Abstract
There has been renewed interest in the role of strategies in sensorimotor learning. The combination of new behavioral methods and computational methods has begun to unravel the interaction between processes related to strategic control and processes related to motor adaptation. These processes may operate on very different error signals. Strategy learning is sensitive to goal-based performance error. In contrast, adaptation is sensitive to prediction errors between the desired and actual consequences of a planned movement. The former guides what the desired movement should be, whereas the latter guides how to implement the desired movement. Whereas traditional approaches have favored serial models in which an initial strategy-based phase gives way to more automatized forms of control, it now seems that strategic and adaptive processes operate with considerable independence throughout learning, although the relative weight given the two processes will shift with changes in performance. As such, skill acquisition involves the synergistic engagement of strategic and adaptive processes.
Keywords: motor learning, motor adaptation, motor skills, cognition
Introduction
At a high school track meet in 1963, an athlete from Oregon changed the face of high jumping by falling, figuratively and literally, into a new technique.1 Dick Fosbury had struggled to clear even modest heights using the “Western Roll,” the popular technique at the time, in which the athlete extends his chest over the bar. After several embarrassing performances, Fosbury reverted to an antiquated scissors technique in which he simply hurdled sideways over the bar. On one attempt, he leaned back, thrusting his hips over the bar, and landing on his back. Not only did he clear the bar, but with subsequent jumps, he began to exaggerate this technique, fully throwing his back over the bar. By the end of the tournament, he had increased his personal record by half a foot. Although this improvement initially brought him up to the level achieved by top performers who were using the Western Roll, Fosbury went on to refine the technique over subsequent years, with his crowning achievement being a gold medal at the 1968 Olympics in Mexico City. Within a few years, nearly all jumpers had adopted the technique that to this day bears his name, the Fosbury Flop.
Fosbury’s success led to a paradigm shift in the high jumping world. The impact of his technique was similar to that observed with other major innovations in high jumping (Fig. 1). The progression of world records generally shows a cyclical pattern. After the introduction of a new technique, there is a period in which the world record climbs in a steady manner over a relatively short period, followed by a rather lengthy plateau. Indeed, the current plateau of the Fosbury era has lasted since 1993, when Javier Sotomayor of Cuba cleared 2.45 m.
Figure 1.
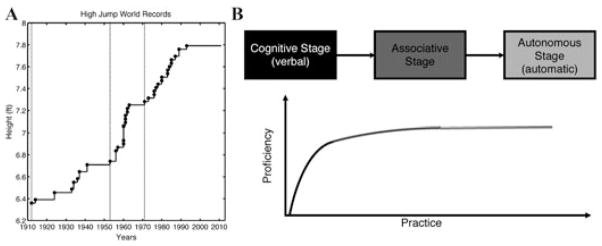
(A) World high jump records over the past century. Dashed vertical lines mark periods in which a particular technique was used by almost all jumpers. (B) Fitts and Posner model of skill acquisition. Their theory posits that skill acquisition follows three sequential stages: cognitive (black), associative (dark gray), and autonomous (light gray). The rate of skill acquisition varies across the three stages.
The history of high jumping establishes the theme for this review. When we think about motor skills, we typically focus on the performer’s ability to execute a movement: how an exceptional quarterback has a rocket arm, or how the star tennis player gets such extraordinary power on her two-handed backhand. Missing from much of this discussion, however, is the role of insight and strategy. What led Fosbury to try going over the bar backward? How does the application of a cognitive strategy change performance and ultimately affect learning?
Studies of motor learning give little consideration to the role of cognitive strategies, in part because such processes are generally hard to formalize and are often variable. We address this limitation in this review and highlight experimental methods that have sought to directly assess the contribution of cognitive strategies in sensorimotor adaptation. We then discuss how computational models can incorporate such processes, and provide a means to understand quantitatively the contribution of cognitive processes to motor learning.
Stages of learning
Fitts and Posner2 proposed a model of skill acquisition that centered on three stages. In their now-classic theory, performance was characterized by three sequential stages, termed the cognitive, associative, and autonomous stages (Fig. 1B). The cognitive stage marks the period in which the task goals are established and used to determine the appropriate sequence of actions to achieve the desired goal. Learning at this stage generally involves the use of explicit knowledge. For Fosbury, the decision to go over the bar backward would constitute the cognitive stage. Once the action sequence has been determined, the learner enters the associative stage in which attention may be focused on specific details of the sequence, determining the appropriate subparts and transitions. This stage may require some exploration of the solution space, perhaps with one segment being overhauled to ensure that the overall action is executed in a smooth and coordinated manner. Although Fosbury pioneered the idea of leading with his back, other jumpers came along to refine this general strategy and develop the proper foot placement, timing, and body orientation. The final stage of learning is the autonomous stage, the phase in which the action is practiced to hone performance into an automatized routine. For high jumping, we might say that Fosbury and his peers guided a generation of jumpers through cognitive and associative stages. But each of these individuals must put in the countless hours of practice required for elite performance that results from the autonomous stage.
