Abstract
Cerebellar pathology is associated with impairments on a range of motor learning tasks including sequence learning. However, various lines of evidence are at odds with the idea that the cerebellum plays a central role in the associative processes underlying sequence learning. Behavioral studies indicate that sequence learning, at least with short periods of practice, involves the establishment of effector-independent, abstract spatial associations, a form of representation not associated with cerebellar function. Moreover, neuroimaging studies have failed to identify learning-related changes within the cerebellum. We hypothesize that the cerebellar contribution to sequence learning may be indirect, related to the maintenance of stimulus–response associations in working memory, rather than through processes directly involved in the formation of sequential predictions. Consistent with this hypothesis, individuals with cerebellar pathology were impaired in learning movement sequences when the task involved a demanding stimulus–response translation. When this translation process was eliminated by having the stimuli directly indicate the response location, the cerebellar ataxia group demonstrated normal sequence learning. This dissociation provides an important constraint on the functional domain of the cerebellum in motor learning.
INTRODUCTION
The cerebellum is widely assumed to play a critical role in motor learning. Damage to the cerebellum is associated with impairments on a range of tasks including eye-blink conditioning (Gerwig et al., 2003), force field adaptation (Smith & Shadmehr, 2005; Maschke, Gomez, Ebner, & Konczak, 2004), prism adaptation (Morton & Bastian, 2004; Martin, Keating, Goodkin, Bastian, & Thach, 1996), and novel anticipatory postural adjustments (Diedrichsen, Verstynen, Lehman, & Ivry, 2005).
Sequence learning is an important domain of motor learning. The serial reaction time task (SRTT) has served as a model task for studying sequence learning (Nissen & Bullemer, 1987). In the typical SRTT, the stimuli appear at one of four locations on a computer screen and responses are made on a keyboard with compatible stimulus–response (S–R) mapping. A consistent finding is that damage to the cerebellum impairs (Doyon et al., 1997; Molinari et al., 1997) or eliminates (Shin & Ivry, 2003; Gomez-Beldarrain, Garcia-Monco, Rubio, & Pascual-Leone, 1998; Pascual-Leone et al., 1993) sequence learning. Based on these observations, it is commonly assumed that the cerebellum is a primary site of plasticity for sequence learning.
However, the neuroimaging literature has failed to provide support for this hypothesis. During the SRTT, learning-related activation increases are consistently observed in the motor cortex, the SMA, and the inferior parietal lobe (see Keele, Ivry, Mayr, Hazeltine, & Heuer, 2003). In contrast, the cerebellar signal remains constant (e.g., van der Graff, Maguire, Leenders, & de Jong, 2006; Seidler et al., 2002) or decreases (e.g., Doyon et al., 2002; Hazeltine, Grafton,&Ivry, 1997) with learning. No studies have reported an increase in cerebellar activity with sequence learning, the commonly accepted signature of a new motor representation (Imamizu et al., 2000).
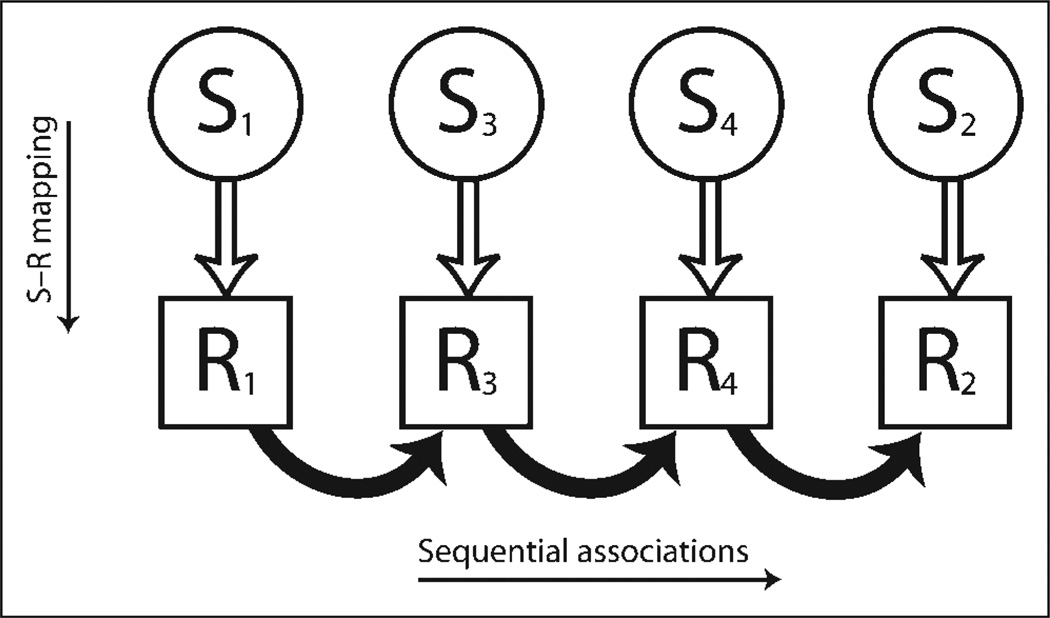
This discrepancy led us to reconsider the role of the cerebellum in sequence learning. Specifically, we hypothesized that the cerebellar contribution might be indirect, working with cortical regions to sustain representations of the S–R mappings, a form of action-based working memory (Figure 1). Working memory models emphasize the establishment of transient links between task-relevant representations; for example, prefrontal goal-based representations modulate activity in perceptual areas of posterior cortical regions to facilitate performance (e.g., Miller & D’Esposito, 2005). A functional account of cerebello-prefrontal pathways may be thought of in a similar manner, with these links maintaining S–R mappings through the preparation of responses or anticipation of the sensory consequences of these responses.
