Abstract
Antinuclear antibodies are a hallmark feature of generalized autoimmune diseases, including systemic lupus erythematosus and systemic sclerosis. However, the processes underlying the loss of tolerance against nuclear self-constituents remain largely unresolved. Using mice deficient in lymphotoxin and Hox11, we report that approximately 25% of mice lacking secondary lymphoid organs spontaneously develop specific antinuclear antibodies. Interestingly, we find this phenotype is not caused by a defect in central tolerance. Rather, cell-specific deletion and in vivo lymphotoxin blockade link these systemic autoimmune responses to the formation of gut-associated lymphoid tissue in the neonatal period of life. We further demonstrate antinuclear antibody production is influenced by the presence of commensal gut flora, in particular increased colonization with segmented filamentous bacteria, and IL-17 receptor signaling. Together, these data indicate that neonatal colonization of gut microbiota influences generalized autoimmunity in adult life.
Keywords: antinuclear antibodies, commensal microbiota, systemic autoimmunity
Introduction
Antinuclear antibodies (ANA) are a hallmark feature of generalized autoimmune diseases (von Muhlen & Tan, 1995). These clinically heterogeneous conditions, such as systemic lupus erythematosus (SLE) and systemic sclerosis (SSc), are characterized by immune-mediated tissue damage in multiple organs, caused by aberrant responses of the adaptive immune system. Immunodominant autoantigens recognized by systemic autoantibodies are often DNA- or RNA-associated protein complexes. Underlying mechanisms that mediate the breach of tolerance against these nuclear autoantigens are only partially understood. In autoimmune-prone strains of mice, antigen-producing cells have been located in secondary lymphoid tissue, and both extrafollicular and germinal center responses have been implicated in the production of such autoantibodies (William et al, 2002).
The lymphotoxin-β receptor (LTβR) functions as receptor for both membrane-bound lymphotoxin (LTα1β2) and LIGHT (TNF superfamily member 14). Lymphotoxin-β receptor signaling controls the development of secondary lymphoid organs and is continuously required in adults for homeostasis and structural architecture of the thymus and secondary lymphoid organs (Futterer et al, 1998). LT-deficient mice thus serve as a prototypic model for studying the influence of secondary lymphoid organs in immune processes. Although antibody responses are impaired in LT-deficient animals due to the absence of follicular dendritic cell networks, germinal center formation and somatic hypermutation can still occur. Based on the presence of perivascular lymphocytic infiltrates in multiple organs as well as organ-specific autoantibodies, an autoimmune phenotype has been defined for the LT-deficient animals (Boehm et al, 2003). However, the role of the disturbed thymic medulla in this autoimmune phenotype is a matter of controversy (Chin et al, 2003; Martins et al, 2008). Whether systemic autoimmune responses do occur in the absence of secondary lymphoid tissues is another area of uncertainty (Boehm et al, 2003; Chin et al, 2003). Given conflicting reports on systemic autoimmune responses in Ltbr−/− mice, we sought to investigate whether autoantibodies directed against nuclear antigens can appear in the absence of secondary lymphoid tissues utilizing Ltbr−/− mice.
Results and Discussion
We found that ∽25% of LTβR-deficient mice developed systemic autoimmune responses by three months of age (Fig1A) using a validated immunodetection system for a broad range of nuclear antigens (Supplementary Fig S1). The immunoassay system identified diverse ANA, including anti-U1RNP, anti-Sm, anti-Scl70/topoisomerase-I, anti-centromere protein B, anti-SSA/Ro52, and anti-Jo1 (Fig1B and C). Antibodies to these nuclear autoantigens are strongly associated with SLE, SSc, and polymyositis (von Muhlen & Tan, 1995). In contrast, no anti-dsDNA was found (Supplementary Fig S2). By six months of age, the prevalence of autoantibodies remained the same, but more mice developed multiple reactivities (Fig1B and C). We semiquantitatively determined the titers of these mice (Fig1B). We could not detect any autoimmune reactivity at six weeks of age despite immune maturation, suggesting a delayed stochastic penetrance of the autoimmune phenotype characteristic in most autoimmune diseases. As LT-deficient animals have a spleen, we sought to determine whether ANA can be generated in asplenic mice by intercrossing Hox-11−/− and Ltbr−/− mice. These double knockout mice still developed the pathological autoantibody responses at the same prevalence, demonstrating that aberrant systemic autoimmune responses can develop in the complete absence of secondary lymphoid organs (Fig1A).
Figure 1.
