Abstract
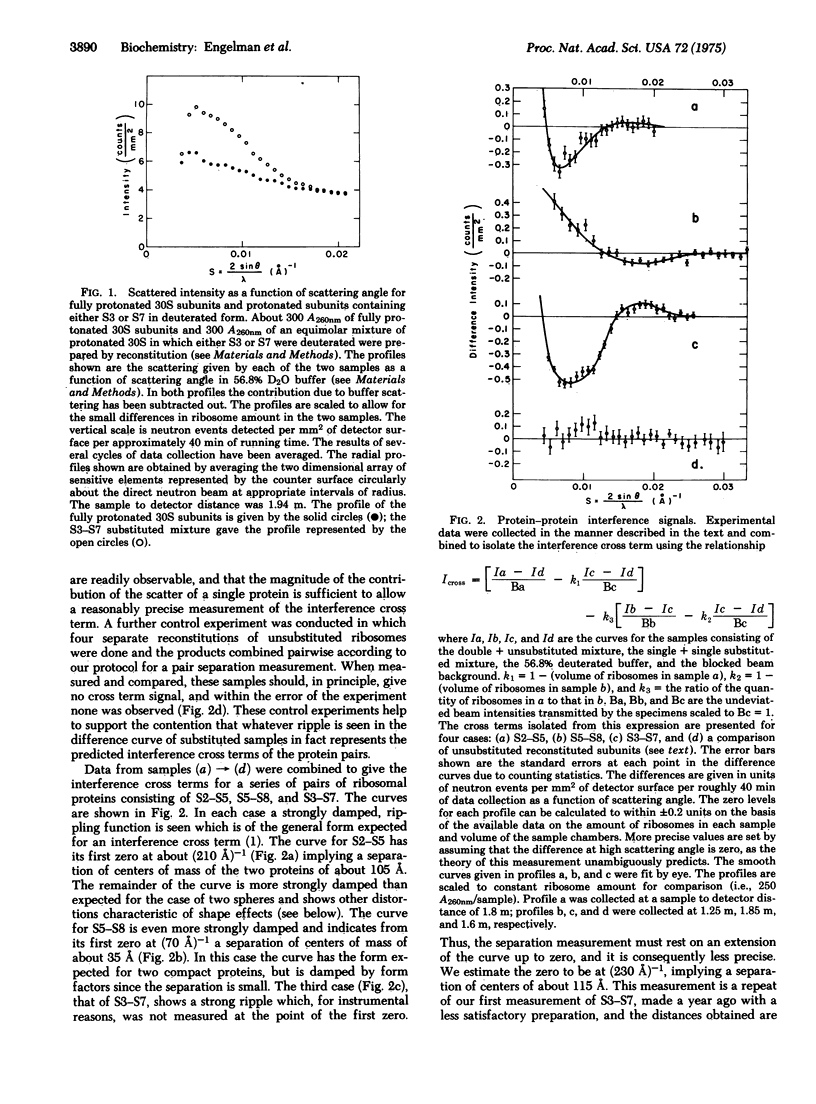
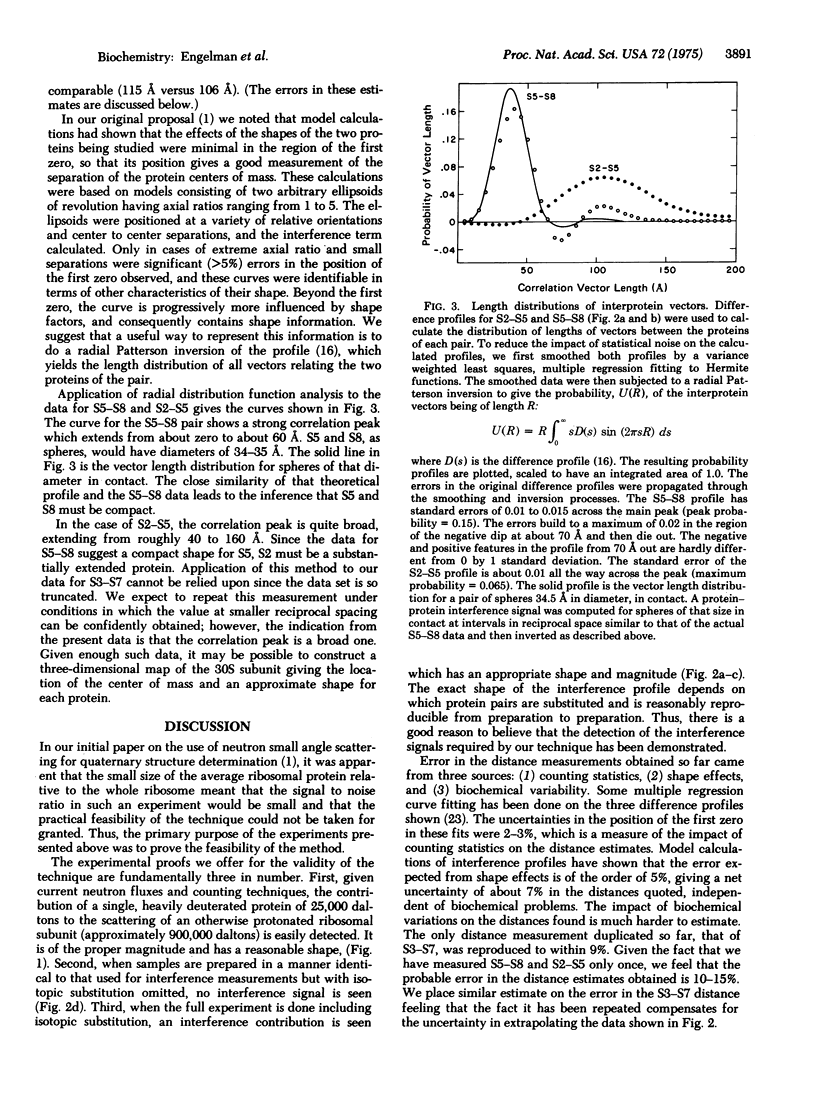
Neutron scattering measurements done on E. coli 30S ribosomal subunit specimens in which specific pairs of proteins were deuterated have enabled us to estimate the distances between the labeled proteins. The distances between centers of gravity of three protein pairs have been determined: S2-S5 (105 A), S3-S7 (115 A), and S5-S8 (35 A). A method for extracting shape information about these proteins from the neutron scattering profiles is demonstrated. The method shows that S5 and S8 are compact and S2 is extended.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Engelman D. M., Moore P. B. A new method for the determination of biological quarternary structure by neutron scattering. Proc Natl Acad Sci U S A. 1972 Aug;69(8):1997–1999. doi: 10.1073/pnas.69.8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held W. A., Ballou B., Mizushima S., Nomura M. Assembly mapping of 30 S ribosomal proteins from Escherichia coli. Further studies. J Biol Chem. 1974 May 25;249(10):3103–3111. [PubMed] [Google Scholar]
- Hoppe W. Letter: the label triangulation method and the mixed isomorphous replacement principle. J Mol Biol. 1973 Aug 15;78(3):581–585. doi: 10.1016/0022-2836(73)90480-4. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. VII. Two-dimensional polyacrylamide gel electrophoresis for fingerprinting of ribosomal proteins. Anal Biochem. 1970 Aug;36(2):401–412. doi: 10.1016/0003-2697(70)90376-3. [DOI] [PubMed] [Google Scholar]
- Lake J. A., Pendergast M., Kahan L., Nomura M. Localization of Escherichia coli ribosomal proteins S4 and S14 by electron microscopy of antibody-labeled subunits. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4688–4692. doi: 10.1073/pnas.71.12.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter L. C., Zeichhardt H., Kurland C. G. Ribosomal protein neighborhoods. I. S18 and S21 as well as S5 and S8 are neighbors. Mol Gen Genet. 1972;119(4):357–366. [PubMed] [Google Scholar]