Figure 1.
Hypothesized indirect contribution of the cerebellum to sequence learning. Cerebello-cortical loops (open arrows) help maintain representations of S–R associations, a form of action-based working memory. These representations provide the input to associative processes involved in sequence learning (filled arrows). Damage to the cerebellum disrupts sequence learning indirectly due to noisy representations of S–R links, even if the cerebellum is not directly involved in the processes required for the formation of sequential associations.
Previous studies have indicated that patients with cerebellar pathology have difficulty in learning arbitrary S–R associations (e.g., Richter et al., 2004; Timmann et al., 2002). What has not been considered previously are the implications of such a deficit for sequence learning. As shown in Figure 1, an impairment in maintaining S–R associations should have indirect consequences for sequence learning. In particular, the input to mechanisms that form sequential predictions would be noisy, even if these learning mechanisms themselves were intact. A corollary of this hypothesis is that the degree of impairment observed in individuals with cerebellar pathology should be modulated by the complexity of the S–R mapping. If these demands are high, sequence learning should be impaired; if the demands are low, sequence learning should be spared.
To date, sequence learning studies involving patients with cerebellar pathology have always used some form of symbolic cues. In most of these, spatial cues have required a translation between stimulus (e.g., varying horizontal position on a monitor) and response space (e.g., finger keyboard). Even when a compatible S–R mapping is employed, studies of bimanual coordination indicate that under such conditions, performance costs related to the translation process persist (Ivry, Diedrichsen, Spencer, Hazeltine, & Semjen, 2004). Such costs are completely abolished with direct cues. Interestingly, studies of sequence learning in primates have used direct cues (e.g., a touchscreen in which the animal is trained to touch the successive stimuli) and sequence learning is observed following bilateral cerebellar lesions (Nixon & Passingham, 2000; Lu, Hikosaka, & Miyachi, 1998).
The current experiments were designed to ask if patients with cerebellar pathology would also benefit on a sequence learning task when the responses were directly cued. We employed two versions of the SRTT in which we manipulated the difficulty of the S–R mapping. In the symbolic cue condition, the responses were based on the color of the stimuli. In the direct cue condition, the response location was directly specified by a stimulus at that location. We hypothesized that patients with cerebellar ataxia would be impaired in sequence learning in the symbolic cueing condition given the demands on working memory for maintaining the S–R mappings. In contrast, we expected that these individuals would show minimal or no impairment in the direct cueing condition because the S–R mappings are directly specified and place minimal demand on working memory. This dissociation, if supported, would specify an important constraint on the contribution of the cerebellum in motor learning.
METHODS
Participants
Eleven individuals with cerebellar degeneration and 15 neurologically healthy adults participated in the experiments (Table 1). All participants were right-handed. The individuals with ataxia had a mixed etiology. Five participants had confirmed genetic subtyping (1 with SCA2, 3 with SCA3, 1 with SCA6). Genetic testing was either negative (n = 3) or had not been conducted (n = 3) for the remaining participants. Two of these individuals had a family history of ataxia. The other four individuals had no family history and a diagnosis of sporadic ataxia of unknown etiology.
Table 1.
Participant Information
| Subj | Exp | Gender | Age | Edu | Onset | Etio | Severity | Limb |
|---|---|---|---|---|---|---|---|---|
| AC08 | 1,2s | M | 55 | 14 | 9 | Unka,b | 32 | 8 |
| AC07 | 1,2s,d | M | 47 | 16 | 15 | SCA2 | 37 | 14 |
| AC06 | 1,2s,d | M | 69 | 17 | 14 | Unka | 43 | 15 |
| AC09 | 1,2d | M | 69 | 20 | 7 | Unk | 18 | 5 |
| AC01 | 1,2d | F | 61 | 18 | 7 | Unka | 34 | 8 |
| AC10 | 1 | M | 78 | 12 | 44 | Unk | 45 | 16 |
| AC11 | 1,2d | F | 47 | 16 | 15 | SCA6 | 54 | 12 |
| AC13 | 2s | M | 73 | 12 | 7 | SCA3 | 65 | 20 |
| AC04 | 2s | M | 52 | 18 | 10 | SCA3 | 50 | 15 |
| AC05 | 2d | F | 47 | 14 | 20 | SCA3 | 39 | 12 |
| AC23 | 2d | F | 58 | 16 | 2 | Unkb | 11 | 2 |
| Controls | 1c | 4F/4M | 65.3 | 16 | ||||
| Controls | 2c | 5F/8M | 67.9 | 17 |
Subj = participant code; Exp = numbers indicate experiment(s) participated in and letters indicate condition(s) tested in Experiment 2 (s = symbolic, d = direct); Edu = years of education; Onset = approximate number of years between initial diagnosis and testing (Unk = unknown); Etio = ataxia group etiology is based on genetic subtyping (Unk = either negative results from genetic testing or no genetic analysis performed); Severity = the total International Cooperative Ataxia Rating Scale (ICARS; Trouillas et al., 1997) score for the individuals with ataxia; Limb = the sum of the ICARS scores for upper limb assessment only (items 10–14).
Inconclusive genetic testing.
Family history with unconfirmed diagnosis.
Six individuals served as controls for both experiments.
As assessed by CT or MRI, all individuals with ataxia showed extensive atrophy of the cerebellar cortex with minimal or no evidence of pathology in extracerebellar structures. The ataxia group was evaluated with the International Cooperative Ataxia Rating Scale (ICARS; Trouillas et al., 1997). All exhibited various degrees of ataxia, including deficits in upper limb movement control, although there was considerable variability in their clinical ratings (see Table 1). The clinical exam also evaluated signs of extracerebellar pathology (cogwheeling, staircasing with the eyes, reflexes, etc.). Most of the participants exhibited no extracerebellar signs. However, one participant with unknown pathology and one SCA3 participant presented mild symptoms of extracerebellar disease.