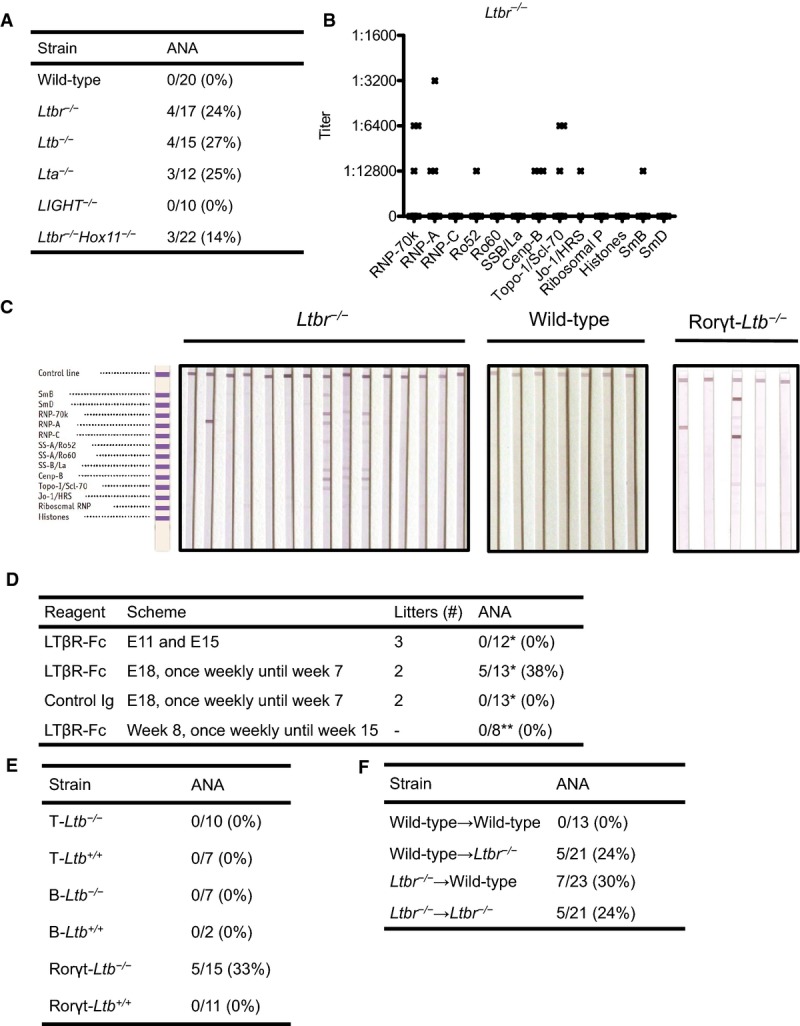
- 3-month-old wild-type (C57BL/6), Ltbr−/−, Lta−/−, LIGHT−/−, and Ltbr−/−Hox11−/− mice; percentage of mice with at least one ANA.
- 6-month-old Ltbr−/− mice (n = 15); titers of individual mice.
- LIA of 6-month-old Ltbr−/− mice, wild-type mice, and 3-month-old Rorγt-Ltb−/− mice.
- LTβR-Fc or control immunoglobulin (Ig)-treated wild-type mice; percentage of mice with at least one ANA. E denotes gestational day. *ANA were determined with LIA at the age of 3 months. **ANA were determined with LIA 3 months after the start of treatment.
- 3-month-old cell-specific LT knockout mice; percentage of mice with at least one ANA.
- Bone marrow cells from wild-type or Ltbr−/− mice were transferred at neonatal age into lethally irradiated wild-type or Ltbr−/− mice; percentage of mice with at least one ANA. ANA were determined with LIA at the age of 3 months.
Histological examination of Ltbr−/− mice confirmed the presence of lymphocytic infiltrates in multiple organs. Given the association of systemic autoimmune responses with generalized autoimmune disease, we specifically looked for characteristic pathological features. However, compared to wild-type mice, no difference was observed in kidney damage, and skin and esophageal sclerosis (Supplementary Fig S3). Furthermore, renal histology and proteinuria were not different between antibody-positive and antibody-negative Ltbr −/− mice (data not shown).
We then evaluated whether structural defects in LT-deficient mice lead to ANA production. To this end, we used an LTβR-Fc fusion protein, which acts as a soluble decoy receptor blocking LTαβ and LIGHT (Rennert et al, 1996). Blocking LTβR signaling at various phases of ontogeny and early postnatally results in the temporally patterned absence of secondary lymphoid organs (lymph nodes, Peyers' patches (PP), and cryptopatches (CP)) (Rennert et al, 1996; Bouskra et al, 2008). We observed that blocking LTβR signaling during late ontogeny through six weeks of age resulted in the appearance of ANA at the age of three months with a comparable spectrum and titers as LTβR-deficient mice (Fig1D and data not shown), and as demonstrated previously, these mice lacked CP, PP, and isolated lymphoid follicles (ILF) (data not shown). In contrast, mice lacking peripheral lymph nodes and PP by blocking LTβR signaling during early ontogeny did not develop autoantibodies. In addition, blocking LTβR signaling during adulthood, which disrupts splenic architecture, also did not result in ANA formation (Fig1D). To rule out a role of LTβR signaling in the thymus during the perinatal window, we performed thymus transplant experiments. Fetal thymi from Ltbr−/− or wild-type mice were depleted of hemato-poietic cells and then grafted under the kidney capsules of nude mice, creating Ltbr−/−→nude mice and wild-type→nude mice. Three months after engraftment, mice were sacrificed and T-cell repopulation was verified in the liver and spleen by flow cytometry (Fig2A and B). Ltbr−/− and wild-type thymi contained approximately equal number of thymocytes, with similar distribution among the different T-cell subsets (Fig2A and B). Levels of total IgG were also not different between the two groups (Fig2C). Importantly, no ANA could be detected in the serum samples of nude mice engrafted with LTβR-deficient thymic lobes (Fig2D). We thus concluded that systemic autoimmune responses can develop in the absence of CP and ILF.
Figure 2.