More generally, learning curves across a wide range of tasks show a general shape that conforms to the basic model of Fitts and Posner.2 There is an initial phase marked by rapid improvements in performance, followed by a more gradual phase in which performance gains accrue much more slowly. Numerous theories have been proposed to account for these functions.3,4 In the Fitts and Posner2 model, the emphasis is on a shift in control in which initial, explicit control gives way to more routinized forms of control. Other models have emphasized that these functions may reflect the parallel operation of multiple processes. Logan5 introduced a theory in which execution reflected a horse race between an algorithmic, explicit process (akin to the cognitive stage) and a memory-retrieval process. Although both processes were assumed to operate at all stages of performance, a shift in their relative contribution naturally arises over time as the memory base builds up.
Psychological theories such as those of Fitts and Posner2 or Logan5 offer a general framework for understanding skill acquisition functions. Similar learning functions are observed in studies of sensorimotor adaptation. This work has spawned a rich computational literature in which performance changes are analyzed from an engineering perspective grounded in ideas related to control systems. However, this new modeling perspective has just begun to address the role of cognitive processes during motor learning, processes that were inherent in the models of Fitts and Posner2 and Logan.5
Sensorimotor adaptation
A common method to study motor learning is to introduce a perturbation into the experimental context. Participants must learn to compensate for these perturbations to re-achieve a high level of performance. The perturbation introduces an error between a motor command and a desired outcome. This error signal serves as input used to update an internal model, a mapping between a desired goal and the motor response necessary to achieve that goal (Fig. 2A). In this manner, the mapping is refined to adjust the motor commands. In general, the goal is assumed to remain constant; for example, the high jumper always wants to clear the bar. Failure to achieve this goal may lead to changes in performance, such as a modification in the takeoff angle or timing of the initial thrust.
Figure 2.

(A) Hypothetical learning curve during adaptation to visuomotor rotation. A 45° rotation is imposed during movements 81–160. Target errors are initially in the direction of the rotation, but with training, adaptation occurs. The rotation is removed on trial 161 and an aftereffect is observed in which target errors are in the direction opposite to the rotation. (B) Virtual reality environments are used to impose systematic transformation between actual and projected hand position. Vision of the limb is occluded. In this example, the target is the gray circle and a 4° rotation clockwise led to displacement of feedback location (black circle).
A wide range of experimental paradigms has been employed to study sensorimotor adaptation. One popular task involves a visuomotor rotation in which the visual feedback indicating hand position is perturbed (Fig. 2B). Visuomotor adaptations are common in everyday life. For example, using a computer mouse requires learning the mapping between the hand-held device and the position of a cursor on a computer screen. In the experimental context, this mapping can be perturbed. In many studies the input–output relationship between a device such as a mouse or joystick is altered. In other conditions, participants make reaching movements in which the hand is not visible, and a cursor is used to provide feedback. The natural mapping between the hand and space is distorted. In a visuomotor rotation, feedback of the hand position is adjusted in a rotational manner. The rotations typically take on values ranging between 30° and 60°.6–9 This task is nicely situated to examine the interaction of action selection and motor execution.
Participants readily adapt to visual perturbations, showing a reduction in target errors with training. Adaptation proceeds in a gradual manner, in which the learning function typically conforms to an exponentially decaying function. This pattern is consistent with the hypothesis that an error signal is used to continuously adjust the visuomotor mapping, with the magnitude of the change proportional to the error. Thus, large errors observed early in training produce relatively large changes in performance compared to the effects of small changes that occur late in training. After training, the visuomotor rotation is removed and the original environment is reinstated. This induces a pronounced aftereffect with errors now occurring in the opposite direction of the initial distortion. If feedback is provided, the learning process is repeated to “wash out” the effects of the altered sensorimotor mapping and restore the original mapping. The presence of an aftereffect is considered the hallmark of true adaptation. Performance gains (e.g., reduced error) are also possible from the implementation of a strategy or a change in the selected action; however, in either case, an aftereffect should either be absent or diminish rapidly. The term “motor learning” generally encompasses changes that may entail a combination of the alteration of a sensorimotor map from adaptation and performance gains resulting from other, nonadaptive processes.
Movement strategies
In the typical visuomotor adaptation study, the perturbation is suddenly introduced after a baseline period of training. On the first trial, the participant will be surprised to see a large error. For example, if feedback is only presented at the endpoint of the movement, the participant suddenly sees feedback indicating an error of 30°. Although one trial may be written off as a chance event, the repetition of this error with subsequent trials leads many participants to become aware that the environment has been perturbed. This awareness suggests an alternative account of visuomotor adaptation: the participant may adopt a strategy to aim their movement in the direction opposite the rotation.
It is generally assumed that strategy-based learning is not a major contributing factor in visuomotor adaptation. First, the learning function during the washout period is similar in form, albeit with a steeper learning rate, than that observed during the initial learning phase. If the participant were employing a strategy, one would expect washout to occur in a more or less categorical manner. That is, the participant could simply choose to not apply the strategy to again establish the normal sensorimotor mapping. Nonetheless, although learning may involve more than the instantiation of a strategy, it is important to recognize that there may be a contribution to performance from strategic processes. More importantly, it is important to consider how a strategy, if employed, influences processes involved in sensorimotor adaptation.