The control group was selected to match the ataxia group in terms of age and education.
Tasks
Experiment 1: Sequential Reaching Task
A 15-in. flat-screen monitor was laid horizontally on a table in front of the participant. The screen displayed five white rings (3-cm diameter) on a black background at all times (Figure 2A). One circle was positioned in the center of the screen and served as the starting position. The other four circles were positioned in the four corners and served as the target locations for the movements. The distance from the center of the starting position to the center of each target was 12.5 cm. A 5 × 5 × 10 mm sensor was secured to the tip of the index finger to provide a continuous record of position relative to a magnetic transmitter (miniBIRD, Ascension Technology, Burlington, VT). The 3-D position of the sensor was sampled at a rate of 137 Hz.
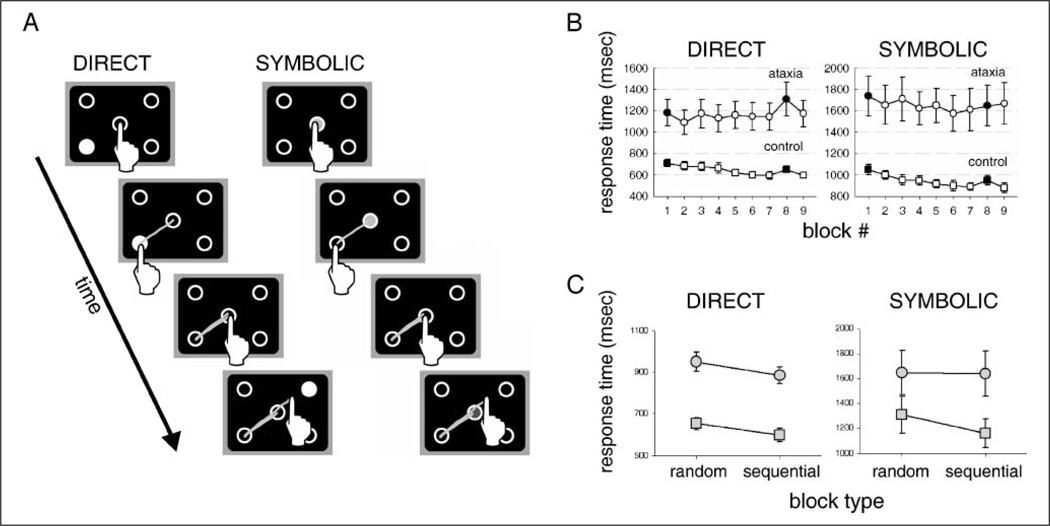
Figure 2.
Individuals with cerebellar ataxia are selectively impaired in sequence learning when the responses are symbolically cued. (A) In Experiment 1, participants reached to one of four targets and then returned to a center position. For direct cues, the target location was illuminated; for symbolic cues, a color presented at the center position indicated the target location. (B) Median RTs, averaged across participants, across blocks (white: sequence blocks; black: random blocks). Note the change in scale for the two tasks. (C) Learning was assessed by comparing RT on the late random probe (Block 8) to the mean RT for the two surrounding sequence blocks (Blocks 7 and 9). Control participants (squares) showed significant learning in both conditions. Individuals with cerebellar ataxia (circles) exhibited learning with direct cues but failed to learn when the responses were cued symbolically. Error bars in B and C represent standard error across participants.
The task required the participant to reach from the starting position to one of the targets. In the direct cue condition, the movement was cued when the black region within one of the target rings turned white. In the symbolic cue condition, the movement was cued when the black region within the starting position turned blue, red, green, or yellow. Prior to the start of the experiment, participants were taught the mapping between the four colors and the four target locations (e.g., red indicates top right). To facilitate learning of the mapping, participants were presented with a diagram illustrating the mapping between the colors and responses.
In both conditions, participants were instructed to reach to the target and return to the starting position as quickly as possible (out-and-back movement). Once the finger re-entered the starting position, a 400-msec inter-response interval was initiated prior to the presentation of the next cue. Seven individuals with ataxia and eight control participants were tested in this experiment.
Experiment 2: Sequential Keypress Task
A response box with four piano-like keys (10.2 cm × 2 cm) was positioned in front of the participant. Minimal force was necessary to activate a microswitch underlying each key. The keys were translucent and a vertical row of red light-emitting diodes (LEDs) was positioned underneath each key. Participants held the index and middle finger of each hand over the four response keys prior to the initiation of a block (Figure 3A).
Figure 3.
Dissociation between sequence learning with symbolic and direct cues using a keypressing task. (A) In Experiment 2, finger movements were used to press keys on a response box. The response for each trial was cued by the onset of LEDs positioned under one of the translucent keys (direct) or by the color of a circle presented on the computer monitor (symbolic). (B) Median RTs, averaged across participants, across blocks (white: sequence blocks; black: random blocks). (C) As in Figure 2C. Control participants (squares) exhibited sequence learning with both types of cues. In contrast, individuals with cerebellar ataxia (circles) only learned in the direct cueing condition. Error bars represent standard error across participants.
In the direct cue condition, the LEDs under one of the response keys were illuminated, causing the selected key to glow in an unambiguous manner. In the symbolic cue condition, the participants were instructed to look at a vertically mounted computer monitor. A white ring was always present at the center of the display. For each trial, the ring was filled with one of four colors. Participants used one of four fingers to press the response key associated with the stimulus. For the direct cue condition, this was the key above the illuminated light; for the symbolic cue condition, this was the key mapped to the presented color. An interval of 450 msec separated each response from the next stimulus.