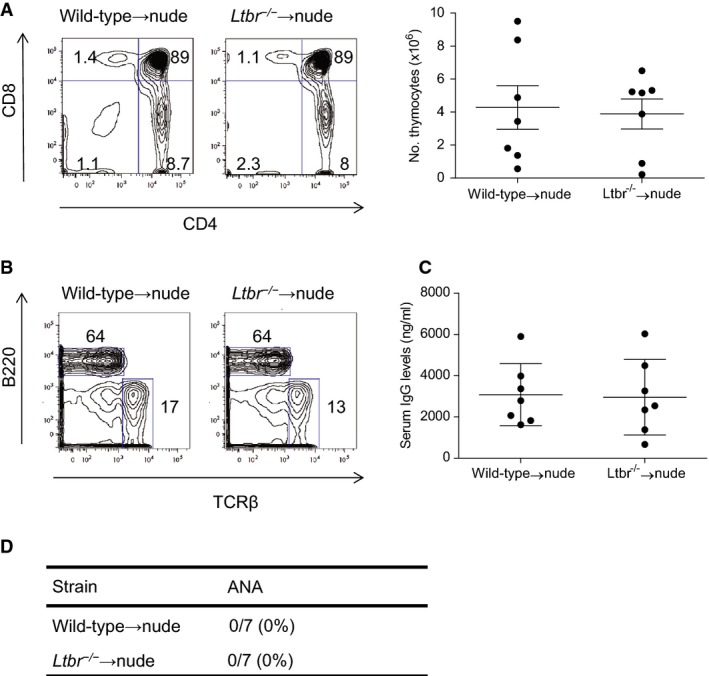
- Thymic reconstitution was analyzed by counting total cell number and flow cytometry for cell percentages. Numbers represent the percentages within the indicated regions (left panel). No significant differences were found. Data represent means ± s.e.m.
- T-cell reconstitution of spleen was assessed by flow cytometry. Numbers represent the percentages within the indicated regions.
- Total IgG from nude mice engrafted with Ltbr−/− or wild-type thymi was determined by ELISA. No significant differences were found. Data represent means ± s.e.m.
- Sera were collected 3 months after transplantation and tested for ANA; percentage of mice with at least one ANA reactivity.
We next wanted to resolve which membrane LT-expressing cell type in the lamina propria of the gut was involved in the maintenance of tolerance against nuclear antigens. To this end, we generated mice deficient in LTβ in T cells (T-Ltb−/−), B cells (B-Ltb−/−) or RORγt+ cells (Rorγt-Ltb−/−), and littermate controls. We could only detect autoantibodies with a similar spectrum as Ltbr−/− in mice lacking membrane LT in RORγt-positive cells (Fig1E). As we were able to test only small sample sizes of cell-specific Ltb−/− mice, we also collected sera from cell-specific Lta−/− mice. Alike cell-specific Ltb−/− mice, only Rorγt-Lta−/− but neither T-Lta−/− nor B-Lta−/− developed ANA at the age of three months (data not shown). To resolve which LTβR-expressing cells in the lamina propria are involved in the maintenance of the tolerance, we performed reciprocal bone marrow transfer experiments between wild-type and Ltbr−/− mice. As shown in Fig1F, both wild-type→Ltbr−/− and Ltbr−/−→wild-type chimeras developed autoantibodies. We thus conclude that communication via the LT-LTβR axis between RORγt+ innate lymphoid cells (ILC) and both radio-resistant and bone marrow-derived cells is essential to maintain tolerance.
RORγt+ ILC have been shown to be essential in the defense of epithelial surfaces and play an important role in the intestinal homeostasis with symbiotic microbiota by the production of IgA, IL-17, and IL-22 production (Vivier et al, 2009). This regulatory control in the gut prompted us to examine the relationship between the gut microbiota and ANA production. Moreover, early postnatal blocking of LTβR signaling leads to a 10-fold expansion of the normal ileal microbiota, including bacteria belonging to the Clostridiales, Bacteroides, and Enterobacteriaceae groups (Bouskra et al, 2008). We first assessed whether elimination of the gut microbiota influenced ANA production. Pregnant mice and their offspring were treated with broad-spectrum antibiotics until the age of 3 months. As previously observed in germfree mice, this caused a significant enlargement of the cecum (data not shown). Ltbr−/− mice receiving antibiotics had a reduced prevalence of ANA compared to a large series of 369 untreated consecutively born three-month-old animals (Fig3A, upper panel). In this series, we observed some variation in the ANA positivity between litters, but no significant differences in prevalence between cages weaned from the same breading cage (data not shown). This points to a maternal but not a cage effect on the phenotype. Therefore, we opted to analyze offspring of at least 2 litters in further experiments. To further substantiate a role for the gut microbiota, we treated germfree C57BL/6 mice with the LTβR-Fc fusion protein from gestational day 18 until six weeks after birth as described above. These experiments were performed in the INRA Anaxem germfree animal facilities. Similarly, germfree animals had a reduced prevalence of ANA (Fig3A, lower panel). Furthermore, we observed a higher frequency of ANA-positive animals compared to the experiments with LTβR-Fc fusion protein performed in the Ghent University vivarium.
Figure 3.
