A hierarchical linear multiple regression model controlling for age was used to test the incremental validity of the TUG-cognition (over and above the AGS falls criteria) on performance of attention/executive function tasks. Age was controlled for because performance on cognitive tests has been shown to decrease with age [22, 23], and raw (rather than age-corrected) test scores were utilized in all computations in order to increase analysis uniformity and sensitivity. A Pearson correlation showed the association between past falls (as a discrete quantitative variable) and TUG-cognition. Further, participants were divided into 3 falls groups: never fallers (zero falls), single fallers (one fall) and multiple fallers (two or more falls). We then created two dummy variables to compare these falls groups: high incidence versus low incidence (never or single = 0, multiple = 1), and single versus never (never = 0, single = 1) to test for differences in TUG-cognition scores between falls groups, using using hierarchical regression models to control for age differences. Next, we examined participants with discrepant risk status on the AGS and TUG-cognition, to determine characteristics of those uniquely identified on the TUG-cognition as at-risk for falls. Finally, we examined whether identification of falls-risk status increased the referrals made to physical therapy from our Memory Assessment Clinic.
Results
Participant Characteristics
The sample of 120 veterans had a mean age of 76.4 (±8.4) years (range 60–90) and was overwhelmingly male (98%). Most were in relatively good physical and emotional health as evidenced by few hospitalizations over the previous 4 months (15%), and a mean depression score in the non-depressed range on the Geriatric Depression Scale-Short Form. Participant characteristics are presented in Table 1.
Table 1.
Veteran Characteristics
| N=120 veterans presenting to a Memory Assessment Clinic | ||||
|---|---|---|---|---|
| N* | Mean | Standard Deviation | Range | |
| Demographics | ||||
| Age in years, Mean (SD) | 120 | 76.4 | 8.4 | 60–90 |
| Male gender, n (%) | 120 | 117 | 98% | Not applicable |
| Education in years, Mean (SD) | 120 | 12.3 | 2.6 | 4–18 |
| Monthly income in US dollars, Mean (SD) | 93 | 2390.0 | 1336.0 | 470–7056 |
| Medical History | ||||
| Number of Psychotropic Medications, Mean (SD) | 119 | 0.5 | 0.8 | 0–3 |
| History of TBI by chart review, n (%) | 120 | 18 | 15% | Not applicable |
| History of MI by chart review, n (%) | 120 | 11 | 9% | Not applicable |
| History of CVA by chart review, n (%) | 120 | 17 | 14% | Not applicable |
| History of arthritis by chart review, n (%) | 120 | 23 | 19% | Not applicable |
| Visual impairment, as reported in medical record, n (%) | 114 | 35 | 31% | Not applicable |
| Number hospitalized in past 4 months by chart review, n (%) | 120 | 18 | 15% | Not applicable |
| Number of self-reported fallers, Mean (SD) | 118 | 1.0 | 2.0 | 0–13 |
| Percentage of self-reported fallers, n (%) | 118 | 48 | 40% | |
| Number of fallers according to medical record, Mean (SD) | 120 | 0.8 | 1.6 | 0–10 |
| Percentage of fallers according to medical record, n (%) | 120 | 44 | 37% | |
| Cognitive and Mood Performance | ||||
| Mini-Mental State Examination (MMSE) | 119 | 25.2 | 3.1 | 16–30 |
| Montreal Cognitive Assessment (MoCA) | 63 | 18.8 | 5.1 | 1–27 |
| Serial Seven’s | 100 | 3.3 | 1.6 | 0–5 |
| World Backwards | 118 | 4.1 | 1.2 | 0–5 |
| RBANS Coding (timed) | 114 | 24.4 | 10.3 | 3–48 |
| RBANS Digit Span | 117 | 8.6 | 2.2 | 4–15 |
| RBANS Figure Copya | 116 | 15.3 | 3.7 | 2–20 |
| RBANS Fluencya (timed) | 119 | 11.9 | 4.8 | 2–23 |
| Semantic Fluency (timed) | 106 | 13.6 | 5.4 | 1–31 |
| Clock Draw | 120 | 0.5 | 0.50 | 0–1 |
| Trail Making Test Ab (score in seconds) | 114 | 66.2 | 44.1 | 20–339 |
| Trails Making Test Bb (score in seconds) | 117 | 274.3 | 194.4 | 46–1383 |
| Score on depression screening measure,c M (SD) | 118 | 3.7 | 3.2 | 0–13 |
N varied slightly depending on time constraints. Some patients worked too slowly to complete all cognitive measures and selected tests were omitted based on clinical judgment. Of note, the substantially lower number of patients completing the MoCA reflects its introduction to the clinic midway through the study.
