Abstract
Bacteriochlorophyll b has the most red-shifted absorbance maximum of all naturally occurring photopigments. It has a characteristic ethylidene group at the C8 position in place of the more common ethyl group, the product of a C8-vinyl reductase, which is carried by the majority of chlorophylls and bacteriochlorophylls used in photosynthesis. The subsequent and first step exclusive to bacteriochlorophyll biosynthesis, the reduction of the C7 = C8 bond, is catalyzed by chlorophyllide oxidoreductase. It has been demonstrated that the enzyme from bacteriochlorophyll a-utilizing bacteria can catalyze the formation of compounds carrying an ethyl group at C8 from both ethyl- and vinyl-carrying substrates, indicating a surprising additional C8-vinyl reductase function, while the enzyme from organisms producing BChl b could only catalyze C7 = C8 reduction with a vinyl substrate, but this product carried an ethylidene group at the C8 position. We have replaced the native chlorophyllide oxidoreductase-encoding genes of Rhodobacter sphaeroides with those from Blastochloris viridis, but the switch from bacteriochlorophyll a to b biosynthesis is only detected when the native conventional C8-vinyl reductase is absent. We propose a non-enzymatic mechanism for ethylidene group formation based on the absence of cellular C8-vinyl reductase activity.
Abbreviations: BChl, bacteriochlorophyll; RC, reaction center; LH, light-harvesting complex; Chl, chlorophyll; E, ethyl; Chlide, chlorophyllide; V, vinyl; 8VR, C8-vinyl reductase; COR, chlorophyllide oxidoreductase; BChlide, bacteriochlorophyllide
Keywords: Bacteriochlorophyll, Chlorophyll, Chlorophyllide oxidoreductase, Photosynthesis, Pathway engineering
Highlights
-
•
We engineer the production of a foreign photopigment in Rhodobacter sphaeroides.
-
•
Native COR-encoding genes are replaced with those from Blastochloris viridis.
-
•
Bacteriochlorophyll b is produced upon additional deletion of conventional 8VR.
-
•
We propose that loss of 8VR activity by COR leads to ethylidene bond formation.
1. Introduction
Bacteriochlorophyll (BChl) b, the most strongly red-shifted (bacterio)chlorin used for light harvesting, was first discovered in 1963 as the major photosynthetic pigment of a Rhodopseudomonas sp. photosynthetic bacterium [1]. BChl b differs from BChl a, the most abundant bacteriochlorin, by the presence of an ethylidene group rather than an ethyl (E) group at the C8 position [2] (Fig. 1A and B), which shifts the Qy absorption band of the unbound pigment 25 nm to the red (from 770 to 795 nm in methanol) [3] (Fig. 1C). BChl b is found in the photosynthetic reaction center (RC)/light-harvesting (LH) 1 complex of Blastochloris (Blc.) viridis (formerly Rhodopseudomonas viridis), first isolated and studied by Drews and Giesbrecht [4], which is able to absorb solar energy approximately 150 nm further into the infrared than an analogous BChl a-containing complex (Fig. 1C). The RC from Blc. viridis was the first membrane protein complex to have its 3D structure determined by X-ray crystallography [5]. The spectral characteristics of BChls allow photosynthetic bacteria to absorb solar energy that is not absorbed by the chlorophyll (Chl)-utilizing cyanobacteria and algae found higher in the water column [6]. The very large red shifts seen for BChl b-containing LH1 complexes, which extend absorption to 1023 nm, sample a region of the solar spectrum well to the red of BChl a-containing LH1 complexes, which absorb in the 800- to 900-nm region. A recent review proposed a re-engineered photosynthetic apparatus for energy capture in which one of the two photosystems found in plants and cyanobacteria was modified so that it could utilize far-red light, while the other photosystem retained the ability to absorb light in the 650- to 700-nm region [7]. Accomplishing such modifications requires control over pigment biosynthesis and photosystem assembly; in this work, we explore the potential of re-routing the biosynthesis of BChl a towards the formation of BChl b.
Fig. 1.
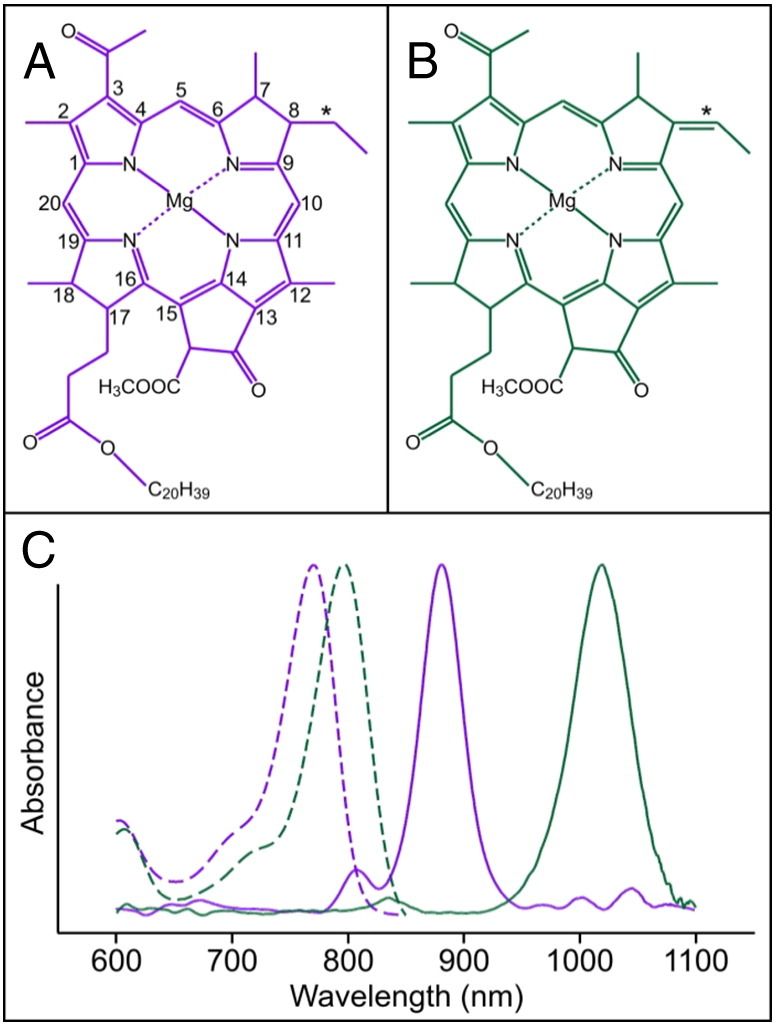
Structures and absorption properties of BChls a and b.
