Abstract
Although working memory impairment has been well-documented among people with schizophrenia (PSZ), the underlying mechanism of this impairment remains unknown. The present study was conducted in a large sample of PSZ and healthy control subjects (HCS) to test the hypothesis that one putative mechanism – vulnerability to distraction from task-irrelevant stimuli – (1) can account for working memory impairment among PSZ, and (2) is associated with other neurocognitive and clinical variables that are highly predictive of functional outcome in schizophrenia. Participants (127 PSZ and 124 HCS) completed a visual change detection task in which a distractor stimulus (mask) was presented on half of the trials during the delay period between sample and test array. PSZ lost proportionately more information from working memory than did HCS, but this effect was small (Cohen’s d = 0.36–0.38), and large differences between groups in working memory capacity remained when differences in distractibility were factored out. Furthermore, vulnerability to distraction was not strongly associated with any clinical or cognitive variables of interest. These results suggest that, although PSZ may be somewhat more susceptible to distraction than HCS, this impairment is unlikely to be a significant factor accounting for the robust capacity deficits observed in this population.
Keywords: Schizophrenia, Working memory, Visual cognition, Distractibility
1. Introduction
Despite a large body of literature demonstrating that people with schizophrenia (PSZ) have reduced working memory (WM) capacity (Lee and Park, 2005), the underlying mechanism of this impairment remains unknown. Recently, the conception that an unusual vulnerability to distraction by task-irrelevant stimuli decreases retention of to-be-encoded items among PSZ has garnered attention (e.g., Anticevic et al., 2012, Mayer et al., 2012). Basic science research has documented a relationship between susceptibility to distraction and variation in working memory capacity among psychiatrically healthy individuals (Fukuda and Vogel, 2011). While a “distractibility hypothesis” of capacity limitations is an appealing framework in this respect, it is also noteworthy that decreased capacity in PSZ has been reported in the absence of any obvious distracting stimuli (Erickson et al., 2014, Gold et al., 2006). Such findings suggest that delineating the specific effects of distraction may be critical for understanding its impact on WM storage in PSZ.
There are two primary forms of distraction that are described in the literature: (1) distraction can take place at the encoding stage if selective attention mechanisms fail to prevent the encoding of task-irrelevant items thereby reducing capacity available for relevant items, or (2) distraction can occur following encoding by disrupting maintenance of the WM representation. PSZ appear to exhibit different levels of susceptibility to these two forms of distraction. For instance, we have previously reported that PSZ exhibit generally intact ability to suppress encoding of salient distractors in a spatial WM paradigm, despite overall reductions in capacity (Erickson et al., 2014). Similarly, Smith et al. (2011) found that PSZ were able to use color cues to guide target words into WM storage and exclude non-target words—again, despite overall reductions in capacity. These recent studies are consistent with earlier work from Gold et al. (2006) demonstrating that PSZ are able to select task-relevant items for WM storage while inhibiting the encoding of task-irrelevant items. One exception may be a failure to filter out extremely salient distractors (especially those that strongly activate the magnocellular pathway) during the encoding of low-salience target items (Hahn et al., 2010, Leonard et al., 2014). Taken together, these results suggest that failures of selective attention during encoding cannot explain the ubiquitous reduction in WM storage capacity in PSZ.
In contrast to findings of generally intact resilience to distractors at the encoding stage, PSZ appear to be vulnerable to distraction by stimuli that occur after the offset of the to-be-encoded stimuli, during either the consolidation phase or the maintenance phase. For instance, Fuller and colleagues (Fuller et al., 2005, Fuller et al., 2009) reported evidence for slowed consolidation in PSZ by presenting masks at various latencies during the delay interval between encoding array and test. It was found that retention of items was impacted by the masks to a greater degree in PSZ compared to healthy control subjects (HCS), even when PSZ were given as long as 800 ms to consolidate the visual array (Fuller et al., 2009). Similarly, Anticevic et al. (2012) found that distractors presented during the maintenance period of a working memory task significantly impaired accuracy in PSZ relative to HCS, and that this vulnerability to distraction was associated with abnormal patterns of connectivity between the dorsolateral prefrontal cortex (DLPFC) and other cortical and subcortical regions.
A critical issue that remains unresolved is the degree to which vulnerability to distraction during maintenance can account for the WM impairment in PSZ. A second, but related issue concerns the extent to which disruption of working memory processes by distraction can account for broader cognitive disturbances. That is, if distraction is the primary mechanism by which working memory fails in PSZ, can it also explain impairment in other forms of cognition or functional outcome? Indeed, the notion that working memory impairment is central to many neurocognitive deficits motivates much of the present work on capacity limitations in PSZ (e.g., Green et al., 2000, Johnson et al., 2013, Lee and Park, 2005).
