Abstract
Serotonin receptors (5-HTRs) are implicated in the pathophysiology of a variety of neuropsychiatric and neurodegenerative disorders and are also targets for drug therapy. In the CNS, most of these receptors are expressed in high abundance in specific brain regions reflecting their role in brain functions. Quantifying binding to 5-HTRs in vivo may permit assessment of physiologic and pathologic conditions, and monitoring disease progression, evaluating treatment response, and for investigating new treatment modalities. Positron emission tomography (PET) molecular imaging has the sensitivity to quantify binding of 5-HTRs in CNS disorders and to measure drug occupancy as part of a process of new drug development. Although research on PET imaging of 5-HTRs have been performed more than two decades, the successful radiotracers so far developed for human studies are limited to 5-HT1AR, 5-HT1BR, 5-HT2AR, 5-HT4R and 5-HT6R. Herein we review the development and application of radioligands for PET imaging of 5-HTRs in living brain.
Keywords: Molecular imaging, PET, 5-HTR, radioligands
INTRODUCTION
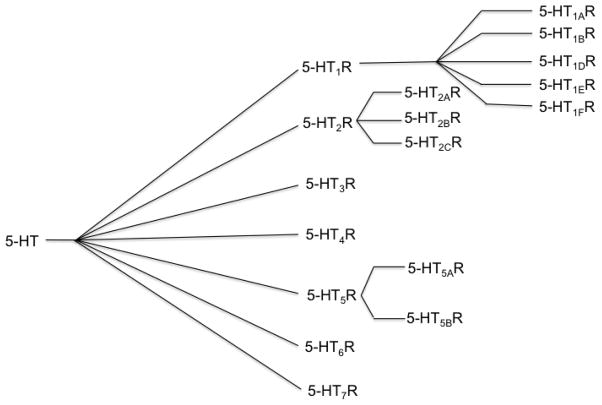
Serotonin or 5-hydroxytryptamine (5-HT) is a neurotransmitter that is widely distributed in animals and plants [1–3]. Serotonin does not cross the blood brain barrier (BBB); the brain synthesizes all its serotonin from tryptophan (from diet) in two steps enzymatically with tryptophan hydroxylase (TPH) and tryptophan decarboxylase (TDC) respectively [4]. Serotonin is found in almost all brain regions and it is implicated in the pathophysiology of a variety of diseases, biological functions and biochemical pathways [1, 2, 5–8]. Serotonin neurons are confined to the brainstem and are located in the raphe nuclei. The neurons project to most of the brain including hippocampus, midbrain, prefrontal, parietal and occipital cortical regions, cingulate cortex and cerebellum, whereas, 5-HT neurons in caudal raphe nuclei project to cerebellum and spinal cord [9–11]. Serotonin effects are mediated through seven 5-HT receptor subtypes, 5-HT1 to 5HT7 (5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, 5-HT1F, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT3, 5-HT4, 5-HT5A, 5-HT5B, 5-HT6, 5-HT7A, 5-HT7B,5-HT7D) and serotonin transporter (SERT) [1, 12]. Among these receptors, 5-HT3R is a ligand gated ion channel receptor (LGICR), whereas, others are coupled with G-protein coupled receptors (GPCR) [1]. The rate of binding of 5-HT varies for 5-HTR subtypes, for example, 5HT1ARs (Ki = 0.2–400 nM), 5-HT1BR (Ki = 1–40 nM), 5-HT7R (Ki = 0.2–8 nM) and 5-HT2AR (Ki = <10 nM) have the highest affinity for 5-HT [13].
Positron Emission Tomography (PET)
PET uses positron-radiolabeled molecules in very low mass amounts to image and measure the function of biological processes with minimum disturbance [14–17]. The primary biological mechanisms that can be studied by PET imaging are specific binding to protein structures (eg: enzymes, transporters and receptors), metabolism, and blood regulation. PET has been used for the quantification of neuroreceptor binding and release of neurotransmitters and measuring enzyme binding in disease pathology, early diagnosis, and evaluation of therapy effects. Furthermore, PET biodistribution studies are able to confirm that the drug candidate/lead ligand reaches the target site and does not accumulate in non-target sites of potential toxicity. The conventional components of PET molecular imaging include production of radioisotopes, radiotracer synthesis, PET scanning and data interpretation. Among the positron emitting isotopes, O-15 (T1/2 = 2.037 minutes), N-13 (T1/2 = 9.96 minutes), C-11 (T1/2 = 20.34 minutes) and F-18 (T1/2 = 109.77 minutes) are the common nuclei produced from cyclotron, reported for neuroimaging with PET.
There are several physiochemical criteria required for successful radioligand development for in vivo imaging [18]. First, the target to be imaged should have adequate density in brain regions of interest and the degree of altered expression in the diseased state must be sufficient to be detectable. In general, PET tracers with a ratio of the target’s concentration (Bmax) to its affinity (Kd or Ki) of at least 10 are expected to have a high probability of providing a reliably quantifiable specific signal in vivo. The radioligand should be safe to use without any adverse toxicology or pharmacology effects. The radioligand must have high affinity (in the nanomolar range) toward the imaging target and good selectivity (at least 20–100 fold, depending on the density of the non-target) over a wide spectrum of biogeneic amines, proteins, receptors, transporters and enzymes in brain. The imaging agent should have preferably low molecular weight (≤450), moderate lipophilicity (logD ~ 1–3) to facilitate BBB permeability [19, 20] and should not have binding affinity to ATP-binding cassette (ABC) transporter family efflux pump (eg: P-glycoprotein (P-gp), multi-drug resistance-associated protein (MDR1a/1b) and breast cancer resistance protein (BCRP) and soluble carrier (SLC) family influx transporters [18]. The chemical structure of the ligand should allow for a rapid radiolabeling that does not alter the pharmacological properties of the molecule and generates adequate specific activity essential to avoid self occupancy (<5%) to the target with unlabeled ligand [21, 22]. A successful radiotracer should have fast clearance from blood, rapid localization to target and fast washout from non-target tissues. The metabolite should be preferably polar, measurable or unlabeled, and should not interfere with the radioligand’s binding kinetics [23]. It is proven that brain uptake of the radiotracer is in direct proportion to the free fraction (fp) of the radioligand. Thus the radioligands should have measurable fp in plasma to allow marginal uptake and reliable measurements of the binding parameters. In general, radioligands with lower lipophilicity have less plasma protein binding and this allows the available fp in blood to diffuse through cell membranes and impacts binding. Furthermore, the plasma clearance rate of radioligand should not be too rapid, because this can affect the accurate determination of the input curve at later time points. The radioligand binding must equilibrate within the imaging time frame and or 3–5 half-lives of the radiotracer [24]. This will also allow reduced radiation exposure to subject. The radioligand should be amenable for quantification with tracer kinetic modeling to obtain binding parameters such as rate constants, volume of distribution (VT), binding potential (BPF or BPP) or specific to nonspecific equilibrium partition coefficient (BPND). An input function is used for kinetic modeling but some methods and outcome measures use data from a reference region instead, or estimate the input function using data from many brain regions. Estimation of binding can be achieved by compartmental, equilibrium or graphical approaches.
To permit measurement of specific binding, the radioligand should have higher target to nontarget ratio (>1.5 and preferably >3–4). The most important parameters in PET tracer development are tracer affinity, selectivity, lipophilicity and metabolism. There are several challenges associated with tracer development. These include limited synthesis time in order to incorporate radionuclei because of the short half life of some PET isotopes like 11C or 18F, the requirement for preparation of a high specific active product in order to keep the injected mass low, metabolite analyses to generate a metabolite-corrected input function, fp measurement, rapid transit across physiological barriers such as BBB, BCRP and cellular uptake to deliver the PET probe to target. Tissue kinetics amenable to mathematical modeling to give quantitative indices are still a challenge for new tracers. The ultimate challenge is to demonstrate whether the tracer distribution is sensitive enough to answer clinical questions relevant to diagnosis, prognosis or target occupancy by medication. In this review, we focus on the radioligands and their applications that are reported mainly in human subjects for PET imaging of the 5-HTRs, based on findings published through June 2014.
5-HT1R
5-HT1Rs have five receptor subtypes with 40–60% sequence homology and these receptors bind 5-HT resulting in activation of the Gi/o class of GPCR and inhibition of adenylate cyclase (AC) resulting in a decrease in cyclic adenosine monophosphate (cAMP) levels [2]. 5-HT1R is an important class of receptors that modulate the communication and functions of 5-HT in brain and are implicated in the pathophysiology of a variety of neurogenenerative and neuropsychiatric disorders. These receptors are expressed in specific brain regions and cortical layers except for 5-HT1FR subtype. 5-HT1AR is the most studied receptor subtype of 5-HT1R that plays a major role in transmission and regulation of 5-HT neuron firing in brain [2].
5-HT1AR
Much is published about the pharmacology and pathophysiology of 5-HT1AR receptors in brain functions [2–4, 25]. In brainstem raphe nuclei, these receptors are somatodendritic autoreceptors, while in other brain regions they are postsynaptic or heteroreceptors on nerve terminals [25, 26]. 5-HT1ARs have been implicated in the pathophysiology of mood and anxiety disorders, suicidal behavior, sexual functions, eating and panic disorders, epilepsy, schizophrenia, Parkinson’s disease (PD) and Alzheimer’s disease (AD). 5HT1AR are involved in the mechanism of action of antidepressants and more recently they have been suggested to mediate trophic and neuroprotecting effects [2, 27–30]. 5-HT1AR agonists and partial agonists have been evaluated as antidepressants, anxiolytics and antipsychotic drugs (APDs) with fewer side effects (31). In vitro and in vivo quantification studies of 5-HT1AR reveal high receptor density in hippocampus (800–3,000 fmol/mg/protein) and in the entire cortical mantle where according to postmortem studies the receptors are densest in layer II (300–1900 fmol/mg/protein) [32, 34]. Lower 5-HT1AR binding levels are found in thalamus and the lowest density is observed in adult striatum, substantia nigra and cerebellum [32, 34].
Development and evaluation of 5-HT1AR PET tracers are documented extensively in literature [35–42]. Among these [carbonyl-11C]WAY100635 (Ki = 2.2 nM, Fig. 3) is the most extensively studied antagonist radiotracer in human subjects. The parent [O-methyl[11C]WAY100635 has a BBB penetrating metabolite [11C]WAY100634, hence it is unsuitable for the quantification of 5-HT1AR [43]. Altered [carbonyl-11C]WAY100635 binding are reported in a variety of psychiatric disorders in comparison to control subjects [35–42]. The advantage of [carbonyl-11C]WAY100635 compared with other 5-HT1AR PET tracers is mainly due to its higher target to nontarget ratio. However, low free fraction, rapid metabolism, complexity in radiosynthesis and low yield make this radiotracer not suitable for routine studies. Furthermore, receptor selectivity assays show that WAY100635 is not a selective 5-HT1AR ligand and it has potential binding to α-1AR (Ki = 16.4 nM, 7.45 times higher than 5-HT1AR) and D4R (Ki =19.9 nM, 9 times higher than 5-HT1AR) [44]. Literature indicates that D4Rs are less abundant in brain and hence D4R binding on PET imaging with [carbonyl-11C]WAY100635 is not significant with a Ki of 19.9 nM [45]. However, the density of α-1AR in brain is sufficient to be detected by PET imaging, and hence there may be binding that may confuse estimation of 5-HT1AR binding in brain regions with higher density of α-1AR using [carbonyl-11C]WAY100635 [46]. The in vivo cross selectivity of [carbonyl-11C] WAY100635 and structurally related radioligands with α-1AR is not studied or reported yet.
Fig. 3.
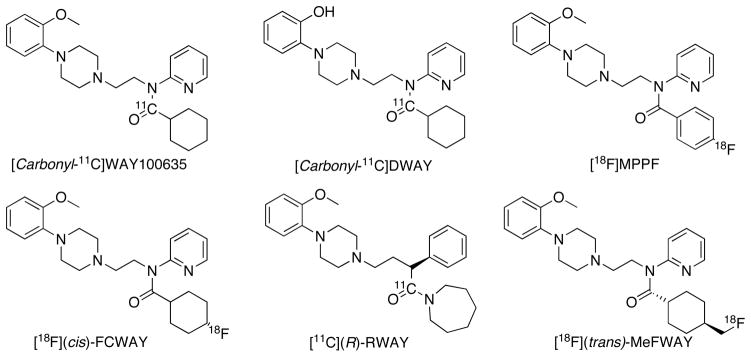
Examples of 5-HT1AR antagonist PET tracers studied in human.
Several other radioligands have been reported that are analogues of WAY100635 [35]. [carbonyl-11C]DWAY (desmethyl-WAY100635) (5-HT1AR Ki = 1.4 nM, Fig. 3) is a minor metabolite of WAY100635 and it provided higher signal to target ratio in various species including human in comparison to [carbonyl-11C]WAY100635 [47, 48]. In contrast with WAY100635, DWAY shows less binding to α-1AR (Ki = 364 nM, Kumar JSD, Mann JJ, unpublished data). [11C](R)-RWAY is a reverse amide of WAY100635 (5-HT1AR Ki = 0.6 nM, Fig. 3), developed to improve the stability of WAY100635. (R)-RWAY possesses significant affinity to 5-HT2BR (Ki = 7.2 nM), α-1AR (Ki =10.35 nM), D2R (Ki = 34.5 nM), D3R (Ki =5.1 nM), and D4R (Ki =15.6 nM). In comparison to [carbonyl-11C]WAY100635 or [carbonyl-11C]DWAY, [11C](R)-RWAY has several radiosynthesis advantages and it showed promising in vivo binding in rodents and non human primates [49,50]. However, the presence of radioactive metabolites in brain and its P-gp binding make this ligand problematic for studies in human subjects [51]. [18F](cis)-FCWAY is a fluoro-analogue of WAY100635 (5-HT1AR Ki = 0.52 nM, Fig. 3) and it has been successfully used to study human disease with PET [52], however, in vivo de[18F]fluorination is the major drawback of this tracer and makes it useful only for quantifying brain structures that are not adjacent to skull. [18F]MPPF (5-HT1AR Ki = 3.3 nM, Fig. 3) is a fluorophenyl analogue of WAY100635, synthesized by the nucleophilic displacement of the corresponding nitro precursor with [18F]fluoride [53]. Although in rodents and cats [18F]MPPF was found to be sensitive enough to measure large changes of intra-synaptic 5-HT levels in vivo [35–37, 54], studies in awake monkeys and human subjects did not show intra synaptic changes of 5-HT with this radiotracer. [18F]MPPF has been used to image 5-HT1AR alteration in a variety of neuropsychatric diseases and it is the only 5-HT1AR PET radioligand tested so far for AD imaging in vivo [55]. Despite the above advantages, [18F]MPPF is a P-gp substrate and this limits the further clinical utility of this radiotracer [35]. [18F]MeFWAY is a fluoromethyl analogue of WAY100635 and of the two isoforms, the trans isomer shows higher binding to 5-HT1AR which is comparable to WAY100635 in vivo. More recently [18F](trans)-MeFWAY has been successfully evaluated in human subjects with no in vivo de[18F]fluorination [58]. However, kinetic analyses with arterial input functions have to be performed for the full quantification of this radiotracer. None of these radiotracers were successful for occupancy measurements of 5-HT1AR drugs even at dose levels higher than that is used in clinics. [carbonyl-11C]WAY100635 and [carbonyl-11C]DWAY are synthesized by reacting [11C]cycloalkyl magnesium chloride with the corresponding amines. [18F](cis)-FCWAY, [18F]MPPF and [18F](trans)-MeFWAY are synthesized via the nucleophilic displacement of their corresponding nitro or tosyl precursors with [18F]fluoride. [11C](R)-RWAY is synthesized by reacting the desmethyl precursor with [11C]CH3I or [11C]MeOTf [36, 37].