(A) IUPAC numbered chemical structure of BChl a. The ethyl group at C8 is indicated by an asterisk. (B) Chemical structure of BChl b. The ethylidene group at C8 is indicated by an asterisk. (C) Absorption spectra of unbound BChls a (dashed purple) and b (dashed green), and RC-LH1 complexes containing BChls a or b from Rba. sphaeroides (solid purple) and Blc. viridis (solid green), respectively.
BChls a and b share a common biosynthetic pathway with Chls up to the precursor chlorophyllide (Chlide) (Fig. 2). The E group at the C8 position of Chls and BChl a results from the reduction of a vinyl (V) group on Chlide, catalyzed by an 8V reductase (8VR) [8], [9], [10], [11], while the first step in true bacteriochlorin biosynthesis, the reduction of the C7 = C8 of Chlide, is catalyzed by Chlide oxidoreductase (COR) [12], [13], [14], [15]. Recently, both in vitro assays using purified COR components (BchX, BchY and BchZ proteins) [16] and genetic complementations [17] have been used to demonstrate that that the enzymes from BChl a-utilizing bacteria (CORa) can catalyze the formation of 8E bacteriochlorophyllide (BChlide) from both 8E and 8V Chlide substrates, indicating a surprising additional 8VR function of the enzyme. However, an in vitro assay using the COR from Blc. viridis (CORb) indicated that this form of the enzyme was only able to catalyze BChlide formation with an 8V Chlide substrate, but this product, found to carry an ethylidene group at the C8 position, was BChlide b rather than BChlide a [16]. No product was formed when CORb was incubated with 8E Chlide, suggesting strict substrate specificity, correlating with the lack of an 8VR-encoding gene in the sequenced genomes of BChl b-producing organisms [16].
Fig. 2.
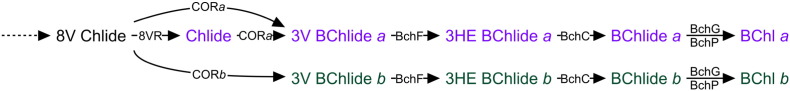
Biosynthetic pathways of BChls a and b from the precursor 8V Chlide.
Pathways highlighted in purple and green are those that utilized by Rba. sphaeroides and Blc. viridis, respectively.
The purple phototrophic bacterium Rhodobacter (Rba.) sphaeroides, which utilizes BChl a in both its light-absorbing antennae and reaction centers, is a model organism for the study of photosynthesis and pigment biosynthesis due to its ability to grow chemotrophically in the dark, allowing deletion of genes involved in these processes [18]. The genome of Rba. sphaeroides contains genes encoding both a conventional 8VR (bciA) and CORa, a mechanism possibly used to ensure that only BChl carrying an 8E group is synthesized [11], [17], [19].
We have replaced the native CORa-encoding genes of Rba. sphaeroides with those encoding the Blc. viridis CORb. Interestingly, our results demonstrate that the CORb replacement mutant retained the ability to synthesize BChl a, while b-type bacteriochlorins could not be detected. This strain was able to photosynthesize, albeit at a reduced rate when compared to the wild type. However, the genetic replacement performed in a strain lacking the conventional 8VR resulted in the biosynthesis of BChl b, yet this second mutation culminated in the loss of photosynthetic viability. Our results lead us to postulate that, rather than catalyzing ethylidene group formation, CORb has lost 8VR activity. We predict that after the enzymatic reduction of C7 = C8, the resulting 8V bacteriochlorin (the existence of which has never been detected in nature) will spontaneously isomerize to the more stable b-type.
2. Materials and methods
2.1. Growth of described strains
Rba. sphaeroides strains were grown microoxically in the dark in a rotary shaker or phototrophically under illumination (90 μmol of photons · m− 2·s− 1) at 34 °C in liquid M22 + medium [20] supplemented with 0.1% casamino acids.
Blc. viridis was grown phototrophically in N2-sparged sodium succinate medium 27 (N medium) [21] under illumination (100 μmol of photons·m− 2·s− 1) at 30 °C as described by Lang and Oesterhelt [22].
Escherichia coli strains JM109 [23] and S17-1 [24] transformed with pK18mobsacB plasmids were grown in a rotary shaker at 37 °C in LB (Luria–Bertani) medium supplemented with 30 μg·ml− 1 kanamycin. All strains and plasmids used in the present study are listed in Supplementary Table 1.
