Abstract
In the human umbilical artery O2 has a direct contractile effect and is also required for induction of contraction by several other agents. Agonist that cause contraction (bradykinin, histamine, and serotonin) cause accumulation of guanosine 3':5'-monophosphate (cGMP) without altering adenosine 3':5'-monophosphate (cAMP). They appear to act through two different mechanisms: one Ca++-dependent, the other Ca++-inhibited. O2 increased the cGMP content of the artery in a Ca++-dependent manner without affecting the cAMP content. Inhibitors of oxidative phosphorylation (oligomycin and 2,4-dinitrophenol) did not diminish this effect of O2. O2 was required for demonstration of the Ca++-dependent accumulation of cGMP in response to bradykinin, histamine, and ionophore A23187. The effect of the phosphodiesterase inhibitor 3-isobutyl-1-methyl xanthine on basal cGMP content and on the bradykinin-induced accumulation was also dependent on the presence of O2. Methylene blue and sodium ascorbate caused cGMP accumulation in O2-deprived arteries. Their effects were not diminished in Ca++-depleted arteries and, in fact, seemed to be inhibited when 2.7 mM Ca++ was present in the medium. The effects of these agents and of serotonin on cGMP, which were inhibited by Ca++, were also inhibited by O2. These non Ca++-, non O2-dependent agents (methylene blue, ascorbate, and serotonin) did not, however, permit demonstration of the effects of the Ca++- and O2-dependent agonists on O2-deprived arteries. It appears that there are in the umbilical artery (and probably in other tissues also) at least two separate mechanisms for control of cGMP synthesis that are influenced differently by Ca++- and O2-linked processes.
Full text
PDF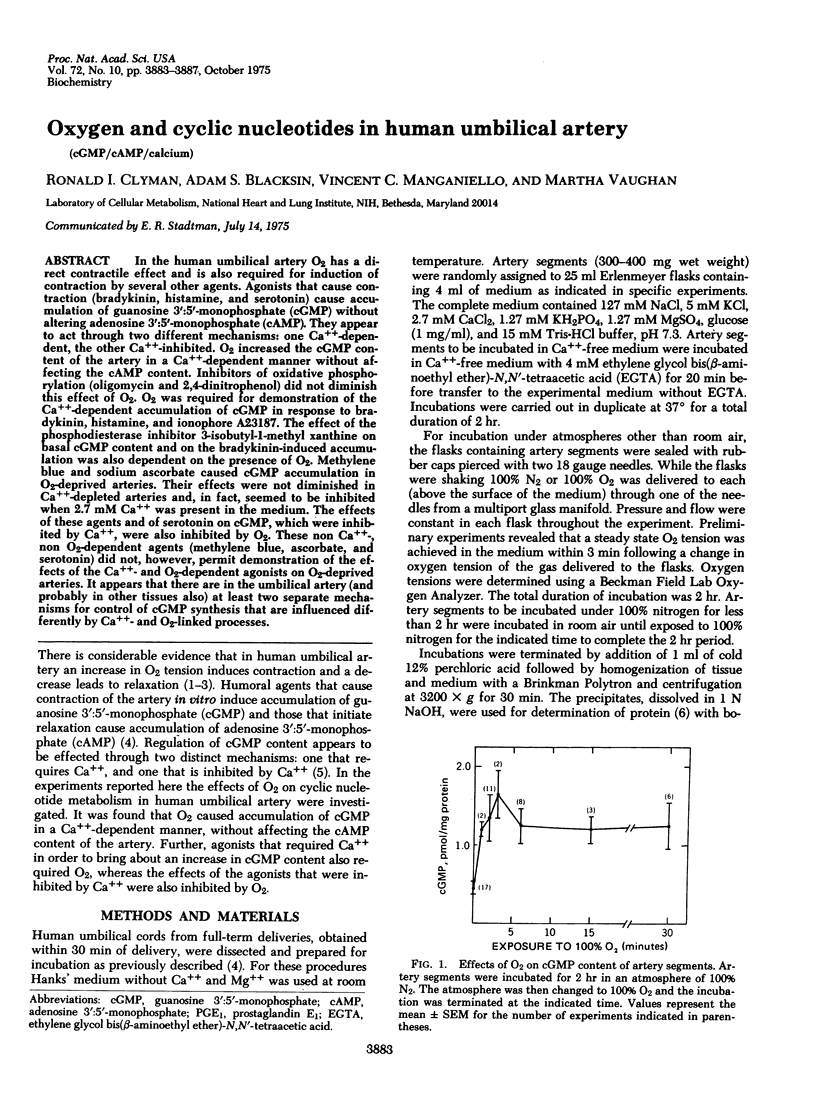


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bigley R. H., Stankova L. Uptake and reduction of oxidized and reduced ascorbate by human leukocytes. J Exp Med. 1974 May 1;139(5):1084–1092. doi: 10.1084/jem.139.5.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor I., Guntheroth W. G. In vitro response to oxygen of human umbilical arteries and of animal ductus arteriosus. Can J Physiol Pharmacol. 1970 Jul;48(7):500–502. doi: 10.1139/y70-077. [DOI] [PubMed] [Google Scholar]
- Chrisman T. D., Garbers D. L., Parks M. A., Hardman J. G. Characterization of particulate and soluble guanylate cyclases from rat lung. J Biol Chem. 1975 Jan 25;250(2):374–381. [PubMed] [Google Scholar]
- Clyman R. I., Blacksin A. S., Sandler J. A., Manganiello V. C., Vaughan M. The role of calcium in regulation of cyclic nucleotide content in human umbilical artery. J Biol Chem. 1975 Jun 25;250(12):4718–4721. [PubMed] [Google Scholar]
