SUMMARY
G-protein coupled receptor 124 (GPR124) is an orphan receptor in the adhesion family of GPCRs and previous global or endothelial-specific disruption of Gpr124 in mice led to defective CNS angiogenesis and blood brain barriergenesis. Similar developmental defects were observed following dual deletion of Wnt7a/Wnt7b or deletion of β-catenin in endothelial cells, suggesting a possible relationship between GPR124 and canonical WNT signaling. Here, we show using in vitro reporter assays, mutation analysis and genetic interaction studies in vivo, that GPR124 functions as a WNT7A/WNT7B specific co-stimulator of β-catenin signaling in brain endothelium. WNT7-stimulated β-catenin signaling was dependent upon GPR124’s intracellular PDZ binding motif and a set of leucine rich repeats in its extracellular domain. This study reveals a vital role for GPR124 in potentiation of WNT7 induced canonical β-catenin signaling with important implications for understanding and manipulating CNS-specific angiogenesis and blood brain barriergenesis.
INTRODUCTION
In vertebrates, the endothelium lining the vasculature of each organ is highly specialized in order to meet the particular physical and metabolic needs of each tissue. CNS endothelium is no exception, displaying a highly diverse and unique set of cell surface proteins including a large number of CNS-specific transporters that contribute to blood-brain barrier (BBB) function (Seaman et al., 2007). Mechanisms regulating developmental CNS angiogenesis are distinct from non-CNS angiogenesis and are tightly coupled to BBB properties that develop concomitantly as vessels invade the neural tube (Daneman et al., 2009; Stenman et al., 2008). Understanding mechanisms that regulate CNS specific angiogenesis and barriergenesis has important ramifications for developing the most selective therapies to combat brain tumors and other cerebrovascular and neurodegenerative disorders.
Angiogenesis in the CNS begins when endothelial cells from the perineural vascular plexus (PNVP) invade the neural parenchyma. WNT7 plays a critical role in this process because Wnt7a/Wnt7b double knockout mice display defective angiogenesis in the forebrain and ventral spinal cord, and fail to establish an effective BBB (Daneman et al., 2009; Stenman et al., 2008). In the afflicted regions, the few vessels that sprout into the neural tissues form unusual tangles called glomeruloids, so named because of their superficial resemblance to kidney glomeruli (Sundberg et al., 2001). Endothelial disruption of β-catenin (Ctnnb1) or simultaneous deletion of the Wnt co-receptors Lrp5 and Lrp6 in endothelium also results in defects in CNS vascular morphogenesis and barriergenesis (Daneman et al., 2009; Stenman et al., 2008; Zhou et al., 2014). Together, these studies suggest canonical β-catenin signaling plays a major role in CNS angiogenesis with WNT7A/WNT7B functioning non-redundantly in specific anatomical locations.
G-protein coupled receptor 124 (GPR124), also known as Tumor Endothelial Marker 5 (TEM5), was originally identified as a gene whose transcripts were elevated in the endothelium derived from tumor versus normal tissues (Carson-Walter et al., 2001; St Croix et al., 2000). GPR124 is an orphan member of the adhesion family of GPCRs, and its involvement in cell signaling has not heretofore been shown. Disruption of Gpr124 in mice led to defects in the developing CNS that share striking similarity to Wnt7a/Wnt7b double knockout mice - that is, blunted angiogenesis with unusual glomeruloid structures formed specifically in the forebrain and ventral spinal cord and a lack of barrier properties in the afflicted regions (Anderson et al., 2011; Cullen et al., 2011; Kuhnert et al., 2010). Based on the striking phenotypic similarity between Gpr124−/− knockout mice and Wnt7a−/−/Wnt7b−/− double knockout mice, we set out to explore a possible connection between GPR124 and WNT7A/WNT7B and found that GPR124 functions as a co-activator of WNT7A/WNT7B induced β-catenin signaling in developing brain endothelium.
RESULTS AND DISCUSSION
GPR124 Is Required for β-Catenin Signaling In Vivo
To determine if the angiogenesis defects in Gpr124−/− mice are caused by abnormal β-catenin signaling, we began by monitoring β-catenin activity in the CNS vasculature of Gpr124−/− embryos that contained a β-galactosidase transgene driven by the β-catenin responsive TCF/LEF promoter, i.e. the BAT-gal reporter. In Gpr124+/+; BAT-gal+ mice, β-galactosidase nuclear staining was prominent in isolectin positive vessels throughout the brain and spinal cord, consistent with earlier reports (Liebner et al., 2008). However, β-galactosidase staining was undetectable in the glomeruloid vessels of Gpr124−/−; BAT-gal+ forebrains, indicating a loss of β-catenin signaling in these regions (Figures 1A and 1B). In the spinal cord of Gpr124−/− mice β-galactosidase staining was readily observed in the normal vessels of the dorsal region but was undetectable in the glomeruloids found in the ventral side, although occasional β-galactosidase positive nuclei were found in the vessel stalks that connected the glomeruloids to the underlying PNVP (Figures 1C and 1D). These results strongly suggest that GPR124 is required for β-catenin signaling in particular anatomical locations of the CNS.
