Introduction
“The heart is the beginning of life, for it is by the heart the blood is moved… the source of all action” wrote William Harvey in 1673. The concept that such action could vary and the heart undergo remodelling in disease stretches back to the classic writings of Corvisart in 1806, when he described “two types of dilatation, active with thick walls and increased force of contraction, and passive with thinning of the walls and a decreased force of contraction.” These notions correspond to current concepts of left ventricular hypertrophy and dilatation as two contrasting types of cardiac remodelling. Yet we have to wait till 1984 before the then novel term remodelling more precisely described the early and later structural changes that occurred in infarcted and non-infarcted ventricular myocardium after coronary artery ligation.1 The next conceptual advance was that disproportionate thinning and dilatation occurred in the infarct region, accompanied by remote remodelling of non-infarcted myocardium, correlated with the extent of expansion. By 2000 the topic was sufficiently prominent to merit a consensus document from the International Forum on Cardiac Remodelling which thoroughly reviewed the concept. Whereas patients with major remodelling underwent progressive worsening of cardiac function, slowing or reversal of remodelling had become a new goal of heart failure therapy.2
Originally, the term remodelling was proposed to characterise the response of remote myocardium to regional infarction and the progression from acute myocardial infarction to chronic heart failure.1,3 Independently and at about the same time, the term remodelling was also used to characterise the progression of atherosclerotic vascular lesions.4,5 In the present seminar, we advocate the concept of remodelling in a broader and more general sense to characterise the responses of myocardium and vasculature to potentially noxious haemodynamic, metabolic and inflammatory stimuli, a process which is initially functional, compensatory and adaptive in nature but, when sustained, progresses to structural alterations which become self-perpetuating and pathogenic per se. Remodelling involves intrinsic responses of the specific cardiovascular cells – cardiomyocytes, endothelium, smooth muscle cells - but also the interstitial cells and matrix.
Endothelial remodelling: where it starts
The endothelial cell (EC), positioned at the interface between the blood vessels and tissues, stands poised to sense the environment and signal modulations of vascular function to maintain homeostasis and host defenses against microbial invaders and injury.6 Inappropriate signalling from vascular ECs can also contribute to common diseases characterized by arterial remodelling, notably atherosclerosis and hypertension. ECs sense the environment in two major ways: (1) local hydrodynamics, and (2) responses to circulating chemical signals. Mediators released by the ECs in turn modulate the function of the subjacent vascular smooth muscle cells (SMCs) in a manner that decisively influences vascular remodelling.
Many risk factors for atherosclerosis impinge on ECs uniformly throughout the circulation (e.g., low-density lipoprotein [LDL]), yet lesions of atherosclerosis tend to occur segmentally, particularly at branch points of arteries. The laminar shear stress that prevails in normal portions of arteries elicits from ECs a protective programme that mitigates the effects of risk factors such as LDL and tonically combats vasoconstriction by releasing nitric oxide, the endothelial-derived relaxing factor. These effects include suppression of vasoconstrictor, inflammatory and prothrombotic gene expression through increasingly well-understood molecular mechanisms. At flow dividers, disturbed flow impedes such atheroprotective functions, yielding activation of the proinflammatory transcription factor nuclear factor kappa B to provoke recruitment of inflammatory cells, and impaired vasodilator activity. The consequent accumulation of leukocytes, mainly mononuclear phagocytes, in the arterial intima sets the stage for foam cell formation due to engulfing of modified lipoproteins that build up in the intima exposed to excess LDL (Figure 1).7
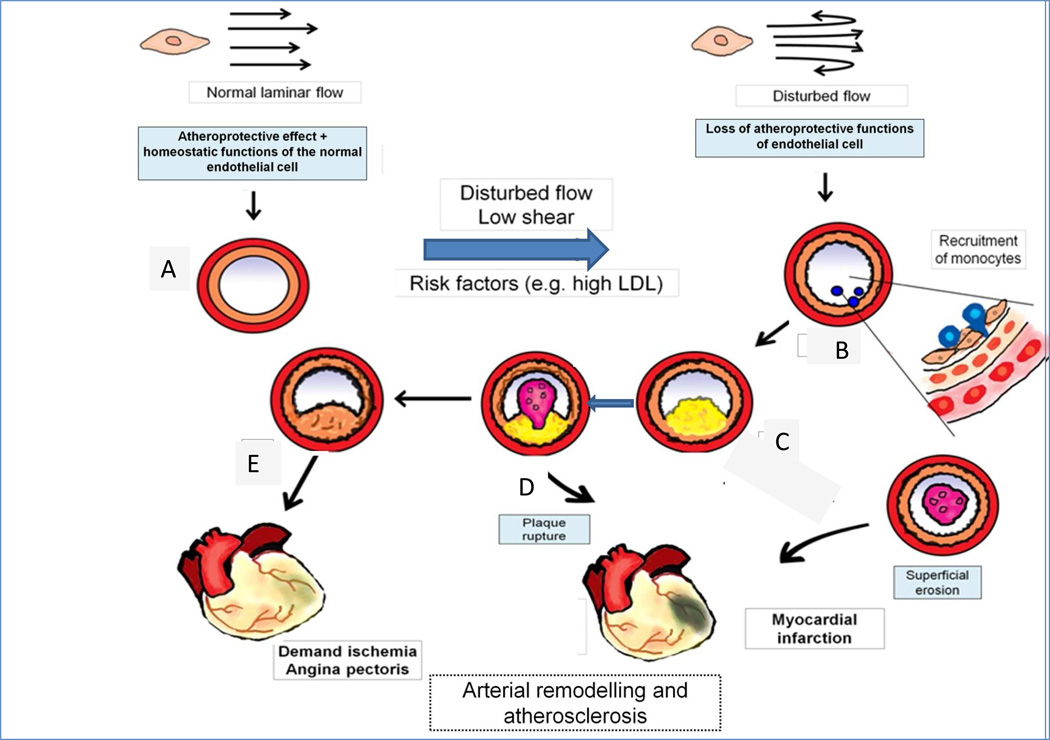
Figure 1. Positive and negative arterial remodelling influences the clinical consequences of atherosclerosis.
Normal laminar shear stress (upper left) elicits atheroprotective and homeostatic functions of endothelial cells (ECs). These functions maintain normal arterial caliber and properties (A). Disturbed blood flow in endocardial disease is shown by the reversed arrows in the upper right. In (B) the red circle portrays the tunica media, and the orange circle indicates the intima of the artery. Disturbed flow promotes the recruitment of monocytes, as depicted in the nascent plaque (B), where monocyte diapedesis (in blue) penetrates between ECs to form a thin-capped, lipid-rich inflamed plaque (C), which can rupture and cause a thrombus (D), leading to myocardial infarction, indicated by cyanosis in the heart diagram. Alternatively the plaque in E can undergo constrictive remodelling to promote flow-limiting stenosis (E) that can cause demand ischaemia and angina pectoris
Macrophages mediate arterial inflammation
These macrophage foam cells elaborate myriad mediators that amplify and sustain the local inflammatory response that promotes progression and the eventual thrombotic complications of atherosclerosis. Moreover, these messages from macrophages beckon SMCs to enter the arterial intima from the tunica media, where they elaborate extracellular matrix macromolecules that lead to fibrous lesions. These lesions can cause arterial stenoses and impede blood flow, leading to ischaemic conditions such as angina pectoris, intermittent claudication, and cerebrovascular disease. Loss of arterial elasticity increases pulse pressure, a finding associated with aging and heightened risk of cardiovascular events. The arterioles resist the plaque formation that is characteristic of larger arteries, but develop medial hypertrophy and intimal thickening, a form of remodelling associated with high blood pressure and implicated in sustaining and aggravating hypertension.8
Matrix metalloproteinases promote arterial remodelling
Among the extracellular matrix molecules manufactured by intimal SMCs, proteoglycans bind lipoprotein particles, increasing LDL residence time in the intima and favouring oxidative modification in this environment that is sequestered from plasma antioxidants.9 SMCs unlike ECs, do not sense luminal shear stress but experience cyclic circumferential deformation due to arterial pulsations. This force augments the synthesis of proteoglycans by SMCs thereby increasing LDL retention in the intima.10 SMCs in the intima, activated by proinflammatory cytokines produced by macrophages (e.g., interleukin-1) can also produce more collagen, leading to arterial fibrosis characteristic of aging and hypertension, thereby boosting the release of matrix-degrading proteinases (e.g., matrix metalloproteinases). These enzymes can remodel the arterial extracellular matrix structure, including the elastin in the external elastic membrane that forms the artery’s outer perimeter. This matrix remodelling paves the way for abluminal expansion — or outward growth — of the growing atheroma (positive remodelling) that preserves the lumen of the artery and maintains flow.11 Ultimately, plaque growth can outstrip this “compensatory enlargement” of the artery wall, permitting the atheroma to encroach on the lumen and cause stenosis (Fig. 1).
The plaque macrophages themselves secrete many matrix-degrading proteinases when they encounter inflammatory signals. These enzymes can attack on a different front — the plaque’s fibrous cap, a structure that typically overlies the lesion’s macrophage-rich lipid core at the inner perimeter of the artery.12 Collagenolysis due to enzymes overproduced by macrophages can weaken and thin the fibrous cap, rendering it susceptible to disruption.13 Fibrous cap fracture allows coagulation proteins in blood to contact the procoagulant tissue factor (TF) generated by macrophages in response to proinflammatory cytokines, unleashing the thrombotic cascade and promoting local clot formation. This scenario causes a majority of fatal myocardial infarctions (MI).