The present study was conducted to serve two primary purposes. First, we aimed to determine whether enhanced vulnerability to distraction can account for decreased storage capacity in PSZ. Second, we tested the hypothesis that vulnerability to distraction is associated with clinical and cognitive variables that are highly associated with functional outcome in schizophrenia. Two hundred fifty-one participants (127 PSZ and 124 HCS) completed a change detection WM task in which on half of the trials a mask was presented during the retention interval between the cue and test array. To determine the impact of distraction on working memory storage capacity, measured here as K (Cowan, 2001), distractibility was quantified in two ways: first, as the difference in number of items stored between mask- and no-mask trial types (KDIFF), and second as the proportional change in number of items stored between mask- and no-mask trial types (KRATIO). The former index indicates the absolute number of items lost to distractibility, while the latter index quantifies the proportion of WM capacity that is impacted by task-irrelevant stimuli. If PSZ are more vulnerable to distraction during the consolidation/maintenance phase of WM, PSZ should exhibit larger KDIFF and KRATIO compared to HCS. Furthermore, if vulnerability to distraction can account for reduced WM capacity in PSZ, group differences in capacity should be eliminated when distractibility is taken into account as a covariate. In addition to providing sufficient power to detect between-group differences in susceptibility to distraction as it relates to WM storage, the present study design and large sample permit evaluation of the relationship between distractibility and predictors of functional outcome.
2. Methods
2.1. Participants
One hundred twenty-seven individuals with a DSM-IV diagnosis of schizophrenia or schizoaffective disorder (83 male) and 124 psychiatrically healthy individuals (74 male) participated in the present experiment (see Table 1 for demographic information). The groups were statistically similar on gender (χ2 =0.86; p = 0.36), age (t = 0.37; p = 0.71), race (χ2 = 3.85; p =0.57), and parental education, a proxy measure of socioeconomic status (t = 1.42; p = 0.16). However, PSZ had significantly fewer years of education than did HCS (t = 8.31; p < 0.001), and had a significantly lower IQ (t = 6.79; p < 0.001). Diagnosis was confirmed using the Structured Clinical Interview for the DSM-IV (SCID-I/P; First et al., 2002), as well as a review of medical records and informant reports when appropriate. All PSZ were reported to be clinically stable by their mental health providers and had not received any changes in medication dosage for at least four weeks prior to testing. Haloperidol dose equivalents were calculated according to the formula recommended by Andreasen et al. (2010). All HCS were free from any current Axis I diagnosis or Schizotypal Personality Disorder (SPD), were not taking any psychiatric medications, and all denied a family history of psychosis. Participants in both groups were between the ages of 18 and 55, and reported no history of neurological injury. PSZ were recruited from the Maryland Psychiatric Research Center and other community clinics, whereas HCS were recruited by way of random digit dialing, web advertising, and word of mouth. All recruiting methods and experimental procedures were approved by the University of Maryland School of Medicine Institutional Review Board.
Table 1.
Demographic information from full sample (mean ± SD).