As an everyday example, consider how people adjust their behavior when riding on a crowded city bus. If forced to stand, one might cocontract the leg muscles to increase stiffness, making the legs (and person) resistant to small and unexpected changes in acceleration. Although this strategy can be effective, it is energetically wasteful. An alternative, more adaptive procedure is for the motor system to predict upcoming perturbations. Suppose both processes are operative. How does the utilization of the cocontraction strategy influence motor adaptation? If we assume the input to the adaptation system is a motor error, the adaptation system may work in a suboptimal manner because the motor error is significantly reduced by cocontraction. Is the adaptation system able to incorporate information about the level of cocontraction in its computations? Or does this system operate in a modular manner, ignorant of the context created by the decision of the person to stiffen the limbs?
One approach to exploring these questions is to compare conditions in which participants are either aware or unaware of an experimentally induced perturbation.10,11 As noted above, in the standard visuomotor adaptation task, a large rotation is abruptly imposed. This produces both a large error signal and, in many situations, creates a situation in which the participants are aware that the environment has been altered. Alternatively, the perturbation can be introduced in small increments, such as 1° every 10 trials, with the full 90° rotation only achieved after 300 trials (Fig. 3A). Under these conditions, participants generally have no awareness of the perturbation because the induced visual error is within the bounds of the variability associated with the motor system. Adaptation occurs in a continuous manner under these conditions, preventing the small errors from accumulating to a level that is noticeable (Fig. 3B). By the end of training, the performance of the participants is similar. However, when the rotation is switched off, the aftereffect is generally smaller for participants in the abrupt condition.10 Moreover, when participants gain knowledge of the rotation through self-inference or instruction, performance is associated with large trial-by-trial variance and longer reaction times, at least in the early stages of adaptation.12–14
Figure 3.
(A) A 90° visuomotor rotation can be introduced in a single step (thick line) or gradually across multiple steps (thin line). (B) Curvature of trajectories as measured by root mean square error (RMSE) when online feedback is available for the abrupt (thick) and gradual (thin) conditions. By the end of training, the degree of error is similar for the two conditions. Adapted from Kagerer et al.10
These features suggest that the participants’ awareness of the perturbation in the abrupt condition may alter their performance. Some participants may opt to test strategies to offset the large error observed after the onset of the rotation,15 although the use of such strategies is likely to be highly idiosyncratic across individuals, as well as variable across trials for a given individual. Consistent with a strategy-use hypothesis is the observation that participants with higher spatial working memory capacity tend to exhibit faster rates of adaptation.16 Verbal working memory has also been linked to motor adaptation. When sequentially trained with two different sensorimotor mappings, a subsequent verbal learning task only disrupted the memory of the most recent mapping.17 Interestingly, this effect was not observed when the verbal task did not involve learning (e.g., vowel counting), suggesting that the point of overlap was within processes associated with learning per se. The specificity here may reflect interference with a verbal strategy (e.g., “On the next trial, I will push to the left”).
Dual-task manipulations have offered an indirect method for assessing strategic contributions to motor learning. The underlying logic is that the cognitive requirements for maintaining and implementing a strategy would be taxed by a concurrent secondary task. Indeed, when a secondary task is performed concurrently with a sensorimotor adaptation task, performance gains during training are reduced.18–21 Interestingly, even seemingly automatic processes, such as learning a new spatiotemporal walking pattern, are affected by dual-task interference.22 Moreover, the effects of dual-task interference are not limited to conditions in which the participants are aware of the sensorimotor perturbation.21 Thus, dual-task costs may be unrelated to the deployment of strategic processes. Instead, the interference may result from other shared stages of processing such as the sensory processing requirements for the primary and secondary tasks.
Direct interaction of action selection and motor adaptation
In most experimental paradigms, the use of a strategy during motor learning is the prerogative of the participant; the experimenter can only infer strategy use from the behavior. Mazzoni and Krakauer23 introduced a novel method to directly address the effect of strategy use on visuomotor adaptation. The workspace consisted of a display of eight visual landmarks, spaced 45° apart. On each trial, a visual target appeared at one of the landmarks. Participants were initially trained to reach directly to the target. After this baseline phase, a 45° counterclockwise rotation was introduced (Fig. 4A) and large visual errors were experienced for two trials. The experimenter then instructed the participant to use a strategy to counteract the rotation, aiming 45° in the clockwise direction to the neighboring landmark (Fig. 4B). The strategy was immediately effective, counteracting the visual error. Surprisingly, as training continued, performance deteriorated: the movement endpoints drifted over trials in the direction of the strategy. That is, the heading angles were greater than the instructed 45° (Fig. 4C). Thus, the participants’ performance became worse with increasing practice (Fig. 4D).
Figure 4.