Rupturing plaques are the tip of the iceberg
There are many more macrophage- and proteinase-packed plaques than there are those that rupture. The mechanisms that trigger a susceptible plaque to rupture and provoke thrombosis at a particular instant remain poorly understood. Thus, despite all of the newer insights into plaque biology and remodelling, we still have a fragmentary understanding of the precipitants of clinical events and it may not just hinge upon the vulnerable plaque but also the vulnerable patient. Moreover, superficial erosion of the endothelial monolayer — another type of plaque disruption — causes a substantial minority of lethal MIs. The mechanisms of formation and of triggering thrombotic events due to superficial erosions remain even less well defined than the mechanisms for episodes of acute plaque rupture.12–14
Most episodes of plaque disruption probably pass below the clinical threshold and do not cause a sustained total arterial occlusion such as the type of thrombi that lead to ST-segment elevation MI (STEMI). Rather, they cause mural thrombi rooted in the intimal lesion that form a “provisional matrix” that evokes a local “wound healing” response. Platelet products and thrombin can signal SMC proliferation and extracellular matrix synthesis that remodels an initially lipid-rich lesion into a fibrous plaque. Contrary to outward remodelling, the healing process causes constrictive remodelling, restricting the lumen and favoring the formation of flow-limiting stenoses.12 Plaque calcification, previously regarded as a passive degenerative process, we now know to involve a complex biological cascade subject to considerable regulation and associated with inflammation.15
Blood pressure and lipid lowering attenuate arterial remodelling
Thus arterial remodelling, often beginning with altered endothelial function, has many faces and varies throughout the life history of an atheroma or a vessel subjected to chronic hypertension. The biological mechanisms described here not only shed light into the pathogenesis of common cardiovascular diseases, but also have therapeutic implications for daily practice. Control of blood pressure can limit the adverse remodelling of both conduit and resistance arteries, reducing the risk of stroke and aggravation of atherosclerosis. Lipid lowering, particularly with statins — a class of drugs with direct anti-inflammatory effects beyond LDL reduction — can limit plaque progression and in some cases, can regress lesions and alter plaque characteristics that associate with rupture and thrombosis.16
Coronary microvascular dysfunction and remodelling in ischaemic and reperfused myocardium
Remodelling of the epicardial coronary arteries disease has preoccupied clinical cardiologists as constrictive remodelling often yields stenotic plaques that cause chronic myocardial ischaemia, while expansive remodelling characterises plaques that rupture and provoke acute thromboses. The reperfusion era ushered in dramatic changes in the management of acute MI. The relief of symptoms in stable coronary artery disease and the prognosis of acute coronary syndromes have improved substantially with reperfusion and coronary revascularisation, but these successes should not divert attention from the coronary microcirculation which evades direct visualisation and intervention, but ultimately determines the extent of myocardial perfusion and function and the clinical outcomes from ischaemia/reperfusion injury.17,18
Experimentally, multiple and interactive mechanisms mediate coronary microvascular dilation during myocardial ischaemia, including autoregulatory adjustments to reduced perfusion pressure, release of adenosine and nitric oxide, and hypoxia.19 Yet, even during profound myocardial ischaemia, coronary vasodilation is not maximal and there remains a significant residual coronary vasoconstrictor tone in the microcirculation.20 The mechanisms of such persistent vasoconstriction during myocardial ischaemia are largely unclear, but include α-adrenergic coronary vasoconstriction, in particular during exercise and also cardio-cardiac reflexes in coronary interventions.21
Under controlled experimental conditions, recruitment of the persistent dilator reserve by pharmacological agents improves flow and function in ischaemic myocardium.20,22 Vasodilator reserve also persists distal to chronic stenosis, yielding impaired resting flow and function. Experimental models of hibernating myocardium23 have blunted myogenic vasodilatation and enhanced constriction to endothelin.24 Chronically ischaemic hibernating myocardium also displays structural remodelling of the coronary microvasculature with mild hypertrophy of smaller and atrophy of larger microvessels25 and reduced vascular distensibility.24
Reperfusion is a double-edged sword
Reperfusion, i.e. removal of the flow obstruction at the site of the epicardial culprit lesion, does not simply restore coronary microvascular perfusion, but imposes an acute stress, apart from and in addition to any pre-existing chronic remodelling. Stressors include particulate debris and soluble substances originating from the culprit lesion26 as well as functional and structural alterations originating in the coronary microvasculature per se.27 In the most extreme but nonetheless relatively frequent cases, the stress of reperfusion to the coronary microvasculature results in no-reflow despite a patent epicardial coronary artery. Spontaneous and/or traumatic/interventional plaque rupture at the culprit lesion liberates particulate debris from the atherosclerotic lesion which mixes with platelet aggregates and coagulation material which can lodge in the coronary microcirculation (Figure 2).
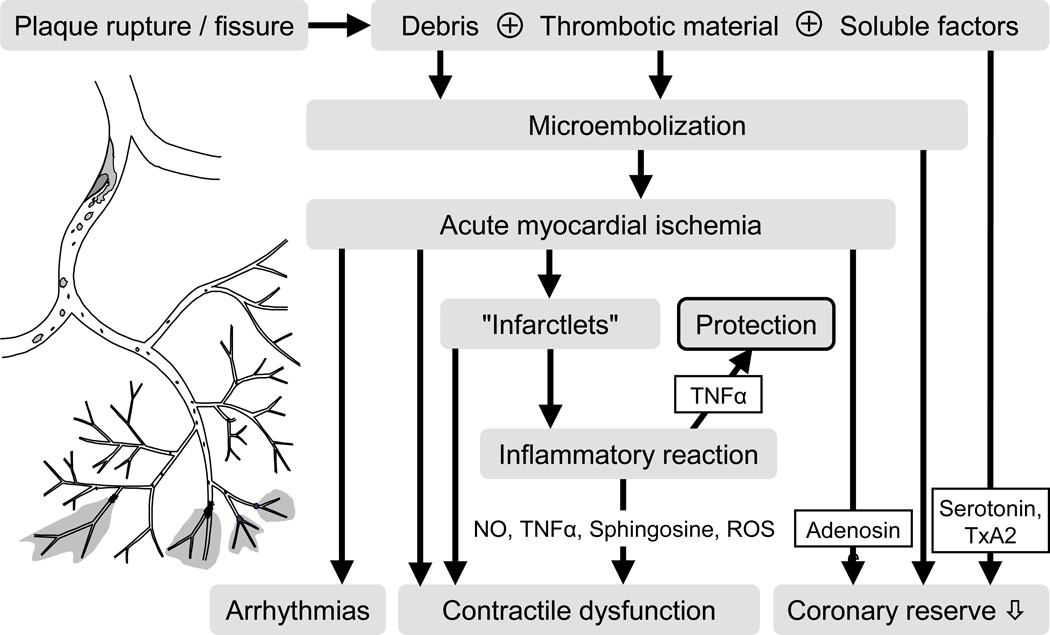
Figure 2. Role of microembolisation in coronary vascular remodelling.
Plaque rupture or fissure without complete epicardial coronary occlusion releases particulate debris from the atherosclerotic culprit lesion which, together with superimposed thrombotic material and soluble substances such as serotonin and thromboxane A 2, is washed into the coronary microcirculation by the residual blood flow. Coronary microembolisation causes microinfarcts with an inflammatory reaction. Inflammatory mediators such as nitric oxide (NO), TNFα and sphingosine induce contractile dysfunction through increased reactive oxygen species (ROS) formation and oxidative modification of the contractile machinery. TNFα in lower concentrations is, however, cardioprotective (upward arrow). Arrhythmias, contractile dysfunction and impaired coronary reserve are the functional consequences of coronary microembolisation. Modified from reference26.
Microvascular remodelling contributes to impaired myocardial perfusion through microembolisation of particulate debris
Experimentally, coronary microembolisation causes patchy microinfarcts with a subsequent inflammatory reaction.28 Autopsy of patients with unstable angina who had died from sudden death can also show embolic material within the coronary microcirculation and patchy microinfarcts.29 Moreover, remodelling with microvascular dysfunction as measured by a variety of techniques strongly influences late mortality in patients with patent epicardial vessels after mechanical or pharmacologic reperfusion.18 Coronary microembolisation not only causes microinfarcts and increases infarct size, but also interferes with potentially protective conditioning strategies.