TBI = traumatic brain injury; MI = myocardial infarction (heart attack), CVA = cerebral vascular accident (stroke) MMSE = MiniMental Status Exam; MoCA = Montreal Cognitive Assessment; RBANS = Repeatable Battery for the Assessment of Neurocognitive Status;
Norms Duff [29] values include M (SD) for 76 year old patients.
Norms Tombaugh [30] values include M (SD) for 76 year old patients.
Depression measured with the Geriatric Depression Scale-Short Form. Scale=0–15; 0–5 points indicating no or minimal symptoms, 6–10 points suggesting mild to moderate depressive symptoms, and 11–15 points consistent with moderate to severe depressive symptoms.
TUG task times and interquartile ranges from the present study as well as from Bohannon [21] and Shumway-Cook et al.’s [13] results are presented in Table 2. Independent sample t-tests comparing TUG task scores from all three studies revealed significant differences. Veterans in the present sample performed the TUG-alone significantly more slowly (X̄ = 13.35, SD = 6.02) than did individuals in Bohannon’s analysis of normal adults in both the 60–69 year old range X̄ = 8.1; t (294) = 7.17, p < .0001, and the 70–79 year old range X̄ = 9.2; t (916) = 3.17, p = .002 (2 tailed). Of interest, the present sample was not significantly different from Bohannon’s group of individuals in the 80–99 year range X̄ = 11.3, t (1220) = 0.98, p = .32 (2 tailed), indicating that our sample performed similarly to healthy individuals of more advanced age. Significant differences were also found between TUG-alone times in the present sample and Shumway-Cook et al.’s scores for non-fallers X̄ = 8.4, SD = 1.7; t (133) = 3.16, p = .002 (2 tailed), as well as for fallers X̄ = 22.2, SD = 9.3; t (133) = −5.01, p < .0001 (2 tailed), with this sample of veterans performing the task significantly more slowly.
Table 2.
Performance on TUG tasks; data from 3 studies
| Current data N = 120, mild to mod. cognitive impairment |
Shumway-Cook et al. (2000) N = 30, neurologically healthy |
Bohannon (2006) N = 4395, Meta-analysis of 21 studies with healthy participants |
|||||
|---|---|---|---|---|---|---|---|
| Age 60–90 | Median (Interquart. Range) | Age > 65 | Age 60–99 | ||||
| Time (in seconds) | M (SD) | Non-fallers | Fallers (>1 fall) | Age 60–99 | 70–79 | 80–99 | |
| M (SD) | M (SD) | M (95%CI) | M (95%CI) | M (95%CI) | |||
| TUG (n = 120) | 13.35 (6.02) | 11.58 (9.24–15.64) | 8.4 (1.7) | 22.2 (9.3) | 9.4 (8.9–9.9) | 9.2 (8.2–10.2) | 11.3(10.0–12.7) |
| TUGman (n = 109) | 15.24 (6.40) | 13.63 (10.45–18.30) | 9.7 (1.6) | 27.2 (11) | |||
| TUGcog n = 118) | 16.73 (8.09) | 15.02 (10.83–20.22) | |||||
Note. TUG = Timed Up and Go Task; TUGman = Timed Up and Go with manual task (holding a cup of water; TUGcog = Timed Up and Go cognitive task (counting backwards from 50).
Interquart. = interquartile
Because a small number of participants performed these timed tasks considerably more slowly than their peers, mean and standard deviation do not necessarily accurately represent the central tendency and range of the sample. Therefore, medians and interquartile ranges are also provided.
CI = confidence interval
Independent sample t-tests comparing scores on the TUG-manual in the present sample (X̄ = 15.24, SD = 6.40) with Shumway Cook et al.’s [13] scores on the same task (participants were 65 years of age or older), revealed significant differences between published data from non-fallers X̄ = 9.7, SD = 1.6; t (122) = 43.33, p = .001 (2 tailed), as well as published data from fallers X̄ = 27.2, SD =11; t (122) = −6.13, p < .0001 (2 tailed). Because the TUG-cognitive task has not been used in other studies, comparisons to other samples could not be conducted.