As in Experiment 1, participants were taught the color–response mapping prior to the start of the symbolic condition. Small colored stickers were attached above the response keys to facilitate learning of the S–R mapping. These were helpful during the instruction period and provided a reminder of the mapping between blocks. Participants were encouraged to maintain fixation on the monitor during the experiment.
Individuals with ataxia (n = 5 for the symbolic condition; n = 7 for the direct condition1) and healthy controls (n = 13) were tested in this experiment.
Procedure
Experiment 1 was composed of nine 56-trial blocks. In Blocks 2–7 and 9, the cues followed an eight-element sequence that repeated seven times. Two grammars were used, one for the direct condition and the other for the symbolic condition, with the pairing counterbalanced across participants. The grammar of one sequence was 2-4-1-3-4-2-3-1; for the other sequence, it was 1-4-2-1-3-2-4-3. The mapping between the elements of the grammar onto to specific S–R was varied across participants (e.g., 1 = upper left for one participant, upper right for another, etc.). The starting position of the sequence for each block was selected at random. On Blocks 1 and 8, the cues were selected randomly with the constraints that a cue was not repeated on successive trials, there were no three-element trills (e.g., 1-3-1, 2-4-2), and each cue occurred an equal number of times during the block. These constraints matched those present in the sequence blocks.
Experiment 2 was composed of 10 blocks. The cues followed a sequence in Blocks 3–7 and 9; on the other blocks, the cues were selected randomly. All other aspects of the procedure were identical to Experiment 1. Although the same grammars were used in Experiment 2, the S–R assignments were randomized. Thus, the participant did not have prior experience with a specific stimulus or response sequence.
The direct and symbolic conditions for each experiment were tested in different sessions. The two sessions were separated by at least 1 week, with the order of the direct and symbolic conditions counterbalanced. The two sessions for Experiment 2 were conducted at least 6 months after Experiment 1. Six control participants and six individuals with ataxia participated in both experiments and those individuals were always tested on the direct cueing condition first.1
Given that participants were tested in multiple sessions, we did not assess sequence awareness. However, based on past work with similar populations and sequence structures, we expect that awareness was low. Participants did not spontaneously offer comments indicating awareness of the sequence nor were the RTs in the range typically observed when people have explicit knowledge of the sequence (see below).
RESULTS
Experiment 1: Sequential Reaching Task
Response time (RT) for the reaching task was defined as the interval between the appearance of the cue and the time when the index finger reached the target. Target arrival was defined as the time at which the kinematic marker was within 1.5 cm of the center of the correct target and velocity dropped below 8 cm/s. Incorrect movements were determined off-line, defined as trials in which the initial trajectory came within 1.5 cm of an incorrect target prior to the correct target. Note that on these trials, the cue remained visible until the participant reached the correct target location.
Errors were slightly higher in the symbolic condition (mean errors/block: ataxia group: 5.1; controls: 4.8) than the direct condition (ataxia group: 4.4; controls: 3.8) but the differences were not reliable [direct: F(1, 13) = 0.21, p = .66; symbolic: F(1, 13) = 0.34; p = .57]. These trials were not included in the RT analyses.
Block-by-block median RTs, averaged across participants within each group, are presented in Figure 2B. As expected, the ataxia group was much slower than the control group, an effect observed with both types of cues [main effect of group, direct: F(1, 117) = 153.0, p < .001; symbolic: F(1, 117) = 145.0, p < .001]. Across the sequence blocks (2–7), the RTs for the control participant tended to become faster in both the direct and symbolic conditions, but these changes were not reliable (F < 1 in both direct and symbolic conditions). The patients also failed to show a consistent reduction in RT across the initial six sequence blocks (again, F < 1 in both conditions).
Changes in performance across the sequence blocks provide a weak measure of sequence learning. Participants may become faster or slower for generic reasons (e.g., more comfortable with the task, fatigue). As such, our primary measure of learning followed the standard convention in the SRTT literature. We assessed sequence learning by comparing RTs on Block 8, in which the stimuli were selected at random, with the two surrounding sequence blocks (Figure 2C). As indicated by the increase in RT on the random block, control participants exhibited sequence learning in both conditions [direct: t(7) = 3.1, p < .01; symbolic: t(7) = 3.9, p < .01]. Consistent with previous reports (Shin & Ivry, 2003; Gomez-Beldarrain et al., 1998; Doyon et al., 1997; Molinari et al., 1997), the individuals with cerebellar ataxia showed no evidence of sequence learning when the responses were symbolically cued. The difference between the random and surrounding sequence blocks was not significant [t(6) = 0.28, p = .39].
However, in the direct cue condition, the individuals with ataxia exhibited significant sequence learning [t(6) = 2.9, p = .01]. Indeed, the magnitude of the increase of RT on the random block was numerically greater than that observed in the controls. When the amount of learning was normalized by calculating the percentage increase of RT on the random block relative to the surrounding sequence blocks, the values were comparable for the control (10%, SD = 11%) and the ataxia (12%, SD = 11%) groups.
Experiment 2: Sequential Keypress Task
Experiment 1 provided a first demonstration of intact sequence learning in individuals with cerebellar ataxia. As predicted, this effect was limited to the direct cue condition. However, compared to previous sequence learning studies in patients with ataxia, Experiment 1 not only employs a novel method of cueing the responses (i.e., direct cues) but also entailed a novel method of responding (i.e., reaching). To ensure that effects were not unique to reaching, we conducted a second experiment using keypress responses. We opted to use color cues for the symbolic condition to restrict the changes with Experiment 1 to just the method of responding.
