Abstract
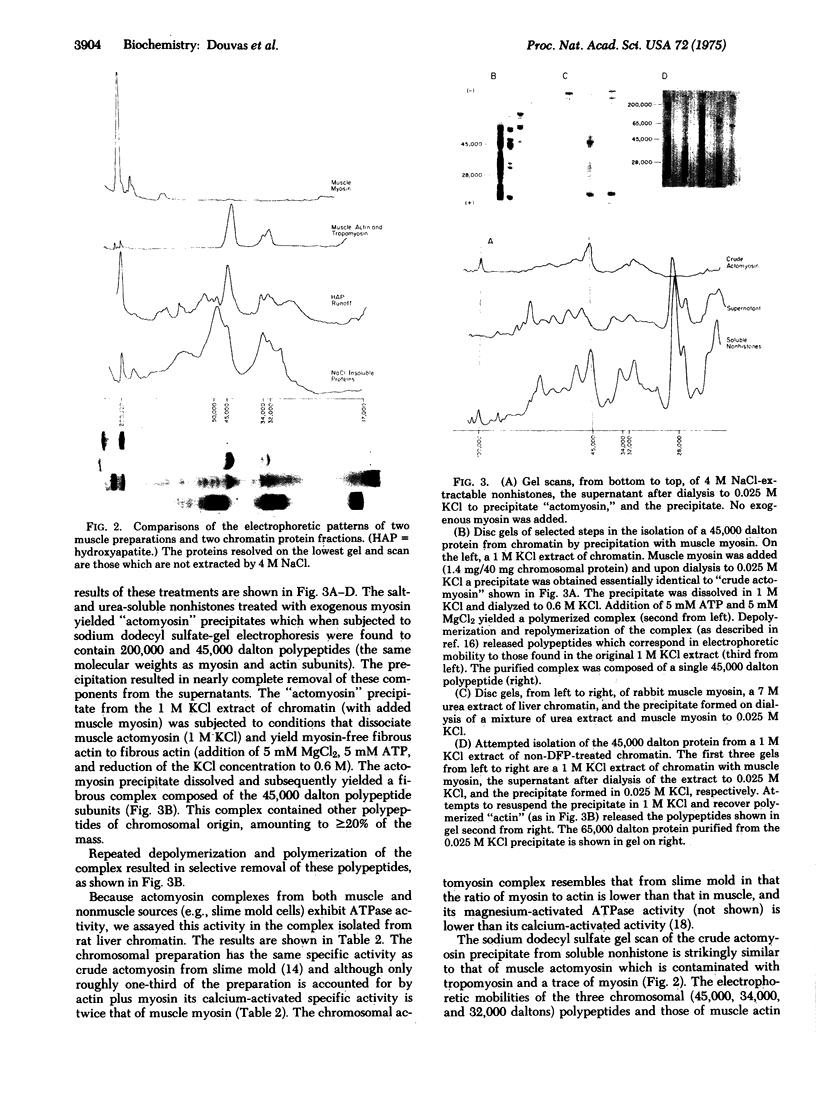
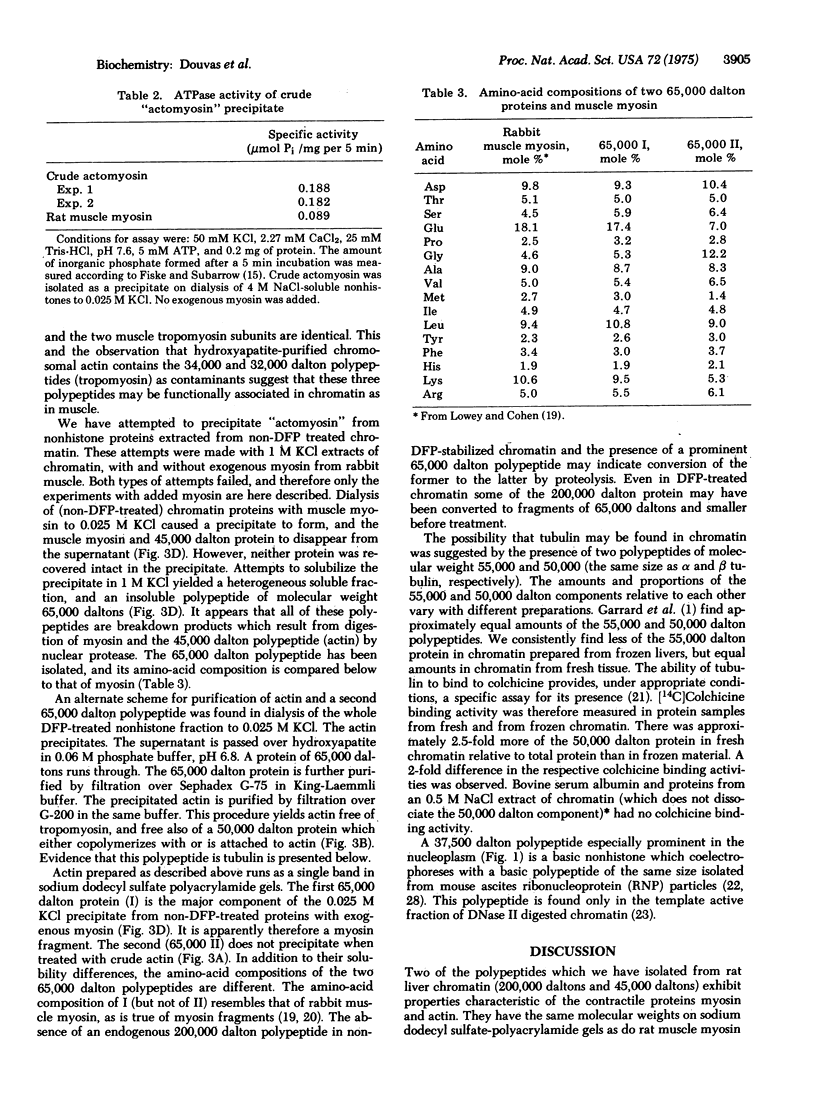
Two major nonhistone polypeptides from rat liver chromatin have been identified as myosin and actin. Preliminary observations indicate that three other chromatin polypeptides of molecular weights 50,000, 34,000, and 32,000 are tubulin and heavy and light tropomyosin, respectively. A sixth component of molecular weight 65,000 which has been purified and electrophoreses as a single band on sodium dodecyl sulfate-polyacrylamide gels may be composed in part of protease-digested myosin. These six polypeptides together account for as much as 38% of the nonhistone protein mass of chromatin in this tissue.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R., Taylor E. W. Isolation of an actomyosin-like protein complex from slime mold plasmodium and the separation of the complex into actin- and myosin-like fractions. Biochemistry. 1969 Dec;8(12):4964–4975. doi: 10.1021/bi00840a046. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Buller A. J., Mommaerts W. F., Seraydarian K. Enzymic properties of myosin in fast and slow twitch muscles of the cat following cross-innervation. J Physiol. 1969 Dec;205(3):581–597. doi: 10.1113/jphysiol.1969.sp008984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae C. B. Reconstitution of chromatin: mode of reassociation of chromosomal proteins. Biochemistry. 1975 Mar 11;14(5):900–906. doi: 10.1021/bi00676a005. [DOI] [PubMed] [Google Scholar]
