Abstract
Background and aims
Esophageal motor disorders are a heterogenous group of conditions identified by esophageal manometry that lead to esophageal dysfunction. The aim of this study was to assess the clinical utility of endoscopic ultrasound in the further evaluation of patients with esophageal motor disorders categorized using the updated Chicago Classification.
Methods
We performed a retrospective, single center study of 62 patients with esophageal motor disorders categorized according to the Chicago Classification. All patients underwent standard radial endosonography to assess for extra esophageal findings or alternative explanations for esophageal outflow obstruction. Secondary outcomes included esophageal wall thickness among the different patient subsets within the Chicago Classification
Key Results
EUS identified 9/62 (15%) clinically relevant findings that altered patient management and explained the etiology of esophageal outflow obstruction. We further identified substantial variability in esophageal wall thickness in a proportion of patients including some with a significantly thickened non-muscular layer.
Conclusions
EUS findings are clinically relevant in a significant number of patients with motor disorders and can alter clinical management. Variability in esophageal wall thickness of the muscularis propria and non-muscular layers identified by EUS may also explain the observed variability in response to standard therapies for achalasia.
Introduction
Esophageal motor disorders are a heterogeneous group of conditions causing esophageal dysfunction. Characteristic symptoms are dysphagia, heartburn or chest pain. The best characterized motor disorder is achalasia, defined by impaired lower esophageal sphincter (LES) relaxation and absent peristalsis1. Using high resolution manometry (HRM), the Chicago Classification has subdivided achalasia into three distinct phenotypes, the clinical relevance of which has thus far been verified in five studies 2–6. Also included in the Chicago Classification is another potential achalasia phenotype classified as esophagogastric junction outflow obstruction (EGJOO), defined by impaired EGJ relaxation (manifest by an elevated integrated relaxation pressure (IRP)) with some degree of preserved peristalsis7. Also novel in the Chicago Classification, spastic and hypercontractile disorders have been divided into two phenotypes: distal esophageal spasm (normal IRP with >20% premature contraction) and jackhammer esophagus (one swallow with distal contractile integral (DCI)>8000 mmHg-s-cm)7.
Although HRM clearly provides detailed physiologic information and a pressure-based topographic map of the esophagus, one must also acknowledge that it is blind to potentially important anatomic correlates of pressure anomalies. Hence, the addition of imaging modalities such as intraluminal and extraluminal ultrasound may be useful adjuncts. Early studies evaluating esophageal wall thickness in patients with achalasia using endoscopic ultrasound (EUS) reported heterogeneous findings with regard to wall and muscle thickness8–10,11. A more recent investigation utilized a novel intraluminal manometry/ultrasound probe and reported marked thickening of the musclaris propria in patients with achalasia and hypercontractile conditions compared to control patients12. That study also reported that hypertrophy of both the circular and longitudinal muscle layers were common findings and that in addition to thickness, overall muscle cross sectional area was greater in patients with achalasia and esophageal spasm compared to controls. A follow-up investigation by the same group evaluated 94 consecutive patients with dysphagia and noted increased esophageal muscle layer thickness in 24% of patients with dysphagia who did not meet conventional criteria for esophageal motor disorders. Furthermore, many patients who did not meet criteria for achalasia or esophageal spasm (ineffective motility, hypertensive LES, etc) were found to have increased muscle wall thickness suggesting this to be a common anatomic finding in patients with non-achalasia esophageal motor disorders 13.
The prevalence of esophageal wall thickening in patients categorized according to the updated Chicago Classification of esophageal motor disorders has not been investigated. Furthermore, the clinical relevance of endosonographic visualization of the esophagus to identify non-mucosal pathology associated with EGJOO is unknown. Thus, the primary aim of this study was to assess the clinical utility of EUS when evaluating esophageal motor disorders categorized by the Chicago Classification.
