Abstract
Psychostimulants such as methylphenidate (MPH) and antidepressants such as fluoxetine (FLX) are widely used in the treatment of various mental disorders or as cognitive enhancers. These medications are often combined, for example, to treat co-morbid disorders. There is a considerable body of evidence from animal models indicating that individually these psychotropic medications can have detrimental effects on brain and behavior, especially when given during sensitive periods of brain development. However, almost no studies investigate possible interactions between these drugs. This is surprising given that their combined neurochemical effects (enhanced dopamine and serotonin neurotransmission) mimic some effects of illicit drugs such as cocaine and amphetamine. Here we summarize recent studies in juvenile rats on the molecular effects in the mid- and forebrain and associated behavioral changes, after such combination treatments. Our findings indicate that these combined MPH+FLX treatments can produce similar molecular changes as seen after cocaine exposure, while inducing behavioral changes indicative of dysregulated mood and motivation, effects that often endure into adulthood.
Introduction
There is increasing use of psychotropic medications in children and adolescents, for example, in the treatment of attention deficit/hyperactivity disorder (ADHD) and major depressive disorder (MDD). Among the most often used medications are selective serotonin reuptake inhibitor (SSRI) antidepressants such as fluoxetine (FLX; Prozac®), which is approved for treatment of pediatric MDD (Iversen, 2006), and psychostimulants such as methylphenidate (MPH; Ritalin®), an effective agent for the management of ADHD (Castle et al., 2007; Kollins, 2008). For example, it was estimated that in 2008 approximately 3 million children between 4 and 17 years of age in the United States alone were treated with psychostimulant medications for ADHD (Swanson et al., 2011). In addition to individual use, MPH and SSRIs are also frequently combined as a treatment strategy for co-morbid ADHD and MDD (Rushton and Whitmire, 2001; Safer et al., 2003), which occurs with up to 40% prevalence in pediatric ADHD populations (Waxmonsky, 2003; Spencer, 2006), as well as in the treatment of other conditions (e.g., Lavretsky et al., 2003; Nelson, 2007; Csoka et al., 2008; Ravindran et al., 2008).
Co-exposure to these drug classes also occurs in patients on antidepressants who use MPH recreationally or as a cognitive enhancer (Kollins, 2008; Swanson and Volkow, 2008; Wilens et al., 2008). This is a particularly uncontrolled form of potentially high-level exposure, as MPH may be snorted or injected (e.g., Teter et al., 2006; see Steiner and Van Waes, 2013). According to surveys, up to 10-20% or more of college students use MPH to improve concentration, stay awake to study, or party (White et al., 2006; Kollins, 2008; Wilens et al., 2008). The 2011 National Survey on Drug Use and Health (NSDUH) reports that approximately 1 million persons age 12 or older in the US admitted current nonmedical use of prescription psychostimulants (SAMHSA, 2012).
Use of psychotropic drugs during development and maturation of the brain is of concern, because studies in animal models show that these drugs can induce maladaptive neurobehavioral changes suggestive of an increased risk for drug addiction and other neuropsychiatric disorders later in life (for reviews, see Carlezon and Konradi, 2004; Andersen, 2005; Carrey and Wilkinson, 2011). However, despite prevalent MPH and FLX use, the neurobiological consequences of their combined use during juvenile periods are unknown (Bhatara et al., 2004; Spencer, 2006). This is particularly striking because together MPH and FLX may have pharmacodynamic properties similar to cocaine. MPH and cocaine both act through inhibition of dopamine transporters (Volkow et al., 2002); differences between these two drugs may be due to inhibition of serotonin transporters by cocaine, but not by MPH, which has little affinity for the serotonin transporter and does not produce serotonin overflow (e.g., Kuczenski and Segal, 1997; Han and Gu, 2006; see Yano and Steiner, 2007). Therefore, combined use of MPH and SSRIs may induce emergent effects by simultaneously inhibiting the reuptake of both dopamine and serotonin.
The role of serotonin in molecular effects of psychostimulants such as cocaine is well established (Steiner and Van Waes, 2013). Thus, while dopamine is critical for cocaine-induced gene regulation in the striatum, serotonin facilitates these effects. For example, attenuation of the serotonin transmission by transmitter depletion (Bhat and Baraban, 1993) or receptor antagonism (Lucas et al., 1997; Castanon et al., 2000) reduces immediate-early gene (IEG) induction by cocaine or its effects on neuropeptide expression (Morris et al., 1988; Walker et al., 1996; Horner et al., 2005) in the striatum. Adding serotonin action (SSRI) to dopamine action (MPH) may thus produce more “cocaine-like” molecular changes than MPH alone. Indeed, a series of studies showed that treatment with SSRIs such as FLX in conjunction with MPH potentiates MPH-induced gene expression, including that of IEGs and neuropeptides, in the striatum (Steiner and Van Waes, 2013). These studies are summarized in the first of the following sections.
The long-term behavioral consequences of juvenile exposure to MPH or FLX have only recently begun to be elucidated in animal models. Exposure to MPH alone in juvenile rats increases sensitivity to stress, while decreasing sensitivity to both natural and drug rewards in adulthood (Andersen et al., 2002; Bolanos et al., 2003). Conversely, FLX treatment during adolescence increases sensitivity to anxiety-eliciting circumstances and to natural rewards, but decreases sensitivity to stress in adulthood (Karpova et al., 2009; Iñiguez et al., 2010b). A recent series of studies investigated the consequences of combined MPH plus FLX treatment and the involved molecular mechanisms within the ventral tegmental area (VTA). These findings are reviewed in the subsequent section.
Effects of juvenile methylphenidate plus fluoxetine treatment on gene regulation in the striatum
There is consensus that changes in gene regulation are critical for psychostimulant addiction (Renthal and Nestler, 2008) and that excessive activation of the dopamine transmission is key for these molecular changes (see Steiner and Van Waes, 2013). Because MPH acts as a dopamine reuptake inhibitor and these drugs are known to produce various molecular changes in the striatum, several labs have investigated effects of MPH on striatal gene regulation and have compared these with the effects of psychostimulants such as cocaine (Steiner and Van Waes, 2013). Early microarray studies in adolescent rats reported that acute and repeated treatment even with a dose as low as 2 mg/kg of MPH (i.p.) altered the expression of hundreds genes in the striatum (Adriani et al., 2006a; Adriani et al., 2006b). Similar to other psychostimulants, MPH affected genes that encode transcription factors (IEGs), neurotransmitter receptors, neuropeptides, postsynaptic density proteins, and other signaling- or plasticity-related molecules (Yano and Steiner, 2007; Carrey and Wilkinson, 2011; Marco et al., 2011). Some of these molecular changes persisted well past the termination of the drug treatment, into the adulthood of the animals (Adriani et al., 2006a; Adriani et al., 2006b; see also Chase et al., 2007; Warren et al., 2011).