5-HT1AR exists in high and low agonist affinity states. The antagonist ligands bind to the high affinity (HA) and low affinity (LA) conformations of 5-HT1AR with similar affinity. Whereas, agonist ligands bind preferentially to the HA state of the receptor, which is coupled to G-protein and therefore agonist binding provides a more meaningful functional measure of the 5-HT1AR that can reflect desensitization and supersensitivity [59, 60]. Although antagonist 5-HT1AR PET tracers can measure the total receptor binding, they cannot detect changes in the high affinity 5-HT1AR binding in disease states or in the context of treatment functionally larger and earlier effects such those of antidepressants that desensitize autoreceptors before they down-regulate total binding [35–40]. Antagonist binding is insensitive to the changes in the intra-synaptic 5-HT concentration and antagonist PET tracers are less sensitive for measuring agonist receptor occupancy in clinical studies to guide new drug development in dose-finding.
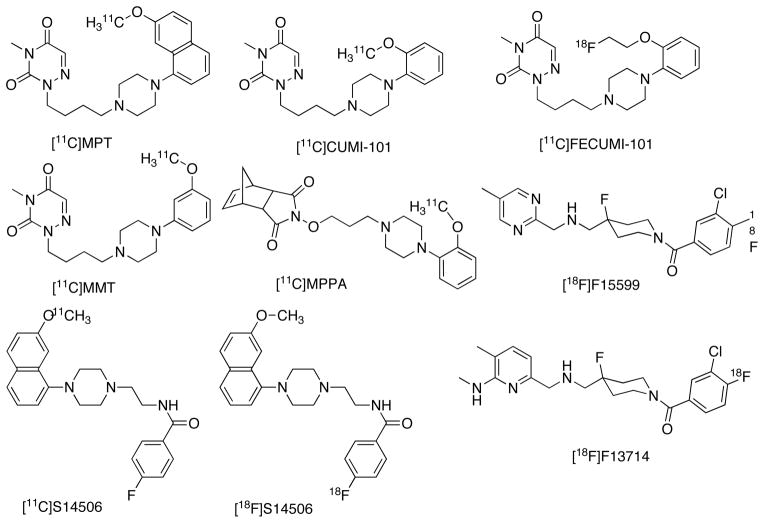
The development of 5-HT1AR agonist PET tracers for the past 2 decades has met with limited success [35]. Most PET imaging studies have been carried out with amino-tetralin, apomorphine, thiochromine and arylpiperazine based 5-HT1AR agonist ligands. Some of these ligands show promising characteristics in vitro but failed in vivo due to a lack of detectable specific binding. [11C]MPT, an arylpiperazine derivative of 3,5-dioxo-(2H,4H)-1,2,4-triazine is the first successful agonist PET tracer reported for 5-HT1AR (Ki = 1.35 nM, Emax = 95%, EC50 = 0.05 nM, Fig. 4) in non human primates [61]. The radiotracer binding in baboon brain was in excellent agreement with the known distribution of 5-HT1AR. The VT of [11C]MPT showed strong correlation with the antagonist tracer [carbonyl-11C]WAY100635. Of note, VT of [carbonyl-11C]WAY100635 was higher compared with [11C]MPT, presumably because [carbonyl-11C]WAY100635, an antagonist, binds to both HA and LA states of 5-HT1AR. Despite the excellent binding profile of [11C]MPT, the slow washout in baboons and unreliable measurement of free fraction, limits this radiotracer from advancing to human studies.
Fig. 4.
Examples of agonist PET tracers for 5-HT1AR.
Several ligands based on 3,5-dioxo-(2H,4H)-1,2,4-triazine were subsequently reported as 5-HT1AR agonist PET tracers [62, 63]. Among these [11C]CUMI-101 or [11C]MMP is the most successful and only radiotracer tested in nonhuman primates and human subjects [64, 65]. [11C]CUMI-101 is a partial agonist (Ki = 0.15 nM, Emax = 80%, EC50 = 0.1 nM, Fig. 4) to 5-HT1AR and it is insensitive to the fluctuations in physiological intra-synaptic concentration of 5-HT and can detect robust pharmacologically induced increases in intrasynaptic 5-HT in baboons and human subjects [66, 67]. However, one study reports that [11C]CUMI-101 did not show significant difference in endogenous 5HT1AR changes in human [68]. The in vivo binding ratios of [11C]CUMI-101 are ~55% less across brain regions in comparison to [carbonyl-11C]WAY100635 and this is in agreement with the previously reported in vitro data of agonist and antagonist binding ratios of 5-HT1AR [69]. Recently, it is reported that [11C]CUMI-101 did not behave as a 5-HT1AR agonist in brain homogenate based assays [70]. The above discrepancy can be partly attributed to the assay conditions because general GTPγS assays have a modest signal/noise ratio in tissue samples and dependent on the concentration of GDP. In another report, [11C]CUMI-101 binding in thalamus and cerebellum in rats and monkeys is partially displaced with the α-1AR ligand prazosin indicating or supporting a cross selectivity of CUMI-101 to α-1AR [71]. However, autoradiography experiments, a much sensitive tool than PET, with [3H]CUMI-101 indicates no significant α1-AR binding in either baboon or human brain and the relative regional brain [3H]CUMI-101 binding is comparable with the known distribution of 5-HT1ARs as defined by [3H]WAY100635 and [3H]8-OH-DPAT [72]. More recently [18F]FECUMI-101, a fluoroethyl analogue of CUMI-101 is reported as a partial agonist radiotracer in nonhuman primates (Ki = 0.1 nM, Emax = 77%, EC50 = 0.85 nM, Fig. 4) [73]. In addition to 5-HT1AR enriched regions, [18F]FECUMI-101 also shows binding in thalamus, which is displaceable with WAY100635. Further investigations of [18F]FECUMI-101 are required to confirm it as a 5-HT1AR partial agonist PET tracer [73]. [18F]15599 and its aminomethyl analogue [18F]13714 are recently reported as 5-HT1AR agonist tracers with nonoptimal target to nontarget ratios [74, 75]. The high affinity ligands [11C]S14506 and [18F]S14506 are also not successful in vivo [76]. All the above radioligands are synthesized via the C-11 methylation of their corresponding phenolates using either [11C]CH3I or [11C]CH3OTf or nucleophilic displacement of aromatic nitro precursors with [18F]F− or [18F]fluoroethylation of the precursor phenolate.
5-HT1BR
The existence of 5-HT1BR was a controversy earlier and it was believed that it was a species homologue of 5-HT1DR [2–5]. However, sequence studies and cloning of receptor confirmed that it is not an analogue of 5-HT1DR. In CNS, 5-HT1BR serves as a presynaptic heteroreceptor on nonserotonergic neurons and serves as an autoreceptor for serotonergic neurons in raphe nucleus. In vitro autoradiography studies show the presence of this receptor at higher levels in the basal ganglia (400–500 fmol/mg/tissue), especially in globus pallidus and substantia nigra followed by superior colliculus, enteropeduncular nuclei, and periaqueductal gray [77]. Lower levels of receptor density were detected in the cerebral cortex, hypothalamus, amygdala, cerebellum and dorsal horn of the spinal cord. 5-HT1BR present in cerebral arteries mediates a major role in 5-HT-induced vasoconstriction in human cerebral arteries (HCA) [2–5]. Pharmacological studies suggest that 5-HT1BR is involved as a regulator for aggression, memory, learning, substance abuse and premature ejaculation. 5-HT1BR and 5-HT1DR are autoreceptors and agonists shut off serotonin release from nerve terminals and this effect may explain their antimigraine properties in man. However, carotid vasoconstriction is an adverse side effect of 5-HT1BR agonists during migraine therapy.
Several high affinity, selective carboxamide analogues of different core chemical structures have been radiolabeled and characterized in vivo with PET [36, 37, 41]. [11C]5-methyl-8-(4-methyl-piperazin-1-yl)-4-oxo-4H-chromene-2-carboxylic acid (4-morpholin-4-yl-phenyl)-amide ([11C]AZ10419369) is a PET ligand for 5-HT1BRs (Ki = 0.8 nM, Fig. 5) [78]. [11C]AZ10419369 PET obtained by the reaction of desmethyl-AZ10419369 with [11C]CH3OTf. PET studies in macaques and human subjects show high tracer uptake (3–4%) in brain. The highest uptake was found in occipital cortex and basal ganglia followed by temporal and frontal cortical regions, less in thalamus and the lowest in cerebellum [79]. Except for palliduum, all other reported brain regions reached equilibrium within the imaging time and 1-TC model with cerebellum as reference region was the optimal modeling method for [11C]AZ10419369 in human subjects [79, 80]. [11C]AZ10419369 shows dose-dependent binding of AZD3783, a 5-HT1BR antagonist with potential antidepressant properties in non-human primates and human subjects indicating its potential for receptor occupancy measurement for drug development and dose-finding in clinical studies [80]. Unlike antagonist radiotracers for 5-HT1ARs, [11C]AZ10419369 was sensitive to fenfluramine-induced increase in synaptic 5-HT. PET studies in rhesus monkeys show 27% and 50% decrease in binding of [11C]AZ10419369 binding after the administration of 1mg/kg and 5 mg/kg fenfluramine respectively [81]. [11C]AZ10419369 PET imaging studies with citalopram shows decreased BPND in the treated monkeys in comparison to controls with highest change (−25%) in raphe nucleus [82]. Human PET studies with clinically relevant citalopram doses show 10% decrease in BPND in raphe nucleus and slight increase in binding (5%) in other regions [82]. Furthermore, pilot studies in human subjects found no correlation between 5-HT or its metabolite 5-hydroxyindole acetic acid (5-HIAA) in the CSF with [11C]AZ10419369 binding in brain. This indicates that [11C]AZ10419369, and perhaps intrasynaptic 5-HT levels, may not correlate with serotonin and 5-HIAA levels in CSF [83]. More recently zolmitriptan, a selective 5-HT1B/1DR agonist has been tested in human with [11C]AZ10419369 and no significant occupancy was detected with a 5 mg dose, whereas 4–5% occupancy was found for 10 mg dose [84]. An agonist 5-HT1BR radiotracer may be more sensitive for occupancy measurement of agonist drugs with PET. [11C]AZ10419369 is also tested in PD and lower binding was observed in orbitofrontal cortex in comparison with control subjects [85]. [11C](R)-1-[4-(2-methoxy-isopropyl)-phenyl]-3-[2-(4-methylpiperazin-1-yl)benzyl]-pyrrolidin-2-one) ([11C]P943) (Ki = 1.2 nM, Fig. 5) is also reported as a selective PET tracer for 5-HT1BR in nonhuman primates and human subjects [86, 87]. Radiosynthesis of [11C]P943 has been achieved by the reaction of [11C]CH3I with desmethyl-[11C]P943. Analogues to [11C]AZ10419369, such as [11C]P943, are sensitive to changes of intra-synaptic 5-HT induced by fenfluramine in monkeys [88]. [11C]P943 has been tested in depression and posttraumatic stress disorder (PTSD) subjects and lower binding was found in ventral palladium and ventral striatum in comparison with control subjects [89, 90]. Greater binding of [11C]P943 was found in subjects with alcohol dependence and pathological gambling [90]. Whereas, lower binding of [11C]P943 uptake was found in cocaine dependent subjects in comparison with healthy controls [91].
Fig. 5.
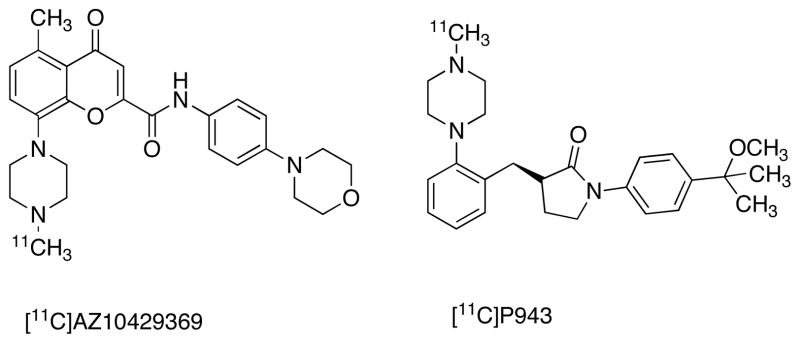
PET radioligands for 5-HT1BR.
5-HT1D,E,FRs
The 5-HT1DR is a heteroreceptor and a serotonin nerve terminal autoreceptor, predominantly expressed in basal ganglia and substantia nigra [2–5]. 5-HT1DR agonists are used for migraine treatment except PNU-142633, a specific 5-HT1DR agonist that proved ineffective for the treatment of migraine [184, 185]. The 5-HT1ER has ~ 60% homology with 5HT1BR. It is concentrated in caudate putamen and hippocampus with lower levels in amygdala, frontal cortex and globus pallidus. The 5-HT1ER is species dependent and is not expressed in rats or mice [2]. There are no selective or high affinity ligands available for this receptor, which limits the elucidation of the function of 5-HTER. Serotonin 5-HT1FRs, previously known as 5-HT1E receptors (70% sequence homology with 5-HT1E) and is located primarily in hippocampus, cortex and dorsal raphe nucleus. The physiological role of 5-HT1FR is currently unknown except its antimigraine properties along with 5-HT1BR and 5-HT1DR affinities. Although selective ligands are available, there is no PET tracers available for 5-HT1D,E,F receptors.