Methods
From January 2008 to January 2013, we identified patients who have undergone both HRM and EUS of the esophagus. We only included patients meeting Chicago Classification criteria for specific esophageal motor disorders. Patients with prior esophageal surgery, esophageal malignancy or previous pneumatic dilation were excluded. All patients underwent standard endoscopy to rule out mucosal lesions of the esophagus or gastric cardia prior to EUS. Most patients were referred for EUS for either endosonographic guided botulinum toxin injection or to evaluate for pseudoachalasia at the discretion of the referring gastroenterologist. The study was approved by the Northwestern University institutional review board.
Esophageal Manometry
A solid-state manometric assembly with 36 circumferential sensors spaced at 1 cm intervals was used (Given imaging, Los Angeles, CA). Studies were done after at least a 6-hour fast. Patients underwent transnasal placement of the manometric catheter positioned to record from the hypopharynx to the stomach. Once in a correct position, the catheter was taped to the nose. Measurements were collected in both supine and sitting positions to assess esophageal and EGJ function. The manometric protocol included at least ten 5-ml water swallows in each posture as well as a 5-minute period to assess basal sphincter pressure. Patients were categorized based on an updated Chicago Classification for esophageal motor disorders summarized in Table 1 14, 15.
Table 1.
Chicago Classification for esophageal motor disorders
| Achalasia | ||
| Type I | IRP>10 with absent esophageal peristalsis |
|
| Type II | IRP≥15 with panesophageal pressurization with ≥20% premature contractions, but no peristaltic propagation |
|
| Type III | IRP≥17 with ≥20% premature contractions, but no peristaltic propagation | |
| EGJOO | IRP≥15 with some instances of intact peristalsis or weak peristalsis with small breaks such that the criteria for achalasia are not met | |
| Jackhammer esophagus | Normal IRP with DCI >8000 | |
| EGJOO with Jackhammer | Elevated IRP with DCI>8000 | |
| Distal Esophageal Spasm | Normal IRP with ≥20% premature contractions | |
Endoscopic Ultrasound
At the time of EUS, all patients underwent standard upper endoscopy and biopsy (if needed) to rule out mechanical obstruction, eosinophilic esophagitis, or malignancy. Radial endosonography was performed using the Olympus Aloka Alpha 5 System. All cases were performed under monitored anesthesia care. Relevant landmarks were recorded and the images saved for later review. The echoendoscope was advanced into the stomach and standard anatomy was assessed. Subsequently, the echoendoscope is withdrawn and the crural diaphragm was identified. Multiple pull-throughs were then performed to identify the LES and assess for malignancy, lymph nodes, vascular compression, or infiltrative disease. Measurements (in mm) were then obtained of the total wall and muscularis propria at the LES, at the thickest segment of the esophagus. All measurements were made at least twice to avoid the potentially confounding effect of esophageal contractions. A second, blinded investigator with advanced training in EUS reviewed measurements and the average of the two observers was used.
Results
Patients
The study period encompassed 3,353 HRM patient studies of which 62 (1.9%) were included. The primary complaints were dysphagia (n=57) and chest pain (n=6). Chicago Classification diagnoses in these patients were: achalasia (24), EGJOO (24), EGJOO with jackhammer esophagus (3), jackhammer (9); DES (2), absent peristalsis (1). Patient characteristics are shown in Table 2. The cohort of achalasics subsequently underwent a variety of treatments (see Table 1) with the most common being endosonographically-directed botulinum toxin injection. The next most common treatments were pneumatic dilation and Heller myotomy. The choice of therapy was made by the referring gastroenterologist. Medical therapies included oral calcium channel blockers (ie. nifedipine) or phosphodiesterase inhibitors (ie. sildenafil). Patients with malignant causes of pseudoachalsia were treated with chemotherapy as determined by their oncologist.
Table 2.