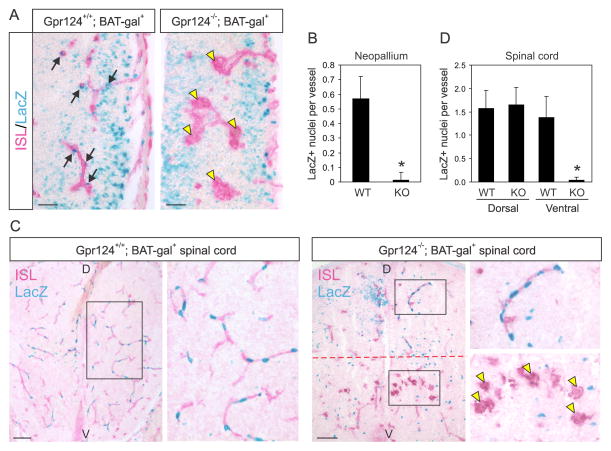
Figure 1. Gpr124 is required for β-catenin signaling in brain endothelium in vivo.
A) β-gal positive nuclei (blue; arrows), indicative of β-catenin signaling, were observed in the isolectin (ISL)-positive vessels (red) that penetrated the lateral ventricular wall in the telencephalon (neopallium) of Gpr124+/+; BAT-Gal+ mice (left panel). Blue nuclei were absent from the glomeruloid vessel structures (yellow arrowheads) found in the lateral ventricular wall of the telencephalon of Gpr124−/−; BAT-Gal+ mice (right panel). Bars: 50 μm.
B) Quantification of the number of β-gal positive nuclei per vessel structure in Gpr124+/+; BAT-Gal+ (WT) or Gpr124−/−; BAT-Gal+ (KO) neopallium. * p<0.0001
C) β-gal positive nuclei (blue) were observed in isolectin (ISL)-positive vessels (red) throughout the spinal cord of Gpr124+/+; BAT-Gal+ mice (left panel). Blue nuclei were observed in the dorsal (D) side of Gpr124−/−; BAT-Gal+ spinal cord but were absent from the glomeruloid vessel structures (yellow arrowheads) found in the ventral (V) side (inset, right panel). Bars: 100 μm.
D) Quantification of the number of β-gal positive nuclei per vessel structure in the ventral or dorsal region of Gpr124+/+; BAT-Gal+ (WT) or Gpr124−/−; BAT-Gal+ (KO) spinal cord. *p<0.0001. The rare β-gal+ nuclei found in Gpr124−/− forebrain (B) or in the ventral region of the spinal cord (D) were located in vessel stalks interconnecting the glomeruloids with the underlying PNVP. Data in (B) and (D) are the mean ± SEM
GPR124 is a Co-Activator of WNT7-Specific β-Catenin Signaling
To determine if GPR124 could also regulate β-catenin signaling in vitro, we began by introducing a TCF/LEF-luciferase reporter into HEK293 cells, a cell system widely used to monitor WNT signaling. These studies revealed up to a 12-fold increase in WNT7-induced luciferase expression upon co-expression of GPR124 (Figure S1). Although HEK293 reporter cells are widely used to monitor β-catenin signaling, we detected a low level of endogenous GPR124 in this cell line which could potentially confound interpretation of results (B.S.C and S.S, unpublished data). To create a more physiologically relevant reporter on a Gpr124 null background, we introduced a TCF/LEF-driven luciferase vector into Gpr124−/− immortalized brain ECs (BECs) derived from the isolectin-positive cell fraction of forebrain micro-dissected from E13.5 embryos (see methods for details). The endothelial origin of the BEC Gpr124 knockout (BKO) reporter cell line was verified by immunofluorescence staining and RT-PCR analysis for the endothelial markers VE-cadherin (cdh5), vascular endothelial growth factor receptor 2 (vegfr2) and laminin (Figure S2). The BKO cells also stained positive for isolectin B4 (Figure S2D). All 10 frizzled Wnt receptors were also detected in BKO cells by RT -PCR analysis (Figure S2A). BKO cells displayed a 2-fold increase in luciferase activity in response to WNT7A expression alone, but luciferase activity increased dose-dependently a further 6-fold upon co-transfection of WNT7A and GPR124 (Figure 2A). Similar results were obtained using a stable GPR124 inducible reporter cell line, called BKO-124i, which was engineered to express GPR124 upon exposure to doxycycline (Figures S2C and S3A). WNT7B showed similar activity to WNT7A stimulating luciferase activity up to 5-fold in combination with GPR124 (Figure 2B). Mouse GPR124 and human GPR124 also showed similar co-stimulatory activity, and addition of an N-terminal FLAG tag or a C-terminal myc tag to GPR124 did not interfere with its ability to potentiate WNT signaling (Figures 2C and 2D). GPR124 expression alone was unable to provoke luciferase activity and GPR125, the closest homologue of GPR124, was unable to augment WNT7-induced luciferase activity (Figures 2A–B and 2D).