Visuomotor adaptation with landmarks. (A) Displays contain eight empty circles, indicating possible target locations arranged along an invisible ring. One circle turns gray, indicating the target for that trial. Feedback indicates the position of the cursor at the time the movement amplitude exceeds the radius of the ring. During rotation phases of the study, the feedback is presented 45° in the counterclockwise direction of the true hand position. (B) In the rotation with strategy block, participants were instructed to move to the neighboring target landmark to offset for the rotation. (C) By the end of rotation + strategy block, the reaches had drifted in the direction of the aiming target, resulting in increased target error. (D) Target errors are centered around zero during the baseline block (1). Large errors are observed when the rotation is unexpectedly introduced (2). When instructed to use the strategy, movements are initially very accurate but, over time, performance deteriorates with error drifting in the direction of the strategy (3). Aftereffect is observed when participants are instructed to stop using the strategy (4). Adapted from Mazzoni and Krakauer.23
What can account for this puzzling effect? Why would the system continue to change despite good on-target performance? Mazzoni and Krakauer23 proposed that this phenomenon reflected the ongoing operation of an implicit motor adaptation system. Importantly, the error signal used for adaptation is based on the difference between the desired aiming location and the visual feedback of the cursor. That is, the adaptation process does not take into account the difference between the movement goal (e.g., the target) and movement feedback, despite the fact that this information defines task success. As emphasized by Mazzoni and Krakauer,23 the drift phenomenon provides strong evidence that sensorimotor adaptation processes are segregated from goal-based movement strategies.
We set out to further explore the interaction, or lack thereof, between strategic and adaptation processes. In their original study, Mazzoni and Krakauer23 limited training to 80 trials, and at the end of this period, the endpoint error had increased to over 25°, presumably because the adaptation system continued to compensate for the mismatch between the aiming location and the feedback location. Would this process continue to operate until the error was eliminated, resulting in an observed endpoint error of 45°? To test this, we quadrupled the training period to 320 trials.24 With this extended training, the basic drift effect was observed over the first part of the training period, followed by a reversal, with performance becoming near perfect by the end of training.
To quantify this nonmonotonic behavior, we developed a novel state space model to capture trial-by-trial changes in performance.24 The key idea in the model is that the output is the result of two learning processes, one associated with movement execution and the other with action planning. Moreover, these two processes use distinct error signals. First, the difference between the desired movement and the actual outcome, what we call aiming error, is used by the adaptation system to recalibrate an internal model. This component is similar to standard state space models of sensorimotor adaptation.25–27 However, in most studies, the desired movement is usually directed at the target; in the strategy-use variant, the desired movement is now directed at an aiming location. Second, the difference between the target and feedback location defines a target error, a signal that is used to adjust the movement strategy (Fig. 5A).
Figure 5.
(A) Implicit adaptation is based on an aiming error signal (black), defined as the difference between the aiming location and feedback location. Strategy adjustment is based on target error (gray), the difference between the target location and feedback location. (B) Aiming targets present. When a strategy is implemented to offset a rotation, target error is initially small because performance is accurate. However, aiming error is large, and adaptation leads to deterioration in accuracy. When target error becomes large, the effect of strategy adjustment becomes more prominent, leading to a reversal of the drift. The system eventually stabilizes even though the two learning processes continue to operate throughout training. The aftereffect observed when the rotation is removed reveals the magnitude of implicit adaptation. Circles: observed data for the group. Solid curve: model fit. (C) Disappearing aiming target group. The aiming target was turned off at movement onset. (D) No aiming target group. The aiming targets were never visible.
By simulating the parallel operation of these two processes, the model produces an excellent match to the function produced by our participants. Implementing a strategy immediately offsets the rotation. Over time, the target error increases, drifting in the direction of the strategy, and then the function reverses to stabilize on correct performance (Fig. 5B). Importantly, the model does not entail any sort of “stages” in which control shifts between “strategy-based” and “adaptation-based” phases. Rather, both processes are always functional. Drift is prominent during the early phase of the training period after strategy implementation because the aiming error is large and target error is small. This results in large changes in the adaptation system and small adjustments of the strategy. As target error becomes large, strategy adjustments become more prominent. Importantly, even when performance stabilizes with minimal target error, the two processes continue to operate, achieving a stable tension. This tension is evident in the aftereffect observed when the rotation is removed and participants are told to stop using the strategy.
In addition to highlighting the parallel operation of two learning processes, the model also provides a fresh take on how strategic and adaptation processes interact. Consistent with the conjecture of Mazzoni and Krakauer,23 the implicit adaptation system appears to be completely isolated from the strategy. The aiming error signal that is used to recalibrate this system to ensure accurate movement execution is based on the difference between the predicted and actual movement outcome; this signal does not incorporate the participant’s strategy, leading to the paradoxical drift phenomenon. In contrast, the strategy is adjusted by an error signal that reflects the movement goal, which is defined as the difference between where the target is located and where the movement ended. Learning within this system modifies a representation relevant for action planning, focused on ensuring that the outcome of the action achieves the desired goal.