Baseline demographics
| Total patients | 62 |
| Sex | Male:34 |
| Female:28 | |
| Symptom | Dysphagia:57 |
| Chest pain:5 | |
| IRP (mean, range) | 23.5 (4–40) |
| Manometric diagnosis | Achalasia I: 4 |
| Achalasia II:12 | |
| Achalasia III:11 | |
| EGJ outflow obstruction:20 | |
| EGJ outflow obstruction/jackhammer:3 | |
| Jackhammer:9 | |
| Spasm:3 | |
| Indication for EUS | rule out mechanical obstruction: 40 |
| Botox therapy: 22 | |
| Subsequent treatment | Pneumatic dilation: 7 |
| Botox: 27 | |
| Heller myotomy: 7 | |
| POEM: 4 | |
| Medical: 5 | |
| Other: 2 | |
| Unknown: 10 |
EGJ: Esophagogastric junction
EUS: Endoscopic ultrasound
IRP: Integrated relaxation pressure
POEM: Peroral endoscopic myotomy
Endoscopic ultrasound wall thickness
In most cases, the primary indication for EUS was to evaluate for pseudoachalasia or extrinsic compression of the EGJ. In patients with an IRP≥15 mmHg, the median maximum wall thickness was 6.1 mm with a median maximum muscularis propria thickness of 2.8 mm. In patients with IRP<15 mmHg, the median wall thickness was 7.3 mm with muscularis propria thickness of 2.9 mm. When evaluating the LES, the total wall thickness, but not the muscularis propria thickness was significantly greater in patients with IRP≥15 mmHg compared to patients with IRP<15 mmHg (p=0.01). These measurements are considerably greater than reported in historical controls8–11, 16 (mean total wall thickness 3.3 mm and muscularis propria thickness 1.0 mm). Overall, among patients with IRP≥15, those with EGJOO were found to have the greatest esophageal wall, muscularis propria, and LES thickness whereas among patients with IRP<15, those with DES had greatest wall thickness. These data are summarized in Tables 4 and 5. Representative images are shown in figure 1.
Table 4.
Wall thickness in patients with IRP≥15 mmHg
| HRM diagnosis | Esophageal body (mm) (95% CI) |
Muscularis propria (mm) |
LES (mm) | Muscularis propria at LES (mm) |
|---|---|---|---|---|
| Achalasia II | 5.9 (5.8–6.0) | 2.5 | 4.5 | 2.6 |
| Achalasia III | 6.1 (5.5–6.7) | 2.3 | 5.2 | 2.7 |
| EGJOO | 6.7 (6.6–6.8) | 3.7 | 5.6 | 4.4 |
| EGJOO/jackhammer | 6.1 (6.0–6.2) | 2.3 | 6.2 | 1.2 |
| Median | 6.1 | 2.8 | 5.1 | 3.0 |
Table 5.
Wall thickness in patients with IRP <15
| HRM diagnosis | Esophageal body (mm) (95% CI) |
MP (mm) | LES (mm) |
|---|---|---|---|
| Achalasia I | 2.1 (2.1–2.2) | 2.2 | 0.8 |
| DES | 8.1 (8.0–8.2) | 4.1 | 2.1 |
| Jackhammer | 7.4 (7.2–7.5) | 4.0 | 4.8 |