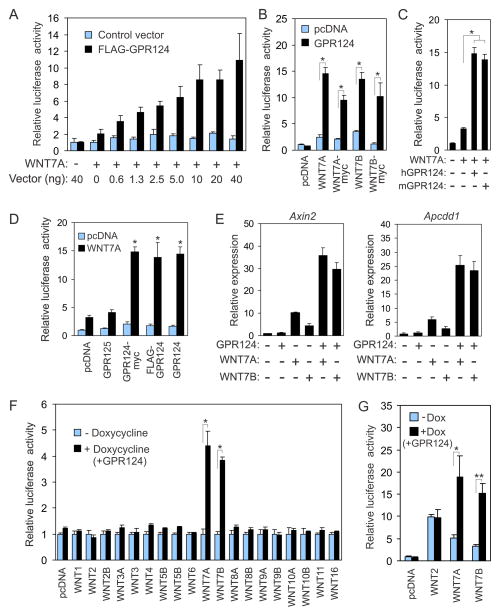
Figure 2. GPR124 is a ligand-specific co-activator of canonical β-catenin signaling.
A) Transfection of BKO reporter cells with increasing concentrations of FLAG-GPR124 caused a dose-dependent increase in luciferase activity in response to WNT7A.
B) WNT7A and WNT7B with and without a myc tag stimulated luciferase activity in BKO cells in combination with GPR124. *p<0.001.
C) Mouse and human GPR124 both enhanced WNT7A induced luciferase activity in BKO cells.
D) N-terminal FLAG or C-terminal myc tags did not alter GPR124’s ability to co-activate WNT7A signaling. GPR125 did not enhance WNT7A signaling. p<0.01 vs pcDNA plus WNT7A.
E) QPCR analysis revealed higher levels of Axin2 and Apcdd1 mRNA expression in doxycycline induced BKO-124i cells.
F) GPR124 selectively enhanced WNT7A and WNT7B induced luciferase activity in BKO-124i cells. *p<0.004
G) GPR124 enhanced the activity of ectopically-expressed WNT7 in BKO-124i cells. HEK293 cells were transfected with pcDNA3.1 (pcDNA), WNT2, WNT7A or WNT7B then 24h later mixed with BKO-124i reporter cells and co-cultured for 24h in the presence or absence of doxycycline (Dox) prior to luciferase analysis. *P=0.04, **p=0.005.
Data are the mean ± SEM. See also Figure S2 and S3.
In order to verify that the luciferase reporter was accurately measuring β-catenin transcription in BKO cells, we expressed WNT7A, WNT7B and GPR124 (Figure S2) and used real-time quantitative PCR (QPCR) to monitor changes in mRNA levels of Axin2 and Apcdd1, genes known to be activated by β-catenin (Jho et al., 2002; Takahashi et al., 2002). As shown in Figure 2E, the expression of Axin2 and Apcdd1 was stimulated by co-expression of GPR124 with WNT7A or WNT7B, demonstrating that GPR124 enhances the WNT7A/WNT7B-induced expression of β-catenin target genes.
Previously described co-activators of canonical WNT signaling such as LRP5 and LRP6, potentiate the activity of many WNTs (Tamai et al., 2004). To determine whether GPR124 is also a broad-spectrum co-activator or if it preferentially activates WNT7A and WNT7B, we measured reporter activity in BKO-124i cells in response to all 19 WNTs. RT-PCR analysis verified that each of the 19 WNTs was expressed following transient transfection of reporter cells (Figure S3B). Strikingly, WNT7A and WNT7B were the only WNTs that displayed significantly elevated activity in combination with GPR124, although some WNTs, such as WNT1, WNT2, WNT3 and WNT3a were able to stimulate luciferase activity independent of GPR124 (Figure 2F and S3C). These results indicate that GPR124 is a ligand-specific co-activator of canonical β-catenin signaling in brain endothelium.
GPR124 could potentially enhance WNT7 activity by aiding in the proper folding, biosynthesis or secretion of WNT7. Alternatively, GPR124 may act at the cell surface to augment the activity of functionally mature WNT7. To distinguish between these possibilities, we transfected HEK293 cells with empty pcDNA3 vector (a negative control), WNT2 (a positive control), WNT7A or WNT7B, mixed each of the transfectants with BKO-124i reporter cells, co-cultured them for 24h in the presence or absence of doxycycline, and then measured luciferase activity. Ectopically produced WNT7A and WNT7B stimulated luciferase activity about 4 to 5-fold above the control vector, but GPR124 expression in BKO-124i cells potentiated signaling a further 3-fold. The WNT2 positive control also stimulated luciferase activity in the reporter cells, but in a GPR124 independent manner (Figure 2G). These results suggest that GPR124 is involved in WNT7 signaling that occurs after WNT7 binds to target cells.
Mature WNT7A and WNT7B share 82% amino acid identity and WNT7B can compensate for loss of WNT7A in the developing CNS (Daneman et al., 2009; Stenman et al., 2008). However, homozygous disruption of Wnt7a alone results in limb defects (Parr and McMahon, 1995) and homozygous missense mutations in WNT7A in humans cause Fuhrmann Syndrome and Al-Awadi/Raas-Rothschild syndrome which are also characterized by limb abnormalities (Woods et al., 2006). It is currently unclear if the limb defects caused by mutation in WNT7A are a result of alterations in canonical and/or non-canonical signaling, although indirect evidence has been provided for both pathways (Adamska et al., 2004; Kengaku et al., 1998). Because GPR124 overexpression in BKO reporter cells provides a highly sensitive readout for measuring WNT7A induced canonical β-catenin signaling, we tested each of the previously reported WNT7A mutants in our reporter assay. Western blotting revealed each of the mutant proteins was expressed at a similar level following transfection into BKO cells (Figure 3A). All 6 mutations resulted in complete or nearly complete loss of GPR124-induced luciferase activity (Figure 3B). These results support the possibility that WNT7A induced canonical β-catenin signaling is important for limb morphogenesis.