2.2. Construction of mutants of Rba. sphaeroides
The Rba. sphaeroides bchX, bchY and bchZ genes were deleted using the allelic exchange vector pK18mobsacB [25]. Sequences up- and downstream of the three clustered genes were amplified with UpF (5′-CCGGAATTCCCGAAACTGATCGACAGTCTGG-3′; EcoRI restriction site underlined) and UpR (5′-CATGATCGCTCATTGCGTCATGCGGTGGCCCTCCAAT CC-3′), and DownF (5′-GGCCACCGCATGACGCAATGAGCGATCATGCCGTCAACACG-3′) and DownR (5′-CCCAAGCTTCGTGGGTTACGGTAAGGCACC-3′; HindIII restriction site underlined) primers, respectively. The up- and downstream PCR products were fused by overlap extension PCR, and the resulting product was digested with EcoRI and HindIII restriction enzymes and ligated into cut pK18mobsacB. Sequenced clones were conjugated into Rba. sphaeroides from E. coli S17-1, and transconjugants in which the clone had integrated into the genome by homologous recombination were selected on M22 + medium supplemented with kanamycin. Transconjugants that had undergone a second recombination event were then selected on M22 + supplemented with 10% (w/v) sucrose, lacking kanamycin. Sucrose-resistant kanamycin-sensitive colonies had excised the allelic exchange vector through the second recombination event [26]. The deletion of bchX, bchY and bchZ was confirmed by colony PCR using CheckF (5′-CCGCTCGATCTACGATGCGTC-3′) and CheckR (5′-CAGCCATGCTATCCTCCGG-3′) primers.
The bchX, bchY and bchZ genes from Blc. viridis were integrated into the genomes of Rba. sphaeroides strains at their original loci in strains that lack their native COR-encoding genes. A fragment of DNA containing the 3′ end of the Rba. sphaeroides bchC gene, the COR-encoding genes from Blc. viridis and the Rba. sphaeroides pufQ gene was synthesized by Genewiz (South Plainfield, NJ, USA). The sequence was edited to mimic the intergenic regions found between the Rba. sphaeroides COR-encoding genes, and silent base changes were introduced to remove restriction sites required for subcloning and to retain the essential upstream promoter region [27], ribosome binding site and start codon for pufQ (Supplementary Fig. 1). The synthesized fragment was sub-cloned into pK18mobsacB and conjugated into ΔbchXYZ background strains of Rba. sphaeroides as detailed above. Integration of the synthetic sequence, mediated by the presence of the bchC and pufQ sequences, was confirmed by colony PCR using the CheckF and CheckR primers detailed above.
2.3. Extraction of pigments
Pigments were extracted from cell pellets after washing in 20 mM HEPES pH 7.2 or from clarified growth medium by adding 9 pellet volumes of 0.2% (v/v) ammonia in methanol, vortex-mixing for 30 s and incubating on ice for 20 min. The extracts were clarified by centrifugation (15000g for 5 min at 4 °C), and the supernatants were immediately analyzed on an Agilent 1200 HPLC system.
2.4. Analysis of pigments by HPLC
BChl extracts were separated on a Supelco Discovery HS C18 reverse-phase column (5 μm particle size, 120 Å pore size, 250 x 4.6 mm) using a method modified from that of Frigaard et al. [28]. Solvents A and B were 42:33:25 (v/v/v) methanol/acetonitrile/H2O and 39:31:30 (v/v/v) methanol/acetonitrile/ethyl acetate, respectively. Pigments were eluted at 1 ml·min− 1 at 40 °C on a linear gradient of 70%–85% solvent B over 20 min, increasing to 100% to wash the column. Elution of BChl a and BChl b species were monitored by checking absorbance at 770 nm and 795 nm, respectively.
Chlide species were separated on a YMC30 C30 reverse-phase column (3 μm particle size, 250 × 4.6 mm) using a method modified from that of Kruk and Myśliwa-Kurdziel [29] as described previously [19].
3. Results and discussion
Due to the high sequence similarity between the COR-encoding genes from Rba. sphaeroides and Blc. viridis, and thus the high probability of introducing unwanted mutations during replacement via homologous recombination, the native bchX, bchY and bchZ genes were deleted in both WT and ΔbciA backgrounds prior to the integration of the Blc. viridis orthologs. The resulting strains, ΔbchXYZ and ΔbciA/ΔbchXYZ, were unable to synthesize BChl and therefore could not grow under photosynthetic conditions. The precursor pigments accumulated by these strains were analyzed by HPLC (Fig. 3). Both the WT and ΔbciA strains were able to synthesize BChl a, and accordingly, no precursor pigments were detected (Fig. 3, traces A,B). The ΔbchXYZ strain accumulated a species of Chlide, identifiable by the presence of a Qy absorption band at ~ 660 nm; however, this species had a blue-shifted Soret band (Fig. 3, trace C). The Chlide species accumulated by ΔbciA/ΔbchXYZ also had a blue-shifted Soret band (Fig. 3, trace D), although this shift was less pronounced than that of the pigment from ΔbchXYZ. The step in BChl biosynthesis subsequent to C7 = C8 reduction is the hydration of the 3V group of BChlide, catalyzed by the gene product of bchF [30], yielding a hydroxyethyl (HE) group. However, it has been documented that disruption of the genes encoding COR can result in the accumulation of a chlorin with a HE group at C3 [31], [32], indicating that BchF displays relaxed substrate specificity. N22, a non-photosynthetic strain of Rba. sphaeroides, was found to accumulate 3HE Chlide, while the mutation was mapped to a CORa-encoding gene [33]. HPLC analysis of this strain indicated that the pigment had the same absorption spectrum and retention time as the pigment extracted from ΔbchXYZ, which we have assigned as 3HE,8E Chlide (Fig. 3, trace E). The red shift in the Soret band of the pigment accumulated by ΔbciA/ΔbchXYZ, when compared to that of 3HE,8E Chlide, can be accounted for by the lack of both 8VRs; an 8- to 10-nm shift in this band is consistent with 8V/8E substitutions of chlorins [34]. We have therefore assigned this pigment as 3HE,8V Chlide.