| Median | 7.3 | 2.9 | 3.9 |
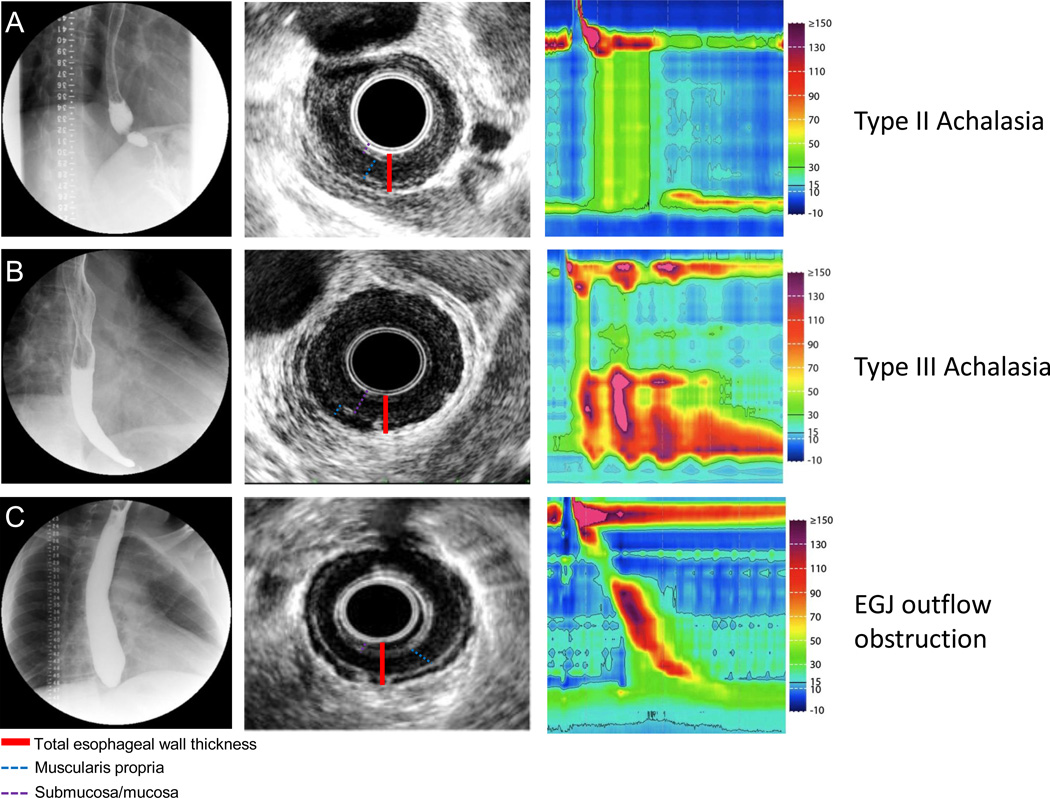
Figure 1. Representative EUS images in patients with achalasia.
Row (A) Fluoroscopy, EUS and HRM are shown in a patient with typical type II achalasia. The fluoroscopy revealed retained barium with narrowing toward the EGJ. Endosonographically, the esophageal wall was thickened with contribution from a hypoechoic muscular layer and isoechoic submucosal layer. Row (B). Type III achalasia. Note the typical early latency contraction shown on HRM. Retained contrast was noted on fluoroscopy. EUS revealed a thickened esophageal wall with the majority comprised of an isoechoic mucosal/submucosal layer. Row (C). A patient with EGJ outflow obstruction. High resolution manometry revealed intact peristalsis with elevated IRP. Fluoroscopy revealed a high column of retained barium. EUS revealed a predominately thickened hypoechoic muscular layer.
Submucosal thickness
We noted that an isoechoic, non-muscular layer contributed significantly to the total wall thickness in some patients. Historical control data from normal subjects and achalasia patients revealed that this non-muscularis propria layer was typically about 2 mm thick. In our cohort, we found considerable variability in this, with some patients having submucosal non-muscularis propria thickness of up to 8 mm. The median non-muscle layer thickness in patients with IRP≥15 mmHg was 2.9 mm (range 1-8 mm). In patients with IRP<15 mmHg, the median was 2.2 mm (range 1-7 mm).
Endosonographic Findings
EUS identified 9 (15%) clinically significant lesions detailed in Table 3 with representative images shown in Figure 2. In two cases, submucosal carcinoma not seen on standard endoscopy was identified as a cause of psuedoachalasia. There were three cases in which EUS identified a dilated and ectatic descending aorta that was compressing the distal esophagus. In one patient, EUS revealed intramucosal sarcoid and one patient was found to have a congenital distal esophageal muscular ring that was ultimately surgically resected. Another patient had a submucosal leiomyoma at the cardia resulting in extrinsic compression. Finally, in one patient with equivocal manometry, EUS guided core biopsy revealed absent ganglion and perineural inflammation consistent with achalasia. All of these findings were associated with EGJOO and likely accounted for the pattern seen on HRM. There were no pathologic findings on EUS in patients with DES or hypercontractility.