Further comparisons indicated that some genes, including those encoding the opioid peptides dynorphin and enkephalin, were less affected by MPH than by drugs such as cocaine (Yano and Steiner, 2005b; Steiner and Van Waes, 2013). Given the importance of serotonin for addiction-related gene regulation and the lack of serotonin effects for MPH (see above), MPH’s reduced impact on gene regulation might not be surprising; this would also be consistent with a lower addiction liability for MPH compared with other psychostimulants (Svetlov et al., 2007). On the other hand, these observations led to the question whether increasing serotonin action (by SSRIs) in conjunction with MPH treatment would increase MPH-induced gene regulation and addiction liability. We (H.S., V.V.W. and colleagues) investigated, in adolescent rats, whether SSRIs modified MPH effects on gene regulation in the striatum.
Effects of acute and repeated MPH+FLX treatment on striatal IEG expression
We first assessed the expression of IEGs (Zif268, c-Fos), as these are useful markers for cell activation by neuronal activity and drug treatments due to their rapid and transient induction (Sharp et al., 1993; Chaudhuri, 1997). These IEGs are also of interest because they encode transcription factors and thus directly participate in neuroplasticity (e.g. Knapska and Kaczmarek, 2004). Gene expression was measured by in situ hybridization histochemistry in a total of 23 striatal sectors that represent all major functional domains of the striatum (Willuhn et al., 2003; Yano and Steiner, 2005a).
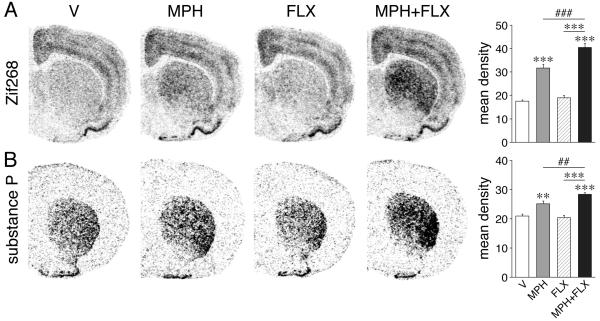
Our results show that acute treatment with SSRIs (fluoxetine or citalopram, 5 mg/kg, i.p.) in conjunction with MPH (2-5 mg/kg) robustly potentiates MPH-induced expression of Zif268 and c-Fos throughout the striatum (Steiner et al., 2010; Van Waes et al., 2010; Fig. 1A). These SSRIs by themselves had no effect. Our regional analysis indicates that this potentiation of IEG induction is most pronounced in the lateral (sensorimotor) striatum. However, significant but smaller potentiation of gene regulation is also seen in select subregions of the nucleus accumbens (medial core, lateral shell) (Van Waes et al., 2010).
Figure 1.
Fluoxetine potentiation of acute methylphenidate-induced gene expression in the striatum (Van Waes et al., 2010; Van Waes et al., 2012). Illustrations of film autoradiograms depict expression of Zif268 (A) and substance P (B) in coronal sections from the middle striatum in animals that received an injection of vehicle (V), methylphenidate (MPH, 5 mg/kg), fluoxetine (FLX, 5 mg/kg) or MPH+FLX and were killed 40 min (Zif268) or 90 min after drug administration. Shown on the right are mean density values (mean±SEM) measured in the whole striatum in these groups. FLX potentiated Zif268 and substance P induction by MPH, but had no effect by itself. FLX potentiation was most robust in the lateral striatum. **p<0.01, ***p<0.001 versus V or FLX; ##p<0.01, ###p<0.001, MPH+FLX versus MPH (potentiation).
In the clinic, these psychotropic medications are administered chronically, and the consequences of repeated treatment with MPH and FLX are thus more relevant for long-term behavioral effects. Repeated psychostimulant treatments produce various molecular changes, including upregulation of expression for some genes and repression for others (McClung and Nestler, 2003; Yuferov et al., 2005; Heiman et al., 2008). One of the best-established gene regulation effects of repeated psychostimulant treatment is blunting (repression) of IEG inducibility; a psychostimulant challenge after repeated pretreatment will produce attenuated (blunted) IEG induction compared with acute induction (see Steiner and Van Waes, 2013). This effect is the result of epigenetic modifications (Renthal et al., 2008) or other neuroadaptations produced by the repeated drug treatment (Steiner and Van Waes, 2013). IEG blunting in the striatum is also seen after repeated MPH treatment (e.g., Brandon and Steiner, 2003; Cotterly et al., 2007). Thus, it was of interest to investigate whether such IEG blunting is modified by coadministration of FLX together with MPH.
We assessed IEG induction (Zif268, Homer1a) by a cocaine challenge (25 mg/kg) one day or 14 days after a 5-day repeated treatment with MPH (5 mg/kg) and/or FLX (5 mg/kg) in adolescent rats. Zif268 encodes a transcription factor (see above). Homer1a is a synaptic plasticity modulator (Thomas, 2002) that is implicated in drug-induced neuroplasticity related to addiction (for review, see Szumlinski et al., 2008). Previous studies had shown that repeated cocaine treatment (5 days) reliably blunts the induction of both Zif268 and Homer1a in the striatum (Unal et al., 2009), while repeated treatment with MPH alone produced blunting of Zif268 induction (Brandon and Steiner, 2003; Cotterly et al., 2007), but had minimal effects on Homer1a expression (Cotterly et al., 2007). Gene expression was again mapped throughout the striatum in order to identify the functional domains affected by these treatments.