In pigs, coronary microembolisation several hours before sustained coronary occlusion augments myocardial tumor necrosis factor (TNF) expression and protects through the Survivor Activating Factor Enhancement (SAFE) pathway,30,31 possibly explaining in part the clinical association of pre-infarction angina with protection from infarction.32,33 Some interventionalists abstain from use of ischaemic postconditioning for fear of coronary microembolisation from further manipulation of the culprit lesion. However, even if coronary microembolisation occurring at the time of reperfusion adds to infarct size in pigs, ischaemic postconditioning still largely reduces infarct size, and this protection is not offset by coronary microembolisation.34 The only way to prevent embolisation of particulate debris in patients may be its capture and removal with protection devices, but the results of these strategies have been mixed.26
Microvascular remodelling contributes to impaired myocardial perfusion through soluble vasoconstrictor, thrombogenic and inflammatory substances
In patients undergoing elective interventional revascularisation, the epicardial culprit lesion upon its rupture not only releases particulate debris but also soluble vasoconstrictor, thrombogenic and inflammatory factors that all contribute to microvascular flow impairment (Figure 2). The nature of these soluble factors depends somewhat on the underlying lesion and situation (native vs. saphenous vein graft, acute coronary syndrome vs. elective intervention), but serotonin, thromboxane A2 and endothelin are important vasoconstrictors35,36 and TNF impairs endothelial dilator function.35 Aspiration devices may be salutary by removal of thrombus and atherosclerotic debris which may generate these vasoconstrictors and other soluble factors. Their functional antagonism is another attractive target when treating microvascular obstruction and no-reflow phenomena after percutaneous coronary interventions. Indeed, nitroprusside and verapamil effectively relieve such microvascular obstruction, whereas the protection by adenosine has remained elusive.35,37
Apart from particulate and soluble factors originating from the upstream culprit lesion, the microcirculation suffers directly from myocardial ischaemia/reperfusion, even when a virgin coronary artery experiences experimental occlusion/reperfusion.27 Endothelial cell swelling and eventual sloughing together with platelet aggregates obstruct the capillary bed, and interstitial oedema compresses the vasculature. More advanced capillary destruction goes along with intramural bleeding.38 These severe structural alterations localize to the area of infarcted myocardium and possibly represent a consequence rather than a cause of MI. Interventions which protect from infarction usually also lessen microvascular obstruction and no-reflow. Lack of reflow in reperfused MI associates with poor recovery of function and prognosis.39 However, no-reflow phenomena are only the tip of the iceberg and reflect the most severe form of microvascular impairment. Direct and indirect measures of microvascular function such as a calculated index of coronary microvascular resistance, ECG ST-segment resolution, or myocardial blush grade predict prognosis in patients undergoing primary percutaneous coronary intervention (PPCI).40,41
In pigs with myocardial infarction, altered vasomotion extends beyond the infarcted myocardium to the remote remodelled myocardium where vascular growth does not keep up with myocardial hypertrophy, but the vasoconstrictor impact of angiotensin and endothelin is blunted to minimize a potential impairment of myocardial oxygen supply.42
Remodelling after myocardial reperfusion injury
Acute MI is a major cause of death and disability worldwide. Much of this morbidity and mortality relates to the remodelling that occurs post-infarction. Although cardiac remodelling often associates with events that occur in the weeks and months following an acute MI, its consequences invariably relate to the initial size of the associated MI. Therefore optimal positive remodelling, following a severe acute ischaemia/reperfusion event, can improve patient outcomes.
The first treatment priority for patients presenting with an acute ST-segment elevation MI is timely reperfusion to reduce the extent of myocardial ischaemic injury and limit the size of the evolving infarction. This is achieved by either PPCI or thrombolysis. However, myocardial reperfusion not only saves the majority of the ischaemic cells but paradoxically has a downside called ‘myocardial reperfusion injury’ with further fresh myocardial injury and cardiomyocyte death, in part from microvascular injury and obstruction (see above). Although optimal reperfusion, using novel antiplatelet and antithrombotic agents to maintain the patency of the infarct-related coronary artery is standard practice, there is currently no well-established therapy for protecting the heart against myocardial reperfusion injury (Figure 3). As lethal reperfusion injury accounts for up to 50% of the final infarct size,43 preventing or limiting such injury could improve the outcome of patients substantially, both by decreasing the size of the eventual infarct and thereby limiting the severity of consequent heart failure.44
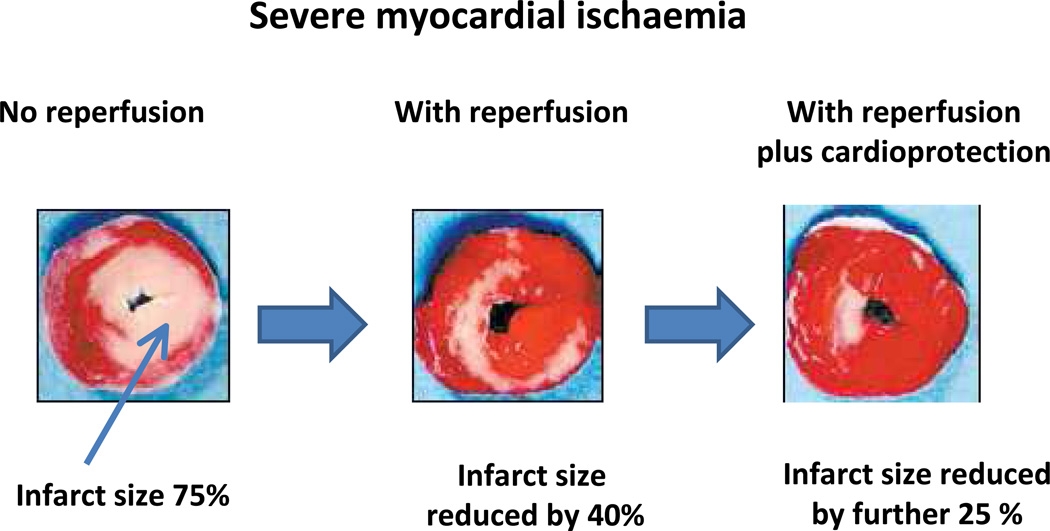
Figure 3. Reduction of infarct size.
In acute myocardial ischaemia rapid reperfusion decreases infarct size variably, roughly by about 40%, leaving 30% still damaged by lethal reperfusion injury. With molecular cardioprotection or pharmacologic agents that inhibit reperfusion injury, the final myocardial infarct size may be rescued by a further 25% thereby achieving a much smaller final infarct. Remote Ischaemic conditioning is a simple non-invasive method of reducing final infarct size.
Experimental studies have identified an endogenous self-protective programme which can be activated by brief cycles of myocardial ischaemia/reperfusion which precede (ischaemic preconditioning)45 or follow the sustained myocardial ischaemia during early reperfusion (ischaemic postconditioning).46 A cardioprotective programme can even be activated at a distance,47 by brief cycles of ischaemia/reperfusion in organs remote from the heart.48
Reperfusion injury is attenuated through activation of cardioprotective signalling pathways
Many signalling steps of such cardioprotective programmes have been identified in numerous experimental studies. There are extracellular trigger molecules (neurohormones, autacoids, cytokines) which activate through sarcolemmal receptors an intracellular cascade of proteins and ultimately converge on the mitochondria.49 Yellon and colleagues introduced the “Reperfusion Imaging Salvage Kinase” (RISK) pathway,50,51 including a range of anti-apoptotic kinases such as Akt and the p42/p44 mitogen activated protein kinase. Unexpectedly, this protective pathway was diminished in animals with features of the “metabolic syndrome” or in diabetic obese mice,52–54 and in human muscle taken from diabetic patients undergoing coronary artery bypass grafts (CABG).55 Insufficient RISK activation52–54 accounted for this impairment, illustrating how animal experiments can simulate the major co-morbid pathologies present in patients with coronary heart disease.
Strong experimental data were followed by evidence from smaller clinical trials that supported the notion of cardioprotection by other interventions, namely: (1) cyclosporine which inhibits opening of the mitochondrial permeability transition pore;56,57 (2) ischaemic postconditioning during PPCI;48,58–60 (3) remote ischaemic preconditioning (RIP) by intermittent limb ischaemia-reperfusion especially in elective CABG and in PPCI;61–63 (4) activation of STAT-364 or STAT-565 that are part of the SAFE pathway;31 and, most recently, (5) the anti-diabetic incretin mimetic exanitide66 and the β-adrenergic blocking agent metoprolol.67 Approaches to limiting adverse remodeling also include clinical benefit from glucose-insulin-potassium (GIK) given intravenously in the ambulance to patients with acute coronary syndrome68 (an intervention that has yielded mixed outcomes when given too late).
Remote ischaemic conditioning is feasible, safe and effective
The translation of the many encouraging experimental studies advocating cardioprotective strategies to reduce infarct size has not yet resulted in robust evidence-based medicine with improvement of patient outcome. There are many reasons for such difficulty in translation, including the reductionist nature of many experimental models on the one hand and the many co-morbidities and co-medications in patients of advanced age on the other hand.44 Ischaemic preconditioning has been translated to patients in elective settings of interventional and surgical coronary revascularisation, but is not feasible in acute myocardial infarction due to its unpredictable onset.32 Ischaemic postconditioning has been successfully translated in smaller studies on selected patients undergoing primary PCI for acute myocardial infarction under tightly controlled conditions;58–60,69 however, the duration of coronary occlusion, presence of collaterals, eventual spontaneous reperfusion before the intervention, and use of direct stenting are important variables which contribute to the difficulty to make general use of ischaemic postconditioning in a real-world scenario.70
The simplest and most successfully translated cardioprotection strategy is RIP. Recent trials reported not only reduced infarct size, as assessed from biomarkers or imaging, but also reduced major adverse cardiovascular events and all-cause mortality in patients undergoing elective interventional71 or surgical72 coronary revascularisation with prior RIP and in patients with acute MI when undergoing RIP during transport to the hospital for PPCI.73 If ongoing larger trials demonstrate benefit on clinical endpoints, guidelines might recommend its use in all ambulances transporting patients with acute chest pain and suspected acute coronary syndromes to the nearest hospital emergency room.