Correlation of Attention/Executive scores with TUG task parameters
Bivariate correlations between measures of attention and executive function and 3 TUG task parameters are presented in Supplemental Table A. Relative to the TUG-alone and the TUG-manual, the TUG-cognition demonstrated the strongest correlations with individual measures of attention and executive function. Correlations between the Attention/Executive Function composite score and the TUG alone (r = −0.36), the TUG-manual (r = −0.28), and the TUG-cognition (r = −0.39) further reflected the TUG-cognition’s robust relationship with cognitive function.
Regression Analyses Predicting Attention/Executive Function: Comparison of Performance Based Measures and AGS Criteria
Hierarchical multiple regression analyses are reported in Table 3 and suggest that TUG-cognition accounts for significant variance in Attention/Executive Function composite scores, after controlling for both age and AGS risk status. Age was entered at Step 1, explaining 10 % of the variance in executive functioning. After the entry of the dichotomous variable “Meets AGS Criteria,” the model explained 15 % of the total variance, with the significance test for the change in R2 indicating that AGS accounted for a significant amount of variance in executive functioning after controlling for age. When TUG-cognition was entered into the model, the total variance in executive functioning explained was 20%, again a significant gain in variance explained. In the final model, Age, AGS criteria and TUG-cognition emerged as statistically significant with Age recording the highest beta value, followed by TUG-cognition.
Table 3.
Hierarchical Regression Analysis of AGS and TUG-cognition Predicting Composite Executive Functioning After Controlling for Age
| Entered | β | R2 | R2 Change | F change | df1 | df2 | p |
|---|---|---|---|---|---|---|---|
| 1. Age | −0.31 | 0.10 | 0.10 | 12.53 | 1 | 116 | 0.001 |
| 2. AGS | −0.24 | 0.15 | 0.06 | 7.47 | 1 | 115 | 0.007 |
| 3. TUG-cognition | −0.26 | 0.20 | 0.05 | 6.71 | 1 | 114 | 0.011 |
Note. AGS = meets AGS criteria for falls risk (1 = yes; 0 = no).
Relationship Between Falls and TUG Tasks
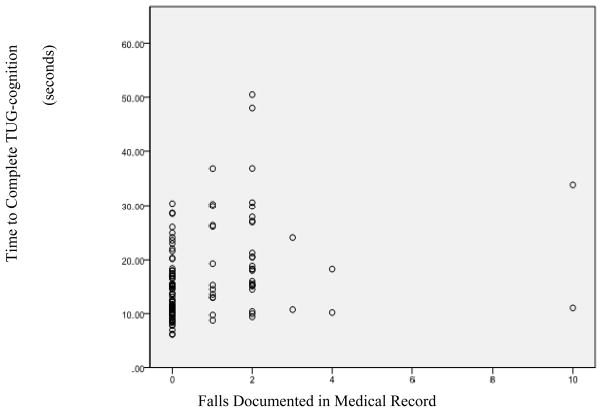
The Pearson correlation between the number of falls reported at the MAC visit and TUG-cognition was not statistically significant (r = 0.12, p = 0.20). However, TUG-cognition tasks demonstrated moderate correlations with falls documented in the medical record (r = 0.27, p = 0.003), establishing a significant relationship between prior falls and TUG scores. Dot plots of TUG-cognition scores compared to falls in the medical record revealed three distinct groups of scores: never fallers (zero falls), single fallers (one fall), and multiple fallers (two or more falls). Figure 1 depicts the relationship between falls and TUG-cognition scores.
Figure 1.
Scatter Plot of TUG-cog Scores Compared with Falls documented in the medical record.
Hierarchical regression analysis using dummy variables to represent differences in falls incidence (high versus low, single versus never) revealed significant differences in TUG-cognition scores. Age was entered at Step 1, explaining 17.8% of the total model variance F (1, 116) = 25.14, p < 0.001. After entry of the two variables comparing falls groups at Step 2, the total variance explained by the model as a whole was 27.7%, F (2,114) = 7.76, p = 0.001. These two variables explained an additional 10% of the variance in TUG-cognition after controlling for age, R2 change = 0.10, F change (3, 114) = 14.53, p < 0.001. In the final model, only the comparison between high versus low incidence (non-fallers + single fallers and multiple fallers) was statistically significant (B = 3.72, p = 0.03), indicating that those in the multiple falls group required an additional 3.72 seconds to complete the TUG-cognition than those in the never- and single falls groups.