| Healthy Controls | Schizophrenia Patients | Effect Size (Cohen’s d) | |
|---|---|---|---|
| Gender (M: F) | 74: 50 | 83: 44 | – |
| Age | 38.14 ± 10.45 | 38.64 ± 10.89 | 0.05 |
| Race (AA: C: Other) | 49: 68: 7 | 47: 70: 10 | – |
| Education (years) | 14.92 ± 2.00 | 12.65 ± 2.32* | 1.05 |
| Parental Education | 13.90 ± 2.52 | 13.40 ± 2.96 | 0.18 |
| Haloperidol dose equivalent (mg/day) | – | 11.75 ± 7.97 | – |
| BPRS Total Score | – | 36.03 ± 7.02 | – |
| BPRS Positive Symptoms (mean) | – | 2.29 ± 1.08 | – |
| BPRS Negative Symptoms (mean) | – | 1.80 ± 0.67 | – |
| BPRS Disorganized Symptoms (mean) | – | 1.35 ± 0.36 | – |
| SANS Total Score | – | 25.88 ± 11.56 | – |
| WASI | 112.52 ± 21.02 | 93.36 ± 23.58* | 0.86 |
| WRAT-4 | 107.15 ± 14.04 | 95.21 ± 13.46* | 0.87 |
| WTAR | 109.90 ± 13.02 | 98.31 ± 15.57* | 0.81 |
| MATRICS Total Score | 52.24 ± 10.50 | 30.55 ± 13.15* | 1.82 |
| MATRICS Processing Speed | 53.61 ± 10.40 | 34.48 ± 11.53* | 1.74 |
| MATRICS Attention/Vigilance | 51.19 ± 8.79 | 38.90 ± 10.55* | 1.27 |
| MATRICS Working Memory | 53.08 ± 9.51 | 38.30 ± 10.53* | 1.47 |
| MATRICS Verbal Learning | 49.08 ± 15.24 | 37.43 ± 12.86* | 0.83 |
| MATRICS Visual Learning | 44.98 ± 14.78 | 33.30 ± 15.08* | 0.78 |
| MATRICS Problem Solving | 51.90 ± 10.17 | 41.57 ± 9.89* | 1.03 |
| MATRICS Social Cognition | 52.42 ± 10.50 | 30.55 ± 13.15* | 1.84 |
| Level of Functioning Total Score | – | 19.83 ± 7.19 | – |
| Level of Functioning: Social | – | 4.60 ± 2.45 | – |
| Level of Functioning: Occupational | – | 2.80 ± 2.63 | – |
MCCB = MATRICS Consensus Cognitive Battery; WASI = Wechsler Abbreviated Scale of Intelligence; WTAR = Wechsler Test of Adult Reading; WRAT = Wide Range Achievement Test; BPRS = Brief Psychiatric Rating Scale.
p < 0.001.
2.2. Neuropsychological & symptom measures
Several standardized neuropsychological measures were administered to examine current and premorbid cognitive functioning in PSZ and HCS: (1) the MATRICS Consensus Cognitive Battery (MCCB; Nuechterlein et al., 2008); (2) the Wide Range Achievement Test 4 (WRAT-4; Wilkinson and Robertson, 2006); (3) the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001); and (4) the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). Finally, the Brief Psychiatric Rating Scale (BPRS; Overall and Gorham, 1962) and Scale for the Assessment of Negative Symptoms (SANS; Andreasen, 1989) were used to measure symptom severity, and the Level of Functioning Scale (LOFS; Hawk et al., 1975) was administered to assess social and occupational activities.
2.3. Stimuli and procedure
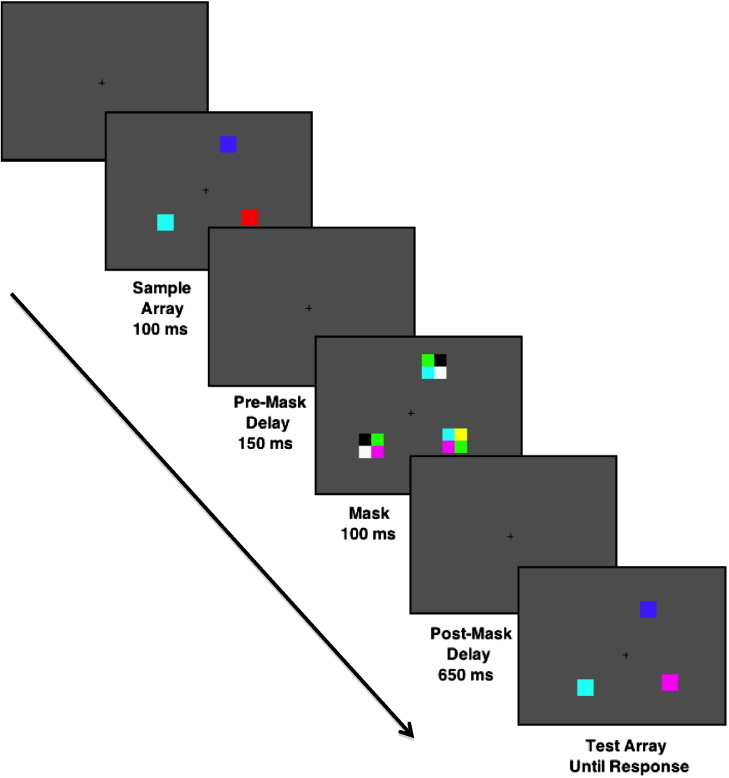
Stimuli were presented on a cathode ray tube monitor with a gray background (x = 0.36, y = 0.33, 2.78 cd/m2) and a continuously visible central fixation cross at a viewing distance of 75 cm. Each trial began by presenting a sample array that consisted of three colored squares, each subtending 0.66° × 0.66° of visual angle, pseudorandomly distributed around an invisible circle with a radius of 4.1° at a minimum distance of 2.12° separation (see Fig. 1). The colors of the squares in the sample array were selected randomly and without replacement from a list of highly discriminable colors. The sample array appeared for 100 ms. After a 900-ms delay interval, a test array was presented. This array was identical to the sample array on 50% of trials (no-change trials), and on the other 50% one of the items changed to a color that was not present in the preceding sample array (change trials). Participants were asked to indicate whether the test array was the same or different from the sample array by making an unspeeded button-press response, and the test array was visible until the subject responded. The next trial began after a 2000-ms intertrial interval. Each subject received 120 trials.