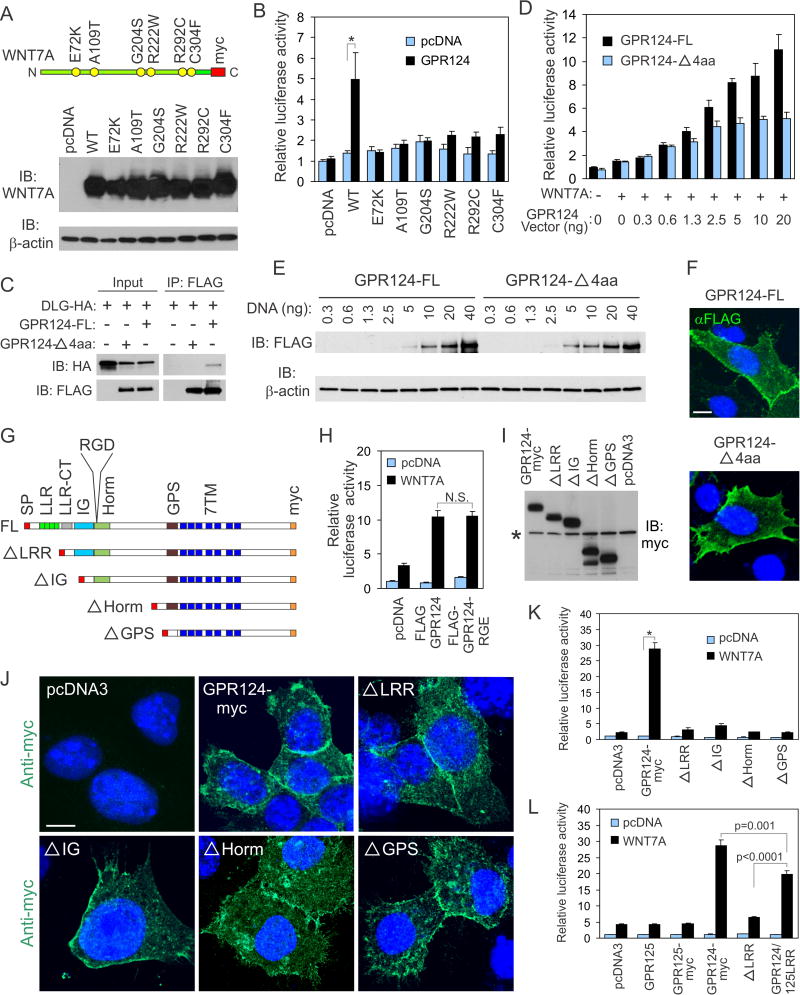
Figure 3. Mutation analysis identifies amino acids of WNT7A and GPR124 important for β-catenin signaling.
A) Western blotting reveals that each of the mutant WNT7A proteins was expressed in BKO cells at a level similar to the wildtype (WT) WNT7 protein. The schematic on top depicts the relative location of each mutation as well as the C-terminal myc tag.
B) Luciferase activity in BKO cells increased in response to wildtype (WT) WNT7A upon co-expression of GPR124 but was attenuated by each of the WNT7A mutations. *p<0.002
C) Co-immunoprecipitation (IP) studies revealed binding of HA-tagged DLG1 (DLG-HA) to full-length FLAG-tagged GPR124 (GPR124-FL) but not the 4 amino acid deletion (GPR124-Δ4aa). IB: Immunoblotting antibody.
D) WNT7A-induced luciferase activity in BKO reporter cells in response to increasing amounts of either GPR124-Δ4aa or the full-length vector (GPR124-FL). A constant amount of WNT7 (40 ng) was transfected into samples marked “+”.
E) Immunoblotting with anti-FLAG antibodies revealed similar levels of protein following transient transfection of cells with the full-length FLAG-tagged GPR124 receptor (GPR124-FL) and the corresponding 4 amino acid deletion (GPR124-Δ4aa). βactin was used as a loading control.
F) Immunofluorescence staining of non-permeabilized cells with anti-FLAG antibodies (green) revealed similar expression of GPR124-FL (full-length) and GPR124-Δ4aa in transfected BKO cells. The nuclei (blue) were counterstained with DAPI. Bar: 10μm.
G) Schematic diagram of full-length (FL) GPR2124 and the N-terminal deletion mutants. SP: signal peptide, 7TM: 7 pass transmembrane domains.
H) Incorporation of an RGE motif in place of the RGD site did not alter GPR124’s ability to activate WNT7A β-catenin signaling. N.S: non-significant.