Descriptive data for individuals with discordant risk status as defined by AGS criteria and TUG-cognition
Analysis of discrepant risk status on falls risk measures revealed 14 veterans uniquely identified as at-risk for falls by the TUG-cognition, and 21 veterans uniquely identified as at-risk by AGS criteria. The group uniquely identified by the TUG-cognition task was significantly older (mean difference of six years; p = .007), than those solely identified by AGS. In addition, in the previous 4 months, significantly fewer individuals identified by TUG-cognition had been hospitalized compared to those identified by AGS criteria ( p = .011), suggesting a lower level of frailty in patients whose risk was identified by TUG-cognition. Finally, the group uniquely identified by the TUG-cognition included three times the rate of Mild Cognitive Impairment diagnoses compared to the group labeled at-risk by AGS, although this difference did not reach statistical significance in this relatively small sample (p = 0.14). In summary, the group uniquely identified by the TUG-cognition was older, less frail, and exhibited a trend toward more mild forms of cognitive impairment than those uniquely identified by the AGS criteria.
Increased Referrals to Physical Therapy
Finally, records obtained from physical therapy service indicated that referral numbers from the MAC more than doubled in frequency after the TUG-cognition was implemented. For example, there was an average of one referral per month in 2007, and more than two referrals per month by 2010. These data suggest that use of the TUG-cognition may facilitate further evaluation of veterans with questionable mobility status.
Discussion
This study compared two methods of identifying individuals at-risk for falls (as documented in the medical record): the AGS criteria and two well established TUG performance-based measures, the TUG alone and the TUG-manual, as well as a modified version of the test, the TUG-cognition. Results suggested that while both the AGS and TUG falls risk criteria (all three forms) were associated with cognitive impairment, performance on the TUG-cognition predicted variance in attention/executive functioning over and above that accounted for by the AGS falls risk criteria. Moreover, the TUG-cognition successfully discriminated between never/single fallers and multiple fallers. Finally, the TUG-cognition may be sensitive to falls risk in a subgroup of patients not detected using the AGS criteria. Those uniquely identified by the TUG-cognition were older and less physically frail, with a trend toward more mild cognitive impairment than those identified solely by the AGS criteria. Altogether these analyses suggested that the TUG tasks, particularly the TUG-cognition, are a necessary supplement to the widely used AGS criteria for older veterans with cognitive impairment. By relying on performance rather than recall of recent falls, the TUG-cognition can more effectively identify individuals whose falls risk is associated with cognitive impairment.
Performance Based Measures Detect Falls Risk Associated with Attention/Executive Function
After controlling for age and risk-status as defined by the AGS criteria, the TUG-cognition demonstrated relationships with attention/executive function tasks. Numerous studies have confirmed the relationship between executive dysfunction and falls. The ability to maneuver in complex environments depends on intact attention/executive function, and deficits in these areas represent risk factors for gait dysfunction and falls [3–7]. This may be especially true in patient populations with neurological disorders, in whom gait patterns may already be subtly altered [24]. Moreover, failure to appropriately identify patients who are at-risk for falling likely results in a corresponding failure to implement necessary intervention strategies. Patients may persist in engaging in hazardous activities or maintain exposure to suboptimal environments because they overestimate their abilities or are unaware of the risk to their safety [25]. Altogether, these data emphasize the importance of using performance-based assessments for patients whose risk for falls is associated with executive dysfunction.
The present data are consistent with recent findings by Herman et al., [26] revealing correlations between the TUG, executive function and multiple falls, whereas two other measures of mobility, the Berg balance test and the Dynamic Gait Index, did not show significant correlations with either cognition or falls. While future studies will help elucidate the relationships between measures of falls risk, cognition and prospective falls, it is clear that both gait and executive dysfunction constitute falls risk factors and opportunities for intervention.