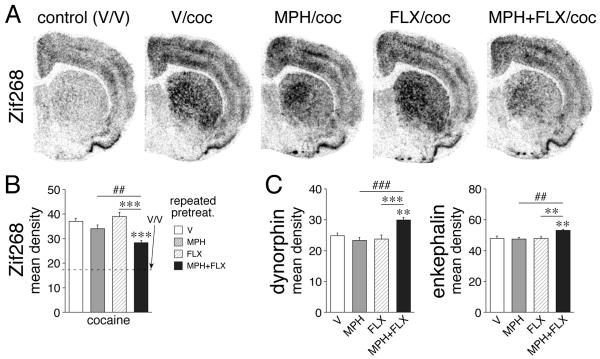
Our findings demonstrate that the 5-day repeated pretreatment with MPH alone produced minor IEG blunting, while FLX alone had no effect. In contrast, adding FLX to MPH strongly potentiated blunting of Zif268 (Fig. 2A, B) and Homer1a induction by the cocaine challenge (Van Waes et al., 2013). This SSRI potentiation of MPH-induced gene repression was present in most striatal sectors, but was again maximal in the lateral, sensorimotor striatum. Overall, there was a positive regional correlation between potentiation of acute IEG induction (Van Waes et al., 2010) and potentiation of blunting after repeated treatment (Van Waes et al., 2013), demonstrating that such acute gene regulation effects can predict the presence and magnitude of neuroadaptations after repeated treatment (see Steiner and Van Waes, 2013). Further results show that this SSRI potentiation of gene blunting endures for at least 14 days after the repeated treatment (in preparation), similar to gene blunting by repeated cocaine treatment (Unal et al., 2009). These findings demonstrate more robust neuroadaptations in striatal gene regulation when FLX is chronically given in combination with MPH.
Figure 2.
Fluoxetine potentiation of repeated methylphenidate-induced changes in gene expression in the striatum (Van Waes et al., 2013). (A, B) Effects on Zif268 induction by a subsequent cocaine challenge. Illustrations of film autoradiograms (A) depict expression of Zif268 in the middle striatum in rats that received 5 daily injections of vehicle (V), methylphenidate (MPH, 5 mg/kg), fluoxetine (FLX, 5 mg/kg) or MPH+FLX, followed on day 6 by a vehicle (V) injection or a cocaine challenge (coc, 25 mg/kg). Mean density values (mean±SEM) for Zif 268 expression are also shown for these treatment groups (B). Repeated MPH pretreatment produced some blunting of Zif268 induction by cocaine. Repeated FLX potentiated this MPH-induced Zif268 blunting (MPH+FLX), but had no effect by itself. (C) Effects on dynorphin and enkephalin expression in the striatum. Mean density values (mean±SEM) are shown for rats that received 5 daily injections of V, MPH (5 mg/kg), FLX (5 mg/kg) or MPH+FLX, and were killed 2 h later. Repeated MPH+FLX treatment produced increased expression of both opioid peptides, while neither MPH nor FLX alone had a significant effect. **p<0.01, ***p<0.001 versus V or FLX; ##p<0.01, ###p<0.001, MPH+FLX versus MPH (potentiation).
Effects of acute and repeated MPH+FLX treatment on striatal neuropeptide expression
In other studies, the impact of acute and repeated treatment with MPH and FLX on the expression of neuropeptides in the striatum was evaluated. The neuropeptides substance P, dynorphin, and enkephalin are selectively expressed by neurons of the direct (substance P, dynorphin) and indirect (enkephalin) striatal output pathways (i.e., striatonigral and striatopallidal projection neurons, respectively) and thus serve as cell type markers for drug actions in these neurons. These neuropeptides also function as neurotransmitters (Steiner and Gerfen, 1998) and are thought to participate in addiction processes (Shippenberg et al., 2007). For example, there is evidence indicating that increased dynorphin function in striato-nigral/VTA neurons after repeated psychostimulant exposure contributes to anhedonia and depression during withdrawal (Nestler and Carlezon, 2006; Wiley et al., 2009). Notably, increased dynorphin expression has also been found in human cocaine addicts (Hurd and Herkenham, 1993; Frankel et al., 2008).
Previous findings showed that acute treatment with MPH alone has robust stimulatory effects on substance P, relatively minor effects on dynorphin and minimal or no effects on enkephalin expression (Yano and Steiner, 2007). Adding FLX to acute MPH confirmed differential effects for these neuropeptides. FLX potentiated MPH-induced expression of substance P (Fig. 1B) and, to some degree, dynorphin, but had no effect on enkephalin (Van Waes et al., 2012). These findings thus suggested some selectivity for the direct pathway by the acute drug treatment.
A further study determined the effects of 5-day repeated coadministration of MPH and FLX on neuropeptide expression in the striatum. Results showed that neither repeated treatment with MPH (5 mg/kg) alone, nor with FLX (5 mg/kg) alone produced changes in dynorphin or enkephalin expression. In contrast, combined MPH+FLX treatment significantly increased the expression of both neuropeptides (Fig. 2C). These findings indicate that repeated combined treatment produces molecular changes in both striatal output pathways, unlike repeated treatment with MPH alone (Brandon and Steiner, 2003) or acute combined treatment (Van Waes et al., 2012), which both favor the direct pathway. These effects of repeated combined treatment are thus also more “cocaine-like” than those of MPH alone (Yano and Steiner, 2007).
Effects of juvenile methylphenidate plus fluoxetine treatment on molecular signaling in the midbrain and behavior
In a series of studies, we (C.B., B.W. and colleagues) investigated the behavioral consequences of juvenile exposure to MPH and FLX. These studies revealed that exposure to either MPH or FLX alone during early life altered sensitivity to stress, anxiety-eliciting situations, and natural and drug rewards in adulthood (Andersen et al., 2002; Bolanos et al., 2003; Karpova et al., 2009; Iñiguez et al., 2010b). Thus, it was of interest to determine how combined MPH plus FLX treatment impacted these behavioral parameters and to assess which neurobiological changes could be mediating these behaviors. While the mechanisms underlying the long-lasting effects of MPH and FLX are unknown, brain derived neurotrophic factor (BDNF)’s downstream target extracellular signal-regulated protein kinase (ERK) 1/2 and its targets cAMP response element-binding protein (CREB) and mammalian target of rapamycin (mTOR) have been implicated in these cellular processes (Mutschler et al., 2000; Carlezon et al., 2005; Covington et al., 2005; Fumagalli et al., 2005; Lu et al., 2006; Iñiguez et al., 2010c; Subramaniam and Unsicker, 2010). Therefore, the studies presented below assessed the effects of juvenile MPH+FLX treatment on adult responsiveness to stress and the activation of ERK-related molecules within the VTA, a neural substrate implicated in regulation of mood (Bolaños et al., 2003; Eisch et al., 2003; Nestler and Carlezon, 2006; Iñiguez et al., 2010a).