Table 3.
Characteristics of patients found to have alternative etiologies of esophageal outflow obstruction
| Manometric diagnosis | EUS Finding | Treatment |
|---|---|---|
| Achalasia I | Aortic compression | Aortic aneurysm repair |
| Achalasia II | Aortic compression | Conservative |
| Achalasia II | Intramural mass | Chemotherapy/XRT |
| Achalasia II | Leiomyoma | Surgical resection |
| EGJOO | Intramural mass | Chemotherapy/XRT |
| EGJOO | Congenital muscular ring | Surgical resection |
| EGJOO | Aortic compression | Follow-up pending |
| EGJOO | Sarcoidosis | Corticosteroids |
| EGJOO | Absent ganglion, perineural inflammation on core biopsy | Heller myotomy |
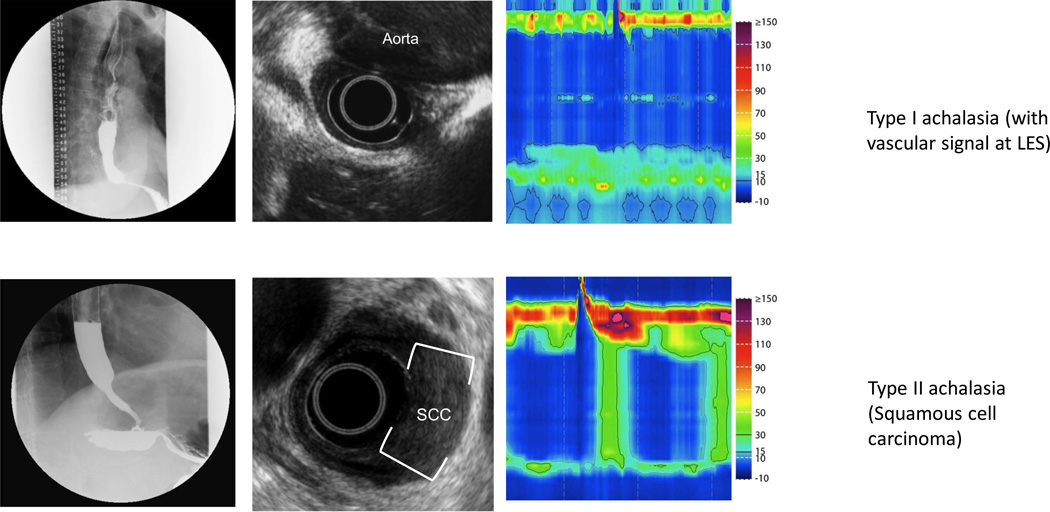
Figure 2. Pseudoachalasia identified on EUS.
Row (A) Fluoroscopy reveals a typical achalasia like pattern. High resolution manometry confirmed absent peristalsis with elevated IRP, however, careful inspection revealed a vascular artifact. EUS confirmed compression of the EGJ by the aorta with loss of the typical plane between the aorta and esophagus. CT confirmed a massively dilated thoracic aneurysm. Row (B) Fluoroscopy and manometry reveal achalasia (type II pattern). This patient had normal standard endoscopy. EUS revealed asymmetric thickening of the distal esophagus with an isoechoic lesion. FNA confirmed squamous cell carcinoma.