- Moore P. B., Engelman D. M., Schoenborn B. P. A neutron scattering study of the distribution of protein and RNA in the 30 S ribosomal subunit of Escherichia coli. J Mol Biol. 1975 Jan 5;91(1):101–120. doi: 10.1016/0022-2836(75)90374-5. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Engelman D. M., Schoenborn B. P. Asymmetry in the 50S ribosomal subunit of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jan;71(1):172–176. doi: 10.1073/pnas.71.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. B. Reaction of N-ethyl maleimide with the ribosomes of Escherichia coli. J Mol Biol. 1971 Aug 28;60(1):169–184. doi: 10.1016/0022-2836(71)90456-6. [DOI] [PubMed] [Google Scholar]
- Morgan J., Brimacombe R. A preliminary three-dimensional arrangement of the proteins in the Escherichia coli 30-S ribosomal sub-particle. Eur J Biochem. 1973 Sep 3;37(3):472–480. doi: 10.1111/j.1432-1033.1973.tb03008.x. [DOI] [PubMed] [Google Scholar]
- Nomura M., Mizushima S., Ozaki M., Traub P., Lowry C. V. Structure and function of ribosomes and their molecular components. Cold Spring Harb Symp Quant Biol. 1969;34:49–61. doi: 10.1101/sqb.1969.034.01.009. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Bollen A., Kahan L., Traut R. R. Topography of ribosomal proteins of the Escherichia coli 30S subunit as studied with the reversible cross-linking reagent methyl 4-mercaptobutyrimidate. Biochemistry. 1974 May 21;13(11):2334–2340. doi: 10.1021/bi00708a015. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of E. coli ribosomes. V. Reconstitution of functionally active 30S ribosomal particles from RNA and proteins. Proc Natl Acad Sci U S A. 1968 Mar;59(3):777–784. doi: 10.1073/pnas.59.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]