I) Western blotting revealed expression of the N-terminal GPR124 deletions. The anti-myc antibody also cross-reacted with a product present in all lanes (asterisk) providing a convenient internal loading control.
J) Immunofluorescence staining of permeabilized BKO cells with anti-myc antibodies (green) 48h post-transfection revealed a similar membrane staining pattern for GPR124-myc (full-length GPR124 with a C-terminal myc tag) and each of the N-terminal deletions. The nuclei (blue) were counterstained with DAPI. Bar: 10μm.
K) BKO reporter assays revealed a loss of β-catenin stimulation by all of the N-terminal GPR124 deletions. *p=0.0001
L) BKO reporter assays revealed that the LRR domain of GPR125 is able to partially rescue luciferase activity in the ΔLRR mutant.
Data are the mean ± SEM.
GPR124’s Activity is Regulated by its Extracellular LRR domain and its Intracellular PDZ Binding Motif
To better understand the role of GPR124, next we began a search for interacting partners using a mass spectrometry approach. For this, we immunoprecipitated GPR124 from immortalized human cerebral microvascular endothelial cells (hCMECs) engineered to express a doxycycline-inducible FLAG-tagged GPR124 expression vector (hCMEC/GPR124i) (Cullen et al., 2011). Immunoprecipitations from non-induced hCMEC/GPR124i and parent hCMECs served as negative controls. Mass spectrometry analysis identified Disks large homolog 1 (DLG1) as one of the most abundant interactors with 43 peptides in the doxycycline treated hCMEC/GPR124 group versus zero peptides in each of the control groups. DLG1 has previously been shown to bind APC, an essential component of the β-catenin destruction complex, and Yamamoto and co-workers previously identified GPR124 as an interacting partner for DLG1 using a yeast two-hybrid approach that employed DLG1 as bait (Yamamoto et al., 2004). In that study the authors demonstrated that the PDZ domain of DLG1 bound to the last 4 amino acids of GPR124, ETTV, which represents a canonical PDZ-binding motif. Using co-immunoprecipitation studies we verified that the last 4 amino acids of GPR124 were essential for binding DLG1 (Figure 3C). To determine if this interaction could be involved in GPR124 mediated co-activation of WNT7 signaling, we compared the 4 amino acid deletion (GPR124-Δ4aa) with the full-length receptor in dose-response studies and found that it displayed a consistent reduction in activity compared to full-length receptor (Figure 3D). GPR124 and GPR124-Δ4aa were expressed at similar levels (Figure 3E) and both receptors were located at the cell surface (Figure 3F) indicating that the reduced potentiation was not caused by reduced expression or trafficking to the cell surface. These results demonstrate that the PDZ binding motif is required for maximal potentiation of WNT7-induced canonical β-catenin signaling (Figure 3D).
GPR124 also has several structural domains in its N-terminal region that could be involved in the co-activation of WNT7 signaling including a set of leucine rich repeats (LRRs), a LRR C-terminal (LRR-CT) domain, an immunoglobulin (IG) domain, a hormone (Horm) domain containing an RGD (Arg-Gly-Asp) motif and a membrane proximal GPS domain (Figure 3G). Proteolytic exposure of the cryptic RGD site in GPR124 has been shown to mediate binding to integrin αvβ3, and it has been postulated that this interaction regulates adhesion and migration during angiogenesis (Vallon et al., 2011; Vallon et al., 2010) To determine if the RGD site was important for GPR124 mediated potentiation of WNT7 signaling, we co-transfected BKO cells with WNT7A and a mutant GPR124 receptor containing an integrin blocking D357E point mutation (RGD → RGE), but found no difference in co-activation compared to wildtype GPR124 (Figure 3H). Next, we generated a series of N-terminal deletions, removing one additional domain with each construct (Figure 3G). Western blot and immunofluorescence analysis demonstrated that the full-length protein and each of the truncated proteins were expressed at comparable levels (Figure 3I) with a similar cell surface staining (Figure 3J). However, when expressed in BKO cells, each of the truncated proteins was non-functional indicating that the most N-terminal deletion, lacking only the LRRs, was sufficient to completely block GPR124 mediated potentiation of WNT7 signaling (Figure 3K). Swapping the LRR in the extracellular domain of GPR124 with the LRR domain of GPR125 resulted in a fusion protein, called GPR124/125LRR that largely rescued potentiation of WNT7 signaling (Figure 3L). Because the full-length GPR125 receptor is unable to potentiate signaling, there are likely other regions outside of the LRR domain that are also required for potentiation but are missing in GPR125. These results also suggest that conserved residues within the LRR domains of GPR124 and GPR125 are important for signaling potentiation.