Fig. 1.
Task sequence for a Mask trial type.
2.3.1. Mask trial type
On 50% of trials, a mask array was presented 150 ms after the offset of the sample array. This inter-stimulus interval was selected on the basis of previous reports indicating that mask onset 100–200 ms after the offset of the sample array yields the most robust between-group differences (Fuller et al., 2005, Fuller et al., 2009). The mask array contained three individual mask objects, one at the location of each of the sample stimuli. Each mask object consisted of four colored squares, each 0.66° × 0.66° visual angle, arranged into a larger square that was centered at the sample stimulus location. The colors that made up the mask for each item were randomly selected from the list of possible sample array colors without replacement, but with the caveat that no mask square color matched the color of the corresponding sample item. The mask appeared for 100 ms, followed by a 650 ms delay, for a total delay period of 900 ms between sample and test array.
2.3.2. No-mask trial type
No mask was presented on the other 50% of trials. On these trials, there was simply a 900-ms delay between the offset of the sample array and the onset of the test array. Mask and no-mask trials were unpredictably intermixed. The number of items stored in working memory, or K, was estimated for each trial type using the formula K = (hit rate −false alarm rate) * set size (Cowan, 2001).
3. Results
The number of items stored in working memory (K) for each group and trial type is presented in Fig. 2a. HCS performed more accurately on both mask and no-mask trials, indicated by a significant main effect of group in a two-way ANOVA (F1,249 = 68.34; p < 0.001) with condition (mask vs. no-mask trials) as a within-subject factor. This group effect was quite large (Cohen’s d = 1.05), and consistent with available literature indicating that the effect size of working memory impairment in PSZ tends to be just over 1.0 (Lee and Park, 2005). The ANOVA also revealed a significant main effect of condition (F1,249 = 510.32; p < 0.001), indicating that the presence of a mask had a deleterious effect on change detection for both groups. The size of the masking effect, collapsed across groups, was very large (Cohen’s d = 2.86).
Fig. 2.
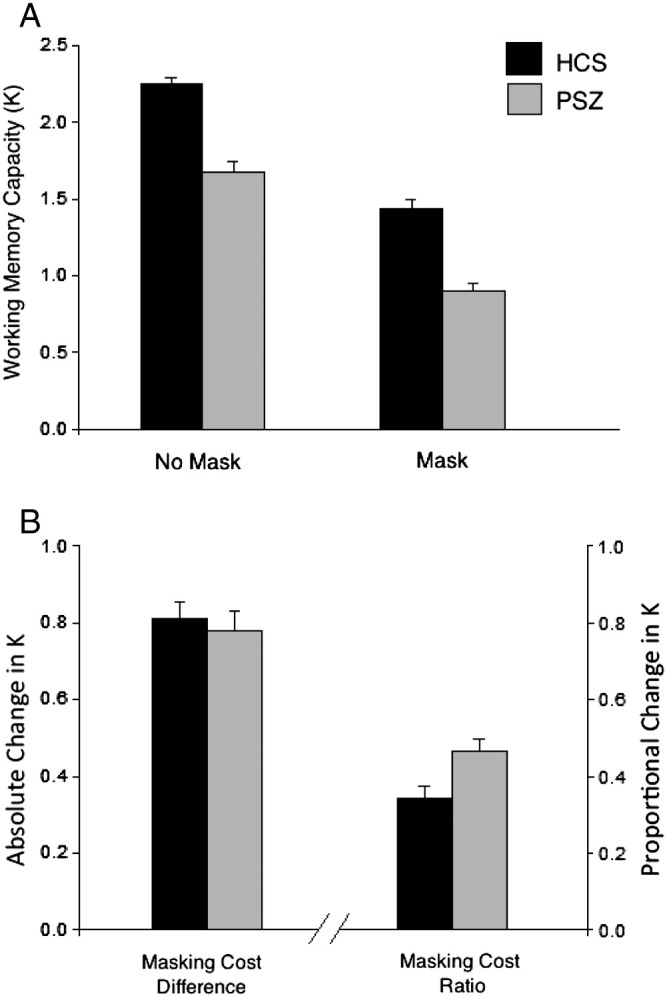
Working memory capacity estimate K (± SEM) and Masking Cost from the full sample. Panel A: Number of items stored (K) for each condition. Panel B: Masking Cost (KDIFF and KRATIO).