Effects of juvenile MPH, FLX, and MPH+FLX exposure on responses to aversive stimuli
The forced swim test (FST) was used to assess rats’ responsiveness to stress (behavioral despair) (Fig. 3). Rats received vehicle (VEH), MPH, FLX, or MPH+FLX daily as juveniles (postnatal days 21-34) and were tested in adulthood (after postnatal day 60) as previously described (Warren et al., 2011). Latency to become immobile and total immobility varied as a function of juvenile drug pretreatment. MPH decreased latency to immobility and increased total immobility, while FLX pretreatment decreased total immobility without affecting latency to immobility when compared to the VEH-pretreated controls. MPH+FLX pretreatment significantly decreased latency to immobility, without influencing total immobility, relative to the VEH-pretreated controls.
Figure 3.
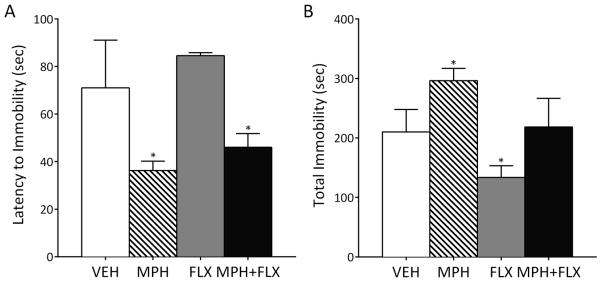
Effects of juvenile exposure to vehicle (VEH), MPH, FLX, or MPH+FLX on forced swimming behaviors. Long-term (2 months after the last injection; n= 6-8/group): (A) MPH and MPH+FLX decreased latency to immobility (p<0.05), whereas FLX had no effect when compared to the VEH-pretreated controls. (B) MPH increased (p<0.05), FLX decreased (p<0.05), and MPH+FLX had no effect on total immobility when compared to the VEH-pretreated controls. *Significantly different from the VEH-pretreated controls (p<0.05). Data are presented as total immobility (in seconds; mean ± SEM).
Effects of juvenile MPH, FLX, and MPH+FLX exposure on ERK signaling in the VTA
The activity of ERK signaling 2 months after juvenile VEH or MPH+FLX exposure was also assessed. ERK signaling was inferred from the phosphorylation of ERK2 protein and two downstream targets, CREB and mTOR (Fig. 4A-C). MPH+FLX exposure increased levels of phosphorylated ERK2, CREB, and mTOR protein within the VTA when compared to the VEH-pretreated controls (all normalized to GAPDH and presented as % of phosphorylated protein). No changes in levels of total ERK2, total CREB, total mTOR, or GAPDH protein were detected. Taken together, these findings indicate that MPH+FLX exposure increased ERK2 activity in the VTA.
Figure 4.
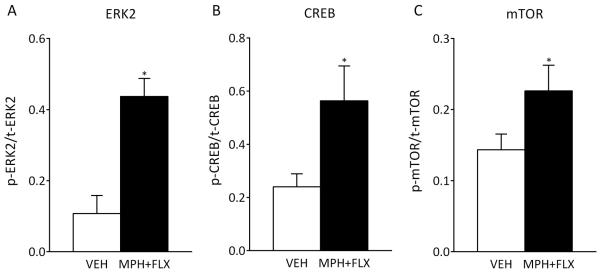
Long-term effects of juvenile exposure to VEH, MPH, FLX, or MPH+FLX on protein phosphorylation within the VTA 2 months after the last injection (n= 8/group). Exposure to MPH+FLX significantly increased the levels of phospho- (p−) ERK2 (A), p-CREB (B), and p-mTOR proteins (C) without affecting total (t) levels of protein when compared to the VEH-pretreated controls (p<0.05, respectively). *Significantly different from the VEH-pretreated controls (p<0.05). Data are presented as a ratio of total protein normalized to GAPDH (mean ± SEM).
Reversal of combined MPH+FLX-induced behavioral despair in adulthood
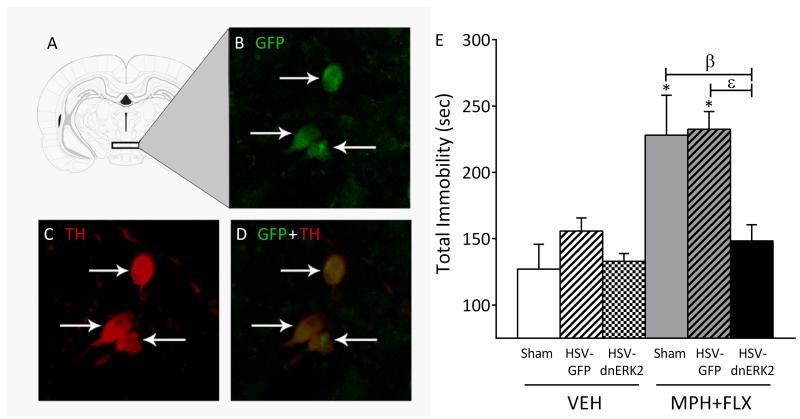
Because juvenile MPH+FLX treatment increases ERK2 signaling within the VTA in adulthood, and ERK activity increases susceptibility to stress (Shen et al., 2004; Iñiguez et al., 2010a), we assessed the consequences of disrupting ERK signaling (by viral constructs) in the VTA on behavioral despair in the FST. Local injection of HSV-dnERK2 (Fig. 5) reduced ERK2 signaling within the VTA of adult rats pretreated with MPH+FLX as juveniles (Iñiguez et al., 2010a). The effects of viral surgeries on day 2 of forced swimming are shown in Figure 5. Analyses indicated that total immobility (Fig. 5) varied as a function of virus treatment. VEH-pretreated rats receiving either microinjections of HSV-GFP (viral control) or HSV-dnERK2-GFP did not differ from the VEH+sham controls. MPH+FLX-pretreated rats receiving sham surgery or HSV-GFP had higher levels of total immobility compared to the VEH+sham controls. Conversely, MPH+FLX-pretreated rats microinjected with HSV-dnERK2 did not differ from the VEH+sham controls, but demonstrated decreased total immobility, when compared to the MPH+FLX-pretreated rats receiving HSV-GFP, indicating that viral-mediated blockade of ERK2 activity reversed the effects of MPH+FLX pretreatment.