Genetic Evidence for a Common GPR124/WNT7 Signaling Pathway In Vivo
To further assess the importance of GPR124 in regulating WNT7 activity in vivo, next we sought evidence for a genetic interaction between Gpr124 and Wnt7. So far no CNS angiogenesis defects have been reported in heterozygous Gpr124+/− mice suggesting that one copy of Gpr124 is sufficient for normal development (Anderson et al., 2011; Cullen et al., 2011; Kuhnert et al., 2010). Similarly, mice with 3 out of 4 mutant Wnt7 alleles (Wnt7a−/−; Wnt7b+/−) display minimal, if any, defects in CNS development (Daneman et al., 2009; Stenman et al., 2008). We reasoned that if GPR124 and WNT7 function in the same pathway, then loss of one Gpr124 allele could make mice more sensitive to reductions in WNT7 expression levels. To test this hypothesis, we evaluated the CNS vasculature in compound mutant E13.5 embryos lacking one Gpr124 allele and 3 out of 4 Wnt7 alleles (i.e. genotype: Gpr124+/−; Wnt7a−/−; Wnt7b+/−). These studies revealed a clear defect in vascular morphogenesis in the compound mutants (Figure 4A). However, no CNS defects were observed in the Gpr124+/− or Wnt7a−/−; Wnt7b+/− control groups (Figure S4A) consistent with earlier reports (Cullen et al., 2011; Stenman et al., 2008). Although the defects were less severe than those found in Gpr124 knockout or Wnt7a/Wnt7b double knockout mice, in the forebrain abnormal glomeruloids were consistently observed in the region of ganglionic eminence (Figure 4A) (100% penetrance, n=6). We also observed glomeruloids in the ventral region of the spinal cord of compound heterozygotes (Figure 4B). Staining for the erythrocyte marker TER119 (Ly76) revealed sporadic hemorrhaging from the glomeruloids (Figure S4B), although the extent of hemorrhage was much less than in Gpr124 null and Wnt7a/Wnt7b null mice. Residual expression of both GPR124 and WNT7A from the remaining wildtype allele presumably accounts for the less severe defects compared to Gpr124 or Wnt7a/Wnt7b null mice.
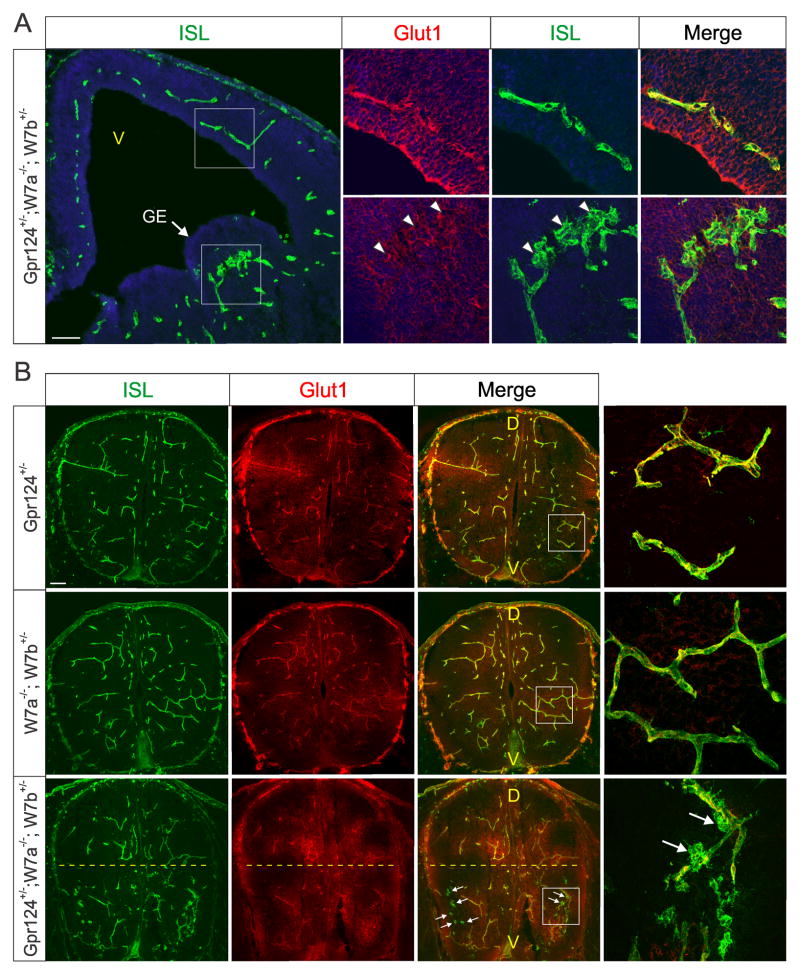
Figure 4. Genetic interaction studies reveal co-operation between Gpr124, Wnt7a and Wnt7b in vivo.
A) Isolectin (ISL) staining revealed unusual glomeruloid structures (arrowheads) in the ganglionic eminence (GE) of Gpr124+/−; Wnt7a−/−; Wnt7b+/− forebrains. Note the reduced level of Glut1 in the glomeruloid structures. V: ventricle. Bar: 100μm.
B) Isolectin (ISL) staining revealed unusual glomeruloid structures (arrows) in the ventral (v) region of Gpr124+/−; Wnt7a−/−; Wnt7b+/− spinal cords. Bar: 100μm.
See also Figure S4.