Fig. 3.
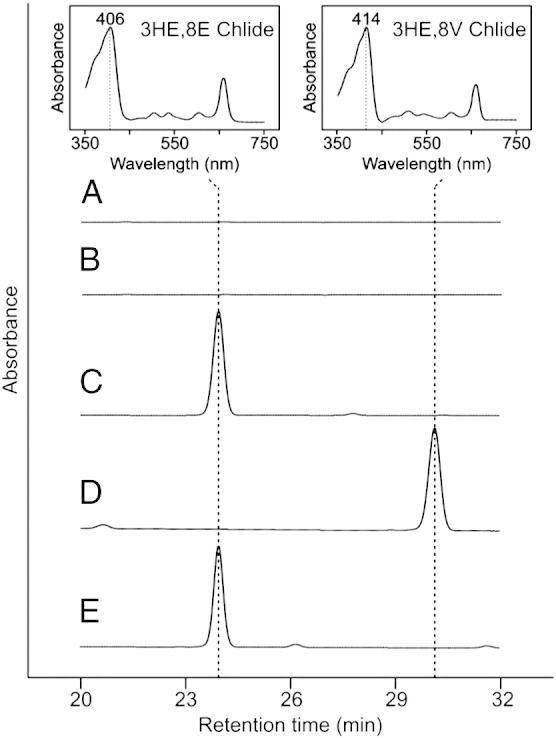
HPLC analysis of precursor pigments extracted from Rba. sphaeroides strains.
HPLC elution profiles of extracts from (A) WT, (B) ΔbciA, (C) ΔbchXYZ, (D) ΔbciA/ΔbchXYZ and (E) mutant N22. Retention times of 23.9 and 30.2 min, and Soret absorption maxima at 406 and 414 nm (inset), are indicative of 3HE,8E Chlide and 3HE,8V Chlide, respectively, in the HPLC solvents. Traces are normalized to major peak height for clarity.
The CORb-encoding genes from Blc. viridis were introduced into the genomes of ΔbchXYZ and ΔbciA/ΔbchXYZ at the loci of the original CORa-encoding genes, yielding bchXYZBv and ΔbciA/bchXYZBv, respectively (Fig. 4). These strains, along with the WT, were grown under microoxic conditions in the dark and were tested for BChl species production by HPLC analysis (Fig. 5). WT Rba. sphaeroides accumulates BChl a exclusively (Fig. 5, trace A). We have previously demonstrated that the ΔbciA strain, lacking the conventional 8VR, produces BChl a, identical to the WT [11]. The bchXYZBv strain also demonstrated BChl a accumulation (Fig. 5, trace B). This result was unexpected since it had already been shown that CORb could not reduce the C7 = C8 of 8E Chlide in vitro, and that this form of the enzyme catalyzes ethylidene group formation [16]. However, the species extracted from the ΔbciA/bchXYZBv strain displayed a shorter retention time than BChl a and a red-shifted Qy absorption band (Fig. 5, trace C) and was identical in these respects to BChl b, extracted from WT Blc. viridis cells (Fig. 5, trace D). These data indicate that the lack of the conventional 8VR in the CORb-producing strain was essential for the biosynthesis of BChl b in vivo. When this 8VR is present, bchXYZBv produces BChl a, albeit in low amounts, while BChl b is undetectable. This result suggests that the substrate for CORb does not bypass BciA and therefore carries an 8E group prior to C7 = C8 reduction. Although Tsukatani et al. [16] demonstrated that no product was formed when CORb was incubated with 8E Chlide in vitro, it is possible that the enzyme is able to reduce the C7 = C8 of 3HE,8E Chlide in vivo. Studies with Rhodobacter spp. mutants have shown that COR demonstrates substrate flexibility and can reduce Chlide species carrying a 3HE group [32], [35], [36]. It is likely that the heterologous assembly of the CORb complex in Rba. sphaeroides provides a favorable environment for enzyme activity in terms of interactions with neighboring enzymes in the BChl pathway, allowing the formation of an 8E bacteriochlorin that is subsequently converted to BChl a.
Fig. 4.
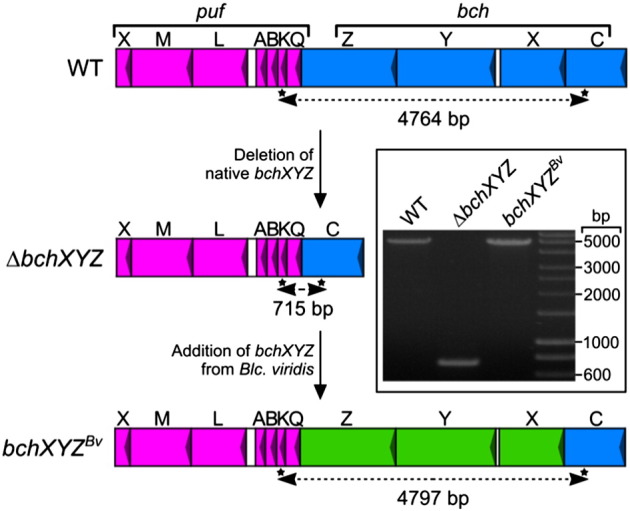
Construction of Rba. sphaeroides mutants designed to express the genes encoding CORb from Blc. viridis.