Discussion
This study evaluated the clinical utility of EUS as an adjunctive evaluation in patients with HRM findings of EGJOO, DES, or hypercontractility. The major finding was that EUS identified clinically significant lesions that altered patient management in 15% of these patients suggesting it to be clinically useful to further evaluate patients with these HRM findings with potential mechanical or anatomical causation. Furthermore, we found marked variability in esophageal wall thickness of these patients, with heterogeneity in both the muscularis propria and non-muscle layers. The non-muscle layer was markedly thickened in some patients with achalasia, and was most abnormal in those with type III (spastic) achalasia. The implications of these findings are not entirely clear, but may partially explain the variability in presentation and response to therapy.
Esophageal motor disorders are primarily defined by manometry. The updated Chicago Classification of motor disorders defines three clinically relevant phenotypes of achalasia, a fourth potential achalasia phenotype with impaired EGJ relaxation, but some degree of intact swallow propagation (EGJOO), DES, and hypercontractility. Using these diagnoses, we assessed the utility of evaluating esophageal wall anatomy with EUS. Our primary finding was that in 8/62 patients (13%) EUS identified an anatomically-based etiology for EGJ obstruction. This expands upon previous studies of EUS in patients with motility disorders that have reported malignancy in some patients. Barthet et al reported 2 cases of intramural carcinoma while Ziegler reported one case of carcinoma out of 16 patients evaluated. To date, ours is the largest series of patients with motility disorders categorized according to the Chicago Classification who have undergone diagnostic EUS. We report a considerably higher rate of extra-esophageal findings. In addition, we identified 3 cases of outflow obstruction secondary to compression from the aorta (dysphagia aortica). These data suggest that EUS should be considered in the diagnostic algorithm of patients with EGJOO. As our cohort was selective, we suspect that EUS will have the highest yield in those patients with a history of malignancy, accentuated vascular signal on HRM, or other clinical parameters that may result in anatomic obstruction of the EGJ.
Previous studies have reported conflicting findings with regard to esophageal wall thickness in achalasia patients8–12, 17. This variability may be attributable to differences in EUS technique, artifactual measurements based on probe placement, esophageal contraction during image capture, or tortuosity of the esophagus. Barthet et al measured total esophageal wall thickness and 4th layer (muscularis propria) thickness in patients with achalasia and controls reporting total esophageal wall thickness at the EGJ of 3.15 mm with muscularis propria thickness of 1.0 mm. These are comparable to the values noted in our study in patients with type I and II achalasia, but markedly less than seen in EGJOO, DES, and hypercontractility. Van Dam et al reported a wall thickness of roughly 4.2 mm in patients with achalasia and a tortuous esophagus. Others did not report any substantive increase in esophageal wall thickness using EUS. However, those early studies used conventional manometry and did not include patients with hypercontractility or EGJOO; in our investigation, we found marked esophageal thickening in patients with type III achalasia and EGJOO. These patients may represent a phenotype related to underlying outflow obstruction from the thickened wall at the EGJ, or perhaps, evolving achalasia prior to the loss of peristalsis.
The use of HRM has identified three distinct clinically significant phenotypes of achalasia based on the pressure topography of the esophageal body5. In addition to variability in pressure topography, recent data suggest differences in longitudinal muscle contraction and esophageal emptying among the three different subtypes further distinguishing them6. We now add to this anatomic variability between type II and type III achalasia, albeit of unknown etiology. A possible explanation for the dense non-muscular layer seen in type III achalasia could be an inflammatory process within the submucosa. Our group has noted this increased submucosal thickness in other esophageal motor disorders as well, specifically DES and Jackhammer esophagus (data not shown.) As such, submucosal thickening may be a cause or consequence of circular muscle dysfunction.