Despite these large main effects of group and condition, there was no significant group × condition interaction (F1,249 = 0.18; p = 0.67). That is, the mask did not differentially impact performance of PSZ as compared with HCS. To explore this null effect further, masking cost was calculated in two ways: first as a difference score (No-Mask K − Mask K, or KDIFF), and second as a proportion of overall K (difference score divided by No-Mask K, or KRATIO; see Fig. 2b). We found that KDIFF was statistically indistinguishable between groups (p = 0.67; Cohen’s d = 0.05), while KRATIO was significantly larger in PSZ than in HCS (p < 0.01; Cohen’s d = 0.37). That is, a greater proportion of patients’ no-mask K was lost due to disruption by the mask. To test the hypothesis that enhanced distractibility as measured by KRATIO accounts for overall capacity limitations in PSZ, KRATIO was entered as a covariate into an ANCOVA with K as the dependent variable. Between-group differences in K remained robust even when accounting for variance in susceptibility to distraction (F1,246 = 55.92; p < 0.001; Cohen’s d = 0.95). This provides strong positive evidence that distractibility is not the sole determinant of reduced working memory capacity in PSZ.
The different patterns for KDIFF and KRATIO can be easily understood when considering the fact that there are substantial between-group differences in baseline, or no-mask performance. Identical decreases in K between no-mask and mask conditions will be proportionally larger for individuals with low baseline K. We therefore conducted a secondary analysis to test the hypothesis that group differences in baseline performance masked true group differences in KDIFF. A subsample of HCS and PSZ pairs was matched one-to-one on no-mask K (see Table 2 for subsample demographic information). The K values for the matched sample (N = 74 per group) are presented in Fig. 3a. A two-way ANOVA revealed a main effect of condition (F1,146 = 398.11; p < 0.001), indicating that performance in both groups was impaired by the presence of the mask. Importantly, the matched sample exhibited a significant group × condition interaction (F1,146 = 5.20; p < 0.05), indicating that the cost in performance in the Mask condition was greater for PSZ than for HCS. This interaction effect is further illustrated by a significantly larger KDIFF (t = 2.28; p < 0.05; Cohen’s d = 0.38) and KRATIO (t =2.20; p < 0.05; Cohen’s d = 0.36) in PSZ compared to HCS (see Fig. 3b).
Table 2.
Demographic information from matched sub-sample (mean ± SD).
| Healthy Controls | Schizophrenia Patients | Effect Size (Cohen’s d) | |
|---|---|---|---|
| Gender (M: F) | 41: 33 | 45: 29 | – |
| Age | 39.70 ± 9.92 | 36.88 ± 11.36 | 0.26 |
| Race (AA: C: Other) | 32: 39: 3 | 29: 41: 4 | – |
| Education (years) | 14.88 ± 2.17 | 12.55 ± 2.50** | 1.00 |
| Parental Education | 14.07 ± 2.73 | 13.41 ± 2.76 | 0.24 |
| Haloperidol dose equivalent (mg/day) | – | 10.50 ± 6.93 | – |
| BPRS Total Score | – | 35.42 ± 7.11 | – |
| BPRS Positive Symptoms (mean) | – | 2.24 ± 1.09 | – |
| BPRS Negative Symptoms (mean) | – | 1.70 ± 0.64 | – |
| BPRS Disorganized Symptoms (mean) | – | 1.31 ± 0.35 | – |
| SANS Total Score | – | 24.21 ± 11.01 | – |
| WASI | 109.84 ± 25.53 | 94.99 ± 26.81** | 0.57 |
| WRAT-4 | 105.42 ± 14.82 | 97.33 ± 14.52* | 0.55 |
| WTAR | 108.32 ± 14.17 | 100.13 ± 16.27* | 0.54 |
| MATRICS Total Score | 50.73 ± 10.70 | 34.45 ± 13.12** | 1.36 |
| MATRICS Processing Speed | 51.90 ± 10.76 | 36.71 ± 12.02** | 1.33 |
| MATRICS Attention/Vigilance | 49.96 ± 8.82 | 42.01 ± 8.94** | 0.90 |
| MATRICS Working Memory | 52.84 ± 8.69 | 41.89 ± 9.98** | 1.17 |
| MATRICS Verbal Learning | 47.74 ± 15.19 | 39.20 ± 14.67* | 0.57 |
| MATRICS Visual Learning | 43.35 ± 14.92 | 34.28 ± 15.45** | 0.60 |
| MATRICS Problem Solving | 50.79 ± 10.81 | 43.44 ± 10.25** | 0.70 |
| MATRICS Social Cognition | 52.63 ± 8.86 | 41.66 ± 12.91** | 0.99 |
| Level of Functioning Total Score | – | 10.12 ± 11.30 | – |
| Level of Functioning: Social | – | 4.75 ± 2.51 | – |
| Level of Functioning: Occupational | – | 2.86 ± 2.69 | – |
MCCB = MATRICS Consensus Cognitive Battery; WASI = Wechsler Abbreviated Scale of Intelligence; WTAR = Wechsler Test of Adult Reading; WRAT = Wide Range Achievement Test; BPRS = Brief Psychiatric Rating Scale.