Diagram depicting the deletion of native CORa-encoding genes (bchX, bchY and bchZ) and their subsequent replacement with orthologs from Blc. viridis in the region of the photosynthesis gene cluster containing RC-LH1 ORFs in the puc operon. Isolation of mutants was checked with colony PCR (inset) by amplifying regions between asterisks and confirmed by sequencing of the amplicons. The fragments amplified from WT, ΔbchXYZ and bchXYZBv were 4764 bp, 715 bp and 4797 bp, respectively.
Fig. 5.
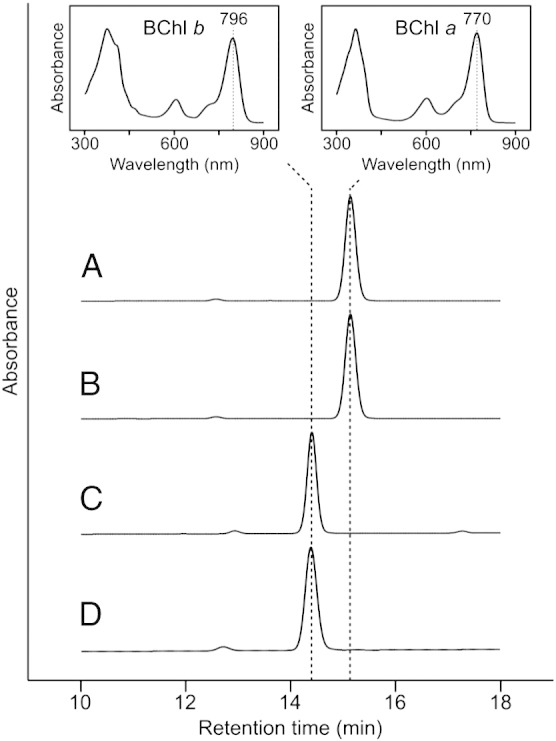
HPLC analysis of BChls extracted from Rba. sphaeroides strains.
HPLC elution profiles of extracts from (A) WT, (B) bchXYZBv, (C) ΔbciA/bchXYZBv and (D) Blc. viridis. Retention times of 14.4 and 15.1 min, and Qy absorption maxima at 796 and 770 nm (inset), are indicative of BChl b and BChl a, respectively, in the HPLC solvents. Traces are normalized to major peak height for clarity.
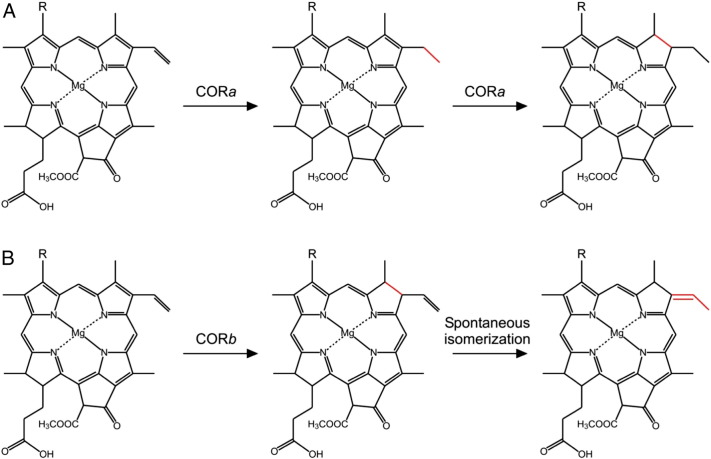
The formation of the ethylidene group, found on BChl b, is observed in the ΔbciA/bchXYZBv strain but not in bchXYZBv. This result leads us to postulate that, rather than CORb having an ethylidene synthase function, this enzyme has lost the ability to reduce the 8V group of Chlide and that after the enzyme-catalyzed reduction of the C7 = C8, the short-lived 8V bacteriochlorin spontaneously isomerizes to the more stable b-type. We also hypothesize that CORa must catalyze the reduction of the 8V group of Chlide prior to the reduction of the C7 = C8 to prevent this isomerization and subsequent production of b-type bacteriochlorins in BChl a-utilizing organisms. These proposed mechanisms are summarized in Fig. 6.
Fig. 6.
Proposed catalytic mechanisms of CORs with an 8V substrate.
(A) CORa reduces the 8V group before reducing the C7 = C8. (B) CORb, having lost its 8VR activity, reduces C7 = C8 to produce an 8V bacteriochlorin which spontaneously isomerizes to the more stable b-type bacteriochlorin carrying an ethylidene group. R may represent either a V (HC = CH2) or an HE (HOC-CH3) group, depending on whether the pigment has undergone hydration catalyzed by the gene product of bchF.
It has been demonstrated that, when exposed to ascorbic acid photoreduction, a V group external to a newly reduced isobacteriochlorin ring is prone to “inward” double-bond migration, yielding a stable ethylidene-isobacteriochlorin [37], [38]. It has also been shown that a further inward migration is possible, with the isomerization of ethylidene groups on BChls b and g resulting in the production of chlorins with intact C7 = C8 bonds [39], [40], [41]. A similar acidic reduction may account for the presence of modified chlorins containing hydroxyl groups at C81 in heliobacteria, BChl g producing organisms that have b-type CORs [42], [43]. Although it has been proposed that 8V Chlide and 3V BChlide b are possible substrates for this modified chlorin [43], an alternative mechanism involving hydration of the 8V group, accounting for hydroxylated Chl, and subsequent dehydration leading to ethylidene group formation has been suggested [30].