5-HT2Rs
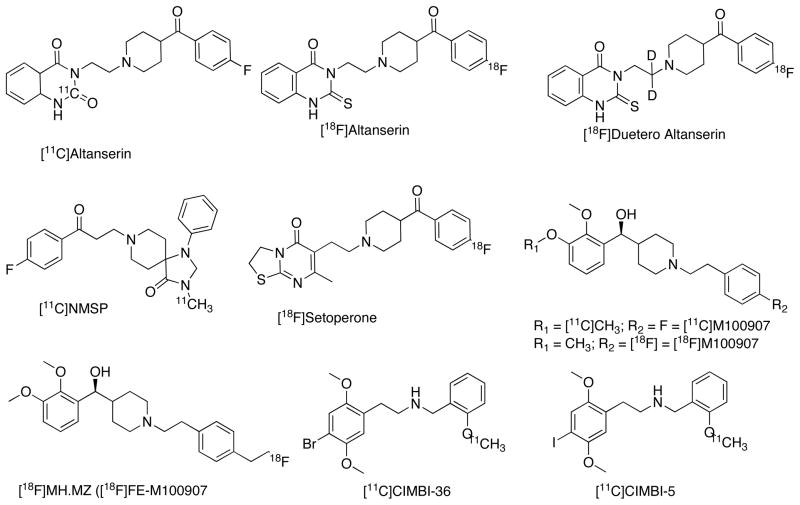
5-HT2Rs are coupled to Gq/11 class of G proteins that stimulate phospholipase C (PLC) producing inositol triphosphate (IP3) and intracellular Ca2+ release [2–5]. 5-HT2Rs are comprised of 3 subtypes namely 5-HT2AR, 5-HT2BR and 5-HT2CR that have 70–80% sequence homology [2–5]. Among these 5-HT2AR is the most predominant subtype and are expressed in central and peripheral tissues [2–5]. In CNS these receptors are principally located in cortex (Bmax about 500 fmol/mg/protein in neocortex), claustrum and basal ganglia [92, 93]. 5-HT2ARs can be in high affinity agonist state but predominantly exist in a low affinity state under normal physiological conditions [2, 94]. Alteration of 5-HT2AR binding is reported in schizophrenia, suicide, stress, PTSD and major depression and this receptor is a target for atypical or new generation antipsychotic drugs [2, 3, 95, 96]. In addition, 5-HT2ARs are also implicated in learning, appetite control, glaucoma, cardiovascular functions and muscle contractions [2, 3]. Preclinical studies show that 5-HT2AR antagonists have antipsychotic, antidepressant and antianxiety properties, whereas agonist ligands possess hallucinogenic properties. Although there are several PET tracers developed to date, their utility for imaging 5-HT2AR in vivo is limited due to high nonspecific binding, tracer kinetics marked by slow washout and inadequate pharmacology [36–39, 97]. [11C]Ketanserin (Ki = 2.3 nM, Fig. 6) is the first 5-HT2AR PET tracer studied in man, despite its affinity for histamine 1R (H1R) (Ki = 2 nM) and α-1AR (Ki = 40 nM) [98]. [11C]Ketanserin is synthesized via the reaction of [11C]COCl2 with the corresponding aminobenzamide precursor [181]. Due to the low target to nontarget ratio and rapid metabolism [11C]ketanserin did not advance for further development [99]. Subsequently, [18F]FEK, a fluoroethyl analogue of ketanserin has been developed and it showed better in vivo characteristics in nonhuman primates [100]. [18F]Setoperone and [11C]NMSP are less selective ligands, whereas, [18F]altanserin and [11C](R)M100907, have high selectivity for 5-HT2AR [36–39]. [18F]setoperone shows significant affinity for D2/3Rs (Ki = 24 nM) in addition to 5-HT2AR (Ki = 2.3 nM, Fig. 6). Both [18F]altanserin and [18F]setoperone are synthesized via the nucleophilic displacement reaction using [18F]F− with the corresponding nitro precursors. [18F]setoperone in vivo imaging is reported in a variety of conditions such as mood disorders, schizophrenia, AD, migraine, stroke, and depression and also explored in the estimation of 5-HT2AR occupancy by antipsychotics [36–39]. In a recent study with [18F]setoperone, lower cortical 5-HT2AR binding was found in adult autism spectrum disorder (ASD) [101]. However, less thalamic binding and no significant difference were found in other brain regions with [18F]setoperone in high functioning adult autistic patients [101]. [11C]NMSP ([11C]MSP), a methyl analogue of spiperone, is a highly selective 5-HT2AR (IC50 = 1.3 nM, Fig. 6), D2/3R (IC50 = 0.23 nM) ligand with low affinity for α–1AR (IC50 = 10.1 nM) [186]. It is synthesized by the [11C]methylation of the corresponding desmethyl precursor. Similar to [18F]setoperone, [11C]NMSP is a dual tracer and has been studied for D2/3R and 5-HT2AR measurements in normal and pathological conditions and for occupancy measurements of antipsychotic drugs including inverse agonists of 5-HT2AR [36–39]. [11C]NMSP shows age-related decline of dopamine D2R and 5-HT2AR in human subjects, higher striatal binding in patients with PD and schizophrenic patients in comparison to controls. Lower [11C]NMSP binding was found in putamen of Huntington patients. [11C]NMSP is also used to evaluate the response of bromocriptine, D2 agonist in pituitary tumors [101]. [18F]Altanserin has acceptable pharmacological specificity for 5-HT2Rs (Ki = 0.13 nM, Fig. 6) but quantitative in vivo imaging of 5-HT2Rs with [18F]altanserin has been hindered by lipophilic radiometabolites that cross the BBB and slow kinetics [36–39]. Better quantification of [18F]altanserin binding can be achieved using a bolus plus constant infusion protocol to overcome slow equilibrium and a multicompartmental model to account for metabolites. [18F]altanserin has been studied in several neuropsychiatric and neurodegenerative disorders. Notable findings are lower [18F]altanserin binding in AD (neocortical regions), medication free depressed patients (right posterolateral orbitofrontal and anterior insular cortices), schizophrenia (frontal cortex), higher binding after sleep deprivation and in obsessive compulsive disorder [36–39]. The replacement of hydrogen with deuterium in [18F]altanserin reduces the rate of undesirable radiometabolite formation. [18F]deuteroaltanserin shows better characteristics and 26% higher brain uptake than [18F]altanserin itself in nonhuman primates and human [102, 103]. Although higher binding in estrogen replacement and lower binding in cortical regions of AD are reported using [18F]deuteroaltanserin, not many studies are available at present to thoroughly assess its potential [104].
Fig. 6.
Examples of PET radioligands for 5-HT2AR.
[11C](R)M100907 aka [11C](R)MDL100907 is the highly selective and widely studied 5-HT2AR (Ki = 0.36 nM, Fig. 6) ligand in rodents, nonhuman primates and human [36–39]. Although [11C]M100907 shows promising characteristics, slow off-rate kinetics makes the tracer quantification difficult unless it can be labeled with a longer half life isotope. [11C]M100907 has been studied in few pathological conditions and greater binding reported in medication free depressed subjects and lower binding in drug naïve OCD patients [105, 106]. Receptor occupancy was found in M100907-medicated schizophrenia patients using [11C]M100907 [107]. Several efforts have been made to develop [18F]M100907 to permit longer duration of scanning. Muhlhausen et al reported racemic [18F]M100907 by the coupling of (2,3-dimethoxyphenyl)(piperidin-4-yl)methanol with [18F]fluoroethyl bromide [108]. More recently, Hooker et al reported an elegant nickel mediated radiofluoronation to synthesize [18F]M100907 and compared its binding with [11C]M100907 [109]. Two groups reported [18F]fluoroethyl-M100907 ([18F]FEM100907 aka [18F]MH.MZ), a fluoroethyl analogue of M100907 with faster off-rate than [11C]M100907 [110,111]. [18F]MH.MZ has higher cortex to cerebellum ratio in nonhuman primates and pigs [112]. Further studies are required to establish the utility of [18F]MH.MZ in human studies.
There are several reports regarding 5-HT2AR agonist radiotracers [113–117]. An agonist ligand may be sensitive to intra-synaptic 5-HT and allow estimation of 5HT release, and measures the GPCRs and thereby provides a more meaningful functional measure of 5-HT2AR binding. [11C]CIMBI-5 ([11C]IDMe aka [11C]NBMeO) (Ki = 0.15 nM, Emax = 91%, Fig. 6) was the first 5-HT2AR agonist tracer studied in pigs and nonhuman primates in vivo [113,114, 183]. In general, a markedly lower BPp is observed for [11C]CIMBI-5 in comparison with [11C]M100907 in baboon brain [114]. [11C]CIMBI-5 BPp values are an average 25% of total binding of [11C]M100907 in the brain regions examined [114]. This finding is consistent with the high affinity site binding ratio of [125I]DOI (agonist) with [3H]ketanserin and [3H]M100907 (antagonists) measured by in vitro autoradiography and in vitro saturation binding studies [118, 119]. More recently [11C]CIMBI-36, a bromo-analogue of CIMBI-5 has been reported to have higher target to non target ratio than [11C]CIMBI-5 in pig and nonhuman primates [116]. CIMBI-36 has affinity for 5-HT2BR (Ki = 0.5 nM) and 5-HT2CR (Ki = 1.5 nM) in addition to its 5-HT2AR affinity (Ki = 0.5 nM, Emax = 87%, Fig. 6) and it is the only agonist PET ligand tested so far in human [117]. The specificity of [11]CIMBI-36 binding in human has been confirmed by the reduced tracer binding in challenge studies predosed with ketanserin. Radiosynthesis and in vivo evaluation of [11C]AC-90179 (Ki = 2.1 nM), an inverse agonist of 5-HT2AR, is reported [120], and PET studies in baboon showed that the tracer penetrates the BBB, however no specific binding was observed.
5-HT2BR
Outside the brain, 5-HT2BRs are involved in muscle contractions and are located in cardiovascular tissues and intestines [2–5]. These receptors are less abundant in CNS and some evidence suggests that 5-HT2BRs are involved in anxiety, cognition, food intake and neuroendocrine regulation in rodents. There are not many selective ligands known for 5-HT2BR, and hence no PET ligands are currently available for this receptor subtype.
5-HT2CR
5HT2CR is expressed in the choroid plexus; Bmax 6.76 pmol/mg/protein in human, 688 fmol/mg protein in pig and 130 pmol/mg protein in rat; and spinal cord [121–123]. The major function of 5-HT2CRs in choroid plexus is to regulate ion exchange between the brain and the cerebrospinal fluid [2–5]. Low levels of 5-HT2CR are expressed in basal ganglia, hippocampus, cortex and amygdala. These receptors may have clinical value in the treatment for depression, panic anxiety, OCD, bulimia, and obesity. Except for choroid plexus, the abundance of 5-HT2CR are low in other brain regions, make this receptor less viable as an imaging target with PET. There are several attempts recently made for the development of 5-HT2CR PET tracers with limited success. These include a low affinity azetidine analogue of pyrimidoazepine, a 5-HT2C agonist (Ki = 75 nM) and antagonists WAY-163909 (Ki = 10 nM) and vabicaserin (Ki = 3 nM) [124, 125]. The radiosynthesis of [11C]pyrimidoazepine has been achieved via [11C]methylation using [11C]CH3I, whereas the antagonist ligands were synthesized via a novel C-11 Pictet-Spengler cyclization with [11C]CH2O ([124, 125]. Although these radiotracers penetrate the BBB, no specific binding was found in vivo in baboon.
5-HT3R
Unlike all the 5-HTRs, the 5-HT3R belongs the class of LGICRs with a close resemblance to nicotinic acetylcholine receptor (nAChR) [2–5]. There are five subtypes of 5-HT3Rs, which allow the permeability of mono (Na+, K+), divalent (Ca2+), ammonium and choline cations. The major functions of these receptors are to rapidly activating and desensitizing inward currents for synaptic transmission. 5-HT3Rs are expressed in central and periphery tissues [2–5]. Studies with [3H]GR65630, a selective 5-HT3R ligand in postmortem human brain revealed specific binding, however, the concentration of these receptors are lower in comparison with other 5-HTRs [126]. Highest expressions of 5-HT3Rs were found in some areas of brain stem (Bmax = 13.1 fmol/mg/protein), striatum (Bmax = 4.8 fmol/mg/protein) and low level expression in amygdala, hippocampus, and various cortical regions [126]. 5-HT3Rs have also been reported in dorsal horn, dorsal root ganglion (DRG) and vagal terminations in the digestive tract [188]. Several radioligands have been studied in various species for the quantification of 5-HT3Rs, and majority of the findings show lower Bmax in brain [127–134]. The distribution and pharmacology of 5-HT3R in brain is species dependent [134, 188]. 5-HT3R ligands have been used in antiemetic therapy for patients undergoing chemo and radiation therapy, and irritable bowel syndrome (IBS) [2–5, 188]. Preclinical data suggests that 5-HT3R could be potential drug target for mood disorder, cognitive impairment, substance abuse, addiction, bulimia, pruritis and autism [2–5, 188]. Several PET radioligands with diverse class has been reported for 5-HT3Rs, however in vivo studies found these ligands are not successful in rodents and primates due to nonspecific binding and poor brain uptake [36,135–140]. The failure of the radioligands for in vivo imaging may also contribute to the low abundant of 5-HT3Rs in brain. Most of the radioligands radioligands reported for 5-HT3Rs (Fig. 8) were synthesized via [11C]methylation of the corresponding precursor amine molecules. [11C]S21007 is synthesized by the reaction of [11C]benzyl iodide with N-nor-S21007 precursor [182].
Fig. 8.
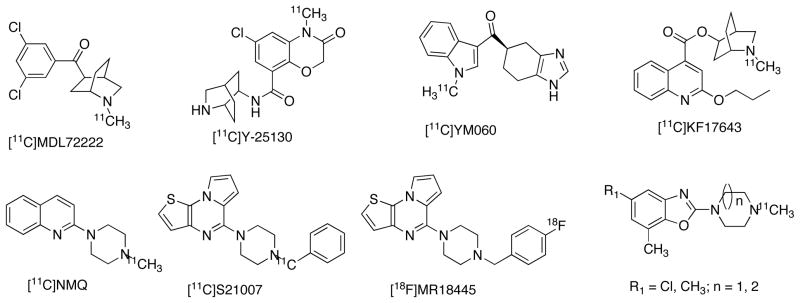
Examples of PET ligands for 5-HT3BR.
5-HT4R
5-HT4Rs are coupled to Gs form of GPCR and promote cAMP formation. 5-HT4Rs have 9 splice variants and are expressed in GI tract, urinary bladder, heart, adrenal gland and centrally located predominantly in striatum (Bmax= 223 fmol/mg/protein), neocortex, thalamus, hippocampus and brainstem [2–5, 141, 142]. In periphery, 5-HT4Rs are involved in the GI and cardiac functions [2–5]. Centrally, these receptors are postulated in the cognitive, memory, depression, ADHD, anorexia and obesity [2–5]. The receptor subtypes of 5-HT4Rs have different degree of internalizations, hence, nonselective ligands for this receptors may cause multiple pathological effects. Several agonist and antagonist ligands are known for this receptor, however splice variant specific ligands are limited [36–38]. Regarding PET imaging, [11C]SB207145 is the only antagonist radiotracer tested so far in human for 5-HT4R [143–146]. [11C]SB207145 belongs to the benzodioxine class of compounds with high affinity for 5-HT4R (Ki = 0.3 nM, Fig. 9) and show heterogeneous binding that corresponds to the known distribution of 5-HT4R binding in rodents, primates, pigs and human. The radioligand did not show sensitivity to endogenous competition studies with citalopram in comparison to control subjects [147]. In regards to 5-HTTLPR, [11C]SB207145 shows 9% higher binding for S allele in neocortex in comparison to LL homozygote [148]. No significant difference of [11C]SB207145 was found in a small group of AD patients in comparison to controls [149]. However, [11C]SB207145 binding was positively correlated to Aβ burden and negatively correlated to MMSE score of AD patients. Several radioligands were emerged based on [11C]SB207145 and among these, [18F]MNI-698 and [18F]MNI-699, the fluoroalkyl analogues of SB207145, were tested in primates [150, 151]. [18F]MNI-698 exhibits excellent in vivo characteristics and it is used for the measurement of 5-HT4R in monkey [151]. Several C-11 labeled analogues of SB207145 derivatives were synthesized for 5-HT4R, however, no in vivo data are reported for these tracers [152]. More recently [11C]prucalopride, a potent 5-HT4R agonist has been developed and in vivo studies in rats show low uptake of the radioligand which is likely due to inadequate lipophilicity and possibility of being a P-gp substrate [153].