Of note, self-reported falls and falls reported in the medical record differed in this study. Because patients with cognitive impairment may incorrectly report their falls, it is suggested that falls reported in the medical record may more accurately represent actual falls. We speculate that compared to the report on falls gathered during a typical appointment check-in process, the medical record may document falls temporally closer to their occurrence, i.e., the report is less affected by impaired recall than a report based on patients’ recall of falls occurring in the past year. This may especially true for individuals with cognitive impairment. In this study, falls reported in the medical record (but not self-reported falls) were significantly related to TUG performance. Because the AGS criteria depend on self-report, the TUG-cognition may provide a useful supplement to the AGS-based risk assessment, especially among patients with impaired cognition.
TUG Tasks Distinguished Between Infrequent-Fallers and Recurrent Fallers
The TUG-cognition distinguished between two sub-groups of fallers, specifically, never and single fallers, and multiple fallers. In other words, multiple fallers demonstrated significantly worse performance on TUG-cognition than single fallers and never fallers, supporting the idea that multiple fallers are more likely to fall due to enduring underlying difficulties, including cognitive impairment. Whereas a single fall may reflect nothing more than unfortunate circumstances, multiple falls are more likely to signal a breakdown in the complex walking physiology or neuromuscular circuitry. The additional cognitive load inherent in counting backwards (TUG-cognition) was sufficient to significantly alter the performance of those people for whom the mechanics of walking were already compromised.
TUG Tasks and AGS Criteria Identified Unique Groups of Potential Fallers
The group uniquely identified as at-risk by the TUG-cognition included individuals in better physical health, with a trend toward higher rates of mild cognitive impairment than those identified solely by AGS criteria. As such, TUG-cognition may be sensitive to more subtle cognitive dysfunction than the AGS criteria, and it may be more adept at detecting falls risk due to cognitive dysfunction earlier in the neurodegenerative process. From a clinical standpoint, this is important because it suggests that early determination of falls risk due to cognitive impairment can be ascertained without a full neuropsychological evaluation. Administration of the TUG tasks can be completed in five minutes in a primary care setting, and provides a determination of falls risk sufficient to refer for further evaluation if necessary. Screening results could facilitate earlier referrals for intervention and the generation of falls prevention strategies while patients are best able to operationalize and benefit from them.
TUG-cognition shows promise as a means of screening fallers with cognitive impairment
The TUG-alone and TUG-manual are well-validated measures of falls risk [10, 12, 21, 26–28]. The current data suggest that a modified version of the TUG, the TUG-cognition, shows promise in identifying patients at-risk for falls due to cognitive impairment, including those who might not be detected by AGS criteria. Not only was the TUG-cognition significantly correlated with tests of attention and executive function (see Supplemental Table A), it also effectively discriminated between falls groups in this cross sectional analysis. These results suggest that the addition of the cognitive challenge (counting backwards from 50 by 1’s) achieved the desired result: producing TUG scores that index executive functioning as well as mobility. We speculate that the TUG-cognition may identify individuals with even mild levels of cognitive decline, and may differentially identify individuals with similar scores on cognitive screening tests (e.g. the MMSE) based on their level of executive function. An important goal for future research includes a prospective study of falls risk using the TUG-cognition. With a sufficient sample size, cut scores can be established (using receiver operant characteristics curves) that optimize predictive accuracy. While the AGS guidelines remain the standard for falls risk, the TUG-cognition is proposed as a complement to AGS screening procedures, and should be especially useful in cases where cognitive impairment is contributing to risk.
In this VA Setting, TUG Measures Increased Referrals to Physical Therapy
Finally, as an incidental finding, it is noted that performance on the TUG measures generated more than double the rate of referrals to physical therapy from the VA Memory Assessment Clinic during the period of the project implementation than the AGS criteria had in the six months prior to beginning the project. Although cognitive impairment is a risk factor for falls, it is not practicable to conduct a comprehensive falls evaluation on every individual who demonstrates cognitive deficits. At the same time, because the consequences of falling are so great, we wished to avoid allowing any potentially at-risk patient to “slip through the cracks” through lack of detection. By utilizing performance-based measures, we were able to identify cognitively impaired individuals at higher risk for falls and to provide them with a more comprehensive evaluation through physical therapy (PT). While an analysis of actual falls prevented as a result of this intervention was beyond the scope of this study, future research should longitudinally assess the relationship of identification of at-risk status by the TUG-cogntion, referral to physical therapy, and future falls.