Accuracy was again higher in the direct condition (overall = 94%) compared to the symbolic condition (overall = 91%). The means for the ataxia group (direct: ataxia = 92%; symbolic: ataxia = 88%) were lower than for the controls (direct: 95%; symbolic 92%), although these differences were not reliable [direct: F(1, 18) = 0.08, p = .78; symbolic: F(1, 16) = 0.10, p = .76]. Incorrect trials were not included in the RT analyses.
RTs for this task were defined as the interval from the presentation of the cue to the time at which the microswitch was activated. The median RT values, averaged across participants within each group, are presented in Figure 3B. The ataxia group was again slower than the healthy control group in both conditions [direct: F(1, 180)=45.9, p<.001; symbolic: F(1, 160)=83.7, p< .001]. Neither group showed a significant reduction in RT across the sequence blocks (3–7) (all Fs < 1).
We assessed sequence learning by comparing median RTs on random Blocks 8 and 10 to the average of the median RTs from the two surrounding sequence blocks (Figure 3C). As indicated by the increase in RT on the random blocks, the control participants exhibited significant sequence learning in both conditions [direct: t(12) = −2.9, p = .006; symbolic: t(12) = 1.7, p = .05]. Similar to Experiment 1, the ataxia group was selectively impaired in sequence learning in the symbolic condition. When the responses were cued symbolically, no difference was observed between the random probes and surrounding sequence blocks [t(4) = −2.6, p = .97], and in fact, the mean RT was actually slower in the sequence block than on the random block. In contrast, the individuals with ataxia exhibited significant learning in the direct cue condition [t(6)=2.6, p=.02]. The magnitude of learning was similar for the ataxia and control groups in this condition [F(1, 18) = 1.2, p = .29].
DISCUSSION
The goal of this study was to re-evaluate the role of the cerebellum in sequence learning. Individuals with cerebellar ataxia exhibited a severe impairment on the SRTT when the responses, whether reaches or keypresses, were cued by the stimulus color. This deficit is consistent with previous neuropsychological studies (Shin & Ivry, 2003; Gomez-Beldarrain et al., 1998; Molinari et al., 1997; Pascual-Leone et al., 1993), indicating that the integrity of the cerebellum is essential for sequence learning.
However, we also found that sequence learning was intact for individuals with cerebellar lesions when the responses were cued directly. Indeed, in this condition, the magnitude of sequence learning was similar for the ataxic and control groups for sequential reaching and keypressing, despite substantial differences in overall RT. The results from the direct cueing conditions provide the first demonstration of spared sequence learning in patients with cerebellar ataxia. It is noteworthy that monkeys with cerebellar lesions also exhibit intact sequence learning when movements are directly cued (Nixon & Passingham, 2000; Lu et al., 1998). We are not aware of any studies in which nonhuman primates were tested with symbolic cues, but would predict that, similar to studies with human participants, cerebellar lesions would disrupt learning under such conditions.
Evidence against a Direct Cerebellar Role for Sequence Learning
The dissociation between the symbolic and direct cues provides an important constraint on the role of the cerebellum in sequence learning. Specifically, this dissociation challenges the assumption that the cerebellum contributes directly to the formation of novel sequential associations. Based on this assumption, we would expect learning impairments independent of the type of movement cue. Although one might posit that the associative processes for sequence learning include the cerebellum when the responses are cued symbolically and extracerebellar when cued directly, a more parsimonious interpretation is that the cerebellum is not directly involved in learning sequential associations. As noted in the Introduction, neuroimaging studies also question a direct role for the cerebellum in sequence learning: In contrast to learning-related changes observed in cortical regions such as the motor cortex, the SMA, and the inferior parietal lobe, cerebellar activation either remains constant or shows learning-related decreases in activation.
Indeed, the discrepancy between the patient and imaging data was a primary motivation for the current work. Computational considerations also led us to reconsider the cerebellar role in sequence learning. First, sequence learning shows a high degree of transfer across effectors (e.g., Keele, Jennings, Jones, Caulton, & Cohen, 1995), suggesting that what is learned, at least in the early stages, is a representation of abstract spatial goals. This form of representation has generally been associated with parietal function (Grafton, Hazeltine, & Ivry, 1998) and not the cerebellum. Second, models of cerebellar learning typically involve the utilization of on-line error signals. Error-based learning is not an essential part of the SRTT. Rather, sequence learning utilizes the generation of expectancies of the forthcoming stimulus and/or its response.
These computational issues can help define a principled basis for specifying the functional domain of the cerebellum in motor learning. Unlike sequence learning, the acquisition of many motor skills such as eye-blink conditioning or VOR adaptation involves the use of online error signals to modify the timing or dynamics of a movement. A wealth of lesion, physiological, and neuroimaging evidence supports a cerebellar locus of plasticity in such tasks (see Raymond, Lisberger, & Mauk, 1996). Similarly, patients with cerebellar degeneration have difficulty learning to move in a novel force field (Smith & Shadmehr, 2005), a task in which on-line error signals are used to modify spatio-temporal control signals.
Indirect Cerebellar Contribution to Sequence Learning as Part of Action-based Working Memory
We propose that the cerebellum is part of a network that represents and maintains the task-relevant S–R mapping (Figure 1). The PFC and the premotor cortex are essential for maintaining S–R mappings (see Wise, di Pellegrino, & Boussaoud, 1996, for a review). Cerebellar interactions with these regions may help sustain these representations, perhaps through the preparation of the required movements and anticipation of their sensory consequences. Consistent with this hypothesis, patients with cerebellar degeneration have been shown to have difficulty on S–R associative learning tasks with color cues (Richter et al., 2004; Timmann et al., 2002).