Fig. 9.
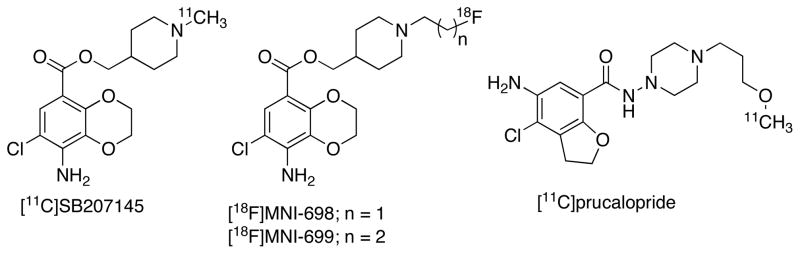
Examples of PET ligands for 5-HT4R.
5-HT5R
5-HT5R is a Gs-coupled GPCR and the least explored 5-HT receptor [2–5]. Of the two known subtypes, 5-HT5AR only been identified in human [2–5]. 5-HT5AR mRNA is expressed in cerebral cortex, hippocampus, hypothalamic area, amygdala and cerebellum [154, 155]. However, the physiological role of this receptor in normal brain function is still unknown and there is a lack of selective ligands for 5-HT5R. To date there is no PET ligands are reported for 5-HT5R.
5-HT6R
5-HT6R is Gs-coupled GPCR having effects via cAMP formation. 5-HT6Rs are localized almost exclusively in the CNS [2–5]. Postmortem studies demonstrated that highest 5-HT6R densities are in striatum (Bmax = 215 fmol/mg/protein), nucleus accumbens and olfactory tubercle, and moderate densities in amygdala, hypothalamus, thalamus, hippocampus and cerebral cortex [156–158]. Roles for 5-HT6R are postulated in cognition, seizures, feeding behavior, anxiety, epilepsy, dementia psychosis, addiction and mood disorders [3–5]. The utility of the currently developed radiotracers for in vivo imaging of 5-HT6R are limited in scope. The [18F]-labeled 5-HT6R ligand [18F]12ST05 (Ki = 4 nM) did not reveal any specific binding to 5-HT6R in rats and cats [159]. The radioligand [11C]SB399885 (Ki=1 nM, Fig. 10) is unsuitable for in vivo studies due to low uptake and its regional binding not in agreement with the known distribution of 5-HT6R [160]. [11C]GSK224558 rapidly enters the porcine brain, but undergoes rapid metabolism with peak regional tissue concentrations reached at approximately 20 min post-injection [161]. The nonselective ligand [11C]GSK215083 (5-HT6R Ki = 0.16 nM and 5-HT2AR Ki = 0.79 nM) has been tested in pigs, nonhuman primates and human [162–165]. [11C]GSK215083 demonstrated uptake and retention in the human brain, and the highest BP was observed in caudate and putamen followed by frontal cortex. Striatal binding and cortical binding of [11C]GSK215083 are mainly attributed to 5-HT6R and 5-HT2AR respectively. A recent patent reports radiolabeled quinolone derivatives for 5-HT6R, structurally similar to GSK215083 with 5-HT6R and 5-HT2AR Kis as 0.339 and 0.395 nM respectively [166, 167].
Fig. 10.
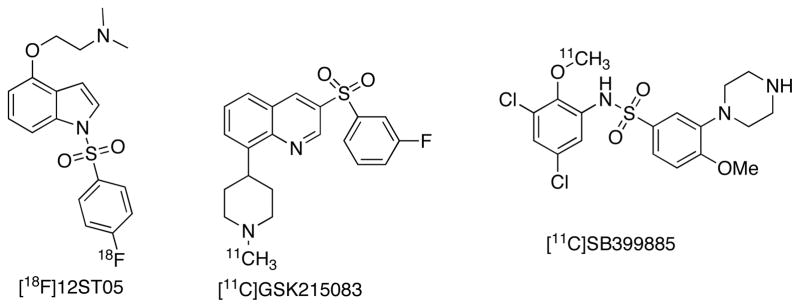
Examples of PET ligands for 5-HT6R.
5-HT7R
5-HT7R is coupled to Gs form of GPCR to activate 5-HT [2–5]. 5-HT7R is expressed in brain, gastrointestinal tract, blood vessels and heart. In brain, higher expression of 5-HT7R is found in thalamus (Bmax = 68 fmol/mg/protein), hypothalamus, hippocampus and cortex [168, 169]. There are three splice variants of 5-HT7R reported in human and all of them show similar pharmacology [170]. 5-HT7R are involved in thermoregulation, cardiac circadian rhythm, learning, memory, mood regulation, autism, neuropathy pain and sleep [2–5]. There is a 49% sequence homology between 5-HT7R and 5-HT1AR [171, 172]. Therefore development of highly selective ligands for one or other of these two targets is a potential challenge. The anatomical distribution of the binding densities of the two receptors is significantly different from each other. For example, 5-HT7R has higher density in thalamus, whereas, the density of 5-HT1AR in thalamus is low [32, 33, 169]; higher density of 5-HT1AR is reported in temporal cortex, whereas, 5-HT7R is very low density in this region [32, 33, 169]. Among the 5-HTRs, 5-HT7R has the highest affinity for 5-HT, which makes this receptor an ideal target for PET imaging [13].
There are limited reports available for the evaluation of 5-HT7R with PET [36]. Although the pioneer 5-HT7R PET ligand [11C]DR4446 (Ki = 9.7 nM, Fig. 11) was tested in monkeys, it did not prove successful in vivo despite its excellent BBB penetration and metabolic stability [173]. Several 18F- ligands based on SB-269970, a selective 5-HT7R has been reported [174–178]. Among these, [18F]2FP3 (Ki = 8.4 nM, Fig. 11) and [18F]4FP3 (Ki = 14 nM, Figure 11) show specific binding in vitro in brain sections of rats [175]. In vivo studies in cats show excellent brain uptake, regional distribution and specific binding for [18F]2FP3 [176]. [11C]CIMBI-806, a dimethoxy biphenyl analogue (Ki = 8.6 nM, Fig. 11) shows excellent in vitro binding in pig brain but did not show specific binding in vivo in pig despite its high brain uptake [179]. More recently [11C]CIMBI-712 (Ki = 1.1 nM, Fig. 11) and [11C]CIMBI-717 (Ki = 2.6 nM, Fig. 11), the two selective phenylpiperazinyl butyloxindole derivatives have been studied as 5-HT7R ligands in pigs [180] and the latter shows higher uptake and specific binding than [11C]CIMBI-712. In summary, [18F]2FP3 and [11C]CIMBI-717 are the two PET ligands proven successful in vivo in cats and pigs, respectively.
Fig. 11.
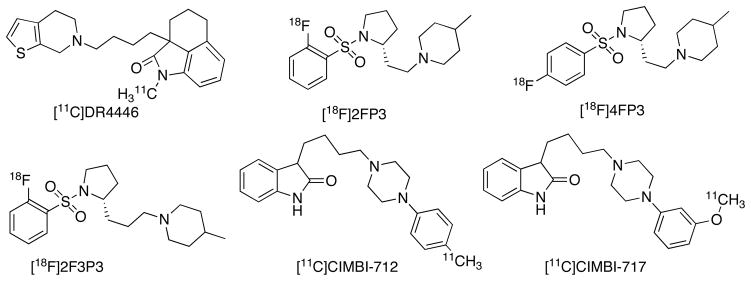
Examples of PET ligands for 5-HT7R.
CONCLUSIONS
PET imaging of 5-HTRs has been progressing for almost three decades and several selective radiotracers have been developed to quantify 5-HT receptor subtypes in normal and pathological conditions in human subjects (Table 1).
Table 1.
List of PET ligands available for 5-HTR imaging in human.
| Receptor subtype | PET Ligands | Advantages | Limitations |
|---|---|---|---|
| 5-HT1AR antagonist | [Carbonyl-11C]WAY-100635 | High target to non-target ratio | Complex radiosynthesis, fast metabolism, difficulty of quantification with arterial input functions. |
| [11C]cis-FCWAY | Radiodefluorination | ||
| [11C]MPPF | P-gp substrate, low target to non target ratio | ||
| [11C]trans-MeFWAY | High target to non-target ratio | Quantification with arterial input data is currently not available | |
| 5-HT1AR partial agonist | [11C]CUMI-101 | Binding to high affinity site in human and NHP | Partial agonist, a-1AR cross selectivity is reported in rodents and monkey. |
| 5-HT1BR antagonist | [11C]AZ10419369 | ||
| [11C]P943 | Reported altered binding in a variety of pathological conditions | ||
| 5-HT2AR antagonist | [18F]Altanserin | Interference of radiometabolite in quantification, need bolus infusion paradigm for injection. | |
| [18F]Setoperone | Nonselective for D2R | ||
| [11C]NMSP | Cross affinity for D2, a-1R, 5-HT1AR, and 5-HT2CR | ||
| [11C](R)-M100907 | Slow kinetics | ||
| 5-HT2AR agonist | [11C]CIMBI-36 | ||
| 5-HT4R antagonist | [11C]SB207145 | Not sensitive to endogenous 5-HT changes, Altered binding in 5-HTTLPR | |
| 5-HT6R antagonist | [11C]GSK215083 | Cross selectivity to 5-HT2AR |
The best validated PET radiotracers developed so far for serotonin receptors are for 5-HT1AR and 5-HT2AR. [Carbonyl-11C]WAY100635, [18F]MPPF and [18F](cis)FCWAY, [18F]altanserin and [11C](R)M100907 are the best 5-HT1AR and 5-HT2AR ligands available but have limitations such as complex radiochemistry, fast metabolism (WAY100635), P-gp substrate (MPPF), radiodefluorination (FCWAY), interference of a radiometabolite (altanserin) and slow off-rate requiring an 18F-label to image long enough to capture equilibrium binding (M100907). The emerging tracers for 5-HTRs include [11C]trans-MeFWAY (5-HT1AR antagonist), [11C]AZ10419369, [11C]P943 (both 5-HT1BR), [11C]SB207145 (5-HT4R) and [11C]GSK215083 (5-HT6R) which are successfully tested in man and reported in few brain disorders. The partial agonist ligand [11C]CUMI-101 is promising in human and nonhuman primates to measure the HA state of 5-HT1AR, however, no data is available so far to support its potential to image pathological conditions. The recently developed [18F](R)M100907 and [18F](R)MH-MZ can circumvent the disadvantages of [11C](R)M100907 and are potential 5-HT2AR PET tracers for diagnosis and drug development. The 5-HT2A/2CR agonist ligand [11C]CIMBI-36 is promising for quantifying high agonist affinity receptor. Despite significant efforts, no PET tracers are available for 5-HT3Rs, possibly due to the low Bmax as well as intracellular localization of these receptors in brain. Similarly, due to the lack of adequate receptor density for 5-HT2BR and lack of available ligands for 5-HT5R, the development of PET tracers for these targets are hindered. [11C]CIMBI-717 and [18F]2FP3 have promising characteristics to image 5-HT7R in human. Future development of suitable PET 5-HTRs agonist/antagonist radiotracers is needed to study more serotonin receptor subtypes and their role in pathophysiology of diseases and to study receptor occupancy of promising therapeutic medications for dose finding as a preliminary step before implementing clinical trials.
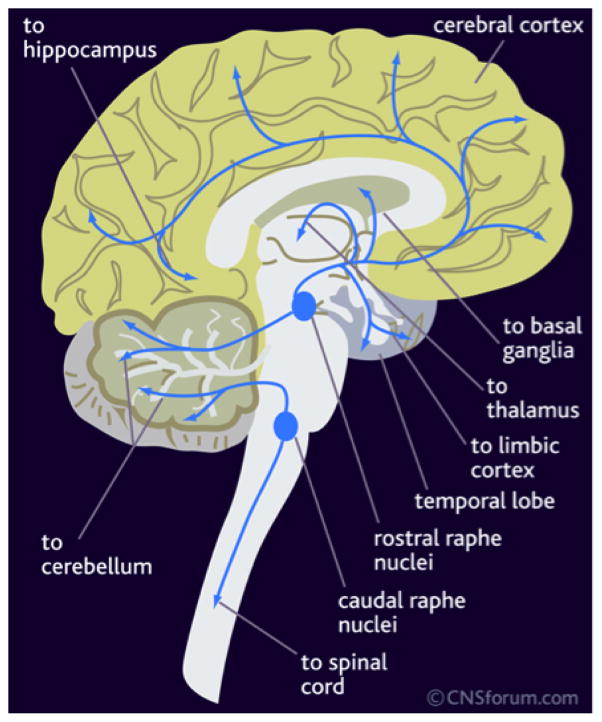
Fig. 1.
Serotogenic pathways in human brain (adapted from CNS forum).
Fig. 2.
Serotonin receptors and subtypes.
Fig. 7.
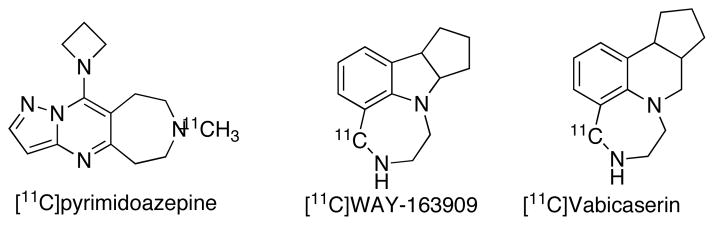
Examples of PET ligands for 5-HT2CR.
Acknowledgments
This work was partially supported by NIMH grant MH040695.
LIST OF ABBREVIATIONS
- Ab
Amyloid beta
- ABC
ATP-binding cassette
- AC
Adenylate cyclase
- AD
Alzheimer’s disease
- ADHD
Attention deficient hyperactivity syndrome
- APD
Antipsychotic drug
- ASD
Autism spectrum disorder
- a-1AR
Adrenergic Alpha1 receptor
- BBB
Blood brain barrier
- BCRP
Brest cancer resistance protein
- Bmax
Binding maxima
- BP
Binding potential
- C
Carbon
- Ca
Calcium
- cAMP
Cyclic AMP
- CH3I
Methyl iodide
- CH2O
Formaldehyde
- CH3OTf
Methyl triflate
- COCl2
Carbonyl chloride (Phosgene)
- CSF
Cerebrospinal fluid
- D2R
Dopamine 2 receptor
- DOI
2,5-dimethoxy-4-iodoamphetamine
- DRG
Dorsal root ganglion
- Emax
Maximum efficacy
- EC50
Half maximal effective concentration
- F
Fluorine
- F−
Fluoride
- fp
Free fraction
- fmol
Femptomole
- GDP
Guanosine diphosphate
- GTPgS
Guanosine 5′-O-[gamma-thio]triphosphate
- HA
High affinity
- HCA
Human cerebral artery
- 5-HIAA
5-Hydroxyindole acetic acid
- H1R
Histamine 1 receptor
- 5-HTR
5-Hydroxytryptamine receptor (serotonin receptor)
- 5-HT
5-Hydroxytryptamine (Serotonin)
- 5-HTTLPR
5-Hydroxytriptamine transporter linked polymorphic region
- GI
Gastrointestinal
- GPCR
G protein-coupled receptor
- IC50
Inhibitory concentration half maximal
- IBS
Irritable bowel syndrome
- IP3
Inositol triphosphate
- K
Potassium
- Kd
Kinetics of dissociation
- Ki
Kinetics of inhibition
- LA
Low affinity
- LGIC
Ligand-gated ion channel
- logD
Partition coefficient or distribution coefficient
- MDR
multi-drug resistance-associated protein
- mg
Milligram
- MMSE
Mini mental state examination
- MSP
Methyl spiperone
- mRNA
Messenger ribonucleic acid
- nAChR
nicotinic acetylcholine receptor
- N
Nitrogen
- Na
Sodium
- nM
nano molar
- NMSP
N-methyl spiperone
- O
Oxygen
- OCD
Obsessive compulsive disorder
- PD
Parkinson’s disease
- PET
Positron emission tomography
- P-gp
P-glycoprotein
- PLC
Phospholipase C
- PTSD
Post traumatic stress disorder
- SERT
Serotonin transporter
- SLC
Soluble carrier
- T1/2
Half life
- TC
Tissue compartment
- TDC
Tryptophan decarboxylase
- TPH
Tryptophan hydroxylase
- VT
Volume of distribution
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest related to the content of the review.