Figure 5.
Viral-mediated blockade of ERK2 in the rat VTA regulates behavioral responses to forced swimming. (A) Region of VTA to which microinjections of HSV vectors were targeted (Paxinos and Watson, 1998). (B) Cells expressing dnERK2 (green: cyanine 2) fluorescence. (C) Cells expressing tyrosine hydroxylase (TH) (red: cyanine 3) fluorescence. (D) Merged image of B and C showing dual-labeled neurons in the VTA (magnification, 400X; ~5 mm caudal to Bregma). Arrows indicate labeled cells. (E) Microinjections of HSV-GFP or HSV-dnERK2 into the VTA in VEH pretreated rats had no effect on total immobility when compared to VEH+sham controls (p>0.05). MPH+FLX-pretreated rats receiving sham or HSV-GFP had increased total immobility when compared to the VEH+sham and VEH+HSV-GFP controls (p<0.05). MPH+FLX-pretreated rats receiving HSV-dnERK2 did not differ when compared to the VEH+sham controls (p>0.05, respectively; n= 6-10/group). *Significantly different from the VEH+sham controls (p<0.05). β Significantly different from MPH+FLX+sham (p<0.05). ε Significantly different from MPH+FLX+HSV-GFP (p<0.05). Data are presented as total immobility (in seconds; mean ± SEM).
In summary, this study (Warren et al., 2011) assessed the neurobiological consequences of concomitant exposure to MPH and FLX, two drugs that are often combined for the management of co-morbid ADHD and depression in pediatric populations (Bhatara et al., 2004). We show that chronic juvenile administration of MPH, FLX, or MPH+FLX alters responsiveness to both rewarding (see Warren et al., 2011) and aversive situations (FST), and disrupts VTA ERK2 expression/signaling. Blockade of VTA ERK2 activity rescued the MPH+FLX-induced behavioral deficits in forced swimming, like administration of antidepressants (Lucki, 1997; Iñiguez et al., 2010b). Together, our findings show that juvenile exposure to combined MPH+FLX increases sensitivity to reward, anxiety- and stress-eliciting situations later in life, and implicate dysregulated ERK signaling within the VTA as a potential mechanism underlying these effects.
Discussion and conclusions
The findings reviewed here show that juvenile treatment with SSRIs in conjunction with psychostimulants such as MPH produces more robust neuroadaptations in the dopamine pathways and their projection targets. These effects include potentiated changes in gene regulation in neurons of the striatum and the nucleus accumbens, and molecular changes in the midbrain areas that give rise to the dopamine projections (VTA). Importantly, at least some of these neuronal changes endured into the adulthood of the animals.
The observed gene regulation effects in the striatum to some degree mimic molecular effects of psychostimulants such as cocaine, which are considered part of the molecular basis of psychostimulant addiction (see Steiner and Van Waes, 2013). Changes in gene regulation were present across most functional domains of the striatum, but were most robust in the lateral, sensorimotor striatum. The sensorimotor striatum is critical for stimulus-response (habit) learning, and drug-induced molecular changes in this part of the striatum are implicated in aberrant habit formation and compulsive behavior in drug addiction, as well as in relapse to drug seeking after previous drug exposure (e.g., Everitt and Robbins, 2005; see Steiner and Van Waes, 2013, for discussion). The molecular changes observed in the nucleus accumbens and VTA may contribute to deficient reward function.
As reviewed here, MPH alone can induce some of these molecular effects (Steiner and Van Waes, 2013), but the addition of SSRIs to MPH typically enhances such gene regulation. These findings thus suggest an enhanced addiction liability for the combination treatment, especially with MPH use as a cognitive enhancer or recreational drug, which often involve higher doses (see Steiner and Van Waes, 2013 for discussion). It is unclear from clinical studies whether MPH alone (in ADHD treatment) increases the risk for subsequent substance use disorders (Barkley et al., 2003; Wilens et al., 2003; Kollins, 2008). Preclinical studies in animal models are also ambiguous. On the one hand, there is evidence that pretreatment with MPH alone facilitates subsequent cocaine seeking and taking in the cocaine self-administration model (Brandon et al., 2001; Crawford et al., 2011), which would suggest an increased risk (O’Connor et al., 2011). On the other hand, behavioral models such as the conditioned place preference (CPP) task indicate reduced rewarding effects of cocaine after repeated MPH pretreatment (Andersen et al., 2002; Wiley et al., 2009; Warren et al., 2011). Although speculative, these observed differences in preclinical models may be due, at least in part, to variables such as age at time of drug exposure, and/or dose and length of treatment. It is not known whether pretreatment with SSRIs in conjunction with MPH will modify subsequent cocaine self-administration behavior. However, pretreatment with SSRIs plus MPH reverses the cocaine-conditioned place aversion seen with MPH pretreatment and produces stronger cocaine preference than without drug pretreatment (Warren et al., 2011). This effect is especially robust in adult rats pretreated as juveniles (Warren et al., 2011). These findings would suggest an increased addiction liability for such combination treatments.
However, adverse behavioral effects may not be limited to the addiction risk. Results presented here show deficits in mood-related behavior as a result of combined MPH+FLX exposure. Forced swimming results showed that MPH and MPH+FLX did not influence behavioral despair, whereas FLX induced a stress-resistant phenotype 24 h after treatment (Iñiguez et al., 2010b). As adults, MPH- and MPH+FLX-pretreated rats showed enhanced susceptibility to swimming stress, whereas FLX-pretreated rats showed decreased vulnerability. These data support evidence that juvenile exposure to MPH induces enduring vulnerability to stress (Lagace et al., 2006; Halladay et al., 2009; Wiley et al., 2009), while FLX exposure results in a stress-resistant behavioral phenotype in adulthood (Karpova et al., 2009; Iñiguez et al., 2010b). Long-term, MPH+FLX pretreated rats did not show substantial change in total immobility, but did show a “depression-like” behavioral profile in latency to immobility. Furthermore, a separate group of MPH+FLX-pretreated rats receiving sham or HSV-GFP surgeries showed increased total immobility. Taken together, these findings seem to indicate not only enhanced sensitivity to cocaine and sucrose reward (place preference conditioning; Warren et al., 2011), but also enhanced reactivity to stressful situations that may be due, in part, to changes in ERK signaling within the VTA.