At present, there are five independent studies that reveal that the Chicago Classification of achalasia subtypes have variable clinical outcomes after therapy; patients with type II achalasia have the best outcome, and type III achalasia has the worst outcome. Although the exact reason for this is unclear, it has been suggested that longitudinal muscle contraction in type II achalasia can aid in esophageal emptying, while uncoordinated longitudinal muscle contraction results in symptoms despite adequate esophageal emptying in type III achalasia6. Based on our current findings, we further speculate that subpopulations of patients with type III achalasia and EGJOO exist in whom the predominate etiology of EGJ obstruction is not muscle hypertrophy, but rather a dense submucosa. Consistent with that speculation, previous studies using high resolution ultrasound probes immediately after pneumatic dilation noted that the submucosa was injured, not the muscularis propria, suggesting that remodeling of the submucosa, not tearing of the muscularis propria after pneumatic dilation may relieve outflow obstruction18.
There are limitations to this study. First, given its retrospective nature, referral for EUS was not standardized and, hence, subject to bias based on the gastroenterologist’s clinical suspicion of an extra-esophageal finding. This may have upwardly biased our yield of EUS in this patient population, as our patient cohort was selected and not a generalized cohort of patients with esophageal dysmotility Secondly, despite having a second endosonographer review images of the EUS to verify measurements, there can be considerable variability with regard to measurements of esophageal wall thickness. As pointed out by Van Dam et al, timing of the image during a contraction, tangential imaging and dilatation of the esophagus can alter the perceived thickness of the esophageal wall. We attempted to standardize our protocol and avoid measurements during an esophageal contraction to minimize these technical issues. Similarly, the degree to which the radial echoendoscope balloon was inflated, can lead to variability in wall measurements such that over-inflation makes the wall appear thin. However, our findings were of increased thickness, negating this potentially confounding effect. Additionally, a control group of patients without dysmotility could provide an informative comparison group. Finally, with regard to our finding of submucosal pathology in a subset of patients, not having histological correlation, we can only assume the constitution of this layer. The endosonographic appearance is isoechoic and distinct from the muscularis propria layer. We could not definitively determine whether this layer was primarily mucosal or submucosal.
In conclusion, we found that EUS was a useful adjunct when evaluating select patients with HRM findings potentially attributable to anatomic anomalies. In our series, EUS led to a change in primary management in 15% of patients. Furthermore, EUS identified variability in esophageal wall thickness, with EGJOO and DES patients having the greatest thickness. We further note that a subset of achalasia patients had markedly thickened submucosa, which may explain the variability in treatment response when comparing patients with type II and type III achalasia. While prospective studies are needed, we suggest that EUS should be considered in the diagnostic algorithm when evaluating select patients with appropriate clinical parameters (ie history of malignancy or vascular signal on HRM) with primary esophageal motor disorders, specifically those with EGJOO.
Key Message.
Esophageal motor disorders are a heterogeneous group of conditions that result in dysfunctional deglutition
Endoscopic ultrasound provides unique anatomic information regarding the esophagogastric junction
EUS provides clinically relevant information in 15% of the patients with motility disorders in this series and identified a unique finding of submucosal/mucosal thickening in a subset of them.