Send Orders for Reprints to reprints@benthamscience.net
References
- 1.Rapport MM, Green AA, Page H. Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem. 1948;176:1243–1251. [PubMed] [Google Scholar]
- 2.Nichols DE, Nichols CD. Serotonin receptors. Chem Rev. 2008;108:1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 3.Bush ES. Serotonin Receptors. Humana Press Inc; New Jersey: 2011. [Google Scholar]
- 4.Tyce GM. Origin and metabolism of serotonin. J Cardiovas Pharm. 1990;16:S1–S7. [PubMed] [Google Scholar]
- 5.Glennon RA, Dukat M. Foye’s Medicinal Chemistry, Chapter 11. In: Williams DA, Zito SW, Lemke TL, Roche VF, editors. Serotonin Receptors and Drugs Affecting Serotonergic Neurotransmission. Lippincott, Williams, & Wilkins; Philadelphia: 2012. [Google Scholar]
- 6.Barnes NM, Sharp TA. Review of central 5-HT receptors and their function. Neuropharmacol. 1999;38:1083. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt JA, Wingen M, Ramaekers JG, Evers EA, Riedel WJ. Serotonin and human cognitive performance. Curr Pharm Des. 2006;12:2473–2486. doi: 10.2174/138161206777698909. [DOI] [PubMed] [Google Scholar]
- 8.Michelsen KA, Prickaerts J, Steinbusch HW. The dorsal raphe nucleus and serotonin: Implications for neuroplasticity linked to major depression and Alzheimer’s disease. Prog Brain Res. 2008;172:233–264. doi: 10.1016/S0079-6123(08)00912-6. [DOI] [PubMed] [Google Scholar]
- 9.Rubinstein JLR. Development of serotonergic neurons and their projections. Biol Psychiatry. 1998;4(3):145–150. doi: 10.1016/s0006-3223(98)00133-4. [DOI] [PubMed] [Google Scholar]
- 10.Törk I. Anatomy of the Serotonergic System. Ann New York Acad Sci. 1990;600:9–34. doi: 10.1111/j.1749-6632.1990.tb16870.x. [DOI] [PubMed] [Google Scholar]
- 11.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Ann Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 13.Paterson LM, Tyacke RJ, Nutt DJ, Knudsen GM. Measuring endogenous 5-HT release by emission tomography: promises and pitfalls. J Cereb Blood Flow Metab. 2010;30:1682–1706. doi: 10.1038/jcbfm.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valk PE, Bailey DE, Townsend DW, Maisey MN. Positron Emission Tomography Basic Science and Clinical Practice. Springler; New York: 2002. [Google Scholar]
- 15.Dunphy MPS, Lewis JS. Radiopharmaceuticals in preclinical and clinical development for monitoring therapy PET. J Nuc Med. 2009;50:106S–121S. doi: 10.2967/jnumed.108.057281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coenen HH, Elsinga PH, Iwata R, Kilbourn MR, Pillai MRA, Rajan MGR, Wagner HN, Jr, Zaknun JJ. Fluorine-18 radiopharmaceuticals beyond [18F]FDG for use in oncology and neurosciences. Nuc Med Biol. 2010;37(7):727–740. doi: 10.1016/j.nucmedbio.2010.04.185. [DOI] [PubMed] [Google Scholar]
- 17.Phelps ME. Positron emission tomography provides molecular imaging of biological processes. Proc Natl Acad Sci USA. 2010;97:9226. doi: 10.1073/pnas.97.16.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pike VW. PET radiotracers: crossing the blood-brain barrier and surviving metabolism. Trends Pharmacol Sci. 2009;30:431–440. doi: 10.1016/j.tips.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waterhouse RN. Determination of lipophilicity and its use as a predictor of blood-brain barrier penetration of molecular imaging agents. Mol Imag Biol. 2003;5:376–389. doi: 10.1016/j.mibio.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Kessler RM, Ansari T, de Paulis DE, Schmidt JA, Clanton HE, Smith RG, Manning D, Gillespie Ebert MH. High affinity dopamine D2 receptor radioligands. 1. Regional rat brain distribution of iodinated benzamides. J Nucl Med. 1999;32:1593–1600. [PubMed] [Google Scholar]
- 21.Fujita M, Innis RB. In Vivo Molecular Imaging: Ligand Development and Research [Google Scholar]
- 22.Davis KL, Cherney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Lippincott, Williams, & Wilkins; Philadelphia: 2002. Applications; pp. 411–425. [Google Scholar]
- 23.Bergstrom M, Grahnen A, Langstrom B. Positron emission tomography microdosing: a new concept with application in tracer and early clinical drug development. Eur J Clin Pharmacol. 2003;59:357–366. doi: 10.1007/s00228-003-0643-x. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee J, Shi B, Narayanan TK, Christian BT, Zhi-Ying Y. Chapter 5-Radiopharmaceuticals for Imaging the Brain. In: Miles NW, John PD, Aarsvold N, editors. Emission Tomography. Academic Press; San Diego: 2004. pp. 89–101. [Google Scholar]
- 25.Laruelle M, Slifstein M, Huang Y. Relationships between radiotracer properties and image quality in molecular imaging of the brain with positron emission tomography. Mol Imag Biol. 2003;5:363–375. doi: 10.1016/j.mibio.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT1A receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 27.Hensler JG. Regulation of 5-HT1A receptor functions in brain following agonist or antidepressant administration. Life Sci. 2003;72:1665–1682. doi: 10.1016/s0024-3205(02)02482-7. [DOI] [PubMed] [Google Scholar]
- 28.Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJ, Mann JJ. Serotonin1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacol. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 29.Neumeister A, Bain E, Nugent AC, Carson RE, Bonne O, Luckenbaugh DA, Eckelman W, Herscovitch P, Charney DS, Drevets WC. Reduced serotonin type 1A receptor binding in panic disorder. J Neurosci. 2004;24:589–591. doi: 10.1523/JNEUROSCI.4921-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiihonen J, Keski-Rahkonen A, Löppönen M, Muhonen M, Kajander J, Allonen T, Någren K, Hietala J, Rissanen A. Brain serotonin1A receptor binding in bulimia nervosa. Biol Psychiatry. 2004;55:871–873. doi: 10.1016/j.biopsych.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Lai MK, Tsang SW, Francis PT, Esiri MM, Keene J, Hope T, Chen CP. Reduced serotonin 5-HT1A receptor binding in the temporal cortex correlates with aggressive behavior in Alzheimer disease. Brain Res. 2003;974:82–87. doi: 10.1016/s0006-8993(03)02554-x. [DOI] [PubMed] [Google Scholar]
- 32.Savitz J, Lucki I, Drevets WC. 5-HT(1A) receptor function in major depressive disorder. Prog Neurobiol. 2009;88(1):17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burnet PWJ, Eastwood PL, Harrison PJ. [3H]WAY100635 for 5-HT1A receptor autoradiography in human brain: a comparison with [3H]8-OH-DPAT and demonstration of increased binding in the frontal cortex in schizophrenia. Neurochem Int. 1997;30:565–574. doi: 10.1016/s0197-0186(96)00124-6. [DOI] [PubMed] [Google Scholar]
- 34.Hall H, Lundkvist C, Halldin C, Farde L, Pike VW, McCarron JA, Fletcher A, Cliffe IA, Barf T, Wikström H, Sedvall G. Autoradiographic localization of 5-HT1A receptors in the postmortem human brain using [3H]WAY100635 and [11C]WAY100635. Brain Res. 1997;745:96–108. doi: 10.1016/s0006-8993(96)01131-6. [DOI] [PubMed] [Google Scholar]
- 35.Kumar JSD, Mann JJ. PET tracers for 5-HT1AR and uses thereof. Drug Develop Today. 2007;12:748–756. doi: 10.1016/j.drudis.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Paterson LM, Kornum BR, Nutt DJ, Pike VW, Knudsen GM. 5-HT Radioligands for Human Brain Imaging With PET and SPECT. Med Res Rev. 2013;33(1):54–111. doi: 10.1002/med.20245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmer L, Le Bars D. Current status of positron emission tomography radiotracers for serotonin receptors in humans. J Label Compd Radiopharm. 2013;56:105–113. doi: 10.1002/jlcr.3001. [DOI] [PubMed] [Google Scholar]
- 38.Paterson LM, Tyacke RJ, Nutt DJ, Knudsen GM. Measuring endogenous 5-HT release by emission tomography: promises and pitfalls. J Cer Blood Flow Metab. 2010;30:1682–1706. doi: 10.1038/jcbfm.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saulin A, Savli M, Lanzenberger R. Serotonin and molecular neuroimaging in humans using PET. Amino Acids. 2012;42(6):2039–2057. doi: 10.1007/s00726-011-1078-9. [DOI] [PubMed] [Google Scholar]
- 40.Borg J. Molecular imaging of the 5-HT1A receptor in relation to human cognition. Behav Brain Res. 2008;195(1):103–111. doi: 10.1016/j.bbr.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 41.Billard T, Le Bars D, Zimmer L. PET Radiotracers for Molecular Imaging of Serotonin 5-HT1A Receptors. Curr Med Chem. 2014;21:70–81. doi: 10.2174/09298673113209990215. [DOI] [PubMed] [Google Scholar]
- 42.Lothe A, Bouvard S, Ryvlin P. PET Imaging of the Serotoninergic 5-HT1A System, in Positron Emission Tomography - Recent Developments in Instrumentation. In: Misciagna S, editor. Research and Clinical Oncological Practice. Intech; Croatia: 2013. [Google Scholar]
- 43.Osman S, Lundkvist C, Pike VW, Halldin C, McCarron JA, Swahn C-G, Ginovart N, Luthra SK, Bench CJ, Grasby PM, Wikström H, Barf T, Cliffe IA, Fletcher A, Farde L. Characterisation of the radioactive metabolites of the 5-HT1A receptor radioligand, [O-methyl-11C]WAY1000635, in monkey and human plasma by HPLC: comparison of the behaviour of an identified radioactive metabolite with parent radioligand in monkey using PET. Nucl Med Biol. 1996;23:627–634. doi: 10.1016/0969-8051(96)00061-3. [DOI] [PubMed] [Google Scholar]
- 44.Chemel BR, Roth BL, Armbruster B, Watts VJ, Nichols DE. WAY-100635 is a potent dopamine D4 receptor agonist. Psychopharmacology (Berl) 2006;188(2):244–251. doi: 10.1007/s00213-006-0490-4. [DOI] [PubMed] [Google Scholar]
- 45.Sumiyoshi T, Stockmeier CA, Overholser JC, Thompson PA, Meltzer HY. Dopamine D4 receptors and effects of guanine nucleotides on [3H]raclopride binding in postmortem caudate nucleus of subjects with schizophrenia or major depression. Brain Res. 1985;681(1–2):109–116. doi: 10.1016/0006-8993(95)00301-6. [DOI] [PubMed] [Google Scholar]
- 46.Isseroff GR, Dillon KA, Fieldust SJ, Biegon A. Autoradiographic analysis of alpha-1-noradrenergic receptors in the human brain postmortem - Effect of suicide. Arch Gen Psychiatry. 1990;47:1049–1053. doi: 10.1001/archpsyc.1990.01810230065010. [DOI] [PubMed] [Google Scholar]
- 47.Pike VW, Halldin C, McCarron JA, Lundkvist C, Hirani E, Olsson H, Hume SP, Karlsson P, Osman S, Swahn C-G, Hall H, Wikström H, Mensonidas M, Poole KG, Farde L. [Carbonyl-11C]Desmethyl-WAY100635 (DWAY) is a potent and selective radioligand for central 5-HT1A receptors in vitro and in vivo. Eur J Nucl Med. 1998;25:338–346. doi: 10.1007/s002590050230. [DOI] [PubMed] [Google Scholar]
- 48.Andrée A, Halldin C, Pike VW, Gunn RG, Olsson H, Farde L. The PET radioligand [carbonyl-11C]desmethyl-WAY-100635 binds to 5-HT1A receptors and provides a higher radioactive signal than [carbonyl-11C]WAY-100635 in the human brain. J Nucl Med. 2002;43:292–303. [PubMed] [Google Scholar]
- 49.Yasuno F, Zoghbi SS, McCarron JA, Hong J, Ichise M, Brown A, Gladding RL, Bacher J, Pike VW, Innis RB. Quantification of serotonin 5-HT1A receptors in monkey brain with [11C](R)-(−)-RWAY. Synapse. 2006;60:510–520. doi: 10.1002/syn.20327. [DOI] [PubMed] [Google Scholar]
- 50.Liow J-S, Lu S, McCarron JA, Hong J, Musachio JL, Pike VW, Innis RB, Zoghbi SS. Effect of a P-glycoprotein inhibitor, cyclosporin A, on the disposition in rodent brain and blood of the 5-HT1A receptor radioligand [11C](R)- (−)-RWAY. Synapse. 2006;61:96–105. doi: 10.1002/syn.20348. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X-Y, Yasuno F, Zoghbi SS, Liow J-S, Hong J, Mccarron JA, Pike VW, Innis RB. Quantification of serotonin 5-HT1A receptors in human with [11C](R)-(2)-RWAY: radiometabolite(s) likely confound brain measurements. Synapse. 2007;61:469–477. doi: 10.1002/syn.20392. [DOI] [PubMed] [Google Scholar]