Limitations
This study presents with some limitations. The majority of participants were male, so validity for female patients remains to be tested. However, because the veteran population increasingly includes women, we wished to ensure that our sample was representative of the veterans presenting to memory clinic. In addition, the veteran population may have unique medical and demographic characteristics, also limiting generalizability of finding to non-veteran populations. Nevertheless, a comparison of our data with other published TUG times suggests that our results are indeed generalizable to other samples. The cross sectional single group design is less informative than a longitudinal design, and falls were recorded retrospectively rather than prospectively. Thus, the degree to which the TUG-cognition prospectively predicts falls is unknown and is an important goal for future research. The chart review was conducted by a single clinician (BF) in a single medical system, which in cases with ambiguous diagnosis, could lead to the possibility of rater error or bias and the omission of falls, which resulted in treatment outside the VA and were not reported to a VA clinician. However, diagnoses in clinic were based on discussion occurring in multi-disciplinary conferences, during which all clinical data are reviewed by the team of clinicians. Analyses may have been affected by the non-normal distribution of several variables; this may have constrained or attenuated results. Finally, the study was performed before the AGS falls risk criteria were revised, using slightly different criteria for falls risk. However, the revised AGS criteria remain dependent on patient recall of falls to establish falls risk, creating a clear distinction with the performance-based TUG tasks.
Conclusions
This study compared two falls screening measures in a sample of older Veterans. Findings suggested that cognitive impairment predicted falls risk status defined by both the TUG tasks and AGS criteria; however, TUG-cognition accounted for additional variance beyond that predicted by AGS. As such, the TUG-cognition may be more effective in identifying individuals at risk for falls due to executive dysfunction than the AGS screening criteria. For clinicians seeking to identify falls risk associated with cognitive dysfunction, these data highlight the need to utilize performance-based measures. Moreover, using the TUG-cognition to detect individuals at-risk due to compromised executive function ability increases the possibility of intervening more quickly and efficaciously in this subgroup of fallers. This is critical because individuals with mild cognitive deficits may still benefit considerably from physical therapy and other cognitively-based strategies, thereby preventing future injurious falls. Overall, identifying patients who are at risk for future falls and providing effective intervention as early as possible will help avoid significant suffering and lower health care costs, while facilitating retention of functional independence and better quality of life.
Supplementary Material
Acknowledgments
Dr. Gleason’s time was supported by the Wisconsin Alzheimer’s Disease Research Center. Dr. Fischer’s time was supported by a Veteran’s Affairs Geriatric Research Education and Clinical Center Advanced Fellowship.
This study utilized archival data from a quality improvement/quality assurance (QI/QA) demonstration project whose goal was to improve clinical ability to detect potential fallers. This QI/QA project was reviewed by the University of Wisconsin’s Health Science Institutional Review Board (UW IRB), a subcommittee of the William S. Middleton Memorial Veteran Hospital’s Research and Development (VA R&D) Committee, and determined that data collection for the project was exempt research per federal regulations. UW IRB and VA R&D approval was granted to retrospectively analyze the results.
Abbreviations
- AGS
American Geriatric Society
- TUG
Timed Up and Go
- QI/QA
quality improvement/quality assurance
- UW IRB
University of Wisconsin’s Health Science Institutional Review Board
- VA R&D
William S. Middleton Memorial Veteran Hospital’s Research and Development
- GRECC
Geriatric Research Education and Clinical Center
- MAC
Memory Assessment Clinic
- RBANS
Repeatable Battery for the Assessment of Neuropsychological Status
- pca
principal components analysis
- PT
physical therapy
- VA
Veterans Affairs
Appendices
Appendix 2
American Geriatric Society Falls Prevention Guidelines, 2001 and 2010
Guideline for the Prevention of Falls in Older Persons.
American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Falls Prevention Panel, 2001.
Specific Recommendations: Assessment
All older individuals who are under the care of a health professional (or their caregivers) should be asked at least once a year about falls.
All older persons who report a single fall should be observed as they stand up from a chair without using their arms, walk several paces, and return. Those demonstrating no difficulty or unsteadiness need no further assessment.
Persons who have difficulty or demonstrate unsteadiness require further assessment.
Older persons who present for medical attention because of a fall, report recurrent falls in the past year, or demonstrate abnormalities of gait and/or balance, require a falls evaluation. This evaluation should be performed by a clinician with appropriate skills and experience, which may necessitate referral to a specialist (e.g. geriatrician).