In order to investigate the fate of BChl b in Rba. sphaeroides, strains were standardized by OD710 and used to inoculate fresh medium in which photosynthetic growth rates were monitored (Table 1). As expected, the WT and ΔbciA strains displayed similarly high growth rates, while the photosynthetic growth of bchXYZBv was greatly reduced. The ΔbciA/bchXYZBv strain was incapable of photosynthetic growth, demonstrating that the biosynthesis of BChl b in Rba. sphaeroides poses problems for the cellular machinery that mediates assembly of the photosynthetic apparatus. The absorption spectra of whole cells indicate that this foreign pigment is not incorporated into RC-LH1 complexes (Fig. 7, spectrum D). It is possible that the native system used to deliver pigments to the photosynthetic apparatus is incapable of handling a foreign BChl, and thus assembly of a functional unit is perturbed. Alternatively, the modification to the C8 group could lead to steric hindrance when the pigment is bound within the photosystem architecture, causing structural instability and leading to the loss of viability when grown phototrophically. The bchXYZBv strain exhibits limited photosynthetic growth, consistent with a reduced amount of BChl a synthesized. The absorption spectrum of this mutant shows that the limited amount of BChl a is mainly used for the assembly of RC-LH1 rather than LH2 complexes, as noticed in earlier studies where BChl biosynthesis was heavily restricted [44]. The accumulation of the 670-nm absorbing pigment, likely Chlide, indicates that the CORb enzyme complex in Rba. sphaeroides operates inefficiently, possibly due to problems interacting with other enzymes in the BChl a pathway.
Table 1.
Photosynthetic growth rates of Rba. sphaeroides strains.
| Strain | Doubling time (h) |
|---|---|
| WT | 5.9 ± 0.3 |
| ΔbciA | 5.7 ± 0.1 |
| bchXYZBv | 15.3 ± 4.2 |
| ΔbciA/bchXYZBv | – |
Values are means of three biological replicates ± SD. Dash (–) indicates data could not be measured.
Fig. 7.
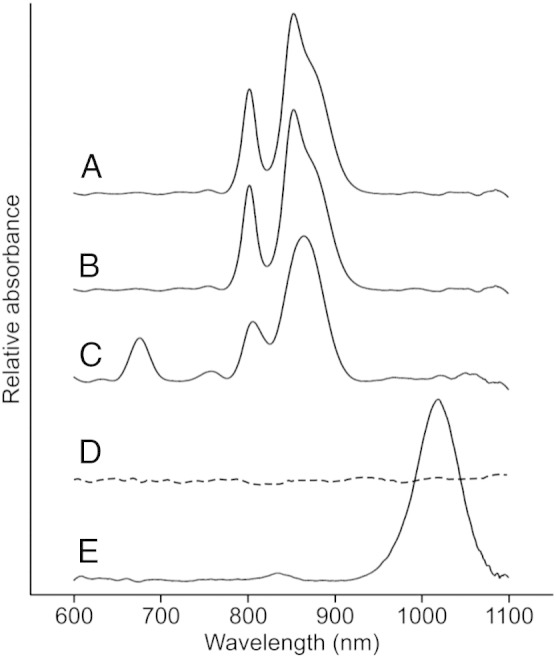
Whole-cell absorption spectra of Rba. sphaeroides strains.
Whole cell absorption spectra of (A) WT, (B) ΔbciA, (C) bchXYZBv, (D) ΔbciA/bchXYZBv and (E) Blc. viridis. Spectra were recorded from cultures grown photosynthetically (solid lines) or microoxically (dashed line) when photosynthetic growth was not possible. Spectra were recorded with samples standardized by OD710 and absorption due to light scattering was removed by baseline subtraction from each data set.
In WT Rba. sphaeroides, BChl a pigments within the peripheral (LH2) and core (LH1) antenna of Rba. sphaeroides funnel energy absorbed in the 800- to 900-nm region of the spectrum, as well as energy absorbed in the visible region absorbed by carotenoid pigments, to the RC [45], [46], [47]. In order to establish a photosystem capable of harvesting a wider range of wavelengths, it will be necessary to assemble lower-energy BChl b-containing RC traps that can benefit from the IR absorption of light by the BChl b-LH1 complexes. Thus, a mixture BChl a/b is required. Fluorescence-excitation experiments have shown that BChl a-containing LH2 from Rhodopseudomonas acidophila can transfer energy to the RC-LH1 complex of Blc. viridis in hybrid reconstituted membranes [48]. The potential ability of our strain to switch between BChl b and BChl a biosynthesis, initiated by the titration of 8VR, may permit the engineering of a system in which excitation energy is funneled from high energy BChl a-containing peripheral antenna (LH2) and lower energy, BChl b-containing core antenna (LH1) into the RC, with no overlap of the absorption bands.
4. Conclusions
We have engineered the biosynthesis of BChl b in the BChl a-utilizing bacterium Rba. sphaeroides. We hypothesize that this has been achieved by removal of the strain's ability to reduce the 8V group on a precursor of the mature pigment. The construction of this strain is an important first step in the re-engineering of photosynthesis in this bacterium.
Acknowledgments
The authors were supported by a grant (BB/G021546/1) from the Biotechnology and Biological Sciences Research Council (UK). C.N.H was also part-funded by an Advanced Award (338895) from the European Research Council. D.P.C wishes to thank Dr. Simon Sedehizadeh (unaffiliated) for useful discussions.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbabio.2014.07.011.
Appendix A. Supplementary data
Supplementary material.