- 52.Choi JY, Lee M, Jeon TJ, Choi S-H, Choi YJ, Lee YK, Kim J-J, Ryu YH. Determination of optimal acquisition time of [18F]FCWAY PET for imaging serotonin 1A receptors in the healthy male subjects. App Rad Isotop. 2014;89:141–145. doi: 10.1016/j.apradiso.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 53.Shiue C, Shiue GG, Mozlev PD, Kung MP, Zhuang ZP, Kim HJ, Kung HF. p-[18F]MPPF: a potential radioligand for PET studies of 5-HT1A receptors in humans. Synapse. 1997;25:147–154. doi: 10.1002/(SICI)1098-2396(199702)25:2<147::AID-SYN5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 54.Aznavour N, Zimmer J. [18F]MPPF as a tool for the in vivo imaging of 5- HT1A receptors in animal and human brain. Neuropharmacol. 2007;52:695–707. doi: 10.1016/j.neuropharm.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 55.Kepe V, Barrio JR, Huang SC, Ercoli L, Siddarth P, Shoghi -JK, Cole GM, Satyamurthy N, Cummings JL, Small GW, Phelps ME. Serotonin1A receptors in the living brain of Alzheimer’s disease patients. Proc Natl Acad Sci. 2006;103:702–707. doi: 10.1073/pnas.0510237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saigal N, Pichika R, Eawaramoorthy B, Collins D, Christian BT, Shi B, Naryana TK, Polkin SG, Mukherji J. Synthesis and biologic evaluation of a novel serotonin 5-HT1A receptor radioligand, [18F]-labeled mefway, in rodents and imaging by PET in a nonhuman primate. J Nucl Med. 2006;47:1697–1706. [PubMed] [Google Scholar]
- 57.Wooten DW, Hillmer AT, Moirano JM, Ahlers EO, Slesarev M, Barnhart TE, Mukherjee J, Schneider ML, Christian BT. Measurement of 5-HT1A receptor density and in-vivo binding parameters of [18F]mefway in the nonhuman primate. J Cereb Blood Flow Metab. 2012;32(8):1546–1558. doi: 10.1038/jcbfm.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bajwa A, Hillmer A, Wooten D, Saigal N, Christian B, Mukherjee J. 18F-Mefway: Efficacy as a PET imaging agent for serotonin 5-HT1A receptors. J Nucl Med. 2014;55(Supplement 1):553. [Google Scholar]
- 59.Clawges HM, Depree KM, Parker EM, Graber SG. Human 5-HT1 receptor subtypes exhibit distinct G protein coupling behaviors in membranes from Sf9 cells. Biochem. 1997;36:12930–12938. doi: 10.1021/bi970112b. [DOI] [PubMed] [Google Scholar]
- 60.Watson J, Collin L, Ho M, Riley G, Scott C, Selkirk JV, Price GV. 5-HT1A receptor agonist-antagonist binding affinity difference as a measure of intrinsic activity in recombinant and native tissue systems. Br J Pharmacol. 2000;130:1108–1114. doi: 10.1038/sj.bjp.0703394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kumar JSD, Majo VJ, Tamir H, Millak MS, Hsing S-C, Prabhakaran J, Simpson NR, Van Heertum RL, Mann JJ, Parsey RV. Synthesis and in vivo validation of [11C]MPT: A potential 5-HT1A receptor agonist PET ligand. J Med Chem. 2006;49:125–134. doi: 10.1021/jm050725j. [DOI] [PubMed] [Google Scholar]
- 62.Mann JJ, Kumar JSD. WO 2006083424 Radiolabeled compounds and uses thereof. 2006
- 63.Mann JJ, Kumar JSD. WO2009–006227 Radiolabeled piperizine compounds and uses thereof. 2009
- 64.Kumar JSD, Prabhakaran J, Majo VJ, Milak MS, Hsiung S-C, Tamir H, Simpson NR, Van Heertum RL, Mann JJ, Parsey RV. Synthesis and in vivo Evaluation of 5-HT1A receptor Agonist Radioligand [O-methyl-11C]2-(4-(2-methoxy- phenyl) piperazin-1-yl)butyl)-4-methyl-1,2,4-triazine-3,5(2H,4H)dione in Nonhuman Primates. Eur J Nuc Med Mol Imaging. 2007;34:1050–1060. doi: 10.1007/s00259-006-0324-y. [DOI] [PubMed] [Google Scholar]
- 65.Milak MS, DeLorenzo C, Zanderigo F, Prabhakaran J, Kumar JSD, Majo VJ, Mann JJ, Parsey RV. In vivo quantification of the human Serotonin 1A receptor using [11C]CUMI-101, an agonist positron emission tomography radiotracer. J Nuc Med. 2010;51:1892–1890. doi: 10.2967/jnumed.110.076257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Milak MS, Severance AJ, Ogden TR, Prabhakaran J, Kumar JSD, Majo VJ, Mann JJ, Parsey RV. In vivo serotonin-sensitive binding of [11C]CUMI-101, a serotonin 1A receptor agonist positron emission tomography radiotracer. J Cer Blood Flow Metab. 2011;31:241–249. doi: 10.1038/jcbfm.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Selvaraj S, Turkheimer F, Rosso L, Faulkner P, Mouchlianitis E, Roiser JP, McGuire P, Cowen PJ, Howes O. Measuring endogenous changes in serotonergic neurotransmission in humans: a [11C]CUMI-101 PET challenge study. Mol Psychiatry. 2012;17(12):1254–1260. doi: 10.1038/mp.2012.78. [DOI] [PubMed] [Google Scholar]
- 68.Pinborg LH, Feng L, Haahr ME, Gillings N, Dyssegaard A, Madsen J, Svarer C, Yndgaard S, Kjaer TW, Parsey RV, Hansen HD, Ettrup A, Paulson OB, Knudsen GM. No change in [11C]CUMI-101 binding to 5-HT1A receptors after intravenous citalopram in human. Synapse. 2012;66(10):880–884. doi: 10.1002/syn.21579. [DOI] [PubMed] [Google Scholar]
- 69.Kumar JSD, Majo VJ, Milak MS, Prabhakaran J, Mali P, Savenkova L, Mann JJ, Parsey RV. Comparison of high and low affinity serotonin 1A receptors by PET in vivo in nonhuman primates. J Pharm Sci. 2012;120:254–257. doi: 10.1254/jphs.12100sc. [DOI] [PubMed] [Google Scholar]
- 70.Hendry N, Christie I, Rabiner EA, Laruelle M, Watson J. In vitro assessment of the agonist properties of the novel 5-HT1A receptor ligand, CUMI-101 (MMP), in rat brain tissue. Nuc Med Biology. 2011;38(2):273–277. doi: 10.1016/j.nucmedbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 71.Shrestha SS, Liow J-S, Lu S, Jenko K, Gladding RL, Svenningsson P, Morse CL, Zoghbi SS, Pike VW, Innis RB. 11C-CUMI-101, a PET radioligand, behaves as serotonin 1A receptor antagonist and also binds to α1 andrenoceptors in brain. J Nuc Med. 2014;55(1):141–146. doi: 10.2967/jnumed.113.125831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar JSD, Parsey RV, Majo VJ, Milak MS, Prabhakaran J, Kassir SA, Underwood M, Mann JJ, Arango V. Autoradiographic evaluation [3H]CUMI-101, a potential 5-HT1A ligand. Brain Res. 2013;1507:11–18. doi: 10.1016/j.brainres.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Majo VJ, Prabhakaran J, Milak MS, Mali P, Parsey RV, Mann JJ, Kumar JSD. Development of [18F]FECUMI-101 as a 5-HT1A agonist radiotracer. Bioorg Med Chem. 2013;17:5598–5604. doi: 10.1016/j.bmc.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lemoine L, Verdurand M, Vacher B, Blanc E, Le Bars D, Tancredi AN, Zimmer L. [18F]F15599, a novel 5-HT1A receptor agonist, as a radioligand for PET neuroimaging. Eur J Nucl Med Mol Imaging. 2010;37:594–605. doi: 10.1007/s00259-009-1274-y. [DOI] [PubMed] [Google Scholar]
- 75.Lemoine L, Becker G, Vacher B, Billard T, Lancelot S, Tancredi AN, Zimmer L. Radiosynthesis and Preclinical Evaluation of 18 F-F13714 as a Fluorinated 5-HT1A Receptor Agonist Radioligand for PET Neuroimaging. J Nucl Med. 2012;53:969–976. doi: 10.2967/jnumed.111.101212. [DOI] [PubMed] [Google Scholar]
- 76.Lu S, Liow J-S, Zoghbi SS, Hong J, Innis RB, Pike VW. Evaluation of [11C]S14506 and [18F]S14506 in rat and monkey as agonist PET radioligands for brain 5-HT1A receptors. Curr Radiopharm. 2010;3(1):9–18. doi: 10.2174/1874471011003010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Varnas K, Hall H, Bonaventure P, Sedvall G. Autoradiographic mapping of 5-HT(1B) and 5-HT(1D) receptors in the postmortem human brain using [(3)H]GR 125743. Brain Res. 2001;915:47–57. doi: 10.1016/s0006-8993(01)02823-2. [DOI] [PubMed] [Google Scholar]
- 78.Andersson JD, Pierson MD, Finnema SJ, Gulyás B, Heys R, Elmore CS, Farde L, Halldin C. Development of a PET radioligand for the central 5-HT1B receptor: radiosynthesis and characterization in cynomolgus monkeys of eight radiolabeled compounds. Nuc Med Biol. 2011;38:261–272. doi: 10.1016/j.nucmedbio.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 79.Katarina Varna K, Nyberg S, Halldin C, Varrone A, Takano A, Karlsson P, Andersson J, McCarthy D, Smith M, Pierson ME, Soderstrom J, Farde L. Quantitative analysis of [11C]AZ10419369 binding to 5-HT1B receptors in human brain. J Cer Blood Flow Metab. 2011;31:113–123. doi: 10.1038/jcbfm.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Magdalena N, Sjoerd JF, Martin S, Christer H, Lars F. Test-retest reliability of [11C]AZ10419369 binding to 5-HT1B receptors in human brain. Eur J Nuc Med Mol Imag. 2014;41(2):301–307. doi: 10.1007/s00259-013-2529-1. [DOI] [PubMed] [Google Scholar]
- 81.Varnäs K, Nyberg S, Karlsson P, Pierson ME, Kågedal M, Cselényi Z, McCarthy D, Xiao A, Zhang M, Halldin C, Farde L. Dose-dependent binding of AZD3783 to brain 5-HT1B receptors in non-human primates and human subjects: a positron emission tomography study with [11C]AZ10419369. Psychopharmacol. 2011;213:533–545. doi: 10.1007/s00213-011-2165-z. [DOI] [PubMed] [Google Scholar]
- 82.Magdalena N, Sjoerd JF, Christer H, Lars F. Effect of a single dose of escitalopram on serotonin concentration in the non-human and human primate brain. Int J Neuropsychopharmacol. 2013;16(7):1577–1586. doi: 10.1017/S1461145712001617. [DOI] [PubMed] [Google Scholar]
- 83.Tiger M, Svenningsson P, Nord M, Jabre S, Halldin C, Lundberg J. No correlation between serotonin and its metabolite 5-HIAA in the cerebrospinal fluid and [11C]AZ10419369 binding measured with PET in healthy volunteers. Synapse. 2014;68(10):480–483. doi: 10.1002/syn.21761. [DOI] [PubMed] [Google Scholar]
- 84.Varnas K, Jucaite A, McCarthy DJ, Stenkrona P, Nord M, Halldin C, Farde L, Kanes S. A PET study with [11C]AZ10419369 to determine brain 5-HT1B receptor occupancy of zolmitriptan in healthy male volunteers. Cephalalgia. 2013:1–8. doi: 10.1177/0333102413476372. [DOI] [PubMed] [Google Scholar]
- 85.Varrone A, Svenningsson P, Forsberg A, Varnäs K, Tiger M, Nakao R, Halldin C, Nilsson L-G, Farde L. Positron emission tomography imaging of 5-HT1BRs receptors in Parkinson’s disease. Neurobiol Aging. 2014;35(4):867–875. doi: 10.1016/j.neurobiolaging.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 86.Nabulsi N, Huang Y, Weinzimmer D, Ropchan J, Frost JJ, McCarthy T, Carson RE, Ding Y-S. High-resolution imaging of brain 5-HT1B receptors in the rhesus monkey using [11C]P943. Nuc Med Biol. 2010;37:205–214. doi: 10.1016/j.nucmedbio.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gallezot J-D, Nabulsi N, Neumeister A, Planeta WB, Williams WA, Singhal T, Kim S, Maguire RP, McCarthy T, Frost JJ. Kinetic modeling of the serotonin 5-HT1B receptor radioligand [11C]P943 in humans. J Cerb Blood Flow Metab. 2010;30(1):196–210. doi: 10.1038/jcbfm.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cosgrove KP, Kloczynski T, Nabulsi N, Weinzimmer D, Lin S-F, Staley JK, Bhagwagar Z, Carson RE. Assessing the sensitivity of [11C]p943, a novel 5-HTIB radioligand, to endogenous serotonin release. Synapse. 2011;65(10):1113–1117. doi: 10.1002/syn.20942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murrough JW, Henry S, Hu J, Gallezot J-D, Planeta WB, Neumaier JF, Neumeister A. Reduced ventral striatal/ventral pallidal serotonin1B receptor binding potential in major depressive disorder. Psychopharmacol. 2011;213(2–3):547–553. doi: 10.1007/s00213-010-1881-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hu J, Henry S, Gallezot J-D, Ropchan J, Neumaier JF, Potenza MN, Sinha R, Krystal JH, Huang Y, Ding Y-S, Carson RE, Neumeister A. Serotonin 1B Receptor Imaging in Alcohol Dependence. Biol Psychiatry. 2010;67(9):800–803. doi: 10.1016/j.biopsych.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matuskey D, Bhagwagar Z, Planeta B, Pittman B, Gallezot J-D, Chen J, Wanyiri J, Najafzadeh S, Ropchan J, Geha P, Huang Y, Potenza MN, Neumeister A, Carson RE, Mallson RT. Reductions in Brain 5-HT1B Receptor Availability in Primarily Cocaine-Dependent Humans. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pazos A, Probst A, Palacios JM. Serotonin receptors in the human brain-IV. Autoradiographic mapping of serotonin-2 receptors. Neurosci. 1987;21:123–139. doi: 10.1016/0306-4522(87)90327-7. [DOI] [PubMed] [Google Scholar]
- 93.Forutan F, Estalji S, Beu M, Nikolaus S, Hamacher K, Coenen HH, Vosberg H, Müller GHW, Larisch R. Distribution of 5HT2A receptors in the human brain: comparison of data in vivo and post mortem. Nuklearmedizin. 2002;41(4):197–201. [PubMed] [Google Scholar]
- 94.Kent RS, De Lean A, Lefkowitz RJ. A quantitative analysis of beta-adrenergic receptor interactions: resolution of high and low affinity states of the receptor by computer modeling of ligand binding data. Mol Pharmacol. 1980;17(1):14–23. [PubMed] [Google Scholar]
- 95.González-Maeso J, Sealfon S. C.Psychedelics and schizophrenia. Trends Neurosci. 2009;32:225–232. doi: 10.1016/j.tins.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 96.Leysen JE. 5-HT2 Receptors. Current Drug Targets - CNS & Neurolog Disord. 2004;3:11–26. doi: 10.2174/1568007043482598. [DOI] [PubMed] [Google Scholar]
- 97.Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psy Res. 2003;37:357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 98.Baron JC, Samson Y, Comar D, Crouzel C, Deniker P, Agid Y. In vivo study of central serotoninergic receptors in man using positron tomography. Revue neurologique. 1985;141(8–9):537–545. [PubMed] [Google Scholar]