Summary of the Updated American Geriatrics Society/British Geriatrics Society Clinical Practice Guideline for Prevention of Falls in Older Persons. Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society, 2010.
Recommendations: Screening and Assessment
All older individuals should be asked whether they have fallen (in the past year).
An older person who reports a fall should be asked about the frequency and circumstances of the fall(s).
Older individuals should be asked whether they experience difficulties with walking or balance.
Older persons who present for medical attention because of a fall, report recurrent falls in the past year, or report difficulties in walking or balance (with or without activity curtailment) should have a multifactorial fall risk assessment.
Older persons who cannot perform or perform poorly on a standardized gait and balance test should be given a multifactorial fall risk assessment.
Older persons who report a single fall in the past year should be evaluated for gait and balance.
Older persons who have fallen should have an assessment of gait and balance using one of the available evaluations.
Older persons who have difficulty or demonstrate unsteadiness during the evaluation require a multifactorial fall risk assessment.
Older persons reporting only a single fall in the past year and reporting or demonstrating no difficulty or unsteadiness during the evaluation do not require a fall risk assessment.
A clinician (or clinicians) with appropriate skills and training should perform the multifactorial fall risk assessment.
Footnotes
Author contributions:
Barbara Fischer: Study conceptualization and design, acquisition of data, analysis and interpretation of data, manuscript preparation
William Hoyt: Data analysis and interpretation and manuscript preparation
Lawrence Maucieri: Data analysis and interpretation and manuscript preparation
Amy Kind: Acquisition of data and manuscript preparation
Gail Gunter Hunt: Acquisition of data and manuscript preparation
Teresa Swader: Acquisition of data and manuscript preparation
Carey Gleason: Study conceptualization and design and analysis and interpretation of data, manuscript preparation
Ronald Gangnon: Consultation and manuscript preparation
References
- 1.National Safety Council. Injury Facts®. 2008. Itasca, IL: 2008. [Google Scholar]
- 2.Panel on Prevention of Falls in Older Persons by the American Geriatric Society, British Geriatrics Society. Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59(1):148–57. doi: 10.1111/j.1532-5415.2010.03234.x. Epub 2011/01/14. [DOI] [PubMed] [Google Scholar]
- 3.Faulkner K, Redfern M, Cauley J, Landsittel D, Studenski S, Rosano C, et al. Multitasking: Association between poorer performance and a history of recurrent falls. J Am Geriatr Soc. 2007;55:570–6. doi: 10.1111/j.1532-5415.2007.01147.x. [DOI] [PubMed] [Google Scholar]
- 4.Holtzer R, Friedman R, Lipton R, Katz M, Xue X, Verghese J. The relationship between specific cognitive functions and falls in aging. Neuropsychology. 2007;21(5):540–8. doi: 10.1037/0894-4105.21.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petterson A, Olsson E, Wahlund L. Effect of divided attention on gait in subjects with and without cognitive impairment. J Geriatr Psychiatr Neurol. 2007;20:58–62. doi: 10.1177/0891988706293528. [DOI] [PubMed] [Google Scholar]
- 6.Springer S, Giladi N, Peretz C, Yogev G, Simon E, Hausdorff J. Dual-tasking effects on gait variability: The role of aging, falls and executive functioning. Movement Disorders. 2006;21(7):950–7. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- 7.Yogev-Seligmann G, Hausdorff J, Giladi N. The role of executive function and attention in gait. Movement Disorders. 2008;23(3):329–42. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackenzie L, Byles J, D’Este C. Validation of self-reported fall events in intervention studies. Clin Rehabil. 2006;20(4):331–9. doi: 10.1191/0269215506cr947oa. Epub 2006/05/25. [DOI] [PubMed] [Google Scholar]
- 9.Collerton J, Kingston A, Bond J, Davies K, Eccles M, Jagger C, et al. The personal and health service impact of falls in 85 year olds: Cross-sectional findings from the Newcastle 85+Cohort Study. Plos One. 2012;7(3):ARTN e33078. doi: 10.1371/journal.pone.0033078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Podsiadlo D, Richardson S. The Timed Up and Go - a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 11.Folstein M, Folstein SE, McHugh P. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. Epub 1975/11/01. 0022-3956(75)90026-6 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Lundin-Olsson L, Nyberg L, Gustavson Y. Attention, frailty, and falls: The effect of a manual task on basic mobility. J Am Geriatr Soc. 1998;46:758–61. doi: 10.1111/j.1532-5415.1998.tb03813.x. [DOI] [PubMed] [Google Scholar]