References
- 1.Eimhjellen K.E., Aasmundrud O., Jensen A. A new bacterial chlorophyll. Biochem. Biophys. Res. Commun. 1963;10:232–236. [Google Scholar]
- 2.Scheer H., Svec W.A., Cope B.T., Studier M.H., Scott R.G., Katz J.J. Structure of Bacteriochlorophyll b. J. Am. Chem. Soc. 1974;96:3714–3716. [Google Scholar]
- 3.Hoogewerf G.J., Jung D.O., Madigan M.T. Evidence for limited species diversity of bacteriochlorophyll b-containing purple nonsulfur anoxygenic phototrophs in freshwater habitats. FEMS Microbiol. Lett. 2003;218:359–364. doi: 10.1016/S0378-1097(02)01195-3. [DOI] [PubMed] [Google Scholar]
- 4.Drews G., Giesbrecht P. Rhodopseudomonas viridis, n. sp., a newly isolated, obligate phototrophic bacterium. Arch. Mikrubiol. 1966;53:255–262. [PubMed] [Google Scholar]
- 5.Deisenhofer J., Epp O., Miki K., Huber R., Michel H. Structure of the protein subunits in the photosynthetic reaction centre of Rhodopseudomonas viridis at 3 Å resolution. Nature. 1985;318:618–624. doi: 10.1038/318618a0. [DOI] [PubMed] [Google Scholar]
- 6.Partensky F., Hess W.R., Vaulot D. Prochlorococcus, a marine photosynthetic prokaryote of global significance. Microbiol. Mol. Biol. Rev. 1999;63:106–127. doi: 10.1128/mmbr.63.1.106-127.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blankenship R.E., Tiede D.M., Barber J., Brudvig G.W., Fleming G., Ghirardi M., Gunner M.R., Junge W., Kramer D.M., Melis A., Moore T.A., Moser C.C., Nocera D.G., Nozik A.J., Ort D.R., Parson W.W., Prince R.C., Sayre R.T. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science. 2011;332:805–809. doi: 10.1126/science.1200165. [DOI] [PubMed] [Google Scholar]
- 8.Granick S. The structural and functional relationships between heme and chlorophyll. Harvey Lect. 1948;44:220–245. [PubMed] [Google Scholar]
- 9.Nagata N., Tanaka R., Satoh S., Tanaka A. Identification of a vinyl reductase gene for chlorophyll synthesis in Arabidopsis thaliana and implications for the evolution of Prochlorococcus species. Plant Cell. 2005;17:233–240. doi: 10.1105/tpc.104.027276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez Maqueo Chew A., Bryant D.A. Characterization of a plant-like protochlorophyllide a divinyl reductase in green sulfur bacteria. J. Biol. Chem. 2007;282:2967–2975. doi: 10.1074/jbc.M609730200. [DOI] [PubMed] [Google Scholar]
- 11.Canniffe D.P., Jackson P.J., Hollingshead S., Dickman M.J., Hunter C.N. Identification of an 8-vinyl reductase involved in bacteriochlorophyll biosynthesis in Rhodobacter sphaeroides and evidence for the existence of a third distinct class of the enzyme. Biochem. J. 2013;450:397–405. doi: 10.1042/BJ20121723. [DOI] [PubMed] [Google Scholar]
- 12.Yen H.-C., Marrs B. Map of genes for carotenoid and bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata. J. Bacteriol. 1976;126:619–629. doi: 10.1128/jb.126.2.619-629.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke D.H., Alberti M., Hearst J.E. The Rhodobacter capsulatus chlorin reductase-encoding locus, bchA, consists of three genes, bchX, bchY, and bchZ. J. Bacteriol. 1993;175:2407–2413. doi: 10.1128/jb.175.8.2407-2413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGlynn P., Hunter C.N. Genetic analysis of the bchC and bchA genes of Rhodobacter sphaeroides. Mol. Gen. Genet. 1993;236:227–234. doi: 10.1007/BF00277117. [DOI] [PubMed] [Google Scholar]
- 15.Nomata J., Mizoguchi T., Tamiaki H., Fujita Y. A second nitrogenase-like enzyme for bacteriochlorophyll biosynthesis: reconstitution of chlorophyllide a reductase with purified X-protein (BchX) and YZ-protein (BchY-BchZ) from Rhodobacter capsulatus. J. Biol. Chem. 2006;281:15021–15028. doi: 10.1074/jbc.M601750200. [DOI] [PubMed] [Google Scholar]
- 16.Tsukatani Y., Yamamoto H., Harada J., Yoshitomi T., Nomata J., Kasahara M., Mizoguchi T., Fujita Y., Tamiaki H. An unexpectedly branched biosynthetic pathway for bacteriochlorophyll b capable of absorbing near-infrared light. Sci. Rep. 2013;3:1217–1223. doi: 10.1038/srep01217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harada J., Mizoguchi T., Tsukatani Y., Yokono M., Tanaka A., Tamiaki H. Chlorophyllide a oxidoreductase works as one of the divinyl reductases specifically involved in bacteriochlorophyll a biosynthesis. J. Biol. Chem. 2014;289:12716–12726. doi: 10.1074/jbc.M113.546739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones M.R., Fowler G.J., Gibson L.C., Grief G.G., Olsen J.D., Crielaard W., Hunter C.N. Mutants of Rhodobacter sphaeroides lacking one or more pigment-protein complexes and complementation with reaction-centre, LH1, and LH2 genes. Mol. Microbiol. 1992;6:1173–1184. doi: 10.1111/j.1365-2958.1992.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 19.Canniffe D.P., Chidgey J.W., Hunter C.N. Elucidation of the preferred routes of C8-vinyl reduction in chlorophyll and bacteriochlorophyll biosynthesis. Biochem. J. 2014 doi: 10.1042/BJ20140163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter C.N., Turner G. Transfer of genes coding for apoproteins of reaction centre and light-harvesting LH1 complexes to Rhodobacter sphaeroides. J. Gen. Microbiol. 1988;134:1471–1480. [Google Scholar]