In the current study, we consider the consequences of these deficits for sequence learning. By this view, the cerebellar contribution to sequence learning is indirect, with S–R links providing the input to associative processes for sequence learning. If the S–R representations are poorly maintained, sequence learning will fail. We assume that demands on this working memory network are high with color cues given that the S–R mapping is arbitrary. As such, sequence learning is absent in individuals with cerebellar ataxia because the associative processes operate on weak inputs. With direct cues, however, demands on this working memory network are minimal.
In previous lesion studies involving the SRTT, spatial cues were presented on a vertically aligned computer monitor and spatially compatible responses were made on a keyboard (Shin & Ivry, 2003; Molinari et al., 1997; Pascual-Leone et al., 1993). The demands on working memory under these conditions are certainly lower than with color cues. Nonetheless, a spatial mapping of this form still requires a translation from stimulus space onto response space. The cost of this translation is apparent in studies of bimanual coordination (Ivry et al., 2004). Whereas intermanual interactions are abolished with direct cues, they remain pronounced with spatially compatible mappings (Albert, Weigelt, Hazeltine, & Ivry, 2007; Hazeltine, Diedrichsen, Kennerley, & Ivry, 2003). Thus, despite their superficial similarity (response to a location indicated by a spatial cue), the translation process from stimulus to response space entails significant processing costs. For bimanual coordination, these costs are associated with response selection; for sequence learning, we propose that these costs are associated with the maintenance of S–R associations in working memory, the inputs to associative processes for sequence learning. That is, we assume that the impairments associated with cerebellar pathology in previous SRTT studies reflect a deficit in maintaining S–R associations rather than a problem in sequence learning per se.
The working memory account advanced here provides a way to reconcile the discrepancy between the neuropsychological and neuroimaging literatures on the cerebellar role in sequence learning. The demands on an action-based working memory process would either remain relatively constant across a scanning session (for both random and sequence blocks) or be reduced as the S–R associations become well established. Both of these patterns have been observed (Seidler et al., 2002; Hazeltine et al., 1997).
More generally, the working memory hypothesis provides a way to link cerebellar contributions to cognition across task domains, emphasizing a role in the preparation of potential responses (reviewed in Ivry & Fiez, 2000). A related action-based working memory model has proven useful in specifying the role of the cerebellum in attention shifting. Individuals with cerebellar lesions are impaired in shifting attention between two dimensions compared to when the focus of attention can be restricted to one dimension (Courchesne et al., 1994; Akshoomoff & Courchesne, 1992). However, subsequent work demonstrated that the cerebellar contribution to this task was related to the demands of maintaining multiple S–R maps in the attention-shifting condition compared to the focused attention condition (Bischoff-Grethe, Ivry, & Grafton, 2002; Ravizza & Ivry, 2001).
The use of individuals with bilateral degeneration precludes inferences about the intracerebellar locus of the observed impairments. Our ataxia group is heterogeneous, both in terms of etiology and symptomatology. We were unable to correlate symptoms with the sequence learning deficit with symbolic cues, not only because most the patients failed to show any evidence of learning but also because the number of individuals with ataxia is small. We note that in studies of visuomotor adaptation, severity of ataxia and learning impairments were also not related (Martin et al., 1996).
It is likely that neuroimaging in healthy individuals will prove useful for evaluating the working memory hypothesis outlined above. Based on anatomical studies of prefrontal–cerebellar connectivity (Ramnani, 2006; Kelly & Strick, 2003), we expect that symbolic cues would lead to greater activation in lateral regions of the neocerebellum. These regions are compromised in our ataxia group, although their pathology certainly encompasses additional regions.
Alternative Interpretations and Limitations of the Current Study
The ataxia group was considerably slower than the controls in both conditions, and this was pronounced with the symbolic cues. This raises the possibility that an RT-based measure of learning may be insensitive, especially when variability is directly related to RT. Two findings argue against this potential limitation. First, the controls showed similar degrees of learning in the direct and symbolic conditions despite having slower RTs in the latter. Thus, for the controls, our measure of sequence learning remained robust across different baseline RTs. Second, in previous studies using spatially compatible cues, patients with ataxia made similar keypressing responses to those of Experiment 2. Overall RTs were just slightly slower than with the direct cues of the present study, yet there was no evidence of learning (Shin & Ivry, 2003; Molinari et al., 1997; Pascual-Leone et al., 1993).
We assume that the slower speed for the cerebellar group primarily reflects their ataxia. Moreover, in both studies, the mean RT for the patients tended to become slower over the test blocks, perhaps due to fatigue (Chaudhuri & Behan, 2004). Although the flat RT functions might indicate a learning deficit in all conditions, we adopted the traditional SRT methodology to assay learning, using a learning probe in which random blocks were sandwiched around sequence blocks. This measure provided evidence that the patients had, in fact, learned the sequence in the direct conditions, even if there was little change in RT across the sequence.
Our study is limited by the heterogeneity of the patient population. As summarized in Table 1, the group was composed of mixed etiologies and individuals with a range of clinical impairments. One concern of note is that we included individuals with SCA3, a subtype that is known to have extracerebellar involvement, at least in the late stages of the disease (Klockgether, 2000). It would, of course, be preferable to restrict testing to individuals with genetic subtypes known to produce “pure” cerebellar syndromes (e.g., SCA6). Nonetheless, concerns with a mixed etiology are mitigated by the fact that the key finding here involves the dissociation between the direct and symbolic cueing conditions, in particular, the normal learning by the patients in the former. If the focus was on impaired performance compared to a control group, it would be problematic to attribute deficits to cerebellar dysfunction if the patients also exhibited extracerebellar symptoms. However, our emphasis here is on the fact that a widely reported learning impairment in individuals with compromised cerebellar function was abolished when the responses were directly cued.