- Chong M. T., Garrard W. T., Bonner J. Purification and properties of a neutral protease from rat liver chromatin. Biochemistry. 1974 Dec 3;13(25):5128–5134. doi: 10.1021/bi00722a012. [DOI] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg E., Kielley W. W. Troponin-tropomyosin complex. Column chromatographic separation and activity of the three, active troponin components with and without tropomyosin present. J Biol Chem. 1974 Aug 10;249(15):4742–4748. [PubMed] [Google Scholar]
- Elgin S. C., Bonner J. Limited heterogeneity of the major nonhistone chromosomal proteins. Biochemistry. 1970 Oct 27;9(22):4440–4447. doi: 10.1021/bi00824a027. [DOI] [PubMed] [Google Scholar]
- Garrard W. T., Bonner J. Changes in chromatin proteins during liver regeneration. J Biol Chem. 1974 Sep 10;249(17):5570–5579. [PubMed] [Google Scholar]
- Garrard W. T., Pearson W. R., Wake S. K., Bonner J. Stoichiometry of chromatin proteins. Biochem Biophys Res Commun. 1974 May 7;58(1):50–57. doi: 10.1016/0006-291x(74)90889-4. [DOI] [PubMed] [Google Scholar]
- Garrels J. I., Elgin S. C., Bonner J. A histone protease of rat liver chromatin. Biochem Biophys Res Commun. 1972 Jan 31;46(2):545–551. doi: 10.1016/s0006-291x(72)80173-6. [DOI] [PubMed] [Google Scholar]
- Gottesfeld J. M., Garrard W. T., Bagi G., Wilson R. F., Bonner J. Partial purification of the template-active fraction of chromatin: a preliminary report. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2193–2197. doi: 10.1073/pnas.71.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenstein E., Rich A. Non-identity of muscle and non-muscle actins. Biochem Biophys Res Commun. 1975 May 19;64(2):472–477. doi: 10.1016/0006-291x(75)90345-9. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- LOWEY S., COHEN C. Studies on the structure of myosin. J Mol Biol. 1962 Apr;4:293–308. doi: 10.1016/s0022-2836(62)80007-2. [DOI] [PubMed] [Google Scholar]