- 13.Shumway-Cook A, Brauer S, Woolacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80(9):896–903. [PubMed] [Google Scholar]
- 14.Nasreddine Z, Phillips N, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment (MoCA): A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53 (4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 15.Goodglass HE. Assessment of aphasia and related disorders. Philadelphia: Lea & Febiger; 1972. [Google Scholar]
- 16.Scanlan J, Brush M, Quijano C, Borson S. Comparing clock tests for dementia screening: naïve judgments vs formal screeenings-what is optimal? Int J Geriatr Psychchiatr. 2002;17:14–21. doi: 10.1002/gps.562. [DOI] [PubMed] [Google Scholar]
- 17.Strauss E, Sherman E, Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary. 3. Oxford: Oxford University Press; 2006. p. 1216. [Google Scholar]
- 18.Partington JEL, RG Partington’s Pathway Test. Psychological Services Center Bulletin. 1949;1:9–20. [Google Scholar]
- 19.Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status manual. San Antonio: The Psychological Corporation; 1998. [Google Scholar]
- 20.Fountoulakis K, Tsolaki M, Iacovides A, Yesavage J, O’Hara R, Kazis A, et al. The validation of the short form of the Geriatric Depression Scale (GDS) in Greece. Aging (Milano) 1999;11(6):367–72. doi: 10.1007/BF03339814. Epub 2000/03/30. [DOI] [PubMed] [Google Scholar]
- 21.Bohannon R. Reference values for the Timed Up and Go Test: A descriptive meta-analysis. J Geriatr Phys Ther. 2006;29(2):64–8. doi: 10.1519/00139143-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Oh H, Madison C, Haight T, Markley C, Jagust W. Effects of age and beta-amyloid on cognitive changes in normal elderly people. Neurobiol Aging. 2012;33(12):2746–55. doi: 10.1016/j.neurobiolaging.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerchner G, Racine C, Hale S, Wilheim R, Laluz V, Miller B, et al. Cognitive processing speed in older adults: Relationship with white matter integrity. Plos One. 2012;7(11):ARTN e50425. doi: 10.1371/journal.pone.0050425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scherder E, Eggermont L, Swaab D, van Heuvelen M, Kamsma Y, de Greef M, et al. Gait in ageing and associated dementias; its relationship with cognition. Neurosci Biobehav Rev. 2007;31(4):485–97. doi: 10.1016/j.neubiorev.2006.11.007. Epub 2007/02/20. S0149-7634(06)00142-4 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Lafargue G, Noel M, Luyat M. In the elderly, failure to update internal models leads to over-optimistic predictions about upcoming actions. Plos One. 2013;8(1):e51218. doi: 10.1371/journal.pone.0051218. Epub 2013/01/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herman T, Giladi N, Hausdorff J. Properties of the ‘Timed Up and Go’ Test: More than meets the eye. Gerontol. 2011;57(3):203–10. doi: 10.1159/000314963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viccaro L, Perera S, Studenski S. Is Timed Up and Go better than gait speed in predicting health, function, and falls in older adults? J Am Geriatr Soc. 2011;59(5):887–92. doi: 10.1111/j.1532-5415.2011.03336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu K, Devine A, Lewis J, Dhaliwal S, Prince R. “Timed Up and Go” test and bone mineral density measurement for fracture prediction. Arch Int Med. 2011;171(18):1655–61. doi: 10.1001/archinternmed.2011.434. [DOI] [PubMed] [Google Scholar]
- 29.Duff K, Patton D, Schoenberg MR, Mold J, Scott JG, Adams RL. Age- and education-corrected independent normative data for the RBANS in a community dwelling elderly sample. Clin Neuropsychol. 2003;17(3):351–66. doi: 10.1076/clin.17.3.351.18082. Epub 2004/01/06. [DOI] [PubMed] [Google Scholar]
- 30.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–14. doi: 10.1016/S0887-6177(03)00039-8. Epub 2004/03/11. S0887617703000398 [pii] [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. Statistical Power Analysis for the behavioral sciences. 2. Hilldale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.