- 99.Moerlein SM, Perlmutter JS. Central serotonergic S2 binding in Papio anubis measured in vivo with N-Ω-[18F]fluoroethylketanserin and PET. Neurosci Lett. 1991;123(1):23–26. doi: 10.1016/0304-3940(91)90149-n. [DOI] [PubMed] [Google Scholar]
- 100.Goldberg J, Anderson GM, Zwaigenbaum L, Hall GBC, Nahmias C, Thompson A, Szatmari PJ. Cortical serotonin type-2 receptor density in parents of children with autism spectrum disorders. Autism Develop Disorders. 2009;39(1):97–104. doi: 10.1007/s10803-008-0604-4. [DOI] [PubMed] [Google Scholar]
- 101.Momose T, Teramoto A, Sasaki Y. Positron emission tomography in the pituitary adenoma. Horumon to Rinsho. 1995;43(7):673–679. [Google Scholar]
- 102.Staley JK, Van Dyck CH, Tan P-Z, Al Tikriti M, Ramsby Q, Klump H, Ng C, Garg P, Soufer R, Baldwin RM. Comparison of [18F]altanserin and [18F]deuteroaltanserin for PET imaging of serotonin2A receptors in baboon brain: pharmacological studies. Nuc Med Biol. 2001;28(3):271–279. doi: 10.1016/s0969-8051(00)00212-2. [DOI] [PubMed] [Google Scholar]
- 103.Soares JC, van Dyck CH, Tan P-Z, Zoghbi SS, Garg P, Soufer R, Baldwin RM, Fujita M, Staley JK, Fu X. Reproducibility of in vivo brain measures of 5-HT2A receptors with PET and [18F]deuteroaltanserin. Psychiatry Res. 2001;106(2):81–93. doi: 10.1016/s0925-4927(01)00071-3. [DOI] [PubMed] [Google Scholar]
- 104.Santhosh L, Estok Kristina M, Vogel RS, Tamagnan GD, Baldwin RM, Mitsis EM, MacAvoy MG, Staley JK, van Dyck CH. Regional distribution and behavioral correlates of 5-HT2A receptors in Alzheimer’s disease with [18F]deuteroaltanserin and PET. Psychiatry Res Neuroimaging. 2009;173(3):212–217. doi: 10.1016/j.pscychresns.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 105.Bhagwagar Z, Hinz R, Taylor M, Fancy S, Cowen P, Grasby P. Increased 5-HT(2A) receptor binding in euthymic, medication-free patients recovered from depression: a positron emission study with [(11)C]MDL 100,907. Am J Psychiatry. 2006;163:1580–1587. doi: 10.1176/ajp.2006.163.9.1580. [DOI] [PubMed] [Google Scholar]
- 106.Perani D, Garibotto V, Gorini A, Moresco RM, Henin M, Panzacchi A, Matarrese M, Carpinelli A, Bellodi L, Fazio F. In vivo PET study of 5HT(2A) serotonin and D(2) dopamine dysfunction in drug-naive obsessive-compulsive disorder. Neuroimage. 2008;42:306–314. doi: 10.1016/j.neuroimage.2008.04.233. [DOI] [PubMed] [Google Scholar]
- 107.Talvik-Lotfi M, Nyberg S, Nordstrom A-L, Ito H, Halldin C, Brunner F, Farde L. High 5HT2A receptor occupancy in M100907-treated schizophrenic patients. Psychopharmacol. 2000;148(4):400–403. doi: 10.1007/s002130050069. [DOI] [PubMed] [Google Scholar]
- 108.Muhlhausen U, Ermert J, Herth MM, Coenen HH. Synthesis, radiofluorination and first evaluation of (±)-[18F]MDL 100907 as serotonin 5-HT2A receptor antagonist for PET. J Labelled Compd Radiopharm. 2009;52:6–12. [Google Scholar]
- 109.Ren H, Wey H-Y, Strebl M, Neelamegam R, Ritter T, Hooker JM. Synthesis and Imaging Validation of [18F]MDL100907 Enabled by Ni-Mediated Fluorination. ACS Chem Neurosci. 2014;5(7):611–615. doi: 10.1021/cn500078e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Herth MM, Markus P, Fabian D, Ulrich S, Hartmut L, Frank R. Preliminary in vivo and ex vivo evaluation of the 5-HT2A imaging probe [18F]MH.MZ. Nuc Med Biol. 2009;36(4):447–454. doi: 10.1016/j.nucmedbio.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 111.Prabhakaran J, Majo V, Milak M, Savenkova L, Parsey R, Mann JJ, Kumar JSD. In vivo quantification of [18F]FE-M100907, a potential serotonin 2A receptor PET ligand. J Nucl Med. 2010;51(Supp 2):1775. [Google Scholar]
- 112.Hansen HD, Ettrup A, Herth MM, Dyssegaard A, Ratner C, Gillings N, Knudsen GM. Direct comparison of [18F]MH.MZ and [18F]altanserin for 5-HT2A receptor imaging with PET5-HT2A Receptor Imaging with PET. Synapse. 2013;67(6):328–337. doi: 10.1002/syn.21643. [DOI] [PubMed] [Google Scholar]
- 113.Ettrup A, Palner M, Gillings N, Santini MA, Hansen M, Kornum BR, Rasmussen LK, Nagren K, Madsen J, Begtrup M, Knudsen GM. Radiosynthesis and evaluation of 11C-CIMBI-5 as a 5-HT2A receptor agonist radioligand for PET. J Nucl Med. 2010;51(11):1763–1770. doi: 10.2967/jnumed.109.074021. [DOI] [PubMed] [Google Scholar]
- 114.Prabhakaran J, Majo V, Milak M, Gillings N, Ettrup A, Prem S, Mann JJ, Parsey RV, Knudsen GM, Kumar JSD. Evaluation of [11C]CIMBI-5 as a 5-HT2A receptor agonist PET tracer in non-human primates. J Nucl Med. 2012;53(Supplement 1):1876. [Google Scholar]
- 115.Ettrup A, Hansen M, Santini MA, Paine J, Gillings N, Palner M, Lehel S, Herth MM, Madsen J, Kristensen J, Begtrup M, Knudsen GM. Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT (2A) agonist PET tracers. Eur J Nucl Med Mol Imaging. 2011;38(4):681–693. doi: 10.1007/s00259-010-1686-8. [DOI] [PubMed] [Google Scholar]
- 116.Ettrup A, Holm S, Hansen M, Wasim M, Santini MA, Palner M, Madsen J, Svarer C, Kristensen JL, Knudsen GM. Preclinical safety assessment of the 5-HT2A receptor agonist PET radioligand [(11)C]Cimbi-36. Mol Imaging Biol. 2013;15(4):376–383. doi: 10.1007/s11307-012-0609-4. [DOI] [PubMed] [Google Scholar]
- 117.Ettrup A, Bang S-C, McMahon B1, Lehel S, Dyssegaard A, Skibsted AW, Jørgensen LM, Hansen M, Baandrup AO, Bache S, Svarer1 C, Kristensen JL, Gillings2 N, Madsen J, Knudsen GM. Serotonin 2A receptor agonist binding in the human brain with [11C]Cimbi-36. J Cer Blood Flow Metab. 2014;34:1188–1196. doi: 10.1038/jcbfm.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McKenna DJ, Peroutka SJ. Differentiation of 5-hydroxytryptamine 2 receptor subtypes using 125I-R-(−)2,5-dimethoxy-4-iodo-phenylisopropylamine and 3H-ketanserin. J Neurosci. 1989;9(10):3482–90. doi: 10.1523/JNEUROSCI.09-10-03482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Palacios JM, Vilaró MT, Mengod G. Multiple conformations of 5-HT2A and 5-HT 2C receptors in rat brain: an autoradiographic study with [125I](±)DOI. Exp Brain Res. 2013;230(4):395–406. doi: 10.1007/s00221-013-3636-8. [DOI] [PubMed] [Google Scholar]
- 120.Prabhakaran J, Majo VJ, Parsey RV, Van Heertum RL, Mann JJ, Kumar JSD. Synthesis and in vivo Evaluation of [11C]ACM: A Potential PET Probe for Imaging 5-HT2AR. J Labeled Comp Radiopham. 2006;49:1069–1077. [Google Scholar]
- 121.Leonhardt S, Gorospe E, Hoffman BJ, Teitler M. Molecular pharmacological differences in the interaction of serotonin with 5-hydroxytryptamine1C and 5-hydroxytryptamine2 receptors. Mol Pharmacol. 1992;42:328–335. [PubMed] [Google Scholar]
- 122.Sharma A, Punhani T, Fone KCF. Distribution of the 5-hydroxytryptamine2C receptor protein in adult rat brain and spinal cord determined using a receptor-directed antibody: effect of 5,7-dihydroxytryptamine. Synapse. 1997;27(1):45–56. doi: 10.1002/(SICI)1098-2396(199709)27:1<45::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 123.Hoyer D, Srivatsa S, Pazos A, Engel G, Palacios JM. [125I]LSD labels 5-HT1C recognition sites in pig choroid plexus membranes. Comparison with [3H]mesulergine and [3H]5-HT binding. Neurosci Lett. 1986;69:269–274. doi: 10.1016/0304-3940(86)90492-1. [DOI] [PubMed] [Google Scholar]
- 124.Granda ML, Carlin SM, Moseley CK, Neelamegam R, Mandeville JB, Hooker JM. Synthesis and Evaluation of Methylated Arylazepine Compounds for PET Imaging of 5-HT2c Receptors. ACS Chem Neurosci. 2013;4:261–265. doi: 10.1021/cn300223d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Neelamegam R, Hellenbrand T, Schroeder FA, Wang C, Hooker JM. Imaging Evaluation of 5HT2C Agonists, [11C]WAY-163909 and [11C]Vabicaserin, Formed by Pictet–Spengler Cyclization. J Med Chem. 2014;57:1488–1494. doi: 10.1021/jm401802f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marazziti D, Betti L, Giannaccini G, Rossi A, Masala I, Baroni S, Cassano GB, Lucacchini A. Distribution of [3H]GR65630 binding in human brain postmortem. Neurochem Res. 2001;26(3):187–90. doi: 10.1023/a:1010939530412. [DOI] [PubMed] [Google Scholar]
- 127.Anissa Abi-Dargham, Laruelle M, Lipska B, Jaskiw GE, Wong DT, Robertson DW, Weinberger DR, Kleinman JE. Serotonin 5-HT3 receptors in schizophrenia: a postmortem study of the amygdala. Brain Res. 1993;616(1–2):53–57. doi: 10.1016/0006-8993(93)90191-o. [DOI] [PubMed] [Google Scholar]
- 128.Rinaldi-Carmona M, Congy C, Pointeau P, Vidal H, Breliere JC, Le Fur G. Identification of binding sites for SR 46349B, a 5-hydroxytryptamine2 receptor antagonist, in rodent brain. Life Sci. 1994;54(2):119–27. doi: 10.1016/0024-3205(94)00782-9. [DOI] [PubMed] [Google Scholar]
- 129.Hewlett WA, Trivedi BL, Zhang Z-J, De Paulis T, Schmidt DE, Lovinger DM, Ansari MS, Ebert MH. Characterization of (S)-des-4-amino-3-[125I]iodozacopride ([125I]DAIZAC), a selective high-affinity radioligand for 5-hydroxytryptamine3 receptors. J Pharm Expt Therap. 1999;288(1):221–231. [PubMed] [Google Scholar]
- 130.Vitalis B, Sebestyen L, Sike M, Solyom S, Harsing LG., Jr Binding characteristics of GYKI-46 903, a non-competitive ligand at 5-HT3 receptors. Pharmacol Res. 2001;43(3):291–299. doi: 10.1006/phrs.2000.0774. [DOI] [PubMed] [Google Scholar]
- 131.Parker RMC, Barnes JM, Ge J, Barber PC, Barnes NM. Autoradiographic distribution of [3H]-(S)-zacopride-labeled 5-HT3 receptors in human brain. J Neurological Sci. 1996;144(1–2):119–127. doi: 10.1016/s0022-510x(96)00211-0. [DOI] [PubMed] [Google Scholar]
- 132.Abi-Dargham A, Laruelle M, Wong DT, Robertson DW, Weinberger DR, Kleinman JE. Pharmacological and regional characterization of [3H]LY278584 binding sites in human brain. J Neurochem. 1993;60(2):730–737. doi: 10.1111/j.1471-4159.1993.tb03208.x. [DOI] [PubMed] [Google Scholar]
- 133.Barnes NM, Costall B, Naylor RJ, Williams TJ, Wischik CM. Normal densities of 5-HT3 receptor recognition sites in Alzheimer’s disease. NeuroReport. 1990;1(3–4):253–254. doi: 10.1097/00001756-199011000-00021. [DOI] [PubMed] [Google Scholar]
- 134.Fletcher S, Barnes NM. Distribution and characterization of the [3H](S)-zacopride labeled 5-HT3 receptor in pig forebrain. Brain Res. 1996;729(2):281–284. [PubMed] [Google Scholar]
- 135.Camsonne R, Barre L, Petit-Taboue MC, Travere JM, Jones R, Debruyne D, Moulin MA, MacKenzie ET, Baron JC. Positron emission tomographic studies of [11C]MDL 72222, a potential 5-HT3 receptor radioligand: Distribution, kinetics and binding in the brain of the baboon. Neuropharmacol. 1993;32:65–71. doi: 10.1016/0028-3908(93)90131-l. [DOI] [PubMed] [Google Scholar]
- 136.Ishiwata K, Ishii K, Ishii S, Senda M. Synthesis of 5-HT3 receptor antagonists, [11C]Y-25130 and [11C]YM060. Appl Radiat Isot. 1995;46:907–910. doi: 10.1016/0969-8043(95)00147-6. [DOI] [PubMed] [Google Scholar]
- 137.Ishiwata K, Saito N, Yanagawa K, Furuta R, Ishii S, Kiyosawa M, Homma Y, Ishii K, Suzuki F, Senda M. Synthesis and evaluation of 5-HT3 receptor antagonist [11C]KF17643. Nucl Med Biol. 1996;23:285–290. doi: 10.1016/0969-8051(95)02079-9. [DOI] [PubMed] [Google Scholar]
- 138.Besret L, Dauphin F, Guillouet S, Dhilly M, Gourand F, Blaizot X, Young AR, Petit-Taboue MC, Mickala P, Barbelivien A, Rault S, Barre L, Baron JC. [11C]S21007, a putative partial agonist for 5-HT3 receptors PET studies. Rat and primate in vivo biological evaluation. Life Sci. 1998;62:115–129. doi: 10.1016/s0024-3205(97)01058-8. [DOI] [PubMed] [Google Scholar]
- 139.Katounina T, Besret L, Dhilly M, Petit-Taboue MC, Barbelivien A, Baron JC, Dauphin F, Barre L. Synthesis and biological investigations of [18F]MR18445, a 5-HT3 receptor partial agonist. Bioorg Med Chem. 1998;6:789–795. doi: 10.1016/s0968-0896(98)00035-2. [DOI] [PubMed] [Google Scholar]
- 140.Thorell JO, Stone-Elander S, Eriksson L, Ingvar M. N-Methylquipazine: Carbon-11 labelling of the 5-HT3 agonist and in vivo evaluation of its biodistribution using PET. Nucl Med Biol. 1997;24:405–412. doi: 10.1016/s0969-8051(97)00003-6. [DOI] [PubMed] [Google Scholar]
- 141.Bockaert J, Claeysen S, Compan V, Dumuis A. 5-HT4 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:39–51. doi: 10.2174/1568007043482615. [DOI] [PubMed] [Google Scholar]