- Lestourgeon W. M., Forer A., Yang Y. Z., Bertram J. S., Pusch H. P. Contractile proteins. Major components of nuclear and chromosome non-histone proteins. Biochim Biophys Acta. 1975 Feb 27;379(2):529–552. [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Martin T., Billings P., Levey A., Ozarslan S., Quinlan T., Swift H., Urbas L. Some properties of RNA:protein complexes from the nucleus of eukaryotic cells. Cold Spring Harb Symp Quant Biol. 1974;38:921–932. doi: 10.1101/sqb.1974.038.01.094. [DOI] [PubMed] [Google Scholar]
- Robson R. M., Goll D. E., Arakawa N., Stromer M. H. Purification and properties of alpha-actinin from rabbit skeletal muscle. Biochim Biophys Acta. 1970 Feb 17;200(2):296–318. doi: 10.1016/0005-2795(70)90173-x. [DOI] [PubMed] [Google Scholar]
- Seraydarian K., Briskey E. J., Mommaerts W. F. The modification of actomyosin by alpha- actinin. I. A survey of experimental conditions. Biochim Biophys Acta. 1967 Apr 11;133(3):399–411. doi: 10.1016/0005-2795(67)90544-2. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Wu F. C., Elgin S. C., Hood L. E. Nonhistone chromosomal proteins of rat tissues. A comparative study by gel electrophoresis. Biochemistry. 1973 Jul 17;12(15):2792–2797. doi: 10.1021/bi00739a003. [DOI] [PubMed] [Google Scholar]
- Wu F. C., Elgin S. C., Hood L. E. The nonhistone chromosomal proteins of vertebrate liver and kidney: a comparative study by gel electrophoresis. J Mol Evol. 1975 Jul 11;5(2):87–101. doi: 10.1007/BF01732514. [DOI] [PubMed] [Google Scholar]
- van den Broek H. W., Noodén L. D., Sevall J. S., Bonner J. Isolation, purification, and fractionation of nonhistone chromosomal proteins. Biochemistry. 1973 Jan 16;12(2):229–236. doi: 10.1021/bi00726a009. [DOI] [PubMed] [Google Scholar]