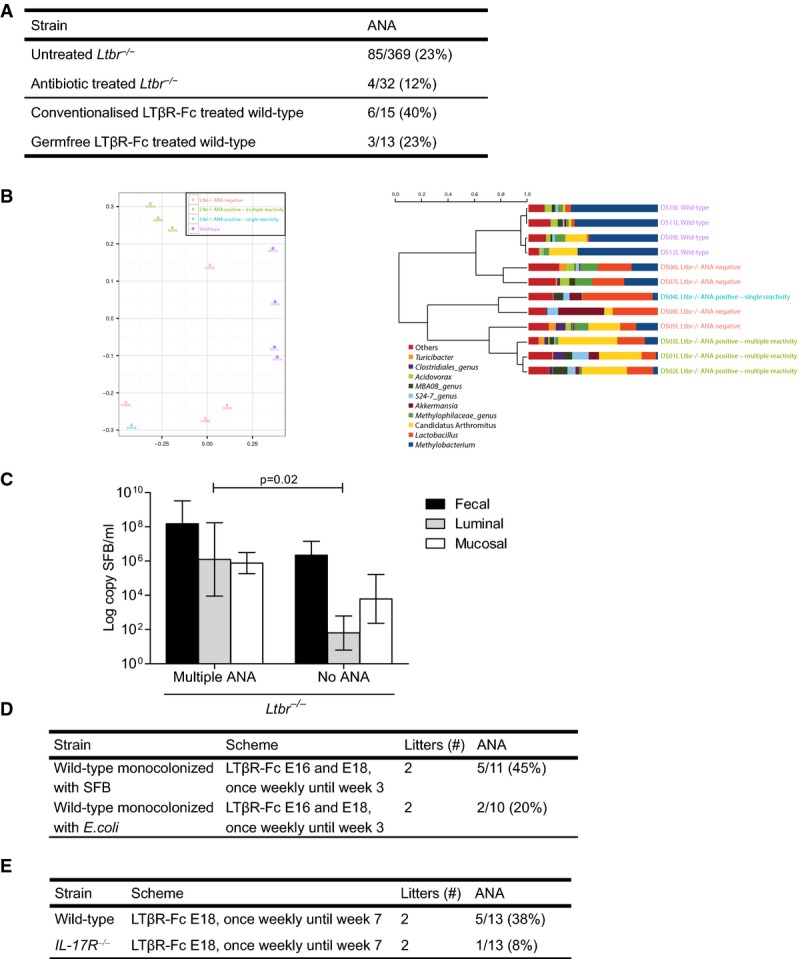
- Ltbr−/− mice were treated or not with antibiotics from birth (upper panel), conventionalized or germfree wild-type mice were treated with the LTβR-Fc fusion protein from gestational day 18 until 6 weeks after birth (lower panel); percentage of mice with at least one ANA. ANA were tested with LIA at the age of 3 months.
- 16S rRNA gene analysis of luminal samples of wild-type, ANA-positive, and ANA-negative Ltbr−/− mice. Left panel: Beta diversity plot using multidimensional scaling on Bray–Curtis dissimilarities distances. Right panel: Hierarchical cluster analysis using Pearson's correlation. Bacteria community compositions differ in the different groups. Please note that Candidatus arthromitus, according to Thompson et al (2012), should be renamed Candidatus savagella.
- Real-time PCR for SFB in luminal, mucosal, and fecal samples of multiple ANA-positive and ANA-negative Ltbr−/− mice. Analysis with ANOVA,P = 0.02 for differences between groups, n = 4 mice per group. Data represent means ± s.e.m.
- C3H/HeN germfree pregnant mice were treated with the LTβR-Fc fusion protein on day 16 and 18 of gestation to prevent the development of PP in their offspring. Pups were also treated in the neonatal period to prevent the development of ILF. Mice were colonized at 8 weeks of age with SFB or E. coli and were sacrificed on d20 or on d60 postcolonization; percentage of mice with at least one ANA. E denotes gestational day.
- LTβR-Fc-treated wild-type or IL17R−/− mice; percentage of mice with at least one ANA. E denotes gestational day. ANA were determined with LIA at the age of 3 months
We next assessed whether gut microbiota composition differed between wild-type, antibody-positive, and antibody-negative LTβR-deficient animals existed. In a first screening, we performed a community profiling on the luminal, mucosal, and fecal microbiome by DGGE (data not shown) and 16S rRNA sequencing, both of which showed the same results. As shown on Fig3B, we observed genotype and antibody-specific clustering of the three animal groups. The most striking differences between groups revealed a species belonging to the segmented filamentous bacteria (SFB), characterizing animals with multiple reactivities as a separate group compared to controls that are in a cluster dominated by Methylobacterium (Fig3B). These data were confirmed by quantitative PCR with primers specific for SFB genes (Fig3C). The presence of the Methylobacterium genus has been associated with contamination in samples with low biomass (Barton et al, 2006; Salter et al, 2014). However, we feel that this is not the case here—the fecal material used in this study has large biomass that should dilute the contaminant rather than allowing for its dominance in the profile. Furthermore, a neighbor-joining tree shows that the observed Methylobacterium forms a separate cluster than the one produced by the reported contaminants (Supplementary Fig S5). Histological analyses of different gut parts comparing ANA-positive and ANA-negative LTβR-deficient animals, however, revealed no inflammatory differences (Supplementary Fig S6). We also directly compared the impact of monocolonization of LTβR-Fc-treated mice with SFB versus the nonadherent and nonvirulent strain of E. coli MG1655 on autoantibody induction in the INRA facilities. We found an increased prevalence of ANA in SFB as opposed to E. coli monocolonized mice (Fig3D). In SFB monocolonized mice, ANA frequency was markedly higher in mice lacking all gut-associated lymphoid tissue compared to mice lacking PP only (Supplementary Fig S4B).
ILF and PP contain the stromal microenvironment for IgA production, a critical antibody that helps maintain gut homeostasis (Eberl, 2007). Furthermore, it was shown that SFB can also induce IgA responses via de novo induction of tertiary lymphoid tissue in contrast to E. coli in mice lacking PP and cryptopatch-derived ILF (Lecuyer et al, 2014). Because an aberrant expansion of SFB was reported in IgA-deficient mice, it could be anticipated that IgA was protective against ANA induction. Yet ANA are present at comparable levels in mice with gut tertiary follicles, which have substantial concentrations of IgA in feces, as in mice lacking all gut lymphoid tissues, which have hardly any IgA detectable in the feces (data not shown). This argues against a critical role for IgA in LT-dependent ANA induction.