Myocardial remodelling in heart failure
Factors such as loading conditions, neurohormonal activation patterns, genetic background and comorbid conditions affect the size, shape and ultrastructure of the heart. In conditions such as pregnancy or endurance exercise terms such as physiological, adaptive, beneficial or compensated remodelling are used, while during pathological stimulation by pressure or volume overload, the condition is described as maladaptive or decompensated remodelling.74 The phenotype, including ventricular structure and mechanical function, is well characterised in patients whereas mechanisms such as signal transduction have mainly been identified in cell and animal models.
Remodelling describes the reorganisation of myocytes, intercellular matrix components and vessels in response to the index stimuli. Clinical remodelling depends on internal diameters and myocardial wall thickness. Either of these can be reduced, normal or increased, resulting in changes in the myocardium that depend on the loading conditions, neuroendocrine activation and genetic factors (Figure 4). In concentric remodelling, the ejection fraction is maintained, ventricular volumes are normal or reduced, and the left ventricular (LV) mass to volume ratio is increased. Clinically, this condition may present as heart failure with preserved ejection fraction.75 In contrast is the pattern of heart failure with reduced ejection fraction and increased end-diastolic volume to wall thickness ratio, also giving heart failure symptoms.76 In both conditions, remodelling involves reorganisation of myocytes, interstitial cells and vessels leading to increased stiffness and/or impaired contractility. Right ventricular remodelling results from increased filling pressures in response to LV failure,77 but also occurs in valve disease and primary pulmonary vascular hypertension.78 Increased LV stiffness results in left atrial remodelling that can promote atrial fibrillation, the most common arrhythmia in the elderly.79 Therefore, remodelling not only affects the LV, but also impairs the function of the right ventricle and atria.
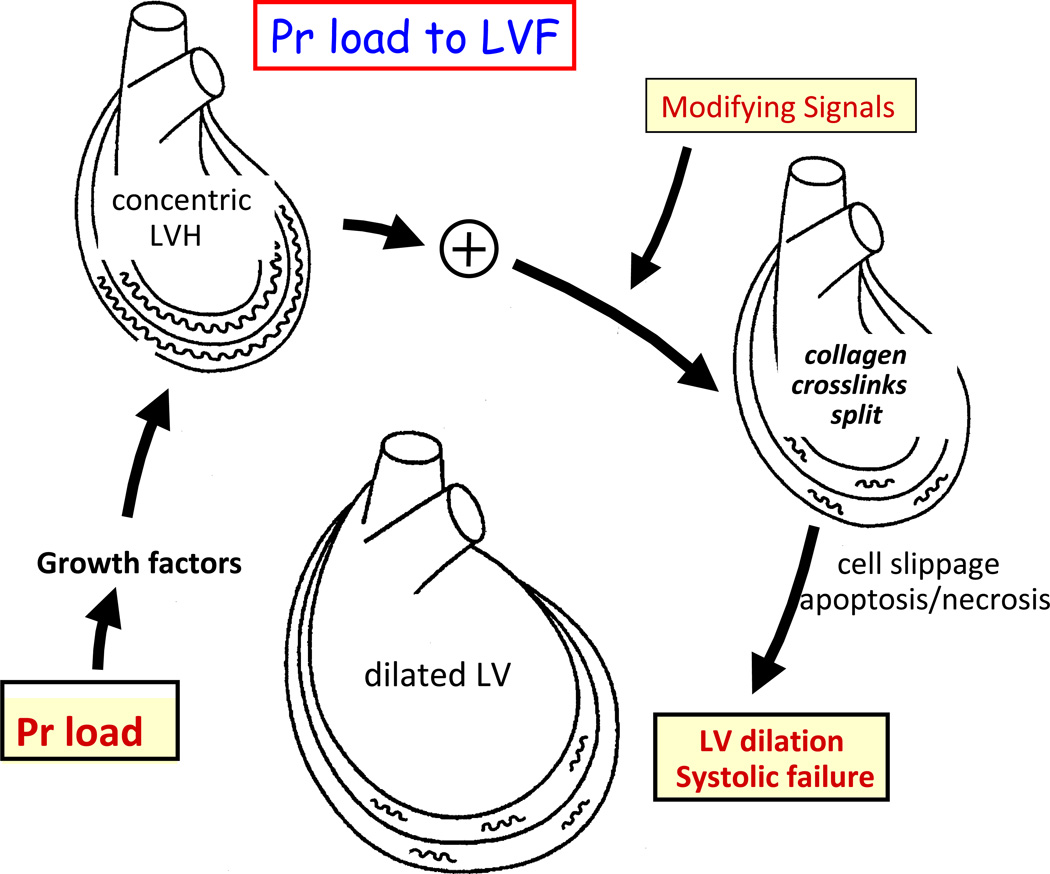
Figure 4. Myocardial remodelling in response to pressure load.
Proposed transition from pressure (Pr) load to concentric hypertrophy to dilated failing left ventricle (LV). Note the role of signals that that break down collagen compared with the opposing role of tissue inhibitors of matrix metalloproteinases (TIMPs). The primary stimulus to the increased collagen are stretch-induced growth factors such as angiotensin II. Concentric remodelled myocardium underoges splitting of the collagen cross-links in response to modifying molecular signals such as metalloproteinases and other signals that disrupt collagen crosslinks to promote LV dilation and systolic heart failure. Adapted from reference.130
Myocardial remodelling is characterised by intrinsic alterations of the cardiomyocyte and the interstitium
The failing myocardium, regardless of its pathogenic origin, has typical features which relate to cardiomyocyte viability, neurohumoral control and excitation-contraction coupling, but also to interstitial cells and matrix. Cardiomyocytes represent 20–30% of cells, but 70–80% of myocardial mass. Myocyte renewal can occur in the human heart80 and is enhanced in experiments in response to exercise or injury.81 Resident stem cells or bone marrow stem cells may transdifferentiate into myocytes.80 Myocytes are capable of undergoing hypertrophy and shape changes accompanied by a myosin isoform shift.82 While pressure overload increases thickness, volume overload increases length.83 In genetic familial hypertrophic cardiomyopathy, fibre disarray occurs.84
The failing human myocardium can contain many apoptotic cells.85 A lesser amount of cell death may relate to various forms of remodelling86 or ischaemia/reperfusion.87 Autophagy is a physiological, often age-related process where myocytes digest damaged and modified proteins during chronic ischaemia,87 hibernation88 and post infarct.89 Inhibition of autophagy aggravates adverse remodelling, while enhanced autophagy improves experimental post MI remodelling.89
Enhanced LV wall stress activates the myocardial renin-angiotensin system resulting in myocardial hypertrophy.90 Aldosterone91 and angiotensin-II further promote interstitial remodelling.92 Alpha-adrenergic stimulation induces myocardial hypertrophy.93 Chronic beta-adrenergic stimulation suppresses beta-adrenergic receptors and augments Giα proteins in hypertensive cardiac hypertrophy94 and in human heart failure,95 while also inducing myocardial apoptosis.96 These mechanisms provide a mechanistic background for evidence-based heart failure treatment with neurohumoral antagonists.76
Transmembrane calcium fluxes trigger calcium release from the sarcoplasmic reticulum (SR). Released calcium re-enters the SR via the calcium ATPase (SERCA2a). In heart failure, excitation-contraction coupling is impaired.97 In particular, SERCA2a is reduced in heart failure in humans98 leading to high cytosolic and low SR calcium concentrations. The resulting decrease in the calcium transient is aggravated by leaky ryanodine receptors,99 experimentally secondary to increased activity of calcium/calmodulin-dependent protein kinase II.97
In the interstitial compartment, fibroblasts modify the extracellular matrix with effects on ventricular size, structure and stiffness. Transforming growth factor-beta-1 is involved in maladaptive remodelling,100 while insulin-like growth factor-1 results in adaptive remodelling.101 The maturation and stability of fibrotic scar tissue are of particular importance in infarct healing.102 Matrix metalloproteinases which degrade extracellular matrix proteins can increase ventricular remodelling in dilated cardiomyopathy or valve disease. Plasma biomarkers reflecting determinants of matrix composition identify the presence of left ventricular hypertrophy and diastolic heart failure in patients.103
The clinical effectiveness of interventions can support the relevance of such mechanisms in humans. Unloading the heart attenuates left ventricular hypertrophy and improves outcomes.104 Renal sympathetic denervation reduces blood pressure105 and myocardial hypertrophy.106 High heart rates indicate poor outcome in patients with heart failure,107 while rate reduction reduces LV remodelling108 and events.109
Impaired mitochondrial oxidative metabolism and adverse energetic remodelling of the failing heart
In addition to structural remodelling, remodelling of cardiac energy metabolism can contribute to the severity of heart failure.110 In particular, both a decrease in energy production and a switch in energy substrate use that occur with remodelling may worsen heart failure.110,111
The progression of heart failure is associated with compromised myocardial energy production indicated by decreased concentrations of both ATP and phosphocreatine.112,113 This depletion appears to result primarily from a decrease in mitochondrial oxidative metabolism110,111 which results in compromise of mitochondrial oxidation of both fatty acids and carbohydrates.110 A compensatory increase in glucose uptake and glycolysis accompanies the reduced oxidative metabolism (Figure 5).