The hemorrhaging observed in the compound mutant suggested that barrier properties may also be compromised. To assess this, we evaluated expression of Glucose Transporter- 1 (Glut1), a widely used indicator of BBB integrity (Bauer et al., 1995). Glut1 levels were low or absent in the glomeruloids of the compound mutant mice (Figures 4A–4B, S4A and S4C). Conversely, we found an increase in plasmalemma vesicle-associated protein (PVLAP) (Figure S4C), a marker of stomatal and fenestral diaphragms that is normally silenced with tightening of the BBB and absent from mature CNS vasculature (Shue et al., 2008). Taken together, these studies suggest that GPR124 and WNT7 co-operate to regulate angiogenesis and establishment of BBB properties in the developing CNS.
In these studies we establish GPR124 as a WNT7-specific co-activator of canonical β-catenin signaling. Zhou et al. also recently demonstrated the importance of GPR124 in promoting WNT7-induced β-catenin signaling using a 293-based luciferase reporter (Zhou and Nathans, 2014). Similar to our study (see Figure S1), in 293 reporter cells they observed a biphasic response to increasing GPR124 levels that included an increase in WNT7-induced β-catenin signaling followed by a partial decrease in signaling at the highest levels of GPR124. Although the physiological relevance of the partial decrease in signaling in 293 reporter cells is currently unclear, in BKO reporter cells we only observed a dose-dependent increase in WNT7-induced β-catenin signaling with increasing levels of GPR124. Using BKO cells we also demonstrate that GPR124 enhanced the WNT7 induction of the endogenous β-catenin response genes Axin2 and Apcdd1. The results we obtained using human GPR124 and WNT7 were similar to those obtained with the murine counterparts, demonstrating that GPR124s role in potentiation of WNT7 signaling has been conserved through evolution. While Zhou et al. demonstrated a genetic interaction between Gpr124 and either β-catenin or Norrin, here we provide evidence for a genetic interaction between Gpr124 and Wnt7a/Wnt7b in vivo.
In our reporter assays we consistently observed some induction (~2–5 fold) of luciferase activity by WNT7 in Gpr124−/− BKO reporter cells. Because GPR124 is not absolutely essential for WNT7 signaling we propose that GPR124 functions as a co-activator, modulating endogenous frizzled/LRP complexes and heightening their sensitivity to WNT7A/WNT7B. We speculate that ligand-specific co-activators may have evolved to help guide cell-specific WNT responses in complex tissue environments where multiple WNTs are competing for multiple frizzled receptors. Although GPR124 is a specific co-activator for WNT7A and WNT7B, it seems likely that multiple frizzleds can mediate these signals. First, all 10 frizzleds were detected in BKO cells. Second, none of the frizzled knockout mice have CNS angiogenesis defects in the forebrain and ventral spinal cord similar to Gpr124−/− or Wnt7a−/−/Wnt7b−/− mice (van Amerongen and Berns, 2006).
Although GPR124 displays widespread expression in CNS vasculature, angiogenesis defects in Gpr124−/− mice were only observed in the forebrain and ventral spinal cord. These locations correspond with the previously described localized expression patterns of Wnt7a and Wnt7b (Daneman et al., 2009; Stenman et al., 2008) and provide a likely explanation for the anatomical specificity of the CNS defects observed in the Grp124−/− mice.
Through mutational analysis we identified several key regions of WNT7 and GPR124 that are involved in potentiation of β-catenin signaling. On the C-terminus of GPR124 we show that the last four amino acids are critical for maximal signaling potentiation and that this region binds DLG1. We also demonstrate the importance of the N-terminal LRR domain of GPR124 in WNT7-induced β-catenin signaling. Our mutational analysis should help facilitate future efforts to further unravel the mechanisms underlying GPR124-selective potentiation of WNT7 signaling.
The adhesion GPCRs represent the second largest family of GPCRs but are currently the least understood. The well-known druggability of GPCRs makes GPR124 an interesting potential target for regulating CNS-specific angiogenesis or barriergenesis. The fact that GPR124 is also upregulated in tumor vasculature suggests that manipulation of GPR124 also holds promise for the development of new anti-angiogenic agents to block tumor growth.
EXPERIMENTAL PROCEDURES
Animals
Mice were housed in a pathogen-free facility certified by the Association for Assessment and Accreditation of Laboratory Animal Care International, and the studies were carried out in accordance with protocols approved by the NCI Animal Care and Use Committee.
Statistical Analysis
A Mann-Whitney test was used to calculate p-values for the β-galactosidase staining and a Student’s t-test was used for all other comparisons. Differences between two groups were presented as the mean ± SEM. All tests were two-sided and p values <0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Terry P. Yamaguchi for helpful discussions during our studies and expression vectors for constitutively active β-catenin and Renilla luciferase. We are grateful to the Open Source WNT project for providing the panel of human WNT plasmids. This work was supported by the Center for Cancer Research Intramural Program, NCI, NIH, a part of the U.S. Department of Health and Human Services (DHSS). The content of this publication does not necessarily reflect the views or policies of the DHHS.
Footnotes
Supplemental Information includes Extended Experimental Procedures four figures.