SFB are important for inducing a robust T-helper cell type 17 population in the small intestinal lamina propria of the mouse gut (Wu et al, 2010) and has the ability to induce such responses inside and outside gut organized lymphoid tissue (Lecuyer et al, 2014). To assess the potential role of IL-17 or IL-25 in the model as an effector cytokine, we treated IL-17R-deficient mice with the LTβR-Fc fusion protein from gestational day 18 until six weeks after birth as described above. In contrast to wild-type mice, this treatment did not induce significant systemic autoimmune responses (Fig3E). In BXD2, a mouse model for lupus, IL-17 was also identified as an important effector cytokine for systemic autoimmune responses (Hsu et al, 2008).
It was reported that intestinal IgA and IgG production plasma cells, while mostly antigen specific, include a relatively high frequency of cells secreting autoreactive and polyspecific antibodies (Benckert et al, 2011; Scheid et al, 2011). This is in contrast to the bone marrow IgG plasma cells where autoreactivity is relatively rare. Because we were unable to find immune-mediated pathology despite the presence of multiple ANA reactivity, this could point to production to polyreactive intestinal B cells due to impaired architecture rather than active regulation of autoantibody responses. In such a scenario, SFB could alter the threshold for induction of ANA given its marked ability to generate potent Th17 responses.
In summary, we report that in the absence of LT IL-17R-dependent systemic autoimmune responses are associated with increased SFB colonization. Overall, our findings enforce a new paradigm that neonatal colonization of the gut impacts systemic autoimmune responses against nuclear antigens in adulthood.
Materials and Methods
Mice
C57BL/6, Rag2−/− (C57BL/6 background) and nude mice (C57BL/6 background) were originally purchased from The Jackson Laboratory. Mice deficient in LTα (De Togni et al, 1994) (backcrossed 8 times on C57BL/6), LTβR (Futterer et al, 1998) (backcrossed 6 times), LIGHT (Scheu et al, 2002) (backcrossed 6 times), HOX11 (Dear et al, 1995) (backcrossed 10 times) and IL-17R (Ye et al, 2001) (backcrossed more than 10 times) have been described previously. Ltb−/−, T-Ltb−/−, B-Ltb−/−, and Rorγt-Ltb−/− were generated by crossing LTβ-floxed mice (Tumanov et al, 2002) with K5-cre (Ramirez et al, 2004), CD4-cre (Lee et al, 2001), mb1-cre (Hobeika et al, 2006), and RORγt-cre (Lochner et al, 2008), respectively. MRL/lpr−/− and (NZB/NZW)F1 mice were purchased from Harlan Europe. Sera from NOD mice were kindly provided by Prof. C. Mathieu (University Hospital Leuven, Belgium). Pristane-induced lupus in C57BL/6 was performed as described previously (Lee et al, 2008). All these mice were housed and bred in the Ghent University vivarium in a specific pathogen-free conditions. Germfree C57BL/6 and C3H/HeN were bred in the INRA Anaxem germfree animal facilities (Jouy-en-Josas, France) and housed in isolators. For colonization, 8- to 9-week-old female germfree mice were gavaged twice at a 24-h interval with 0.5 ml of fresh anaerobic cultures of E. coli MG1655 or of fecal homogenate from SFB monoassociated mice (Gaboriau-Routhiau et al, 2009). Colonization by E. coli was monitored by bacterial counts after culture on nonselective brain heart infusion agar plates (Difco). For SFB monitoring, DNA was extracted from frozen ileal biopsies and 16S rRNA was amplified by qRT–PCR by using specific primer pairs for SFB or total bacteria on an ABI Prism 7300 Sequence Detection System (Life Technologies) as described (Gaboriau-Routhiau et al, 2009). Gnotobiotic mice were sacrificed on day 20 and 60 postcolonization. All animal procedures were approved by the Institutional Animal Care and Ethics Committee of Ghent University.
Antibodies, fusion protein, and antibiotics
Pregnant mice were injected simultaneously i.v. and i.p. with 50 μg of LTβR-Fc on day 11 and day 15 of gestation, or only on day 18 of gestation. When the mother was injected only at day 18, progeny received weekly i.p. injections with 25 μg of LTβR-Fc (continuous) starting at day 7 after birth until the age of 6 weeks. Controls consisted of C57BL/6 mice treated with control IgG. In addition, adult C57BL/6 mice (8 weeks) were injected i.p. weekly with 100 μg of LTβR-Fc or control immunoglobulin for 7 weeks. Prevention of development of gut lymphoid tissues was performed with a slightly different scheme in experiments with monocolonized mice. Pregnant germfree mice were i.v. injected with 150 μg of LTβR-Fc on day 16 and 18 of gestation. Some germfree pups born from LTβR-Fc-treated mothers were further i.p. injected with 10 μg LTβR-Fc once a week during the first 3 weeks of life. Ampicillin, streptomycin, and colistin (Sigma-Aldrich) were supplied to mice in the drinking water at a concentration of 1, 5, and 1 g/l, respectively.