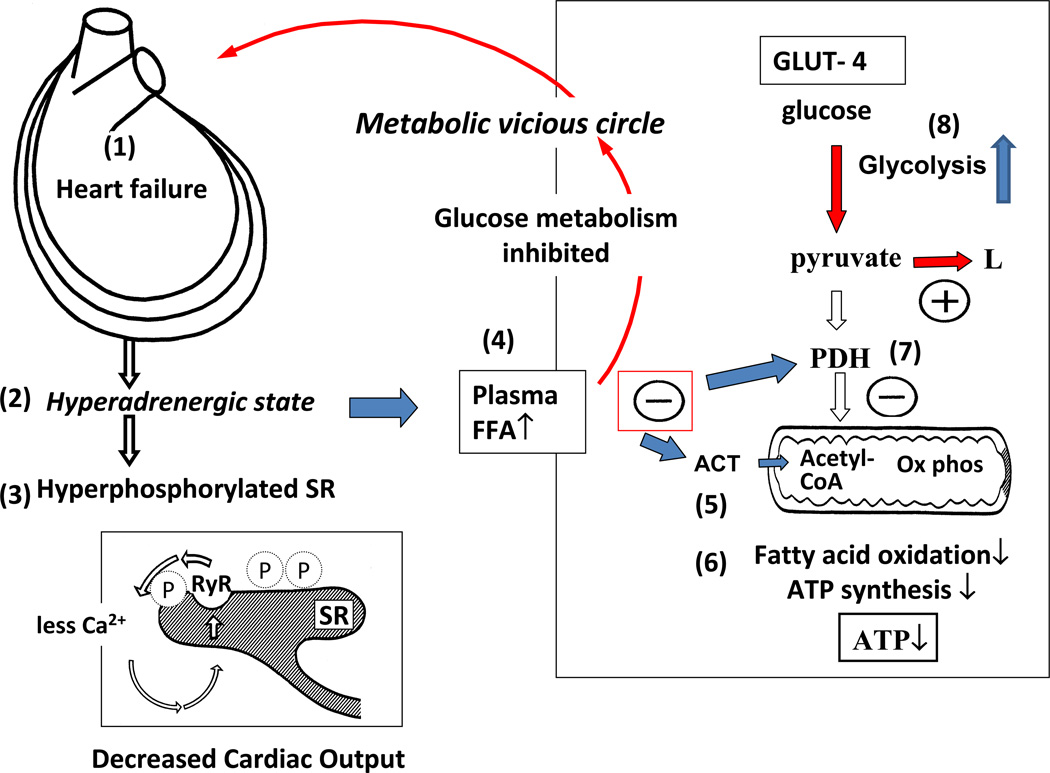
Figure 5. The metabolic vicious circle in heart failure.
Dilation of the myocardium in heart failure (1) leads to adrenergic activation (2) that in turn hyperphosphorylates the sarcoplasmic reticulum (3) and increases levels of circulating free fatty acids (4). The latter inhibit mitochondrial function at the level of acyl carnitine transferase (ACT) (5), thus inhibiting fatty acid oxidation and synthesis of ATP (6). Plasma FFAs also inhibit pyruvate dehydrogenase (PDH, 7) to promote anaerobic glycolysis (8) rather than oxidative metabolism. Adapted from reference131.
As a result of the decrease in fatty acid oxidation and the increase in glucose uptake and glycolysis, the failing heart appears to revert toward a “foetal metabolic phenotype.”110,111 The decrease in oxidative metabolism and rise in glycolysis observed in the failing heart associates with changes in expression and activity of metabolic enzymes consistent with this switch to foetal metabolism. These alterations include the expression of peroxisome proliferator activated receptor alpha and peroxisome proliferator activate receptor gamma cofactor alpha, regulators of mitochondrial biogenesis and fatty acid oxidation enzyme expression, processes which are decreased in the failing heart.114
Adrenergic drive promotes switches in energy metabolism
While the failing heart shifts energy metabolism, there is some confusion as to what exact switch in energy metabolism occurs. The general consensus is that there is a switch from fatty acid to glucose use in the failing heart.111,115 However, clinical studies disagree as to whether fatty acid oxidation is decreased,116 increased117,118 or unchanged119 in the failing heart. Regardless of what occurs with fatty acid oxidation at the level of the myocardium, the hyperadrenergic state that develops in heart failure will elevate blood free fatty acid levels.118 As a consequence, based on the Randle cycle, fatty acids will be preferred over carbohydrates for whatever mitochondrial oxidative capacity the heart retains.
With regard to the alterations in glucose metabolism in heart failure, the heart uses two pathways for glucose metabolism: glycolysis and glucose oxidation (Figure 5). Like fatty acid oxidation, glucose oxidation rates depend on mitochondrial oxidative capacity, and as such a decrease in mitochondrial function in the failing heart not only limits fatty acid oxidation, but also restricts glucose oxidation. Indeed, recent studies have shown that glucose oxidation rates decrease preferentially in the failing mouse heart.120 This decrease in glucose oxidation results from a decrease in the activity of pyruvate dehydrogenase, the rate-limiting enzyme of glucose oxidation. The production of protons and lactate rises because of augmented glycolysis and decreased glucose oxidation in heart failure. This increase in lactate and proton production in the failing heart can decrease cardiac efficiency and is potentially detrimental.110
Optimisation of metabolism attenuates adverse remodelling and worsening of heart failure
Therapeutic strategies include, both increasing energy supply to the heart and switching energy substrate preference to increase cardiac efficiency.110,111 Stimulating glucose oxidation is one approach to improve both energy supply and cardiac efficiency, and it improves cardiac function in experimental models121 and in human heart failure.122 Altering fatty acid oxidation rates offers another approach to improve cardiac function. However, there is not a clear consensus regarding the desirability of increasing or decreasing fatty acid oxidation. Increasing fatty acid oxidation might increase energy supply to the failing heart, but also could decrease glucose oxidation rates. In contrast, inhibiting fatty acid oxidation will increase mitochondrial glucose oxidation and potentially increases cardiac efficiency in heart failure.
Although there is not a clear consensus as to whether fatty acid oxidation is impaired in the failing heart, clinical studies suggest that inhibition of fatty acid oxidation can improve cardiac function in heart failure patients. Trimetazidine is a partial fatty acid oxidation inhibitor123 that improves the symptoms, cardiac function, and clinical outcomes in heart failure patients (see124 for meta-analysis of existing clinical studies). Drugs that inhibit carnitine acyltransferase, a key enzyme involved in mitochondrial fatty acid uptake (Figure 5), have beneficial effects on the function of failing heart.125,126 Decreasing cardiac fatty acid oxidation secondary to lowering circulating fatty acid levels also has potential benefit in heart failure. This may include the use of peroxisome proliferator-activated receptor (PPAR) agonists, which decrease the severity of heart failure in pigs,127 although some agents in this class can aggravate clinical heart failure.
Some of the benefits of β-adrenergic receptor blockers may occur secondary to lowering circulating free fatty acid levels as shown with carvedilol.128 When given chronically to patients with systolic heart failure, carvedilol as compared with atenolol, bisoprolol, metoprolol and nebivolol in randomised direct comparison trials, reduced all-cause mortality.129 Similar metabolic principles may explain the clinical benefit in some studies of GIK given intravenously in the ambulance to patients with acute coronary syndromes68 and the reduction of infarct size with the β-adrenergic blocker metoprolol when given intravenously before reperfusion.67
Summary
Remodelling of the heart and vessels characterizes coronary artery disease, hypertension and heart failure. Remodelling of the coronary arteries starts in the endothelium and progressively advances towards the atherosclerotic plaque that when causing ischemia and infarction provokes myocardial remodelling. The arteriolar microvascular response to hypertension, luminal narrowing, smooth muscle hyperplasia and medial thickening perpetuates elevated blood pressure that predisposes to myocardial ischaemia, infarction, and failure. These biological mechanisms not only shed light on the pathogenesis of common cardiovascular diseases, but have therapeutic implications for daily practice. Control of blood pressure can limit the adverse remodelling of both conduit and resistance arteries. Lipid lowering can modulate the remodelling of atherosclerotic lesions and alter plaque characteristics associated with rupture and thrombosis.
A wealth of sound animal experimental data has identified multiple potential therapeutic targets for the modification of microvascular dysfunction and reperfusion injury. Nonetheless, the results of clinical trials have been disappointing, a discord that may be explained by species differences in microvascular function, the presence of co-morbidities and co-medications in patients as opposed to animals and differences between the evolving and dynamic course of clinical MI in contrast to early rapid reperfusion experimentally. Also in contrast to experimental reperfusion studies, the typical patient with MI presents relatively late in the clinical course by when the ability to make a difference is limited. Thus reperfusion and treatment of reperfusion injury must be initiated as early as possible to avert damage to the myocardium and the coronary microcirculation.
Considerable remodelling of the myocardium occurs in response to reperfusion for acute MI. Decreasing such adverse myocardial remodelling may experimentally be achieved by stimulation of molecular defences such the RISK and SAFE pathways at the time of reperfusion. Activation of protective pathways by cyclosporine A or metoprolol at reperfusion, remote limb conditioning before surgical revascularisation or in the ambulance and early infusion of GIK provide protection in patients. Positive results of ongoing larger clinical trials would justify greater use of such therapies to reduce adverse remodelling during reperfusion.44
The extent of myocardial remodelling relates to the reorganisation of cardiac function and structure in response to physiological (adaptive) or pathological stimuli (maladaptive), involving all tissue components including myocytes, interstitial cells and interstitial matrix. Remodelling is reflected in alterations of excitation-contraction coupling, neurohormonal activation and morphology. The index events are different, such as hypertension, overload hypertrophy, and increased wall stress secondary to myocyte loss after myocardial infarction and myocarditis or tachycardia secondary to neuroendocrine activation.