- 142.Jakeman LB, To ZP, Eglen RM, Wong EH, Bonhaus DW. Quantitative autoradiography of 5-HT4 receptors in brains of three species using two structurally distinct radioligands, [3H]GR113808 and [3H]BIMU-1. Neuropharmacol. 1994;33:1027–1038. doi: 10.1016/0028-3908(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 143.Gee AD, Martarello L, Passchier J, Wishart M, Parker C, Matthews J, Comley R, Hopper R, Gunn R. Synthesis and evaluation of [11C]SB207145 as the first in vivo serotonin 5-HT4 receptor radioligand for PET imaging in man. Curr Radiopharm. 2008;1:110–114. [Google Scholar]
- 144.Kornum BR, Lind NM, Gillings N, Marner L, Andersen F, Knudsen GM. Evaluation of the novel 5-HT4 receptor PET ligand [11C]SB207145 in the Gottingen minipig. J Cereb Blood Flow Metab. 2009;29:186–196. doi: 10.1038/jcbfm.2008.110. [DOI] [PubMed] [Google Scholar]
- 145.Marner L, Gillings N, Comley RA, Baare WF, Rabiner EA, Wilson AA, Houle S, Hasselbalch SG, Svarer C, Gunn RN, Laruelle M, Knudsen GM. Kinetic modeling of 11C-SB207145 binding to 5-HT4 receptors in the human brain in vivo. J Nucl Med. 2009;50:900–908. doi: 10.2967/jnumed.108.058552. [DOI] [PubMed] [Google Scholar]
- 146.Madsen K, Haahr MT, Marner L, Keller SH, Baaré WF, Svarer C, Hasselbalch SG, Knudsen GM. Age and sex effects on 5-HT4 receptors in the human brain: a [11C]SB207145 PET study. J Cereb Blood Flow Metab. 2011;31:1475–1481. doi: 10.1038/jcbfm.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Marner L, Gillings N, Madsen K, Erritzoe D, Baare WF, Svarer C, Hasselbalch SG, Knudsen GM. Brain imaging of serotonin 4 receptors in humans with [11C]SB207145-PET. Neuroimage. 2010;50:855–861. doi: 10.1016/j.neuroimage.2010.01.054. [DOI] [PubMed] [Google Scholar]
- 148.Fisher PM, Holst KK, Mc Mahon B, Haahr ME, Madsen K, Gillings N, Baaré WF, Jensen PS, Knudsen GM. 5-HTTLPR status predictive of neocortical 5-HT4 binding assessed with [(11)C]SB207145 PET in humans. Neuroimage. 2012;62(1):130–136. doi: 10.1016/j.neuroimage.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 149.Madsen K, Neumann W-J, Holst K, Marner L, Haahr MT, Lehel S, Knudsen GM, Hasselbalch SGJ. Cerebral Serotonin 4 Receptors and Amyloid-β in Early Alzheimer’s Disease. J Alzheimer’s Dis. 2011;26(3):457–466. doi: 10.3233/JAD-2011-110056. [DOI] [PubMed] [Google Scholar]
- 150.Caillé F, Morley TJ, Tavares AA, Papin C, Twardy NM, Alagille D, Lee HS, Baldwin RM, Seibyl JP, Barret O, Tamagnan GD. Synthesis and biological evaluation of positron emission tomography radiotracers targeting serotonin 4 receptors in brain: [18F]MNI-698 and [18F]MNI-699. Bioorg Med Chem Lett. 2013;23(23):6243–6247. doi: 10.1016/j.bmcl.2013.09.097. [DOI] [PubMed] [Google Scholar]
- 151.Tavares AAS, Caillé F, Barret O, Papin C, Lee H, Morley TJ, Fowles K, Holden D, Seibyl JP, Alagille D, Tamagnan GD. Nucl Med Biol. 2014;41(5):432–439. doi: 10.1016/j.nucmedbio.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 152.Xu R, Hong J, Morse CL, Pike VW. Synthesis, structure-affinity relationships, and radiolabeling of selective high-affinity 5-HT4 receptor ligands as prospective imaging probes for positron emission tomography. J Med Chem. 2010;53(19):7035–7047. doi: 10.1021/jm100668r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Buiter HJC, Windhorst AD, Huisman MC, De Maeyer JH, Schuurkes JAJ, Lammertsma AA, Leysen JE. Radiosynthesis and preclinical evaluation of [11C] prucalopride as a potential agonist PET ligand for the 5-HT4 receptor. Eur J Nucl Med Mol Imag Res. 2013:3–24. doi: 10.1186/2191-219X-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Grailhe R, Grabtree GW, Hen R. Human 5-HT(5) receptors: The 5-HT(5A) receptor is functional but the 5-HT(5B) receptor was lost during mammalian evolution. Eur J Pharmacol. 2001;418:157–167. doi: 10.1016/s0014-2999(01)00933-5. [DOI] [PubMed] [Google Scholar]
- 155.Nelson DL. 5-HT5 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:53–58. doi: 10.2174/1568007043482606. [DOI] [PubMed] [Google Scholar]
- 156.Woolley ML, Marsden CA, Fone KC. 5-ht6 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:59–79. doi: 10.2174/1568007043482561. [DOI] [PubMed] [Google Scholar]
- 157.East SZ, Burnet PW, Leslie RA, Roberts JC, Harrison PJ. 5-HT6 receptor binding sites inschizophrenia and following antipsychotic drug administration: Autoradiographic studies with [125I]SB-258585. Synapse. 2002;45:191–199. doi: 10.1002/syn.10097. [DOI] [PubMed] [Google Scholar]
- 158.Hirst WD, Minton JA, Bromidge SM, Moss SF, Latter AJ, Riley G, Routledge C, Middlemiss DN, Price GW. Characterization of [(125)I]-SB-258585 binding to human recombinant and native 5-HT(6) receptors in rat, pig and human brain tissue. Br J Pharmacol. 2000;130:1597–1605. doi: 10.1038/sj.bjp.0703458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tang S, Verdurand M, Joseph B, Lemoine L, Daoust A, Billard T, Fournet G, Le Bars D, Zimmer L. Synthesis and biological evaluation in rat and cat of [18F]12ST05 as a potential 5-HT6 PET radioligand. Nucl Med Biol. 2007;34:995–1002. doi: 10.1016/j.nucmedbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 160.Liu F, Majo VJ, Prabhakaran J, Milak MS, Mann JJ, Parsey RV, Kumar JSD. Synthesis and in vivo evaluation of [O-methyl-11C] N-[3,5-dichloro-2-(methoxy)phenyl]-4-(methoxy)-3-(1-piperazinyl)benzenesulfonamide as an imaging probe for 5-HT6 receptors. Bioorg Med Chem. 2011;19(17):5255–5259. doi: 10.1016/j.bmc.2011.06.090. [DOI] [PubMed] [Google Scholar]
- 161.Huiban M, Passchier J, Martarello L, Cunningham VJ, Jakobsen S, Gee AD. [11C]GSK224558 as a potential PET ligand for the delineation of 5-HT6 receptors. Neuroimage. 2006;31:T12–T43. [Google Scholar]
- 162.Parker CA, Martarello L, Rabiner EA, Gunn RN, Laruelle M, Slifstein M, Cunningham VJ. Evaluation of the in vivo ED50 for the PET radioligand. [11C]GSK215083, in papio anubis. Neuroimage. 2010;52:S37. [Google Scholar]
- 163.Martarello L, Cunningham VJ, Matthews JC, Rabiner EA, Jakobsen S, Gee AD. Radiolabelling and in vivo evaluation of [11C]GSK215083 as potential PET radioligand for the 5-HT6 receptor in porcine brain. J Cereb Blood Flow Metab. 2005;25:S598. [Google Scholar]
- 164.Parker CA, Cunningham VJ, Martarello L, Rabiner EA, Searle GE, Gee AD, Davy M, Johnson CN, Ahmed M, Gunn RN, Laruelle M. Evaluation of the novel 5-HT6 receptor radioligand, [11C]GSK215083 in human. Neuroimage. 2008;41:T20. [Google Scholar]
- 165.Parker CA, Gunn RN, Rabiner EA, Slifstein M, Comley R, Salinas C, Johnson CN, Jakobsen S, Houle S, Laruelle M, Cunningham VJ, Martarello L. Radiosynthesis and characterization of 11C-GSK215083 as a PET radioligand for the 5-HT6 receptor. J Nucl Med. 2012;53(2):295–303. doi: 10.2967/jnumed.111.093419. [DOI] [PubMed] [Google Scholar]
- 166.Black LA. US 2013/0343993 Radiolabeled 5-HT6 ligands. 2013
- 167.Rosse G. Quinoline Derivatives as 5-HT6 Receptor PET Ligands. ACS Med Chem Lett. 2014;5 (4):275–276. doi: 10.1021/ml500045k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Thomas DR, Atkinson PJ, Hastie PG, Roberts JC, Middlemiss DN, Price GW. [3H]-SB-269970 radiolabels 5-HT7 receptors in rodent, pig and primate brain tissues. Neuropharmacol. 2002;42:74–81. doi: 10.1016/s0028-3908(01)00151-4. [DOI] [PubMed] [Google Scholar]
- 169.Varnas K, Thomas DR, Tupala E, Tiihonen J, Hall H. Distribution of 5-HT7 receptors in the human brain: a preliminary autoradiographic study using [3H]SB-269970. Neurosci Lett. 2004;367:313–316. doi: 10.1016/j.neulet.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 170.Hedlund PB, Sutcliffe JG. Functional, molecular and pharmacological advances in 5-HT7 receptor research. Trends Pharmacol Sci. 2004;25:481–486. doi: 10.1016/j.tips.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 171.Thomas DR, Hagan JJ. 5-HT7 receptors. Curr Drug Targets CNS Neurol Disord. 2004;3:81–90. doi: 10.2174/1568007043482633. [DOI] [PubMed] [Google Scholar]
- 172.Krobert KA, Bach T, Syversveen T, Kvingedal AM, Levy FO. The cloned human 5-HT7 receptor splice variants: A comparative characterization of their pharmacology, function and distribution. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:620–632. doi: 10.1007/s002100000369. [DOI] [PubMed] [Google Scholar]
- 173.Zhang MR, Haradahira T, Maeda J, Okauchi T, Kida T, Obayashi S. Synthesis and preliminary PET study of the 5-HT7 receptor antagonist [11C]DR4446. J Labelled Compd Radiopharm. 2002;45:857–866. [Google Scholar]
- 174.Andriès J, Lemoine L, Mouchel-Blaisot A, Tang S, Verdurand M, Bars DL, Zimmer L, Billard T. Looking for a 5-HT7 radiotracer for positron emission tomography. Bioorg Med Chem Lett. 2010;20(12):3730–3733. doi: 10.1016/j.bmcl.2010.04.076. [DOI] [PubMed] [Google Scholar]
- 175.Lemoine L, Andries J, Le Bars D, Billard T, Zimmer L. Comparison of 4 radiolabeled antagonists for serotonin 5-HT7 receptor neuroimaging: toward the first PET radiotracer. J Nucl Med. 2011;52:1811–1818. doi: 10.2967/jnumed.111.089185. [DOI] [PubMed] [Google Scholar]
- 176.Colomb J, Becker G, Forcellini E, Meyer S, Buisson L, Zimmer L, Billard T. Synthesis and pharmacological evaluation of a new series of radiolabeled ligands for 5-HT7 receptor PET neuroimaging. Nucl Med Biol. 2014;41:330–337. doi: 10.1016/j.nucmedbio.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 177.Herth MM, Volk B, Pallagi K, Bech LK, Antoni FA, Knudsen GM, Kristensen JL. Synthesis and in vitro evaluation of oxindole derivatives as potential radioligands for 5-HT(7) receptor imaging with PET. ACS Chem Neurosci. 2012;3:1002–1007. doi: 10.1021/cn3001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Andries J, Lemoine L, Le Bars D, Zimmer L, Billard T. Synthesis and biological evaluation of potential 5-HT7 receptor PET radiotracer. Eur J Med Chem. 2011;46:3455–3461. doi: 10.1016/j.ejmech.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 179.Herth MM, Hansen HD, Ettrup A, Dyssegaard A, Lehel S, Kristensen J, Knudsen GM. Synthesis and evaluation of [11C]Cimbi-806 as a potential PET ligand for 5-HT7 receptor imaging. Bioorg Med Chem. 2012;20:4574–4581. doi: 10.1016/j.bmc.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 180.Hansen HD, Herth MM, Ettrup A, Andersen VL, Lehel S, Dyssegaard A, Kristensen JL, Knudsen GM. Radiosynthesis and in vivo evaluation of novel radioligands for PET imaging of cerebral 5-HT7 receptors. J Nucl Med. 2014;55 (4):640–6. doi: 10.2967/jnumed.113.128983. [DOI] [PubMed] [Google Scholar]
- 181.Berridge M, Comar D, Crouzel C, Baron J-C. 11C- Labeled Ketanserin: A Selective Serotonin s2 Antagonist. J Label Compd Radiopharm. 1983;20(1):73–78. [Google Scholar]
- 182.Guillouet S, Barre L, Gourand F, Lasne MC, Rault S. Synthesis of [11C]S21007 A Novel 5-HT3 Partial Agonist As A Potential Tracer for PET Studies. J Label Compd Radiopharm. 1996;38:367–371. [Google Scholar]
- 183.Nichols DE, Frescas SP, Chemel BR, Rehder KS, Zhong D, Lewin AH. High specific activity tritium-labeled N-(2-methoxybenzyl)-2,5-dimethoxy-4-iodophenethylamine (INBMeO): A high-affinity 5-HT2A receptor-selective agonist radioligand. Bioorg Med Chem Let. 2008;16:6116–23. doi: 10.1016/j.bmc.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Villalón CM, Centurión D, DeVries P, Saxena PR. An introduction to migraine: from ancient treatment to functional pharmacology and antimigraine therapy. Proc West Pharmacol Soc. 2002;45:199–210. [PubMed] [Google Scholar]
- 185.Chan KY, Vermeersch V, de Hoon J, Villalón CM, MaassenVanDenBrink A. Potential mechanisms of prospective antimigraine drugs: A focus on vascular (side) effects. Pharmacol Therapeutics. 2011;12(993):332–251. doi: 10.1016/j.pharmthera.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 186.Lyon RA, Titeler M, Frost JJ, Whitehouse PJ, Wong DF, Wagner HN, Jr, Dannals RF, Links JM, Kuhar MJ. 3H-3-N-methylspiperone labels D2 dopamine receptors in basal ganglia and S2 serotonin receptors in cerebral cortex. J Neurosci. 1986;6(10):2941–2949. doi: 10.1523/JNEUROSCI.06-10-02941.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Miyake A, Mochizuki S, Takemoto Y, Akuzawa S. Molecular cloning of human 5-hydroxytryptamine3 receptor: heterogeneity in distribution and function among species. Mol Pharmacol. 1995;48(3):407–416. [PubMed] [Google Scholar]
- 188.Thompson AJ, Lummis SCR. The 5-HT3 receptor as a therapeutic target. Expert Opin Ther Targets. 2007;11(4):527–540. doi: 10.1517/14728222.11.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]