Thymus transplants
Thymi were isolated from newborn Ltbr−/− or wild-type mice and cultured in 1.35 mM 2-deoxyguanosine (Sigma-Aldrich) for 5 days to deplete bone marrow-derived cells. Two thymic lobes were then transplanted under the kidney capsule of adult nude mice (Martins et al, 2008). Mice were bled 6 weeks after the procedure to verify the presence of circulating T cells by flow cytometry. After 3 months, mice were bled and sacrificed. Thymic lobes, liver, and spleen cells were analyzed by flow cytometry.
Bone marrow transfers
Bone marrow was isolated from the femur and tibia of Ltbr−/− or wild-type mice. Three million bone marrow cells were injected in the liver of sublethally irradiated (400 rad) 7-day-old C57BL/6 and LTβR-deficient recipient mice.
Antinuclear antibodies
Specific ANA except for anti-dsDNA were detected by line immunoassay (INNO-LIA ANA Update, Innogenetics NV). The nylon strips were incubated with serum at a 1:200 dilution. Following washing, a 1:2,500 dilution of an alkaline phosphatase-conjugated anti-mouse IgG was added (Chemicon). After washing, the reaction was revealed with the chromogen 5-bromo-4-chloro-3-indolyl phosphate, producing a dark brown color in proportion to the amount of specific autoantibody in the test sample. Sulfuric acid was added to stop the color development. The cutoff of the reactivities was determined by testing 20 serum samples from 6-month-old C57BL/6 mice. None produced any background staining higher than a 1:12,800 dilution of a strong anti-RNP-A reactivity of a MRL/lpr−/− mouse. Thus, we considered a higher intensity as a positive test result. To determine the intensity of the color as quantification for the amount of antibody in the test sample, further dilutions of the reference anti-RNP-A reactivity were used (at least as intense as 1:12,800 was considered 1+, 1:6,400 2+, 1:3,200 3+, 1:1,600 4+, 1:800 5+, and 1:200 6+). The assay contains the following recombinant and natural antigens: SmB, SmD, RNP-A, RNP-C, RNP-70k, Ro52/SSA, Ro60/SSA, La/SSB, CenpB, Topo-I/Scl70, Jo-1, ribosomal P, and histones. Anti-dsDNA IgG antibodies were detected with ELISA according to the manufacturer's instructions (Alpha Diagnostic International).
Histopathology
Tissues for histological examination were fixed in 4% buffered formaldehyde and embedded in paraffin. Sections were stained with hematoxylin and eosin, periodic-acid Schiff, silver staining, and Masson's trichrome using the standard technique. De-identification and coding of slides was done so that a blinded evaluation could be performed. Slides were scored by trained pathologists (LA and CAC for the gut sections and AD for the renal sections). Renal slides were scored as described previously (Keeton et al, 1995). Briefly, for glomerular damage, 10 random glomeruli of each animal were scored using a semiquantitative grading system. In this grading system, a grade 0 corresponds with specimens with no glomerular lesions, grade I lesions show minimal mesangial thickening, grade II lesions contain increases in both mesangium and glomerular cellularity, and grade III lesions contain the preceding features plus superimposed inflammation and/or capsular adhesions. Grade IV lesions show obliteration of glomerular architecture involving more than 70% of the glomeruli. Furthermore, glomerular crescents, interstitial inflammation, and vascular inflammation were described. Dermal sclerosis and esophageal sclerosis was determined using ImageJ to measure the thickness of dermis and submucosa. Inflammatory scores of different part of the gut were performed according to an adapted scoring scheme (Van der Sluis et al, 2006; Supplementary Fig S5B).
Flow cytometry
Liver mononuclear cells were isolated using an adjusted 33% Percoll gradient (GE Healthcare) (Franki et al, 2006). Cell suspensions from thymus and spleen were prepared by conventional methods. Mononuclear cells were isolated from peripheral blood using a Ficoll–Paque Plus gradient (GE Healthcare). Cell stainings were performed as described previously (Franki et al, 2006). Cells were acquired on a FACSCanto II (Becton Dickinson) flow cytometer and analyzed using FlowJo software (Tree Star). Primary antibodies used were from eBioscience: anti-TCRβ (H57-597), anti-CD4 (L3T4), anti-CD8 (Ly-2), anti-FOXP3 (FJK-16s), and B220–APC-Cy7.