p < 0.01.
p < 0.001.
Fig. 3.
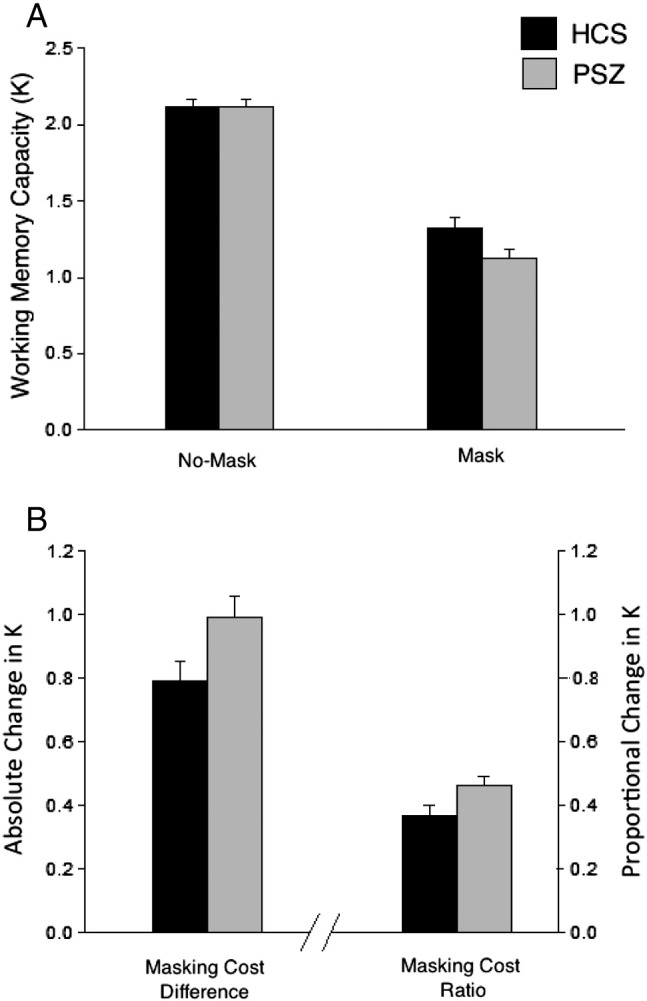
Working memory capacity estimate K (± SEM) from the sub-sample of K-matched participants. Panel A: Number of items stored (K) for each condition. Panel B: Masking Cost (KDIFF and KRATIO).
Finally, we examined the association between masking cost and clinical and cognitive measures to test the hypothesis that greater susceptibility to distraction is associated with poorer cognition and greater symptom severity. The correlations for the KDIFF and KRATIO measures are presented in Supplementary Table 1, and correlations with the original no-mask K values are found in Supplementary Table 2. Owing to the large number of correlations that were examined in the present study, the Benjamini–Hochberg correction for false discovery rate was used (Benjamini and Hochberg, 1995). Broadly, neither index of masking cost was significantly associated with any of the clinical or cognitive variables for HCS or PSZ; the only exception was a modest negative association between the processing speed subtest from the MATRICS battery and KDIFF among HCS. Overall, however, the magnitude of distractibility was not strongly associated with variables related to functional outcome. By contrast, and consistent with correlations reported by Johnson et al. (2013), overall working memory capacity estimated from no-mask K was significantly associated with several measures of neurocognition in both groups (see Supplementary Table 2).