Acknowledgments
This work was supported by R01 DK079902 (JEP) from the Public Health Service
Footnotes
Author Contribution:
Kumar Krishnan: Study design, acquisition of data, drafting the manuscript, critical review
Chen-Yuan Lin: Acquisition of data
Rajesh Keswani: Acquisition of data
John Pandolfino: Study design, critical review, study oversight
Peter Kahrilas: Critical review, study oversight
Srinadh Komanduri: Study design, study oversight
References
- 1.Spechler SJ, Castell DO. Classification of oesophageal motility abnormalities. Gut. 2001;49:145–151. doi: 10.1136/gut.49.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roman S, Zerbib F, Queneherve L, et al. The Chicago classification for achalasia in a French multicentric cohort. Dig Liver Dis. 2012;44:976–980. doi: 10.1016/j.dld.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Pratap N, Kalapala R, Darisetty S, et al. Achalasia cardia subtyping by high-resolution manometry predicts the therapeutic outcome of pneumatic balloon dilatation. J Neurogastroenterol Motil. 2011;17:48–53. doi: 10.5056/jnm.2011.17.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohof WO, Salvador R, Annese V, et al. Outcomes of Treatment for Achalasia Depend on Manometric Subtype. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Pandolfino JE, Kwiatek MA, Nealis T, et al. Achalasia: a new clinically relevant classification by high-resolution manometry. Gastroenterology. 2008;135:1526–1533. doi: 10.1053/j.gastro.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong SJ, Bhargava V, Jiang Y, et al. A unique esophageal motor pattern that involves longitudinal muscles is responsible for emptying in achalasia esophagus. Gastroenterology. 2010;139:102–111. doi: 10.1053/j.gastro.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bredenoord AJ, Fox M, Kahrilas PJ, et al. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil. 2012;24(Suppl 1):57–65. doi: 10.1111/j.1365-2982.2011.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deviere J, Dunham F, Rickaert F, et al. Endoscopic ultrasonography in achalasia. Gastroenterology. 1989;96:1210–1213. doi: 10.1016/0016-5085(89)91644-2. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler K, Sanft C, Friedrich M, et al. Endosonographic appearance of the esophagus in achalasia. Endoscopy. 1990;22:1–4. doi: 10.1055/s-2007-1012776. [DOI] [PubMed] [Google Scholar]
- 10.Van Dam J, Falk GW, Sivak MV, Jr, et al. Endosonographic evaluation of the patient with achalasia: appearance of the esophagus using the echoendoscope. Endoscopy. 1995;27:185–190. doi: 10.1055/s-2007-1005659. [DOI] [PubMed] [Google Scholar]
- 11.Barthet M, Mambrini P, Audibert P, et al. Relationships between endosonographic appearance and clinical or manometric features in patients with achalasia. Eur J Gastroenterol Hepatol. 1998;10:559–564. doi: 10.1097/00042737-199807000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Mittal RK, Kassab G, Puckett JL, et al. Hypertrophy of the muscularis propria of the lower esophageal sphincter and the body of the esophagus in patients with primary motility disorders of the esophagus. Am J Gastroenterol. 2003;98:1705–1712. doi: 10.1111/j.1572-0241.2003.07587.x. [DOI] [PubMed] [Google Scholar]
- 13.Dogan I, Puckett JL, Padda BS, et al. Prevalence of increased esophageal muscle thickness in patients with esophageal symptoms. Am J Gastroenterol. 2007;102:137–145. doi: 10.1111/j.1572-0241.2006.01003.x. [DOI] [PubMed] [Google Scholar]
- 14.Lin Z, Kahrilas PJ, Roman S, et al. Refining the criterion for an abnormal Integrated Relaxation Pressure in esophageal pressure topography based on the pattern of esophageal contractility using a classification and regression tree model. Neurogastroenterol Motil. 2012;24:e356–e363. doi: 10.1111/j.1365-2982.2012.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roman S, Pandolfino JE, Chen J, et al. Phenotypes and clinical context of hypercontractility in high-resolution esophageal pressure topography (EPT) Am J Gastroenterol. 2012;107:37–45. doi: 10.1038/ajg.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Parashar VK, Mittal RK. Asymmetry of lower esophageal sphincter pressure: is it related to the muscle thickness or its shape? Am J Physiol. 1997;272:G1509–G1517. doi: 10.1152/ajpgi.1997.272.6.G1509. [DOI] [PubMed] [Google Scholar]
- 17.Van Dam J. Endoscopic ultrasonography in achalasia. Endoscopy. 1994;26:792–793. doi: 10.1055/s-2007-1009108. [DOI] [PubMed] [Google Scholar]
- 18.Schiano TD, Fisher RS, Parkman HP, et al. Use of high-resolution endoscopic ultrasonography to assess esophageal wall damage after pneumatic dilation and botulinum toxin injection to treat achalasia. Gastrointest Endosc. 1996;44:151–157. doi: 10.1016/s0016-5107(96)70132-3. [DOI] [PubMed] [Google Scholar]