Although the adaptation system is modular in that it does not take into account the strategy (e.g., recognize that the feedback may be displaced because of a strategy adopted to negate a rotation), the impact of the adaptation system on strategy adjustment is unclear. In our model, the strategic process only has access to target error, the difference between the goal and the feedback; it does not have access to changes arising from adaptation. Thus, the influence is indirect.
As reviewed above, certain features of performance—slower RTs, increased variability, and smaller aftereffects—have led to the inference that participants may adopt a strategy to offset a large perturbation in visuomotor adaptation tasks. However, none of these studies has reported the drift phenomenon. This is puzzling if one assumes that by adopting a strategy (e.g., “aim in clockwise direction of target), a mismatch is created between the aiming location and the feedback location. Why should the explicit instruction in the use of a strategy produce drift, whereas self-discovery by the participant does not?
The answer seems to be because of a second important methodological difference between these standard visuomotor adaptation tasks and the variant introduced by Mazzoni and Krakauer.23 The latter included visible landmarks spaced every 45° to provide a reference point for the strategy. In the standard task, these landmarks are absent; participants only see a stimulus at the target location. We propose that the landmarks serve as a proxy for the predicted location of the movement. That is, even though the adaptation system uses an error based on the difference between the predicted and actual movement, the landmarks provide a salient referent for the predicted movement location. When these landmarks are absent, the participant’s sense of the predicted movement (e.g., 45° clockwise from the target) is likely uncertain, and thus the weighting given to the aiming error term is attenuated. We tested this idea by comparing conditions in which the landmarks were always present, disappeared at movement initiation, or were never presented (Figs. 5C and D). Consistent with the certainty hypothesis, the degree of drift was attenuated as uncertainty increased.24 Indeed, when the landmarks were never present, drift was minimal throughout the training block.
Reward-based learning and error-based adaptation
Although our modeling work entails two error terms, error-based learning may not be the most appropriate way to characterize the strategy adjustment process. Rather, learning within the strategic process, with its emphasis on the movement goal, may be better described in terms of models of reinforcement learning. These models are designed to account for how organisms explore different regions of a strategy space, attempting to identify the action policy that results in the largest reward. In our task, a shift in policy might occur when the rise in target error because of adaptation becomes too large. That is, when a chosen action fails to achieve the predicted reward, a new strategy might be adopted. In our study, only a few of the participants exhibited categorical-like changes in performance. For these participants, we observed abrupt reductions in target error, suggesting that the participant either stopped using the strategy or made a categorical change in their strategy (e.g., switched from aiming to a landmark to a position between two landmarks). For the other participants, the data suggest a more gradual change in the strategy with incremental changes that eventually led to movement success.
The relationship between reinforcement learning and sensorimotor adaptation was highlighted in a recent study in which participants were only provided with categorical feedback when learning a rotation.28 In this format, the participants were not given visual feedback of their movement endpoint, but instead observed an explosion of the target on successful trials, those in which the hand passed within a criterion window. Compared to standard adaptation tasks, learning here was characterized by a dramatic increase in trial-by-trial variance, limited generalization to untrained movements, and reduced sensorimotor remapping. Furthermore, the learning function could be accounted for by a reward-based learning model in which action policies (aiming direction) were adjusted to maximize the rate of reward. Similar to the instructed strategy tasks, the Izawa and Shadmehr28 study also suggests that motor learning may be composed of (at least) two processes: a reward-based action-selection process and an error-based adaptation process.
The form and certainty of the visual feedback influences the form of the representational changes associated with the performance changes. Aftereffects are larger when the feedback is continuous during adaptation, compared to conditions in which only endpoint feedback or knowledge of performance is provided.29–31 Continuous visual feedback provides more salient spatiotemporal feedback of the relationship between the movement and feedback. As such, the information is more reliable, with a tight covariance between motor commands and their outcome. Increasing this covariance seems to facilitate adaptation as evident by the larger after-effect. Conversely, decreasing this covariance promotes learning via strategic processes, evident in the attenuated aftereffect. Indeed, in their computational model, Izawa and Shadmehr28 show how the uncertainty of visual feedback can distribute learning across adaptation or action-selection processes. As in our model, both processes seem to operate in parallel.
Movement errors have been thought to provide a signal that is used to incrementally update an internal model, one that predicts the consequences of motor commands given the dynamics of the environment. Generalization, in which movements to novel directions or in novel contexts show effects of learning, have been taken as evidence of the presence of an internal model for motor control. However, motor learning can arise by reinforcement, simply relating the success or failure of an executed movement. This form of learning has been called “model-free” because the feedback does not guide formation of an internal model of task dynamics, but rather only the value of potential actions or movements.32 Model-free learning can also arise from pure repetition,32,33 or what has been called use-dependent plasticity.34 The work on model-based and model-free learning again emphasizes that motor learning is a composite term.