Analysis of the gut flora
The most prominent shifts within the microbiota were monitored via denaturing gradient gel electrophoresis (DGGE). After DNA extraction and PCR, gels were run using an Ingeny PhorU apparatus (Ingeny International). DNA-fragment bands on the DGGE gel that distinguished autoantibody-positive from autoantibody-negative samples on the DGGE gel were identified through sequence analysis. Specific bands were cut from the gel, after which the 180-bp sized fragment was sequenced (AGOWA). Normalization and further analysis of the gels was carried out using BioNumerics software v5.10. To identify the band of interest in more phylogenetic detail, a clone library was made from the samples of interest. PCR was performed on the sample with primers F27/R1492. PCR products were cut from the gel, purified (QIAquick Gel Extraction Kit, QIAGEN), and cloned with a TOPO TA Cloning Kit with PCR2.1-TOPO Vector (Invitrogen). Clone DNA was amplified with primers M13F/M13R, after which nested PCR was followed with DGGE primers GC-338F/518R. Clones from which the DNA band on DGGE matched with the DNA band of interest (distinctive between autoantibody-positive and autoantibody-negative) were sent for sequencing (AGOWA). Sequences (480 bp) were manually inspected and compared with databases at the NCBI website (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Following the identification of segmented filamentous bacteria (SFB) as distinctive DNA band between autoantibody-positive and autoantibody-negative mice, we next performed a quantitative analysis of SFB with qPCR using primers SFB736F and SFB844R as reported previously (Barman et al, 2008). The 16S rRNA gene was characterized by sequencing the V4 variable region. Samples were prepared using a modification of a previously described protocol (Caporaso et al, 2011) using the same primers as the Earth Microbiome Project (515F/806R). Amplification reactions were prepared in triplicate. Agilent Bioanalyzer was used for quantification of the amplicons, and the Illumina MiSeq platform was used for sequencing. Paired-end reads were processed using LotuS (http://psbweb05.psb.ugent.be/lotus/downloads.html), and further analysis was performed using Phyloseq (McMurdie & Holmes, 2012) and R scripts. From the greengenes database (v. 2013), we identify Candidatus arthromitus (ID: 376862) that, according to Thompson et al (2012), should be Candidatus savagella (Thompson et al, 2012). Greengenes reference sequence for Candidatus arthromitus (ID 376862) is 100% identical to EU791150.1 (isolated from mouse distal small intestine and reported as uncultured bacteria) and 99% to several Candidatus arthromitus sp. SFB-mouse sequences (NR_074545.1, NR_074460.1, AP012209.1, AP012202.1, D86305.1, LK932435.1, CP008713.1, EU791182.1). All these sequence entries are annotated as Candidatus arthromitus in the NCBI record. Also, the closest sequence coming from full genomes is that from a SFB-Mouse-NL, a commensal bacterium associated with maturation of gut immune function published recently and reported as Candidatus arthromitus (Bolotin et al, 2014).
Statistical analysis
For categorical data analyses, Fisher's exact tests were used, whereas continuous data were analyzed by Student's t-tests, Mann–Whitney U-tests, or ANOVA with Bonferroni post hoc tests, using PASW 18.0 (IBM). Pearson's correlation and UPGMA (unweighted pair group method using arithmetic mean) clustering were used to calculate dendrograms, using BioNumerics v5.10 (Applied Maths).
Acknowledgments
The authors greatly appreciate the technical assistance of T. Decruy, T. Lacoere, S. Maertens, J. Coudenys, N. Degryse, and E. Verheugen. This work is supported by a fund of Scientific Research—Flanders (FWO) and by a concerted research action grant of the Research Council of Ghent University. DE is also a member of a multidisciplinary research platform (MRP) of Ghent University and is supported by Interuniversity Attraction Pole (IUAP) grant Devrepair from the Belspo Agency (project P7/07). DE is also supported by from the EU's Seventh Framework Programme under EC-GA n° 305266 ‘MIAMI’.
Author contributions
JTVP, ED, and DE conceived of and designed the study. JTVP, ED, IV, MBD, FW, AD, LA, CAC, FvdL, TVdW, PSN, SAN, SR, AAK, RT, JR, VG, NC, and CFW performed the experiments. JTVP, ED, TVdW, GE, VG, NC, and ED analyzed the data. JTVP and DE wrote the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting Information
Supplementary Figures S1–S6
Supplementary Figure Legends
Review Process File
References
- Barman M, Unold D, Shifley K, Amir E, Hung K, Bos N, Salzman N. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun. 2008;76:907–915. doi: 10.1128/IAI.01432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton HA, Taylor NM, Lubbers BR, Pemberton AC. DNA extraction from low-biomass carbonate rock: an improved method with reduced contamination and the low-biomass contaminant database. J Microbiol Methods. 2006;66:21–31. doi: 10.1016/j.mimet.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Benckert J, Schmolka N, Kreschel C, Zoller MJ, Sturm A, Wiedenmann B, Wardemann H. The majority of intestinal IgA+ and IgG+ plasmablasts in the human gut are antigen-specific. J Clin Invest. 2011;121:1946–1955. doi: 10.1172/JCI44447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T, Scheu S, Pfeffer K, Bleul CC. Thymic medullary epithelial cell differentiation, thymocyte emigration, and the control of autoimmunity require lympho-epithelial cross talk via LTbetaR. J Exp Med. 2003;198:757–769. doi: 10.1084/jem.20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolotin A, de Wouters T, Schnupf P, Bouchier C, Loux V, Rhimi M, Jamet A, Dervyn R, Boudebbouze S, Blottiere HM, Sorokin A, Snel J, Cerf-Bensussan N, Gaboriau-Routhiau V, van de Guchte M, Maguin E. Genome sequence of “Candidatus Arthromitus” sp. strain SFB-mouse-NL, a commensal bacterium with a key role in postnatal maturation of gut immune functions. Genome Announc. 2014;2:e00705–e00714. doi: 10.1128/genomeA.00705-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin RK, Lo JC, Kim O, Blink SE, Christiansen PA, Peterson P, Wang Y, Ware C, Fu YX. Lymphotoxin pathway directs thymic Aire expression. Nat Immunol. 2003;4:1121–1127. doi: 10.1038/ni982. [DOI] [PubMed] [Google Scholar]