4. Discussion
The purpose of the present study was twofold: first, we sought to determine whether enhanced vulnerability to distraction during the consolidation/maintenance phase could account for low storage capacity in a large sample of PSZ and HCS. Second, we sought to determine whether susceptibility to disruption of working memory during the consolidation/maintenance phase was related to important clinical and cognitive outcome variables in a different way than working memory capacity limitations observed in the absence of distraction. Here we report evidence that PSZ do indeed exhibit significantly increased vulnerability to distraction, consistent with Fuller et al. Fuller et al. (2005), (2009) and Anticevic et al. (2012). Although this group difference was not detectable with a simple difference score using the full sample, the ratio cost was significantly larger in PSZ. That is, a greater proportion of baseline K was lost as a result of the masks. Converging evidence was provided by an analysis of subgroups matched on No-Mask K, in which PSZ exhibited moderately increased vulnerability to distraction relative to HCS (Cohen’s d = 0.36–0.38).
The primary question that motivates this and other studies (e.g., Mayer et al., 2012) is whether increased vulnerability to distraction can account for the broader cognitive deficits in PSZ. That is, does vulnerability to distraction represent a critical underlying abnormality that has broad ramifications on downstream cognitive functions? To this end, we highlight two important observations that emerge from the present data: first, PSZ do appear to exhibit greater susceptibility to WM disruption by distracting stimuli presented during the consolidation/maintenance interval; second, PSZ exhibit deficits in WM storage even when controlling for the impact of distractibility. That is, group differences in working memory storage remained robust even when the impact of distraction was added as a covariate (Cohen’s d = 0.95). Therefore, it appears that vulnerability to distraction in PSZ reported here and elsewhere (Anticevic et al., 2012, Fuller et al., 2005, Fuller et al., 2009) occurs in the context of another, primary impairment that drives poor WM encoding and retention even in the absence of external distraction.
The hypothesis that vulnerability to distraction is not a primary determinant of WM capacity limitations is further supported by nonsignificant correlations between distractibility and overall cognitive performance. That is, vulnerability to distraction did not appear to capture much variance in broader measures of cognition. While the available data suggest that distractibility may be generally unrelated to clinical and cognitive function measures, the lack of association between these variables should be interpreted with caution. Fluctuations in symptomatology and capacity for independent functioning are characteristic of the disorder, and may consequently have a deleterious impact on estimates of relationship strength between these phenomena. Furthermore, the LOFS is only one broad measure of social and occupational outcome; future studies may consider the use of more behaviorally specific assessments of functional capacity such as the UCSD Performance-Based Skills Assessment (UPSA; Patterson et al., 2001).
In sum, it appears that PSZ do exhibit greater vulnerability to distraction during WM consolidation/maintenance than do HCS; however, the effect of distractibility in PSZ is small compared to overall reductions in K, and exhibits only weak correlations with clinical and cognitive variables of interest. Moving forward, it will be important to identify neural and cognitive models of WM encoding and maintenance that can account for low capacity in PSZ under no-distraction conditions (Leonard et al., 2013). It is possible that this yet undefined primary impairment and a secondary vulnerability to distraction interact to yield an additive impact on general cognitive ability. Alternately, it is possible that PSZ exhibit larger effects of distractibility under real-world conditions, in which they are not given explicit instruction regarding which stimuli constitute distractors and which constitute targets. Future studies will explore these intriguing hypotheses.
Role of funding source
This work was supported by the National Institute of Mental Health (R01 MH065034 and R01 MH080066 to J.G.).
Contributors
Dr. Molly Erickson analyzed the data and wrote the manuscript. Drs. Steven Luck and James Gold were instrumental in the design of the experiment. Drs. Britta Hahn and Carly Leonard assisted with interpretation of the data and the writing of the manuscript. Benjamin Robinson programmed the task, collected the data, and participated in data analysis.
Conflict of interest
None of the authors have any conflicts of interest to report.
Footnotes
Available online 6 October 2014
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.scog.2014.09.001.