Neural systems subserving action selection and motor execution
This two-process interpretation may also provide a new perspective for understanding the consequences of neural pathology on specific learning processes. Consider the effects of cerebellar damage on sensorimotor control and adaptation. Numerous studies have shown that patients with cerebellar ataxia show attenuated adaptation. These findings have been assumed to reflect the operation of a compromised learning system.35–37 Alternatively, the patients’ performance may reflect the implementation of a compensatory process, one in which they have come to rely on alternative forms of control. Lang and Bastian38 observed that patients with cerebellar damage performed surprisingly well when asked to make rapid complex drawing movements, reaching a performance level comparable to that of control participants. However, when the drawing task was performed concurrently with a secondary task, their performance was markedly reduced. These results suggest that the patients may have relied on a strategy-based control system, one that was taxed by the inclusion of the secondary task.
We directly addressed this question by providing an explicit strategy to a group of individuals with bilateral cerebellar degeneration who were presented with a visuomotor rotation.39 The patients had no difficulty implementing the strategy and were successful in immediately counteracting a 45° rotation. However, in comparison to control participants, the patients exhibited attenuated drift; their performance remained accurate across the training block. These results indicate that patients with cerebellar degeneration can use a strategy, and in fact, their use of a strategy remains stable because it is not disrupted by the operation of the adaptation system.
Our results are consistent with prior work showing that patients with cerebellar damage are impaired in sensorimotor adaptation.35,36,40 It is interesting to ask why these individuals do not generate compensatory strategies in tasks using standard adaptation tasks. That is, if the error signal remains stable over trials (because of an impaired learning mechanism), why don’t the patients come up with a strategy, given that they have little difficulty using one when given explicit instructions? It is possible that although the adaptation process is isolated from strategies, the reverse may not be true; adaptation processes may inform strategic processes. For example, visuomotor rotations create a complex pattern of errors. The adaptation system may aid in the formation of a movement strategy by providing detailed error information. Without this information, it may be difficult to generate a successful strategy because the strategy is designed to overcome these errors.
By this view, damage to the cerebellum may not only disrupt sensorimotor adaptation, but may also disrupt the generation of cognitive strategies. This hypothesis may also help to explain why patients with cerebellar degeneration show a greater impairment in learning to compensate for an abrupt, and large, force field perturbation, compared to when the perturbation is introduced gradually.37 When errors are large, the cerebellum may work in concert with frontal areas to aid in an action-selection process required for the generation of a movement strategy.41,42 Extensive reciprocal connections between the cerebellum and prefrontal cortex (PFC) are suggestive of a coordinated network that can integrate motor and cognitive processes.
Lesions of PFC, either virtually with transcranial magnetic stimulation or from naturally occurring lesions, can disrupt performance on sensorimotor learning tasks.43 Patients with PFC lesions exhibit pronounced deficits in visuomotor adaptation.44–46 Interestingly, these patients have difficulty describing the perturbation, or when aware of it, have difficulty reporting what action would be required to compensate for the perturbation.44,45 In a similar vein, older adults show a slower rate of adaptation compared to younger individuals,47 a deficit that is attenuated in older adults who are able to explicitly describe the perturbation.48 Thus, this deficit may, in part, be related to a problem in generating strategies for motor adaptation.
Generating a movement strategy
At present, considerable progress has been made in the development of computational models that describe sensorimotor adaptation. In contrast, we know little about the process of strategy development. What sources of information are used to generate and modify strategies? What are the dynamics of strategy change? Are there signals inherent in action or task-dependent variables that help define the solution space? What drove Fosbury to lean back when he reverted to the scissors technique? His willingness to break with the conventions of his day may have been fueled by the fact that the landing pit was now filled with shock absorbent foam rather than sawdust and wood chips. With softer materials, jumpers no longer had to worry about landing on their feet or hands.
To fully understand strategy development and change, it will be necessary to characterize the inputs to the strategy process. Popular tasks for studying action selection, such as the n-bandit task, are generally limited to a small, fixed set of discrete actions. This limits the search space to a finite set of action–outcome alternatives. In motor control, the search space is continuous and in some sense nearly infinite. It is also unclear if reward is the driving input for the strategy system. Reward in many contexts is discrete—a choice was either correct or incorrect. However, actions, especially when they involve complex sequences of movements, are much more varied. How would reward signals be used to train a strategic process with a nearly infinite action space? A reasonable experimental approach will require a more constrained situation, tasks in which there is a relatively limited set of potential actions to isolate the inputs and characterize the time course of the strategic process.