Remodelling can substantially alter cardiac energy metabolism in the failing heart, manifested as decreased mitochondrial oxidative metabolism and increased glycolysis. Increasing both overall energy metabolism and the source of cardiac energy used by inhibition of fatty acid oxidation are potential therapeutic approaches to treat heart failure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This seminar integrates the contributions of the authors to a symposium held in Cape Town on May 3, 2013 to celebrate the 80th birthday of Lionel Opie.
Reference List
- 1.Roberts CS, MacLean D, Maroko P, Kloner RA. Early and late remodeling of the left ventricle after acute myocardial infarction. Am J Cardiol. 1984;54:407–410. doi: 10.1016/0002-9149(84)90206-6. [DOI] [PubMed] [Google Scholar]
- 2.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer JM, Pfeffer MA, Braunwald E. Influence of chronic captopril therapy on the infarcted left ventricle of the rat. Circ Res. 1985;57:84–95. doi: 10.1161/01.res.57.1.84. [DOI] [PubMed] [Google Scholar]
- 4.Zarins CK, Lu CT, Gewertz BL, Lyon RT, Rush DS, Glagov S. Arterial disruption and remodeling following balloon dilatation. Surgery. 1982;92:1086–1095. [PubMed] [Google Scholar]
- 5.Kadar A, Bjorkerud S. Arterial remodeling following mechanical injury. The role and nature of smooth muscle cells. Pathol Res Pract. 1985;180:342–347. doi: 10.1016/S0344-0338(85)80103-5. [DOI] [PubMed] [Google Scholar]
- 6.Gimbrone MA, Jr., Garcia-Cardena G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc Pathol. 2013;22:9–15. doi: 10.1016/j.carpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 8.Schiffrin EL. Vascular remodeling in hypertension: mechanisms and treatment. Hypertension. 2012;59:367–374. doi: 10.1161/HYPERTENSIONAHA.111.187021. [DOI] [PubMed] [Google Scholar]
- 9.Williams KJ, Tabas I. Lipoprotein retention--and clues for atheroma regression. Arterioscler Thromb Vasc Biol. 2005;25:1536–1540. doi: 10.1161/01.ATV.0000174795.62387.d3. [DOI] [PubMed] [Google Scholar]
- 10.Lee RT, Yamamoto C, Feng Y, et al. Mechanical strain induces specific changes in the synthesis and organization of proteoglycans by vascular smooth muscle cells. J Biol Chem. 2001;276:13847–13851. doi: 10.1074/jbc.M010556200. [DOI] [PubMed] [Google Scholar]
- 11.Sluijter JP, de Kleijn DP, Pasterkamp G. Vascular remodeling and protease inhibition--bench to bedside. Cardiovasc Res. 2006;69:595–603. doi: 10.1016/j.cardiores.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 12.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 13.Libby P. Collagenases and cracks in the plaque. J Clin Invest. 2013;123:3201–3203. doi: 10.1172/JCI67526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falk E, Nakano M, Benton JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists' view. Eur Heart J. 2012;34:728. doi: 10.1093/eurheartj/ehs411. [DOI] [PubMed] [Google Scholar]
- 15.New SE, Goettsch C, Aikawa M, et al. Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ Res. 2013;113:72–77. doi: 10.1161/CIRCRESAHA.113.301036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365:2078–2087. doi: 10.1056/NEJMoa1110874. [DOI] [PubMed] [Google Scholar]
- 17.Lerman A, Holmes DR, Herrmann J, Gersh BJ. Microcirculatory dysfunction in ST-elevation myocardial infarction: cause, consequence, or both? Eur Heart J. 2007;28:788–797. doi: 10.1093/eurheartj/ehl501. [DOI] [PubMed] [Google Scholar]
- 18.Prasad A, Stone GW, Holmes DR, Gersh B. Reperfusion injury, microvascular dysfunction, and cardioprotection: the "dark side" of reperfusion. Circulation. 2009;120:2105–2112. doi: 10.1161/CIRCULATIONAHA.108.814640. [DOI] [PubMed] [Google Scholar]
- 19.Zhang C, Rogers P, Merkus D, et al. Regulation of coronary microvascular resistance in health and disease. Comprehensive Physiology. 2011;12:521–549. [Google Scholar]
- 20.Canty JM, Klocke FJ. Reduced regional myocardial perfusion in the presence of pharmacologic vasodilator reserve. Circulation. 1985;71:370–377. doi: 10.1161/01.cir.71.2.370. [DOI] [PubMed] [Google Scholar]
- 21.Heusch G, Baumgart D, Camici P, et al. a-Adrenergic coronary vasoconstriction and myocardial ischemia in humans. Circulation. 2000;101:689–694. doi: 10.1161/01.cir.101.6.689. [DOI] [PubMed] [Google Scholar]
- 22.Heusch G, Guth BD, Seitelberger R, Ross J., Jr. Attenuation of exercise-induced myocardial ischemia in dogs with recruitment of coronary vasodilator reserve by nifedipine. Circulation. 1987;75:482–490. doi: 10.1161/01.cir.75.2.482. [DOI] [PubMed] [Google Scholar]
- 23.Heusch G, Schulz R, Rahimtoola SH. Myocardial hibernation: a delicate balance. Am J Physiol Heart Circ Physiol. 2005;288:H984–H999. doi: 10.1152/ajpheart.01109.2004. [DOI] [PubMed] [Google Scholar]
- 24.Sorop O, Merkus D, de Beer VJ, et al. Functional and structural adaptations of coronary microvessels distal to a chronic coronary artery stenosis. Circ Res. 2008;102:795–803. doi: 10.1161/CIRCRESAHA.108.172528. [DOI] [PubMed] [Google Scholar]
- 25.Mills I, Fallon JT, Wrenn D, et al. Adaptive responses of coronary circulation and myocardium to chronic reduction in perfusion pressure and flow. Am J Physiol Heart Circ Physiol. 1994;266:H447–H457. doi: 10.1152/ajpheart.1994.266.2.H447. [DOI] [PubMed] [Google Scholar]
- 26.Heusch G, Kleinbongard P, Boese D, et al. Coronary microembolization: from bedside to bench and back to bedside. Circulation. 2009;120:1822–1836. doi: 10.1161/CIRCULATIONAHA.109.888784. [DOI] [PubMed] [Google Scholar]
- 27.Kloner RA, Ganote CE, Jennings RB. The "no-reflow" phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54:1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dörge H, Neumann T, Behrends M, et al. Perfusion-contraction mismatch with coronary microvascular obstruction: role of inflammation. Am J Physiol Heart Circ Physiol. 2000;279:H2587–H2592. doi: 10.1152/ajpheart.2000.279.6.H2587. [DOI] [PubMed] [Google Scholar]
- 29.Falk E. Unstable angina with fatal outcome: dynamic coronary thrombosis leading to infarction and/or sudden death. Autopsy evidence of recurrent mural thrombosis with peripheral embolization culminating in total vascular occlusion. Circulation. 1985;71:699–708. doi: 10.1161/01.cir.71.4.699. [DOI] [PubMed] [Google Scholar]
- 30.Skyschally A, Gres P, Hoffmann S, et al. Bidirectional role of tumor necrosis factor-a in coronary microembolization: progressive contractile dysfunction versus delayed protection against infarction. Circ Res. 2007;100:140–146. doi: 10.1161/01.RES.0000255031.15793.86. [DOI] [PubMed] [Google Scholar]
- 31.Lecour S. Activation of the protective survivor activating factor enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK path? J Mol Cell Cardiol. 2009;47:32–40. doi: 10.1016/j.yjmcc.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Heusch G. Nitroglycerin and delayed preconditioning in humans. Yet another new mechanism for an old drug? Circulation. 2001;103:2876–2878. doi: 10.1161/01.cir.103.24.2876. [DOI] [PubMed] [Google Scholar]
- 33.Rezkalla SH, Kloner RA. Ischemic preconditioning and preinfarction angina in the clinical arena. Nature Clin Pract Cardiovasc Med. 2004;1:96–102. doi: 10.1038/ncpcardio0047. [DOI] [PubMed] [Google Scholar]
- 34.Skyschally A, Walter B, Heusch G. Coronary microembolization during early reperfusion - infarct extension, but protection by ischemic postconditioning. Eur Heart J. 2012;34:3314–3321. doi: 10.1093/eurheartj/ehs434. [DOI] [PubMed] [Google Scholar]
- 35.Kleinbongard P, Boese D, Baars T, et al. Vasoconstrictor potential of coronary aspirate from patients undergoing stenting of saphenous vein aortocoronary bypass grafts and its pharmacological attenuation. Circ Res. 2011;108:344–352. doi: 10.1161/CIRCRESAHA.110.235713. [DOI] [PubMed] [Google Scholar]
- 36.Kleinbongard P, Baars T, Mohlenkamp S, Kahlert P, Erbel R, Heusch G. Aspirate from human stented native coronary arteries vs. saphenous vein grafts: more endothelin but less particulate debris. Am J Physiol Heart Circ Physiol. 2013;305:H1222–H1229. doi: 10.1152/ajpheart.00358.2013. [DOI] [PubMed] [Google Scholar]
- 37.Desmet W, Bogaert J, Dubois C, et al. High-dose intracoronary adenosine for myocardial salvage in patients with acute ST-segment elevation myocardial infarction. Eur Heart J. 2011;32:867–877. doi: 10.1093/eurheartj/ehq492. [DOI] [PubMed] [Google Scholar]
- 38.Robbers LF, Eerenberg ES, Teunissen PF, et al. Magnetic resonance imaging-defined areas of microvascular obstruction after acute myocardial infarction represent microvascular destruction and haemorrhage. Eur Heart J. 2013;34:2346–2353. doi: 10.1093/eurheartj/eht100. [DOI] [PubMed] [Google Scholar]
- 39.Niccoli G, Burzotta F, Galiuto L, Crea F. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54:281–292. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 40.Sorajja P, Gersh BJ, Costantini C, et al. Combined prognostic utility of ST-segment recovery and myocardial blush after primary percutaneous coronary intervention in acute myocardial infarction. Eur Heart J. 2005;26:667–674. doi: 10.1093/eurheartj/ehi167. [DOI] [PubMed] [Google Scholar]