Supplementary data
Supplementary material
References
- Andreasen N.C. Scale for the Assessment of Negative Symptoms (SANS) Br J Psychiatry. 1989;155:53–58. [PubMed] [Google Scholar]
- Andreasen N.C., Pressler M., Nopoulos P., Miller D., Ho B.C. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67(3):255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A., Repovs G., Krystal J.H., Barch D.M. A broken filter: prefrontal functional connectivity abnormalities in schizophrenia during working memory interference. Schizophr Res. 2012;141(1):8–14. doi: 10.1016/j.schres.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate — a practical and powerful approach to multiple testing. J R Stat Soc Series B-Methodological. 1995;57(1):289–300. [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2001;24(1):87-+. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Erickson M.A., Hahn B., Leonard C.J., Robinson B.M., Gray B., Luck S.J., Gold J. Impaired working memory capacity is not caused by failures of selective attention in schizophrenia. Schizophr Bull. 2014 doi: 10.1093/schbul/sbu101. [Epub ahead of print. http://dx.doi.org/10.1093/schbul/sbu101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Biometrics Research, New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) [Google Scholar]
- Fukuda K., Vogel E.K. Individual differences in recovery time from attentional capture. Psychol Sci. 2011;22(3):361–368. doi: 10.1177/0956797611398493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R.L., Luck S.J., McMahon R.P., Gold J.M. Working memory consolidation is abnormally slow in schizophrenia. J Abnorm Psychol. 2005;114(2):279–290. doi: 10.1037/0021-843X.114.2.279. [DOI] [PubMed] [Google Scholar]
- Fuller R.L., Luck S.J., Braun E.L., Robinson B.M., McMahon R.P., Gold J.M. Impaired visual working memory consolidation in schizophrenia. Neuropsychology. 2009;23(1):71–80. doi: 10.1037/a0013854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J.M., Fuller R.L., Robinson B.M., McMahon R.P., Braun E.L., Luck S.J. Intact attentional control of working memory encoding in schizophrenia. J Abnorm Psychol. 2006;115(4):658–673. doi: 10.1037/0021-843X.115.4.658. [DOI] [PubMed] [Google Scholar]
- Green M.F., Kern R.S., Braff D.L., Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the "right stuff"? Schizophr Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Hahn B., Robinson B.M., Kaiser S.T., Harvey A.N., Beck V.M., Leonard C.J. Failure of schizophrenia patients to overcome salient distractors during working memory encoding. Biol Psychiatry. 2010;68(7):603–609. doi: 10.1016/j.biopsych.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawk A.B., Carpenter W.T., Strauss J.S. Diagnostic criteria and five-year outcome in schizophrenia: a report from the international pilot study of schizophrenia. Arch Gen Psychiatry. 1975;32(3):343–347. doi: 10.1001/archpsyc.1975.01760210077005. [DOI] [PubMed] [Google Scholar]
- Johnson M.K., McMahon R.P., Robinson B.M., Harvey A.N., Hahn B., Leonard C.J. The relationship between working memory capacity and broad measures of cognitive ability in healthy adults and people with schizophrenia. Neuropsychology. 2013;27:220–229. doi: 10.1037/a0032060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114(4):599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Leonard C.J., Kaiser S.T., Robinson B.M., Kappenman E.S., Hahn B., Gold J.M. Toward the neural mechanisms of reduced working memory capacity in schizophrenia. Cereb Cortex. 2013;23(7):1582–1592. doi: 10.1093/cercor/bhs148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard C.J., Robinson B.M., Hahn B., Gold J.M., Luck S.J. Enhanced distraction by magnocellular salience signals in schizophrenia. Neuropsychologia. 2014;56:359–366. doi: 10.1016/j.neuropsychologia.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer J.S., Fukuda K., Vogel E.K., Park S. Impaired contingent attentional capture predicts reduced working memory capacity in schizophrenia. PLoS One. 2012;7(11) doi: 10.1371/journal.pone.0048586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein K.H., Green M.F., Kern R.S., Baade L.E., Barch D.M., Cohen J.D. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatr. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Overall J.E., Gorham D.R. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Patterson T.L., Goldman S., McKibbin C.L., Hughs T., Jeste D.V. UCSD Performance-Based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophr Bull. 2001;27(2):235–245. doi: 10.1093/oxfordjournals.schbul.a006870. [DOI] [PubMed] [Google Scholar]
- Smith E.E., Eich T.S., Cebenoyan D., Malapani C. Intact and impaired cognitive-control processes in schizophrenia. Schizophr Res. 2011;126(1–3):132–137. doi: 10.1016/j.schres.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1999. Abbreviated Scale of Intelligence (WASI) [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 2001. Wechsler Test of Adult Reading. [Google Scholar]
- Wilkinson G.S., Robertson G.J. Psychological Assessment Resources; Lutz, FL: 2006. Wide Range Achievement Test 4 Professional Manual. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material