In conclusion, the findings in juvenile rats summarized in this review indicate that MPH plus SSRI combination treatments can produce a variety of molecular changes that mimic, in part, neuroadaptations induced by illicit drugs such as cocaine. Some of these molecular changes are potentiated effects, others are distinct to the combined treatment. These neuronal changes are associated with behavioral changes indicative of deficient reward sensitivity and mood regulation, with some of these effects enduring into adulthood. These findings underscore the need for more in-depth investigation of the effects of such combination therapies, especially when they are used in the developing brain.
Acknowledgements
This work was supported in part by NIDA grants DA031916, DA011261 (to H.S.), DA026854 (to C.B.), and MH093311 (to B.W.).
References
- Adriani W, Leo D, Greco D, Rea M, di Porzio U, Laviola G, Perrone-Capano C. Methylphenidate administration to adolescent rats determines plastic changes in reward-related behavior and striatal gene expression. Neuropsychopharmacology. 2006a;31:1946–1956. doi: 10.1038/sj.npp.1300962. [DOI] [PubMed] [Google Scholar]
- Adriani W, Leo D, Guarino M, Natoli A, Di Consiglio E, De Angelis G, Traina E, Testai E, Perrone-Capano C, Laviola G. Short-term effects of adolescent methylphenidate exposure on brain striatal gene expression and sexual/endocrine parameters in male rats. Ann. N. Y. Acad. Sci. 2006b;1074:52–73. doi: 10.1196/annals.1369.005. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Stimulants and the developing brain. Trends Pharmacol. Sci. 2005;26:237–243. doi: 10.1016/j.tips.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WAJ. Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat. Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher K. Does the treatment of attention-deficit/hyperactivity disorder with stimulants contribute to drug use/abuse? A 13-year prospective study. Pediatrics. 2003;111:97–109. doi: 10.1542/peds.111.1.97. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Baraban JM. Activation of transcription factor genes in striatum by cocaine: role of both serotonin and dopamine systems. J. Pharmacol. Exp. Ther. 1993;267:496–505. [PubMed] [Google Scholar]
- Bhatara V, Feil M, Hoagwood K, Vitiello B, Zima B. National trends in concomitant psychotropic medication with stimulants in pediatric visits: practice versus knowledge. J. Atten. Disord. 2004;7:217–226. doi: 10.1177/108705470400700404. [DOI] [PubMed] [Google Scholar]
- Bolaños CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol. Psychiatry. 2003;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- Bolaños CA, Perrotti LI, Edwards S, Eisch AJ, Barrot M, Olson VG, Russell DS, Neve RL, Nestler EJ. Phospholipase Cgamma in distinct regions of the ventral tegmental area differentially modulates mood-related behaviors. J. Neurosci. 2003;23:7569–7576. doi: 10.1523/JNEUROSCI.23-20-07569.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Steiner H. Repeated methylphenidate treatment in adolescent rats alters gene regulation in the striatum. Eur. J. Neurosci. 2003;18:1584–1592. doi: 10.1046/j.1460-9568.2003.02892.x. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Konradi C. Understanding the neurobiological consequences of early exposure to psychotropic drugs: linking behavior with molecules. Neuropharmacology. 2004;47:47–60. doi: 10.1016/j.neuropharm.2004.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrey N, Wilkinson M. A review of psychostimulant-induced neuroadaptation in developing animals. Neurosci. Bull. 2011;27:197–214. doi: 10.1007/s12264-011-1004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon N, Scearce-Levie K, Lucas JJ, Rocha B, Hen R. Modulation of the effects of cocaine by 5-HT1B receptors: a comparison of knockouts and antagonists. Pharmacol. Biochem. Behav. 2000;67:559–566. doi: 10.1016/s0091-3057(00)00389-0. [DOI] [PubMed] [Google Scholar]
- Castle L, Aubert RE, Verbrugge RR, Khalid M, Epstein RS. Trends in medication treatment for ADHD. J. Atten. Disord. 2007;10:335–342. doi: 10.1177/1087054707299597. [DOI] [PubMed] [Google Scholar]
- Chase T, Carrey N, Soo E, Wilkinson M. Methylphenidate regulates activity regulated cytoskeletal associated but not brain-derived neurotrophic factor gene expression in the developing rat striatum. Neuroscience. 2007;144:969–984. doi: 10.1016/j.neuroscience.2006.10.035. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A. Neural activity mapping with inducible transcription factors. Neuroreport. 1997;8:v–ix. [PubMed] [Google Scholar]
- Cotterly L, Beverley JA, Yano M, Steiner H. Dysregulation of gene induction in corticostriatal circuits after repeated methylphenidate treatment in adolescent rats: Differential effects on zif 268 and homer 1a. Eur. J. Neurosci. 2007;25:3617–3628. doi: 10.1111/j.1460-9568.2007.05570.x. [DOI] [PubMed] [Google Scholar]
- Covington HE, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr., Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Baella SA, Farley CM, Herbert MS, Horn LR, Campbell RH, Zavala AR. Early methylphenidate exposure enhances cocaine self-administration but not cocaine-induced conditioned place preference in young adult rats. Psychopharmacology. 2011;213:43–52. doi: 10.1007/s00213-010-2011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoka A, Bahrick A, Mehtonen OP. Persistent sexual dysfunction after discontinuation of selective serotonin reuptake inhibitors. J. Sex. Med. 2008;5:227–233. doi: 10.1111/j.1743-6109.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Bolaños CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol. Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Frankel PS, Alburges ME, Bush L, Hanson GR, Kish SJ. Striatal and ventral pallidum dynorphin concentrations are markedly increased in human chronic cocaine users. Neuropharmacology. 2008;55:41–46. doi: 10.1016/j.neuropharm.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Molteni R, Calabrese F, Frasca A, Racagni G, Riva MA. Chronic fluoxetine administration inhibits extracellular signal-regulated kinase 1/2 phosphorylation in rat brain. J. Neurochem. 2005;93:1551–1560. doi: 10.1111/j.1471-4159.2005.03149.x. [DOI] [PubMed] [Google Scholar]