- Clyman R. I., Sandler J. A., Manganiello V. C., Vaughan M. Guanosine 3',5'-monophosphate and adenosine 3',5'-monophosphate content of human umbilical artery. J Clin Invest. 1975 May;55(5):1020–1025. doi: 10.1172/JCI108002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detar R., Bohr D. F. Oxygen and vascular smooth muscle contraction. Am J Physiol. 1968 Feb;214(2):241–244. doi: 10.1152/ajplegacy.1968.214.2.241. [DOI] [PubMed] [Google Scholar]
- Eltherington L. G., Stoff J., Hughes T., Melmon K. L. Constriction of human umbilical arteries. Interaction between oxygen and bradykinin. Circ Res. 1968 Jun;22(6):747–752. doi: 10.1161/01.res.22.6.747. [DOI] [PubMed] [Google Scholar]
- Fay F. S. Guinea pig ductus arteriosus. I. Cellular and metabolic basis for oxygen sensitivity. Am J Physiol. 1971 Aug;221(2):470–479. doi: 10.1152/ajplegacy.1971.221.2.470. [DOI] [PubMed] [Google Scholar]
- Joiner P. D. Effects of ascorbate on contractile activity of ileal smooth muscle. J Pharmacol Exp Ther. 1973 Apr;185(1):84–93. [PubMed] [Google Scholar]
- KOVALCIK V. THE RESPONSE OF THE ISOLATED DUCTUS ARTERIOSUS TO OXYGEN AND ANOXIA. J Physiol. 1963 Nov;169:185–197. doi: 10.1113/jphysiol.1963.sp007249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S. M. The identification of prostaglandins in human umbilical cord. Br J Pharmacol Chemother. 1967 Feb;29(2):230–237. doi: 10.1111/j.1476-5381.1967.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H., Murad F. Evidence for two different forms of guanylate cyclase in rat heart. J Biol Chem. 1974 Nov 10;249(21):6910–6916. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehninger A. L. Mitochondria and calcium ion transport. Biochem J. 1970 Sep;119(2):129–138. doi: 10.1042/bj1190129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair X., Dyer D. C. Effect of metabolic inhibitors and oxygen on responses of human umbilical arteries. Am J Physiol. 1973 Nov;225(5):1118–1122. doi: 10.1152/ajplegacy.1973.225.5.1118. [DOI] [PubMed] [Google Scholar]
- Nair X., Dyer D. C. Responses of human umbilical artery to oxygen, bradykinin and histamine. Res Commun Chem Pathol Pharmacol. 1974 Feb;7(2):381–388. [PubMed] [Google Scholar]
- Oberhänsli-Weiss I., Heymann M. A., Rudolph A. M., Melmon K. L. The pattern and mechanisms of response to oxygen by the ductus arteriosus and umbilical artery. Pediatr Res. 1972 Sep;6(9):693–700. doi: 10.1203/00006450-197209000-00001. [DOI] [PubMed] [Google Scholar]
- Otto D. A., Ontko J. A. Regulation of the mitochondrial oxidation-reduction state in intact hepatocytes by calcium ions. Biochem Biophys Res Commun. 1974 Nov 27;61(2):743–750. doi: 10.1016/0006-291x(74)91020-1. [DOI] [PubMed] [Google Scholar]
- Sass M. D., Caruso C. J., Axelrod D. R. Accumulation of methylene blue by metabolizing erythrocytes. J Lab Clin Med. 1967 Mar;69(3):447–455. [PubMed] [Google Scholar]