- De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, Russell JH, Karr R, Chaplin DD. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- Dear TN, Colledge WH, Carlton MB, Lavenir I, Larson T, Smith AJ, Warren AJ, Evans MJ, Sofroniew MV, Rabbitts TH. The Hox11 gene is essential for cell survival during spleen development. Development. 1995;121:2909–2915. doi: 10.1242/dev.121.9.2909. [DOI] [PubMed] [Google Scholar]
- Eberl G. From induced to programmed lymphoid tissues: the long road to preempt pathogens. Trends Immunol. 2007;28:423–428. doi: 10.1016/j.it.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Franki AS, Van Beneden K, Dewint P, Hammond KJ, Lambrecht S, Leclercq G, Kronenberg M, Deforce D, Elewaut D. A unique lymphotoxin {alpha}beta-dependent pathway regulates thymic emigration of V{alpha}14 invariant natural killer T cells. Proc Natl Acad Sci USA. 2006;103:9160–9165. doi: 10.1073/pnas.0508892103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The lymphotoxin beta receptor controls organogenesis and affinity maturation in peripheral lymphoid tissues. Immunity. 1998;9:59–70. doi: 10.1016/s1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, Eberl G, Snel J, Kelly D, Cerf-Bensussan N. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Hobeika E, Thiemann S, Storch B, Jumaa H, Nielsen PJ, Pelanda R, Reth M. Testing gene function early in the B cell lineage in mb1-cre mice. Proc Natl Acad Sci USA. 2006;103:13789–13794. doi: 10.1073/pnas.0605944103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, Le TV, Lorenz RG, Xu H, Kolls JK, Carter RH, Chaplin DD, Williams RW, Mountz JD. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- Keeton M, Ahn C, Eguchi Y, Burlingame R, Loskutoff DJ. Expression of type 1 plasminogen activator inhibitor in renal tissue in murine lupus nephritis. Kidney Int. 1995;47:148–157. doi: 10.1038/ki.1995.17. [DOI] [PubMed] [Google Scholar]
- Lecuyer E, Rakotobe S, Lengline-Garnier H, Lebreton C, Picard M, Juste C, Fritzen R, Eberl G, McCoy KD, Macpherson AJ, Reynaud CA, Cerf-Bensussan N, Gaboriau-Routhiau V. Segmented filamentous bacterium uses secondary and tertiary lymphoid tissues to induce gut IgA and specific T helper 17 cell responses. Immunity. 2014;40:608–620. doi: 10.1016/j.immuni.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- Lee PY, Kumagai Y, Li Y, Takeuchi O, Yoshida H, Weinstein J, Kellner ES, Nacionales D, Barker T, Kelly-Scumpia K, van Rooijen N, Kumar H, Kawai T, Satoh M, Akira S, Reeves WH. TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus. J Exp Med. 2008;205:2995–3006. doi: 10.1084/jem.20080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins VC, Boehm T, Bleul CC. Ltbetar signaling does not regulate Aire-dependent transcripts in medullary thymic epithelial cells. J Immunol. 2008;181:400–407. doi: 10.4049/jimmunol.181.1.400. [DOI] [PubMed] [Google Scholar]
- McMurdie PJ, Holmes S. Phyloseq: a bioconductor package for handling and analysis of high-throughput phylogenetic sequence data. Pac Symp Biocomput. 2012:235–246. [PMC free article] [PubMed] [Google Scholar]
- von Muhlen CA, Tan EM. Autoantibodies in the diagnosis of systemic rheumatic diseases. Semin Arthritis Rheum. 1995;24:323–358. doi: 10.1016/s0049-0172(95)80004-2. [DOI] [PubMed] [Google Scholar]
- Ramirez A, Page A, Gandarillas A, Zanet J, Pibre S, Vidal M, Tusell L, Genesca A, Whitaker DA, Melton DW, Jorcano JL. A keratin K5Cre transgenic line appropriate for tissue-specific or generalized Cre-mediated recombination. Genesis. 2004;39:52–57. doi: 10.1002/gene.20025. [DOI] [PubMed] [Google Scholar]
- Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin alpha/beta complex is required for the development of peripheral lymphoid organs. J Exp Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter S, Cox MJ, Turek EM, Calus ST, Cookson WO, Moffatt MF, Turner P, Parkhill J, Loman N, Walker AW. 2014. Reagent contamination can critically impact sequence-based microbiome analyses. bioRxiv 007187.
- Scheid JF, Mouquet H, Kofer J, Yurasov S, Nussenzweig MC, Wardemann H. Differential regulation of self-reactivity discriminates between IgG+ human circulating memory B cells and bone marrow plasma cells. Proc Natl Acad Sci USA. 2011;108:18044–18048. doi: 10.1073/pnas.1113395108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheu S, Alferink J, Potzel T, Barchet W, Kalinke U, Pfeffer K. Targeted disruption of LIGHT causes defects in costimulatory T cell activation and reveals cooperation with lymphotoxin beta in mesenteric lymph node genesis. J Exp Med. 2002;195:1613–1624. doi: 10.1084/jem.20020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CL, Vier R, Mikaelyan A, Wienemann T, Brune A. ‘Candidatus Arthromitus’ revised: segmented filamentous bacteria in arthropod guts are members of Lachnospiraceae. Environ Microbiol. 2012;14:1454–1465. doi: 10.1111/j.1462-2920.2012.02731.x. [DOI] [PubMed] [Google Scholar]
- Tumanov A, Kuprash D, Lagarkova M, Grivennikov S, Abe K, Shakhov A, Drutskaya L, Stewart C, Chervonsky A, Nedospasov S. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity. 2002;17:239–250. doi: 10.1016/s1074-7613(02)00397-7. [DOI] [PubMed] [Google Scholar]
- Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat Rev Immunol. 2009;9:229–234. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, Littman DR, Benoist C, Mathis D. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32:815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures S1–S6
Supplementary Figure Legends
Review Process File