- 41.Fearon WF, Low AF, Yong AS, et al. Prognostic value of the index of microcirculatory resistance measured after primary percutaneous coronary intervention. Circulation. 2013;127:2436–2441. doi: 10.1161/CIRCULATIONAHA.112.000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duncker DJ, de Beer VJ, Merkus D. Alterations in vasomotor control of coronary resistance vessels in remodelled myocardium of swine with a recent myocardial infarction. Med Biol Eng Comput. 2008;46:485–497. doi: 10.1007/s11517-008-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 44.Heusch G. Cardioprotection: chances and challenges of its translation to the clinic. Lancet. 2013;381:166–175. doi: 10.1016/S0140-6736(12)60916-7. [DOI] [PubMed] [Google Scholar]
- 45.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Z-Q, Corvera JS, Halkos ME, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–H588. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 47.Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic "preconditioning" protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–899. doi: 10.1161/01.cir.87.3.893. [DOI] [PubMed] [Google Scholar]
- 48.Ovize M, Thibault H, Przyklenk K. Myocardial conditioning: opportunities for clinical translation. Circ Res. 2013;113:439–450. doi: 10.1161/CIRCRESAHA.113.300764. [DOI] [PubMed] [Google Scholar]
- 49.Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 50.Schulman D, Latchman DS, Yellon DM. Urocortin protects the heart from reperfusion injury via upregulaiton of p42/p44 MAPK signaling pathway. Am J Physiol Heart Circ Physiol. 2002;283:H1481–H1488. doi: 10.1152/ajpheart.01089.2001. [DOI] [PubMed] [Google Scholar]
- 51.Hausenloy DJ, Yellon DM. New directions for protecting the heart against ischaemia-reperfusion injury: targeting the reperfusion injury salvage kinase (RISK)-pathway. Cardiovasc Res. 2004;61:448–460. doi: 10.1016/j.cardiores.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 52.Wagner C, Kloeting I, Strasser RH, Weinbrenner C. Cardioprotection by postconditioning is lost in WOKW rats with metabolic syndrome: role of glycogen synthase kinase 3beta. J Cardiovasc Pharmacol. 2008;52:430–437. doi: 10.1097/FJC.0b013e31818c12a7. [DOI] [PubMed] [Google Scholar]
- 53.Bouhidel O, Pons S, Souktani R, Zini R, Berdeaux A, Ghaleh B. Myocardial ischemic postconditioning against ischemia-reperfusion is impaired in ob/ob mice. Am J Physiol Heart Circ Physiol. 2008;295:H1580–H1586. doi: 10.1152/ajpheart.00379.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev. 2007;59:418–458. doi: 10.1124/pr.107.06002. [DOI] [PubMed] [Google Scholar]
- 55.Sivaraman V, Hausenloy DJ, Wynne AM, Yellon DM. Preconditioning the diabetic human myocardium. J Cell Mol Med. 2010;14:1740–1746. doi: 10.1111/j.1582-4934.2009.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Piot C, Croisille P, Staat P, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 57.Mewton N, Croisille P, Gahide G, et al. Effect of cyclosporine on left ventricular remodeling after reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:1200–1205. doi: 10.1016/j.jacc.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 58.Staat P, Rioufol G, Piot C, et al. Postconditioning the human heart. Circulation. 2005;112:2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 59.Thibault H, Piot C, Staat P, et al. Long-term benefit of postconditioning. Circulation. 2008;117:1037–1044. doi: 10.1161/CIRCULATIONAHA.107.729780. [DOI] [PubMed] [Google Scholar]
- 60.Mewton N, Thibault H, Roubille F, et al. Postconditioning attenuates no-reflow in STEMI patients. Basic Res Cardiol. 2013;108:383. doi: 10.1007/s00395-013-0383-8. [DOI] [PubMed] [Google Scholar]
- 61.Hausenloy DJ, Mwamure PK, Venugopal V, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomized controlled trial. Lancet. 2007;370:575–579. doi: 10.1016/S0140-6736(07)61296-3. [DOI] [PubMed] [Google Scholar]
- 62.Thielmann M, Kottenberg E, Boengler K, et al. Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol. 2010;105:657–664. doi: 10.1007/s00395-010-0104-5. [DOI] [PubMed] [Google Scholar]
- 63.Botker HE, Kharbanda R, Schmidt MR, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 64.Heusch G, Musiolik J, Gedik N, Skyschally A. Mitochondrial STAT3 activation and cardioprotection by ischemic postconditioning in pigs with regional myocardial ischemia/reperfusion. Circ Res. 2011;109:1302–1308. doi: 10.1161/CIRCRESAHA.111.255604. [DOI] [PubMed] [Google Scholar]
- 65.Heusch G, Musiolik J, Kottenberg E, Peters J, Jakob H, Thielmann M. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans. Circ Res. 2012;110:111–115. doi: 10.1161/CIRCRESAHA.111.259556. [DOI] [PubMed] [Google Scholar]
- 66.Lonborg J, Vejlstrup N, Kelbaek H, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–1499. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 67.Ibanez B, Macaya C, Sanchez-Brunete V, et al. Effect of early metoprolol on infarct size in ST-segment-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention: The effect of metoprolol in cardioprotection during an acute myocardial infarction (METOCARD-CNIC) trial. Circulation. 2013;128:1495–1503. doi: 10.1161/CIRCULATIONAHA.113.003653. [DOI] [PubMed] [Google Scholar]
- 68.Selker HP, Beshansky JR, Sheehan PR, et al. Out-of-hospital administration of intravenous glucose-insulin-potassium in patients with suspected acute coronary syndromes: The IMMEDIATE Randomized Controlled Trial. JAMA. 2012;107:1925–1933. doi: 10.1001/jama.2012.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thuny F, Lairez O, Roubille F, et al. Post-Conditioning Reduces Infarct Size and Edema in Patients With ST-Segment Elevation Myocardial Infarction. J Am Coll Cardiol. 2012;59:2175–2181. doi: 10.1016/j.jacc.2012.03.026. [DOI] [PubMed] [Google Scholar]
- 70.Hahn JY, Song YB, Kim EK, et al. Ischemic Postconditioning during Primary Percutaneous Coronary Intervention: The POST Randomized Trial. Circulation. 2013;128:1889–1896. doi: 10.1161/CIRCULATIONAHA.113.001690. [DOI] [PubMed] [Google Scholar]
- 71.Davies WR, Brown AJ, Watson W, et al. Remote Ischemic Preconditioning Improves Outcome at 6 Years After Elective Percutaneous Coronary Intervention: The CRISP Stent Trial Long-Term Follow-Up. Circ Cardiovasc Interv. 2013;6:246–251. doi: 10.1161/CIRCINTERVENTIONS.112.000184. [DOI] [PubMed] [Google Scholar]
- 72.Thielmann M, Kottenberg E, Kleinbongard P, et al. Cardioprotection and prognosis by remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597–604. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- 73.Sloth AD, Schmidt MR, Munk K, et al. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2013 doi: 10.1093/eurheartj/eht369. [DOI] [PubMed] [Google Scholar]
- 74.Knoll R, Iaccarino G, Tarone G, et al. Towards a re-definition of 'cardiac hypertrophy' through a rational characterization of left ventricular phenotypes: a position paper of the Working Group 'Myocardial Function' of the ESC. Eur J Heart Fail. 2011;13:811–819. doi: 10.1093/eurjhf/hfr071. [DOI] [PubMed] [Google Scholar]
- 75.Ohtani T, Mohammed SF, Yamamoto K, et al. Diastolic stiffness as assessed by diastolic wall strain is associated with adverse remodelling and poor outcomes in heart failure with preserved ejection fraction. Eur Heart J. 2012;33:1742–1749. doi: 10.1093/eurheartj/ehs135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 77.Guazzi M, Arena R. Pulmonary hypertension with left-sided heart disease. Nat Rev Cardiol. 2010;7:648–659. doi: 10.1038/nrcardio.2010.144. [DOI] [PubMed] [Google Scholar]
- 78.Voelkel NF, Quaife RA, Leinwand LA, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 79.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 80.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bostrom P, Mann N, Wu J, et al. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pandya K, Porter K, Rockman HA, Smithies O. Decreased beta-adrenergic responsiveness following hypertrophy occurs only in cardiomyocytes that also re-express beta-myosin heavy chain. Eur J Heart Fail. 2009;11:648–652. doi: 10.1093/eurjhf/hfp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 84.Alpert NR, Mohiddin SA, Tripodi D, et al. Molecular and phenotypic effects of heterozygous, homozygous, and compound heterozygote myosin heavy-chain mutations. Am J Physiol Heart Circ Physiol. 2005;288:H1097–H1102. doi: 10.1152/ajpheart.00650.2004. [DOI] [PubMed] [Google Scholar]