- Halladay LR, Iñiguez SD, Furqan F, Previte MC, Chisum AM, Crawford CA. Methylphenidate potentiates morphine-induced antinociception, hyperthermia, and locomotor activity in young adult rats. Pharmacol. Biochem. Behav. 2009;92:190–196. doi: 10.1016/j.pbb.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suárez-Fariñas M, Schwarz C, Stephan DA, Surmeier DJ, Greengard P, Heintz N. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner KA, Adams DH, Hanson GR, Keefe KA. Blockade of stimulant-induced preprodynorphin mRNA expression in the striatal matrix by serotonin depletion. Neuroscience. 2005;131:67–77. doi: 10.1016/j.neuroscience.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Iversen L. Neurotransmitter transporters and their impact on the development of psychopharmacology. Br. J. Pharmacol. 2006;147(Suppl1):S82–88. doi: 10.1038/sj.bjp.0706428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Vialou V, Warren BL, Cao JL, Alcantara LF, Davis LC, Manojlovic Z, Neve RL, Russo SJ, Han MH, Nestler EJ, Bolaños-Guzmán CA. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J. Neurosci. 2010a;30:7652–7663. doi: 10.1523/JNEUROSCI.0951-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Bolaños-Guzmán CA. Short- and long-term functional consequences of fluoxetine exposure during adolescence in male rats. Biol. Psychiatry. 2010b;67:1057–1066. doi: 10.1016/j.biopsych.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Neve RL, Russo SJ, Nestler EJ, Bolaños-Guzmán CA. Viral-mediated expression of extracellular signal-regulated kinase-2 in the ventral tegmental area modulates behavioral responses to cocaine. Behav. Brain Res. 2010c;214:460–464. doi: 10.1016/j.bbr.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova NN, Lindholm J, Pruunsild P, Timmusk T, Castrén E. Long-lasting behavioural and molecular alterations induced by early postnatal fluoxetine exposure are restored by chronic fluoxetine treatment in adult mice. Eur. Neuropsychopharmacol. 2009;19:97–108. doi: 10.1016/j.euroneuro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog. Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Kollins SH. ADHD, substance use disorders, and psychostimulant treatment: current literature and treatment guidelines. J. Atten. Disord. 2008;12:115–125. doi: 10.1177/1087054707311654. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J. Neurochem. 1997;68:2032–2037. doi: 10.1046/j.1471-4159.1997.68052032.x. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Yee JK, Bolaños CA, Eisch AJ. Juvenile administration of methylphenidate attenuates adult hippocampal neurogenesis. Biol. Psychiatry. 2006;60:1121–1130. doi: 10.1016/j.biopsych.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Lavretsky H, Kim MD, Kumar A, Reynolds CF. Combined treatment with methylphenidate and citalopram for accelerated response in the elderly: an open trial. J. Clin. Psychiatry. 2003;64:1410–1414. doi: 10.4088/jcp.v64n1202. [DOI] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Lucas JJ, Segu L, Hen R. 5-Hydroxytryptamine1B receptors modulate the effect of cocaine on c-fos expression: converging evidence using 5-hydroxytryptamine1B knockout mice and the 5-hydroxytryptamine1B/1D antagonist GR127935. Mol. Pharmacol. 1997;51:755–763. doi: 10.1124/mol.51.5.755. [DOI] [PubMed] [Google Scholar]
- Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav. Pharmacol. 1997;8:523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- Marco EM, Adriani W, Ruocco LA, Canese R, Sadile AG, Laviola G. Neurobehavioral adaptations to methylphenidate: the issue of early adolescent exposure. Neurosci. Biobehav. Rev. 2011;35:1722–1739. doi: 10.1016/j.neubiorev.2011.02.011. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat. Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Reimer S, Hollt V, Herz A. Regulation of striatal prodynorphin mRNA levels by the raphe-striatal pathway. Brain Res. 1988;464:15–22. doi: 10.1016/0169-328x(88)90013-7. [DOI] [PubMed] [Google Scholar]
- Mutschler NH, Miczek KA, Hammer RPJ. Reduction of zif268 messenger RNA expression during prolonged withdrawal following “binge” cocaine self-administration in rats. Neuroscience. 2000;100:531–538. doi: 10.1016/s0306-4522(00)00298-0. [DOI] [PubMed] [Google Scholar]
- Nelson JC. Augmentation strategies in the treatment of major depressive disorder. Recent findings and current status of augmentation strategies. CNS Spectr. 2007;12(Suppl 22):6–9. doi: 10.1017/s1092852900016011. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WAJ. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- O’Connor EC, Chapman K, Butler P, Mead AN. The predictive validity of the rat self-administration model for abuse liability. Neurosci. Biobehav. Rev. 2011;35:912–938. doi: 10.1016/j.neubiorev.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- Ravindran AV, Kennedy SH, O’Donovan MC, Fallu A, Camacho F, Binder CE. Osmotic-release oral system methylphenidate augmentation of antidepressant monotherapy in major depressive disorder: results of a double-blind, randomized, placebo-controlled trial. J. Clin. Psychiatry. 2008;69:87–94. doi: 10.4088/jcp.v69n0112. [DOI] [PubMed] [Google Scholar]
- Renthal W, Carle TL, Maze I, Covington HE, Truong HT, Alibhai I, Kumar A, Montgomery RL, Olson EN, Nestler EJ. Delta FosB mediates epigenetic desensitization of the cfos gene after chronic amphetamine exposure. J. Neurosci. 2008;28:7344–7349. doi: 10.1523/JNEUROSCI.1043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol. Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton JL, Whitmire JT. Pediatric stimulant and selective serotonin reuptake inhibitor prescription trends: 1992 to 1998. Arch. Pediatr. Adolesc. Med. 2001;155:560–565. doi: 10.1001/archpedi.155.5.560. [DOI] [PubMed] [Google Scholar]
- Safer DJ, Zito JM, DosReis S. Concomitant psychotropic medication for youths. Am. J. Psychiatry. 2003;160:438–449. doi: 10.1176/appi.ajp.160.3.438. [DOI] [PubMed] [Google Scholar]
- SAMHSA Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. 2012 NSDUH Series H-44, HHS Publication No. (SMA) 12-4713. http://www.samhsa.gov/data/NSDUH/2011SummNatFindDetTables/Index.aspx. [Google Scholar]
- Sharp FR, Sagar SM, Swanson RA. Metabolic mapping with cellular resolution: c-fos vs. 2-deoxyglucose. Crit. Rev. Neurobiol. 1993;7:205–228. [PubMed] [Google Scholar]