Finally, it is important to consider whether the dissociation between the direct and symbolic cues might be related to differences in awareness. Perhaps the ataxia group was able to learn in the direct condition because they became aware of the sequence and failed to learn in the symbolic condition because they were unaware of the sequence. We did not ascertain participants’ awareness because we did not want to bias them in subsequent sessions. Nonetheless, the participants’ spontaneous comments did not indicate awareness of the sequence. The RT and learning scores also indicate that awareness was low. RTs for the controls (and individuals with cerebellar ataxia) with direct cues are much slower than when awareness is high and the modest increase in RT on the random blocks was similar to that observed when learning is implicit (e.g., Grafton, Hazeltine, & Ivry, 1995). Given these considerations, it seems unlikely that the learning for the ataxia group was due to enhanced awareness.
Moreover, in a previous study, individuals with cerebellar degeneration failed to exhibit learning on an SRTT even when explicitly informed of the sequence (Pascual-Leone et al., 1993). The working memory hypothesis developed here provides a novel interpretation of this puzzling result. Even if participants learn to verbally report the response sequence during pretraining, performance of the actual SRTT requires the on-line maintenance of the S–R mapping. Assuming this representation was noisy, a learning mechanism for forming sequential associations would again be taxed, leading to a dissociation between measures of explicit knowledge (pretraining sequence recall) and performance (sequence learning).
Conclusion
The neuroimaging and neuropsychological literatures offer different conclusions regarding the role of the cerebellum in sequence learning. The results of the current study suggest a resolution. Rather than contribute to sequence learning directly, the cerebellum may work in concert with cortical regions to maintain the representations that are a prerequisite for associative processes. More generally, these results provide an important constraint for models of cerebellar learning. Focusing on the critical representational changes that occur during learning and, as such, on the underlying computations, should serve as a useful guide in specifying the functional domain of the cerebellum in learning and cognition.
Acknowledgments
This work was supported by NIH grants NS30256 and F32 NS048012.
Footnotes
Our main goals in Experiment 2 were twofold. First, we wanted to replicate the finding that the patients exhibited normal sequence learning when the responses were directly cued. Second, we wanted to use a keypressing task because this form of responding had been used in all previous SRTT studies with neurological patients. After obtaining the replication, we decided that we should also include the symbolic condition for completeness. At this time, some of the patients were no longer available for testing (see Table 1). Thus, we do not have equal numbers of participants in the direct and symbolic conditions of Experiment 2 and the order of the two cue types is not counterbalanced.
REFERENCES
- Akshoomoff NA, Courchesne E. A new role for the cerebellum in cognitive operations. Behavioral Neuroscience. 1992;106:731–738. doi: 10.1037//0735-7044.106.5.731. [DOI] [PubMed] [Google Scholar]
- Albert N, Weigelt M, Hazeltine E, Ivry RB. Target selection during bimanual reaching to direct cues is unaffected by the perceptual similarity of the targets. Journal of Experimental Psychology: Human Perception and Performance. 2007;33:1107–1116. doi: 10.1037/0096-1523.33.5.1107. [DOI] [PubMed] [Google Scholar]
- Bischoff-Grethe A, Ivry RB, Grafton ST. Cerebellar involvement in response reassignment rather than attention. Journal of Neuroscience. 2002;22:546–553. doi: 10.1523/JNEUROSCI.22-02-00546.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A, Behan P. Fatigue in neurological disorders. Lancet. 2004;363:978–988. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Townsend J, Akshoomoff NA, Saitoh O, Yeung-Courchesne R, Lincoln AJ, et al. Impairment in shifting attention in autistic and cerebellar patients. Behavioral Neuroscience. 1994;108:848–865. doi: 10.1037//0735-7044.108.5.848. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Verstynen T, Lehman SL, Ivry RB. Cerebellar involvement in anticipating the consequences of self-produced actions during bimanual movement. Journal of Neurophysiology. 2005;93:801–812. doi: 10.1152/jn.00662.2004. [DOI] [PubMed] [Google Scholar]
- Doyon J, Gaudreau D, Laorce R, Castonguay M, Bedard PJ, Bedard F, et al. Role of the striatum, cerebellum, and frontal lobes in the learning of a visuomotor sequence. Brain and Cognition. 1997;34:218–245. doi: 10.1006/brcg.1997.0899. [DOI] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proceedings of the National Academy of Sciences, U.S.A. 2002;99:1017–1022. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwig M, Dimitrova A, Kolb FP, Maschke M, Brol B, Kunnel A, et al. Comparison of eyeblink conditioning in patients with superior and posterior inferior cerebellar lesions. Brain. 2003;126:71–94. doi: 10.1093/brain/awg011. [DOI] [PubMed] [Google Scholar]
- Gomez-Beldarrain M, Garcia-Monco JC, Rubio B, Pascual-Leone A. Effect of focal cerebellar lesions on procedural learning in the serial reaction time task. Experimental Brain Research. 1998;120:25–30. doi: 10.1007/s002210050374. [DOI] [PubMed] [Google Scholar]
- Grafton S, Hazeltine E, Ivry R. Functional mapping of sequence learning in normal humans. Journal of Cognitive Neuroscience. 1995;7:497–510. doi: 10.1162/jocn.1995.7.4.497. [DOI] [PubMed] [Google Scholar]