Work along these lines could provide a new perspective for understanding not only strategy change, but spontaneous strategy development.49 Considerable research has been devoted to understanding the processes underlying spontaneous insight in problem-solving tasks.50,51 Borrowing techniques from the insight literature may offer clues to strategy development in the motor domain and help us understand progress in human performance. As shown in Figure 1, the world record in high jumping has not budged since 1993. Although we may see a new leaper perfect the Fosbury Flop and produce an incremental change in the record, the next ascent of the bar is likely to require the discovery of a radically new technique.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Hoffer R. Something in the Air: American Passion and Defiance in the 1968 Mexico City Olympics. Free Press; New York: 2009. [Google Scholar]
- 2.Fitts PM, Posner MI. Human Performance. Brooks/Cole Pub. Co; Belmont, CA: 1967. [Google Scholar]
- 3.Crossman ERFW. A theory of the acquisition of speed-skill. Ergonomics. 1959;2:143–166. [Google Scholar]
- 4.Seibel R. Discrimination reaction time for a 1,023 alternative task. J Exp Psychol. 1963;66:215–226. doi: 10.1037/h0048914. [DOI] [PubMed] [Google Scholar]
- 5.Logan G. Toward an instance theory of automatization. Psychol Rev. 1988;95:492–527. [Google Scholar]
- 6.Cunningham HA. Aiming error under transformed spatial mappings suggests a structure for visualmotor maps. J Exp Psychol Hum Percept Perform. 1989;15:493–506. doi: 10.1037//0096-1523.15.3.493. [DOI] [PubMed] [Google Scholar]
- 7.Imamizu H, Uno Y, Kawato M. Internal representations of the motor apparatus: implications from generalization in visuomotor learning. J Exp Psychol Hum Percept Perform. 1995;21:1174–1198. doi: 10.1037//0096-1523.21.5.1174. [DOI] [PubMed] [Google Scholar]
- 8.Pine ZM, Krakauer JW, Gordon J, Ghez C. Learning of scaling factors and reference axes for reaching movements. Neuroreport. 1996;7:2357–2361. doi: 10.1097/00001756-199610020-00016. [DOI] [PubMed] [Google Scholar]
- 9.Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci. 2000;20:8916–8924. doi: 10.1523/JNEUROSCI.20-23-08916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kagerer FA, Contreras-Vidal JL, Stelmach GE. Adaptation to gradual as compared with sudden visuomotor distortions. Exp Brain Res. 1997;115:557–561. doi: 10.1007/pl00005727. [DOI] [PubMed] [Google Scholar]
- 11.Hwang EJ, Smith MA, Shadmehr R. Dissociable effects of the implicit and explicit memory systems on learning control of reaching. Exp Brain Res. 2006;173:425–437. doi: 10.1007/s00221-006-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saijo N, Gomi H. Multiple motor learning strategies in visuomotor rotation. PloS One. 2010;5:e9399. doi: 10.1371/journal.pone.0009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Ruiz J, Wong W, Armstrong IT, Flanagan JR. Relation between reaction time and reach errors during visuomotor adaptation. Behav Brain Res. 2011;219:8–14. doi: 10.1016/j.bbr.2010.11.060. [DOI] [PubMed] [Google Scholar]
- 14.Benson BL, Anguera JA, Seidler RD. A spatial explicit strategy reduces error but interferes with sensorimotor adaptation. J Neurophysiol. 2011;105:2843–2851. doi: 10.1152/jn.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin TA, Keating JG, Goodkin HP, et al. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain. 1996;119(Pt 4):1199–1211. doi: 10.1093/brain/119.4.1199. [DOI] [PubMed] [Google Scholar]
- 16.Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Contributions of spatial working memory to visuomotor learning. J Cogn Neurosci. 2010;22:1917–1930. doi: 10.1162/jocn.2009.21351. [DOI] [PubMed] [Google Scholar]
- 17.Keisler A, Shadmehr R. A shared resource between declarative memory and motor memory. J Neurosci. 2010;30:14817–14823. doi: 10.1523/JNEUROSCI.4160-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eversheim U, Bock O. Evidence for processing stages in skill acquisition: a dual-task study. Learn Mem. 2001;8:183–189. doi: 10.1101/lm.39301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor JA, Thoroughman KA. Motor adaptation scaled by the difficulty of a secondary cognitive task. PloS One. 2008;3:e2485. doi: 10.1371/journal.pone.0002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor JA, Thoroughman KA. Divided attention impairs human motor adaptation but not feedback control. J Neurophysiol. 2007;98:317–326. doi: 10.1152/jn.01070.2006. [DOI] [PubMed] [Google Scholar]
- 21.Galea JM, Sami SA, Albert NB, Miall RC. Secondary tasks impair adaptation to step- and gradual-visual displacements. Exp Brain Res. 2010;202:473–484. doi: 10.1007/s00221-010-2158-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malone LA, Bastian AJ. Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol. 2010;103:1954–1962. doi: 10.1152/jn.00832.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci. 2006;26:3642–3645. doi: 10.1523/JNEUROSCI.5317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor JA, Ivry RB. Flexible cognitive strategies during motor learning. PLoS Comput Biol. 2011;7:e1001096. doi: 10.1371/journal.pcbi.1001096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thoroughman KA, Shadmehr R. Learning of action through adaptive combination of motor primitives. Nature. 