- 85.Olivetti G, Abbi R, Quaini F, et al. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 86.Konstantinidis K, Whelan RS, Kitsis RN. Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc Biol. 2012;32:1552–1562. doi: 10.1161/ATVBAHA.111.224915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yan L, Vatner DE, Kim S-J, et al. Autophagy in chronically ischemic myocardium. PNAS. 2005;102:13807–13812. doi: 10.1073/pnas.0506843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elsässer A, Vogt AM, Nef H, et al. Human hibernating myocardium is jeopardized by apoptotic and autophagic cell death. J Am Coll Cardiol. 2004;43:2191–2199. doi: 10.1016/j.jacc.2004.02.053. [DOI] [PubMed] [Google Scholar]
- 89.Kanamori H, Takemura G, Goto K, et al. The role of autophagy emerging in postinfarction cardiac remodelling. Cardiovasc Res. 2011;91:330–339. doi: 10.1093/cvr/cvr073. [DOI] [PubMed] [Google Scholar]
- 90.Patel BM, Mehta AA. Aldosterone and angiotensin: Role in diabetes and cardiovascular diseases. Eur J Pharmacol. 2012;697:1–12. doi: 10.1016/j.ejphar.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 91.Ahokas RA, Warrington KJ, Gerling IC, et al. Aldosteronism and peripheral blood mononuclear cell activation: a neuroendocrine-immune interface. Circ Res. 2003;93:e124–e135. doi: 10.1161/01.RES.0000102404.81461.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Harrison DG, Cai H, Landmesser U, Griendling KK. Interactions of angiotensin II with NAD(P)H oxidase, oxidant stress and cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2003;4:51–61. doi: 10.3317/jraas.2003.014. [DOI] [PubMed] [Google Scholar]
- 93.Jensen BC, O'Connell TD, Simpson PC. Alpha-1-adrenergic receptors: targets for agonist drugs to treat heart failure. J Mol Cell Cardiol. 2011;51:518–528. doi: 10.1016/j.yjmcc.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castellano M, Bohm M. The cardiac beta-adrenoceptor-mediated signaling pathway and its alterations in hypertensive heart disease. Hypertension. 1997;29:715–722. doi: 10.1161/01.hyp.29.3.715. [DOI] [PubMed] [Google Scholar]
- 95.Böhm M, Gierschik P, Jakobs K-H, et al. Increase of G1a in human hearts with dilated but not ischemic cardiomyopathy. Circulation. 1990;82:1249–1265. doi: 10.1161/01.cir.82.4.1249. [DOI] [PubMed] [Google Scholar]
- 96.Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998;98:1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- 97.Neef S, Maier LS. Novel aspects of excitation-contraction coupling in heart failure. Basic Res Cardiol. 2013;108:360. doi: 10.1007/s00395-013-0360-2. [DOI] [PubMed] [Google Scholar]
- 98.Hasenfuss G, Reinecke H, Studer R, et al. Relation between myocardial function and expression of sarcoplasmic reticulum Ca2+-ATPase in failing and nonfailing human myocardium. Circ Res. 1994;75:434–442. doi: 10.1161/01.res.75.3.434. [DOI] [PubMed] [Google Scholar]
- 99.Shan J, Betzenhauser MJ, Kushnir A, et al. Role of chronic ryanodine receptor phosphorylation in heart failure and beta-adrenergic receptor blockade in mice. J Clin Invest. 2010;120:4375–4387. doi: 10.1172/JCI37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Teekakirikul P, Eminaga S, Toka O, et al. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-beta. J Clin Invest. 2010;120:3520–3529. doi: 10.1172/JCI42028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takeda N, Manabe I, Uchino Y, et al. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2009;120:254–265. doi: 10.1172/JCI40295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Christia P, Bujak M, Gonzalez-Quesada C, et al. Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction. J Histochem Cytochem. 2013;61:555–570. doi: 10.1369/0022155413493912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zile MR, Desantis SM, Baicu CF, et al. Plasma biomarkers that reflect determinants of matrix composition identify the presence of left ventricular hypertrophy and diastolic heart failure. Circ Heart Fail. 2011;4:246–256. doi: 10.1161/CIRCHEARTFAILURE.110.958199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Okin PM, Devereux RB, Jern S, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–2349. doi: 10.1001/jama.292.19.2343. [DOI] [PubMed] [Google Scholar]
- 105.Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 106.Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59:901–909. doi: 10.1016/j.jacc.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 107.Böhm M, Swedberg K, Komajda M, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886–894. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 108.Tardif JC, O'Meara E, Komajda M, et al. Effects of selective heart rate reduction with ivabradine on left ventricular remodelling and function: results from the SHIFT echocardiography substudy. Eur Heart J. 2011;32:2507–2515. doi: 10.1093/eurheartj/ehr311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 110.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 111.Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 112.Conway MA, Allis J, Ouwerkerk R, Niioka T, Rajagopalan B, Radda GK. Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet. 1991;338:973–976. doi: 10.1016/0140-6736(91)91838-l. [DOI] [PubMed] [Google Scholar]
- 113.Nascimben L, Friedrich J, Liao R, Pauletto P, Pessina AC, Ingwall JS. Enalapril treatment increases cardiac performance and energy reserve via the creatine kinase reaction in myocardium of Syrian myopathic hamsters with advanced heart failure. Circulation. 1995;91:1824–1833. doi: 10.1161/01.cir.91.6.1824. [DOI] [PubMed] [Google Scholar]
- 114.Finck BN, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation. 2007;115:2540–2548. doi: 10.1161/CIRCULATIONAHA.107.670588. [DOI] [PubMed] [Google Scholar]
- 115.Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res. 2009;81:412–419. doi: 10.1093/cvr/cvn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Davila-Roman VG, Vedala G, Herrero P, et al. Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:271–277. doi: 10.1016/s0735-1097(02)01967-8. [DOI] [PubMed] [Google Scholar]
- 117.Taylor M, Wallhaus TR, DeGrado TR, et al. An evaluation of myocardial fatty acid and glucose uptake using PET with [18F]fluoro-6-thia-heptadecanoic acid and [18F]FDG in Patients with Congestive Heart Failure. J Nucl Med. 2001;42:55–62. [PubMed] [Google Scholar]
- 118.Opie LH, Knuuti J. The adrenergic-fatty acid load in heart failure. J Am Coll Cardiol. 2009;54:1637–1646. doi: 10.1016/j.jacc.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 119.Funada J, Betts TR, Hodson L, et al. Substrate utilization by the failing human heart by direct quantification using arterio-venous blood sampling. PLoS ONE. 2009;4:e7533. doi: 10.1371/journal.pone.0007533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mori J, Basu R, McLean BA, et al. Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: a metabolic contribution to heart failure with normal ejection fraction. Circ Heart Fail. 2012;5:493–503. doi: 10.1161/CIRCHEARTFAILURE.112.966705. [DOI] [PubMed] [Google Scholar]
- 121.Kato T, Niizuma S, Inuzuka Y, et al. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail. 2010;3:420–430. doi: 10.1161/CIRCHEARTFAILURE.109.888479. [DOI] [PubMed] [Google Scholar]
- 122.Bersin RM, Wolfe C, Kwasman M, et al. Improved hemodynamic function and mechanical efficiency in congestive heart failure with sodium dichloroacetate. J Am Coll Cardiol. 1994;23:1617–1624. doi: 10.1016/0735-1097(94)90665-3. [DOI] [PubMed] [Google Scholar]
- 123.Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Circ Res. 2000;86:580–588. doi: 10.1161/01.res.86.5.580. [DOI] [PubMed] [Google Scholar]
- 124.Gao D, Ning N, Niu X, Hao G, Meng Z. Trimetazidine: a meta-analysis of randomised controlled trials in heart failure. Heart. 2011;97:278–286. doi: 10.1136/hrt.2010.208751. [DOI] [PubMed] [Google Scholar]
- 125.Lee L, Campbell R, Scheuermann-Freestone M, et al. Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation. 2005;112:3280–3288. doi: 10.1161/CIRCULATIONAHA.105.551457. [DOI] [PubMed] [Google Scholar]
- 126.Abozguia K, Elliott P, McKenna W, et al. Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation. 2010;122:1562–1569. doi: 10.1161/CIRCULATIONAHA.109.934059. [DOI] [PubMed] [Google Scholar]
- 127.Zhu P, Lu L, Xu Y, Schwartz GG. Troglitazone improves recovery of left ventricular function after regional ischemia in pigs. Circulation. 2000;101:1165–1171. doi: 10.1161/01.cir.101.10.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wallhaus TR, Taylor M, DeGrado TR, et al. Myocardial free fatty acid and glucose use after carvedilol treatment in patients with congestive heart failure. Circulation. 2001;103:2441–2446. doi: 10.1161/01.cir.103.20.2441. [DOI] [PubMed] [Google Scholar]
- 129.Dinicolantonio JJ, Lavie CJ, Fares H, Menezes AR, O'Keefe JH. Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol) Am J Cardiol. 2013;111:765–769. doi: 10.1016/j.amjcard.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 130.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 131.Opie LH. The metabolic vicious cycle in heart failure. Lancet. 2004;364:1733–1734. doi: 10.1016/S0140-6736(04)17412-6. [DOI] [PubMed] [Google Scholar]