- Grafton S, Hazeltine E, Ivry RB. Abstract and effector-specific representations of motor sequences identified with PET. Journal of Neuroscience. 1998;18:9420–9428. doi: 10.1523/JNEUROSCI.18-22-09420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeltine E, Diedrichsen J, Kennerley S, Ivry RB. Bimanual cross-talk during reaching movements is primarily related to response selection, not the specification of motor parameters. Psychological Research. 2003;67:56–70. doi: 10.1007/s00426-002-0119-0. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Grafton ST, Ivry RB. Attention and stimulus characteristics determine the locus of motor-sequence encoding: A PET study. Brain. 1997;120:123–140. doi: 10.1093/brain/120.1.123. [DOI] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, et al. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403:192–195. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Diedrichsen J, Spencer RMC, Hazeltine E, Semjen A. A cognitive neuroscience perspective on bimanual coordination and interference. In: Swinnen SP, Duyens J, editors. Interlimb coordination. Norwell, MA: Kluwer Academic; 2004. pp. 259–295. [Google Scholar]
- Ivry RB, Fiez JA. Cerebellar contributions to cognition and imagery. In: Gazzaniga MS, editor. The new cognitive neurosciences. Cambridge, MA: MIT Press; 2000. pp. 999–1011. [Google Scholar]
- Keele SW, Ivry RB, Mayr U, Hazeltine E, Heuer H. The cognitive and neural architecture of sequence representation. Psychological Review. 2003;110:316–339. doi: 10.1037/0033-295x.110.2.316. [DOI] [PubMed] [Google Scholar]
- Keele SW, Jennings P, Jones S, Caulton D, Cohen A. On the modularity of sequence representation. Journal of Motor Behavior. 1995;27:17–30. [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. Journal of Neuroscience. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klockgether T. Handbook of ataxia disorders. New York: Marcel Dekker; 2000. [Google Scholar]
- Lu X, Hikosaka O, Miyachi S. Role of monkey cerebellar nuclei in skill for sequential movement. Journal of Neurophysiology. 1998;79:2245–2254. doi: 10.1152/jn.1998.79.5.2245. [DOI] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms: I. Focal olivocerebellar lesions impair adaptation. Brain. 1996;119:1183–1198. doi: 10.1093/brain/119.4.1183. [DOI] [PubMed] [Google Scholar]
- Maschke M, Gomez CM, Ebner TJ, Konczak J. Hereditary cerebellar ataxia progressively impairs force adaptation during goal-directed arm movements. Journal of Neurophysiology. 2004;91:230–238. doi: 10.1152/jn.00557.2003. [DOI] [PubMed] [Google Scholar]
- Miller BT, D’Esposito M. Searching for “the Top” in Top–Down Control. Neuron. 2005;48:535–538. doi: 10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Molinari M, Leggio MG, Solida A, Ciorra R, Misciangna S, Silveri MC, et al. Cerebellum and procedural learning: Evidence from focal cerebellar lesions. Brain. 1997;120:1753–1762. doi: 10.1093/brain/120.10.1753. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Prism adaptation during walking generalizes to reaching and requires the cerebellum. Journal of Neurophysiology. 2004;92:2497–2509. doi: 10.1152/jn.00129.2004. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology. 1987;19:1–32. [Google Scholar]
- Nixon PD, Passingham RE. The cerebellum and cognition: Cerebellar lesions impair sequence learning but not conditional visuomotor learning in monkeys. Neuropsychologia. 2000;38:1054–1072. doi: 10.1016/s0028-3932(99)00138-4. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Grafman J, Clark K, Stewart M, Massaquoi S, Lou J-S, et al. Procedural learning in Parkinson’s disease and cerebellar degeneration. Annals of Neurology. 1993;34:594–602. doi: 10.1002/ana.410340414. [DOI] [PubMed] [Google Scholar]
- Ramnani N. The primate cortico-cerebellar system: Anatomy and function. Nature Reviews Neuroscience. 2006;7:511–522. doi: 10.1038/nrn1953. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Ivry RB. Comparison of the basal ganglia and cerebellum in shifting attention. Journal of Cognitive Neuroscience. 2001;13:285–297. doi: 10.1162/08989290151137340. [DOI] [PubMed] [Google Scholar]
- Raymond JL, Lisberger SG, Mauk MD. The cerebellum: A neuronal learning machine? Science. 1996;272:1126–1131. doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]
- Richter S, Matthies K, Ohde T, Dimitrova A, Gizewski E, Beck A, et al. Stimulus–response versus stimulus–stimulus–response learning in cerebellar patients. Experimental Brain Research. 2004;158:438–449. doi: 10.1007/s00221-004-1920-3. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Purushotham A, Kim SG, Ugurbil K, Willingham D, Ashe J. Cerebellum activation associated with performance change but not motor learning. Science. 2002;296:2043–2046. doi: 10.1126/science.1068524. [DOI] [PubMed] [Google Scholar]
- Shin JC, Ivry R. Spatial and temporal sequence learning in patients with Parkinson’s disease or cerebellar lesions. Journal of Cognitive Neuroscience. 2003;15:1232–1243. doi: 10.1162/089892903322598175. [DOI] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. Journal of Neurophysiology. 2005;93:2809–2821. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- Timmann D, Drepper J, Maschke M, Kolb FP, Böring D, Thilmann AF, et al. Motor deficits cannot explain impaired cognitive associative learning in cerebellar patients. Neuropsychologia. 2002;40:788–800. doi: 10.1016/s0028-3932(01)00181-6. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. Journal of Neurological Sciences. 1997;145:205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- van der Graff FHCE, Maguire RP, Leenders KL, de Jong BM. Cerebral activation related to implicit sequence learning in a Double Serial Reaction Time task. Brain Research. 2006;1081:179–190. doi: 10.1016/j.brainres.2006.01.103. [DOI] [PubMed] [Google Scholar]
- Wise SP, di Pellegrino G, Boussaoud D. The premotor cortex and nonstandard sensorimotor mapping. Canadian Journal of Physiology and Pharmacology. 1996;74:469–482. doi: 10.1139/cjpp-74-4-469. [DOI] [PubMed] [Google Scholar]