2000;407:742–747. doi: 10.1038/35037588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng S, Sabes PN. Modeling sensorimotor learning with linear dynamical systems. Neural Comput. 2006;18:760–793. doi: 10.1162/089976606775774651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarahn E, Weston GD, Liang J, et al. Explaining savings for visuomotor adaptation: linear time-invariant state-space models are not sufficient. J Neurophysiol. 2008;100:2537–2548. doi: 10.1152/jn.90529.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izawa J, Shadmehr R. Learning from sensory and reward prediction errors during motor adaptation. PLoS Comput Biol. 2011;7:e1002012. doi: 10.1371/journal.pcbi.1002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shabbott BA, Sainburg RL. Learning a visuomotor rotation: simultaneous visual and proprioceptive information is crucial for visuomotor remapping. Exp Brain Res. 2010;203:75–87. doi: 10.1007/s00221-010-2209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinder MR, Tresilian JR, Riek S, Carson RG. The contribution of visual feedback to visuomotor adaptation: how much and when? Brain Res. 2008;1197:123–134. doi: 10.1016/j.brainres.2007.12.067. [DOI] [PubMed] [Google Scholar]
- 31.Hinder MR, Woolley DG, Tresilian JR, et al. The efficacy of colour cues in facilitating adaptation to opposing visuomotor rotations. Exp Brain Res. 2008;191:143–155. doi: 10.1007/s00221-008-1513-7. [DOI] [PubMed] [Google Scholar]
- 32.Huang VS, Haith A, Mazzoni P, Krakauer JW. Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron. 2011;70:787–801. doi: 10.1016/j.neuron.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verstynen T, Sabes PN. How each movement changes the next: an experimental and theoretical study of fast adaptive priors in reaching. J Neurosci. 2011;31:10050–10059. doi: 10.1523/JNEUROSCI.6525-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diedrichsen J, White O, Newman D, Lally N. Use-dependent and error-based learning of motor behaviors. J Neurosci. 2010;30:5159–5166. doi: 10.1523/JNEUROSCI.5406-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophysiol. 2005;93:2809–2821. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- 36.Rabe K, et al. Adaptation to visuomotor rotation and force field perturbation is correlated to different brain areas in patients with cerebellar degeneration. J Neurophysiol. 2009;101:1961–1971. doi: 10.1152/jn.91069.2008. [DOI] [PubMed] [Google Scholar]
- 37.Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of error affects cerebellar contributions to motor learning. J Neurophysiol. 2010;103:2275–2284. doi: 10.1152/jn.00822.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lang CE, Bastian AJ. Cerebellar damage impairs automaticity of a recently practiced movement. J Neurophysiol. 2002;87:1336–1347. doi: 10.1152/jn.00368.2001. [DOI] [PubMed] [Google Scholar]
- 39.Taylor JA, Klemfuss NM, Ivry RB. An explicit strategy prevails when the cerebellum fails to compute movement errors. Cerebellum. 2010;9:580–586. doi: 10.1007/s12311-010-0201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin TA, Keating JG, Goodkin HP, et al. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain. 1996;119(Pt 4):1183–1198. doi: 10.1093/brain/119.4.1183. [DOI] [PubMed] [Google Scholar]
- 41.Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- 42.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Ann Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 43.Pascual-Leone A, Wassermann EM, Grafman J, Hallett M. The role of the dorsolateral prefrontal cortex in implicit procedural learning. Exp Brain Res. 1996;107:479–485. doi: 10.1007/BF00230427. [DOI] [PubMed] [Google Scholar]
- 44.Slachevsky A, et al. Preserved adjustment but impaired awareness in a sensory-motor conflict following pre-frontal lesions. J Cogn Neurosci. 2001;13:332–340. doi: 10.1162/08989290151137386. [DOI] [PubMed] [Google Scholar]
- 45.Slachevsky A, et al. The prefrontal cortex and conscious monitoring of action: an experimental study. Neuropsychologia. 2003;41:655–665. doi: 10.1016/s0028-3932(02)00225-7. [DOI] [PubMed] [Google Scholar]
- 46.Ivry RB, Schlerf J, Xu J, et al. Soc Neurosci Abstr. Washington, DC: 2008. Strategic and recalibration processes during visuomotor rotation in cerebellar ataxia. [Google Scholar]
- 47.Fernandez-Ruiz J, Hall C, Vergara P, Diiaz R. Prism adaptation in normal aging: slower adaptation rate and larger aftereffect. Brain Res Cogn Brain Res. 2000;9:223–226. doi: 10.1016/s0926-6410(99)00057-9. [DOI] [PubMed] [Google Scholar]
- 48.Heuer H, Hegele M. Adaptation to visuomotor rotations in younger and older adults. Psychol Aging. 2008;23:190–202. doi: 10.1037/0882-7974.23.1.190. [DOI] [PubMed] [Google Scholar]
- 49.Kounios J, Beeman M. The Aha! Moment: the cognitive neuroscience of insight. Curr Dir Psychol Sci. 2009;18:210–216. [Google Scholar]
- 50.Bowden EM, Jung-Beeman M. Aha! Insight experience correlates with solution activation in the right hemisphere. Psychon Bull Rev. 2003;10:730–737. doi: 10.3758/bf03196539. [DOI] [PubMed] [Google Scholar]
- 51.Smith RW, Kounios J. Sudden insight: all-or-none processing revealed by speed-accuracy decomposition. J Exp Psychol Learn Mem Cogn. 1996;22:1443–1462. doi: 10.1037//0278-7393.22.6.1443. [DOI] [PubMed] [Google Scholar]