AUTHOR CONTRIBUTIONS
EP, SS, JR and BSC designed the study. All authors designed and performed experiments and analyzed results. EP and BSC wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamska M, MacDonald BT, Sarmast ZH, Oliver ER, Meisler MH. En1 and Wnt7a interact with Dkk1 during limb development in the mouse. Dev Biol. 2004;272:134–144. doi: 10.1016/j.ydbio.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Pan L, Yang XM, Hughes VC, Walls JR, Dominguez MG, Simmons MV, Burfeind P, Xue Y, Wei Y, et al. Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. Proc Natl Acad Sci U S A. 2011;108:2807–2812. doi: 10.1073/pnas.1019761108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer H, Sonnleitner U, Lametschwandtner A, Steiner M, Adam H, Bauer HC. Ontogenic expression of the erythroid-type glucose transporter (Glut 1) in the telencephalon of the mouse: correlation to the tightening of the blood-brain barrier. Brain Res Dev Brain Res. 1995;86:317–325. doi: 10.1016/0165-3806(95)00044-e. [DOI] [PubMed] [Google Scholar]
- Carson-Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, St Croix B. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res. 2001;61:6649–6655. [PubMed] [Google Scholar]
- Cullen M, Elzarrad MK, Seaman S, Zudaire E, Stevens J, Yang MY, Li X, Chaudhary A, Xu L, Hilton MB, et al. GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishment of the blood-brain barrier. Proc Natl Acad Sci U S A. 2011;108:5759–5764. doi: 10.1073/pnas.1017192108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kengaku M, Capdevila J, Rodriguez-Esteban C, De La Pena J, Johnson RL, Izpisua Belmonte JC, Tabin CJ. Distinct WNT pathways regulating AER formation and dorsoventral polarity in the chick limb bud. Science. 1998;280:1274–1277. doi: 10.1126/science.280.5367.1274. [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Mancuso MR, Shamloo A, Wang HT, Choksi V, Florek M, Su H, Fruttiger M, Young WL, Heilshorn SC, Kuo CJ. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science. 2010;330:985–989. doi: 10.1126/science.1196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature. 1995;374:350–353. doi: 10.1038/374350a0. [DOI] [PubMed] [Google Scholar]
- Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, St Croix B. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007;11:539–554. doi: 10.1016/j.ccr.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shue EH, Carson-Walter EB, Liu Y, Winans BN, Ali ZS, Chen J, Walter KA. Plasmalemmal vesicle associated protein-1 (PV-1) is a marker of blood-brain barrier disruption in rodent models. BMC Neurosci. 2008;9:29. doi: 10.1186/1471-2202-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Sundberg C, Nagy JA, Brown LF, Feng D, Eckelhoefer IA, Manseau EJ, Dvorak AM, Dvorak HF. Glomeruloid microvascular proliferation follows adenoviral vascular permeability factor/vascular endothelial growth factor-164 gene delivery. Am J Pathol. 2001;158:1145–1160. doi: 10.1016/S0002-9440(10)64062-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Fujita M, Furukawa Y, Hamamoto R, Shimokawa T, Miwa N, Ogawa M, Nakamura Y. Isolation of a novel human gene, APCDD1, as a direct target of the beta-Catenin/T-cell factor 4 complex with probable involvement in colorectal carcinogenesis. Cancer Res. 2002;62:5651–5656. [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, He X. A mechanism for Wnt coreceptor activation. Mol Cell. 2004;13:149–156. doi: 10.1016/s1097-2765(03)00484-2. [DOI] [PubMed] [Google Scholar]
- Vallon M, Aubele P, Janssen KP, Essler M. Thrombin-induced shedding of tumor endothelial marker 5 and exposure of its RGD motif are regulated by cell surface protein disulfide isomerase. Biochem J. 2011 doi: 10.1042/BJ20111682. [DOI] [PubMed] [Google Scholar]
- Vallon M, Rohde F, Janssen KP, Essler M. Tumor endothelial marker 5 expression in endothelial cells during capillary morphogenesis is induced by the small GTPase Rac and mediates contact inhibition of cell proliferation. Experimental Cell Research. 2010;316:412–421. doi: 10.1016/j.yexcr.2009.10.013. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Berns A. Knockout mouse models to study Wnt signal transduction. Trends Genet. 2006;22:678–689. doi: 10.1016/j.tig.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Woods CG, Stricker S, Seemann P, Stern R, Cox J, Sherridan E, Roberts E, Springell K, Scott S, Karbani G, et al. Mutations in WNT7A cause a range of limb malformations, including Fuhrmann syndrome and Al-Awadi/Raas-Rothschild/Schinzel phocomelia syndrome. Am J Hum Genet. 2006;79:402–408. doi: 10.1086/506332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Irie K, Asada M, Mino A, Mandai K, Takai Y. Direct binding of the human homologue of the Drosophila disc large tumor suppressor gene to seven-pass transmembrane proteins, tumor endothelial marker 5 (TEM5), and a novel TEM5-like protein. Oncogene. 2004;23:3889–3897. doi: 10.1038/sj.onc.1207495. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Nathans J. Gpr124 controls CNS angiogenesis and blood-brain barrier integrity by promoting ligand-specific canonical wnt signaling. Dev Cell. 2014;31:248–256. doi: 10.1016/j.devcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Tischfield M, Williams J, Smallwood PM, Rattner A, Taketo MM, Nathans J. Canonical WNT signaling components in vascular development and barrier formation. J Clin Invest. 2014;124:3825–3846. doi: 10.1172/JCI76431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.