- Shen CP, Tsimberg Y, Salvadore C, Meller E. Activation of Erk and JNK MAPK pathways by acute swim stress in rat brain regions. BMC Neurosci. 2004;5:36. doi: 10.1186/1471-2202-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol. Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TJ. ADHD and comorbidity in childhood. J. Clin. Psychiatry. 2006;67(Suppl 8):27–31. [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp. Brain Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- Steiner H, Van Waes V. Addiction-related gene regulation: Risks of exposure to cognitive enhancers vs. other psychostimulants. Prog. Neurobiol. 2013;100:60–80. doi: 10.1016/j.pneurobio.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Van Waes V, Marinelli M. Fluoxetine potentiates methylphenidate-induced gene regulation in addiction-related brain regions: Concerns for use of cognitive enhancers? Biol. Psychiatry. 2010;67:592–594. doi: 10.1016/j.biopsych.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S, Unsicker K. ERK and cell death: ERK1/2 in neuronal death. FEBS J. 2010;277:22–29. doi: 10.1111/j.1742-4658.2009.07367.x. [DOI] [PubMed] [Google Scholar]
- Svetlov SI, Kobeissy FH, Gold MS. Performance enhancing, non-prescription use of Ritalin: a comparison with amphetamines and cocaine. J. Addict. Dis. 2007;26:1–6. doi: 10.1300/J069v26n04_01. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Increasing use of stimulants warns of potential abuse. Nature. 2008;453:586. doi: 10.1038/453586a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM, Wigal TL, Volkow ND. Contrast of medical and nonmedical use of stimulant drugs, basis for the distinction, and risk of addiction: comment on Smith and Farah (2011) Psychol. Bull. 2011;137:742–748. doi: 10.1037/a0024898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem. Pharmacol. 2008;75:112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ. Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration. Pharmacotherapy. 2006;26:1501–1510. doi: 10.1592/phco.26.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas U. Modulation of synaptic signalling complexes by Homer proteins. J. Neurochem. 2002;81:407–413. doi: 10.1046/j.1471-4159.2002.00869.x. [DOI] [PubMed] [Google Scholar]
- Unal CT, Beverley JA, Willuhn I, Steiner H. Long-lasting dysregulation of gene expression in corticostriatal circuits after repeated cocaine treatment in adult rats: Effects on zif 268 and homer 1a. Eur. J. Neurosci. 2009;29:1615–1626. doi: 10.1111/j.1460-9568.2009.06691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waes V, Beverley J, Marinelli M, Steiner H. Selective serotonin reuptake inhibitor antidepressants potentiate methylphenidate (Ritalin)-induced gene regulation in the adolescent striatum. Eur. J. Neurosci. 2010;32:435–447. doi: 10.1111/j.1460-9568.2010.07294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waes V, Carr B, Beverley JA, Steiner H. Fluoxetine potentiation of methylphenidate-induced neuropeptide expression in the striatum occurs selectively in direct pathway (striatonigral) neurons. J. Neurochem. 2012;122:1054–1064. doi: 10.1111/j.1471-4159.2012.07852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waes V, Vandrevala M, Beverley J, Steiner H. Selective serotonin re-uptake inhibitors potentiate gene blunting induced by repeated methylphenidate treatment: Zif268 versus Homer1a. Addict. Biol. 2013 doi: 10.1111/adb.12067. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Franceschi D, Maynard L, Ding YS, Gatley SJ, Gifford A, Zhu W, Swanson JM. Relationship between blockade of dopamine transporters by oral methylphenidate and the increases in extracellular dopamine: therapeutic implications. Synapse. 2002;43:181–187. doi: 10.1002/syn.10038. [DOI] [PubMed] [Google Scholar]
- Walker PD, Capodilupo JG, Wolf WA, Carlock LR. Preprotachykinin and preproenkephalin mRNA expression within striatal subregions in response to altered serotonin transmission. Brain Res. 1996;732:25–35. doi: 10.1016/0006-8993(96)00483-0. [DOI] [PubMed] [Google Scholar]
- Warren BL, Iñiguez SD, Alcantara LF, Wright KN, Parise EM, Weakley SK, Bolaños-Guzmán CA. Juvenile administration of concomitant methylphenidate and fluoxetine alters behavioral reactivity to reward- and mood-related stimuli and disrupts ventral tegmental area gene expression in adulthood. J. Neurosci. 2011;31:10347–10358. doi: 10.1523/JNEUROSCI.1470-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxmonsky J. Assessment and treatment of attention deficit hyperactivity disorder in children with comorbid psychiatric illness. Curr. Opin. Pediatr. 2003;15:476–482. doi: 10.1097/00008480-200310000-00006. [DOI] [PubMed] [Google Scholar]
- White BP, Becker-Blease KA, Grace-Bishop K. Stimulant medication use, misuse, and abuse in an undergraduate and graduate student sample. J. Am. Coll. Health. 2006;54:261–268. doi: 10.3200/JACH.54.5.261-268. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, Fusillo S. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47:21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Wiley MD, Poveromo LB, Antapasis J, Herrera CM, Bolaños Guzmán CA. Kappa-opioid system regulates the long-lasting behavioral adaptations induced by early-life exposure to methylphenidate. Neuropsychopharmacology. 2009;34:1339–1350. doi: 10.1038/npp.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Sun W, Steiner H. Topography of cocaine-induced gene regulation in the rat striatum: Relationship to cortical inputs and role of behavioural context. Eur. J. Neurosci. 2003;17:1053–1066. doi: 10.1046/j.1460-9568.2003.02525.x. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Methylphenidate (Ritalin) induces Homer 1a and zif 268 expression in specific corticostriatal circuits. Neuroscience. 2005a;132:855–865. doi: 10.1016/j.neuroscience.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Topography of methylphenidate (Ritalin)-induced gene regulation in the striatum: differential effects on c-fos, substance P and opioid peptides. Neuropsychopharmacology. 2005b;30:901–915. doi: 10.1038/sj.npp.1300613. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Methylphenidate and cocaine: the same effects on gene regulation? Trends Pharmacol. Sci. 2007;28:588–596. doi: 10.1016/j.tips.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Yuferov V, Nielsen D, Butelman E, Kreek MJ. Microarray studies of psychostimulant-induced changes in gene expression. Addict. Biol. 2005;10:101–118. doi: 10.1080/13556210412331308976. [DOI] [PubMed] [Google Scholar]