- 21.Claus D., Schaub-Engels C., editors. Catalogue of strains. 2nd ed. 1977. German collection of microorganisms; pp. 279–280. [Google Scholar]
- 22.Lang F.S., Oesterhelt D. Microaerophilic growth and induction of the photosynthetic reaction center in Rhodopseudomonas viridis. J. Bacteriol. 1989;171:2827–2834. doi: 10.1128/jb.171.5.2827-2834.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mpl8 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 24.Simon R., Priefer U., Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Nat. Biotechnol. 1983;1:784–791. [Google Scholar]
- 25.Schäfer A., Tauch A., Jäger W., Kalinowski J., Thierbach G., Pühler A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- 26.Hamblin P.A., Bourne N.A., Armitage J.P. Characterization of the chemotaxis protein CheW from Rhodobacter sphaeroides and its effect on the behaviour of Escherichia coli. Mol. Microbiol. 1997;24:41–51. doi: 10.1046/j.1365-2958.1997.3241682.x. [DOI] [PubMed] [Google Scholar]
- 27.Hunter C.N., McGlynn P., Ashby M.K., Burgess J.G., Olsen J.D. DNA sequencing and complementation/deletion analysis of the bchA-puf operon region of Rhodobacter sphaeroides: in vivo mapping of the oxygen-regulated puf promoter. Mol. Microbiol. 1991;5:2649–2661. doi: 10.1111/j.1365-2958.1991.tb01974.x. [DOI] [PubMed] [Google Scholar]
- 28.Frigaard N.-U., Larsen K.L., Cox R.P. Spectrochromatography of photosynthetic pigments as a fingerprinting technique for microbial phototrophs. FEMS Microbiol. Ecol. 1996;20:69–77. [Google Scholar]
- 29.Kruk J., Myśliwa-Kurdziel B. Separation of monovinyl and divinyl protochlorophyllides using C30 reverse phase high performance liquid chromatography column: analytical and preparative applications. Chromatographia. 2004;60:117–123. [Google Scholar]
- 30.Chew A.G., Bryant D.A. Chlorophyll biosynthesis in bacteria: the origins of structural and functional diversity. Annu. Rev. Microbiol. 2007;61:113–129. doi: 10.1146/annurev.micro.61.080706.093242. [DOI] [PubMed] [Google Scholar]
- 31.Richards W.R., Lascelles J. The biosynthesis of bacteriochlorophyll. The characterization of latter stage intermediates from mutants of Rhodopseudomonas spheroides. Biochemistry. 1969;8:3473–3482. doi: 10.1021/bi00836a051. [DOI] [PubMed] [Google Scholar]
- 32.Pudek M.R., Richards W.R. A possible alternate pathway of bacteriochlorophyll biosynthesis in a mutant of Rhodopseudomonas sphaeroides. Biochemistry. 1975;14:3132–3137. doi: 10.1021/bi00685a015. [DOI] [PubMed] [Google Scholar]
- 33.Hunter C.N., Coomber S.A. Cloning and oxygen regulated expression of the bacteriochlorophyll biosynthesis genes bch E, B, A and C of Rhodobacter sphaeroides. J. Gen. Microbiol. 1988;134:1471–1480. [Google Scholar]
- 34.Tamiaki H., Kunieda M. In: Handbook of Porphyrin Science: Catalysis and bio-inspired systems, partII. Kadish K.M., Smith K.M., Guilard R., editors. World Scientific; Singapore: 2011. Photochemistry of chlorophylls and their synthetic analogs; pp. 223–290. [Google Scholar]
- 35.Taylor D.P., Cohen S.N., Clark W.G., Marrs B.L. Alignment of genetic and restriction maps of the photosynthesis region of the Rhodopseudomonas capsulata chromosome by a conjugation-mediated marker rescue technique. J. Bacteriol. 1983;154:580–590. doi: 10.1128/jb.154.2.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bollivar D.W., Suzuki J.Y., Beatty J.T., Dobrowolski J.M., Bauer C.E. Directed mutational analysis of bacteriochlorophyll a biosynthesis in Rhodobacter capsulatus. J. Mol. Biol. 1994;237:622–640. doi: 10.1006/jmbi.1994.1260. [DOI] [PubMed] [Google Scholar]
- 37.Smith K.M., Simpson D.J., Snow K.M. Isobacteriochlorophyll b analogues from photoreduction of metallochlorins. J. Am. Chem. Soc. 1986;108:6834–6835. [Google Scholar]
- 38.Simpson D.J., Smith K.M. Ascorbic acid photoreductions of zinc(II) chlorophyll derivatives: access to metal-free isobacteriochlorins. J. Am. Chem. Soc. 1988;110:2854–2861. [Google Scholar]
- 39.Kobayashi M., Yamamura M., Akutsu S., Miyake J., Hara M., Akiyama M., Kise H. Successfully controlled isomerization and pheophytinization of bacteriochlorophyll b by weak acid in the dark in vitro. Anal. Chim. Acta. 1998;361:285–290. [Google Scholar]
- 40.Kobayashi M., Hamano T., Akiyama M., Watanabe T., Inoue K., Oh-oka H., Amesz J., Yamamura M., Kise H. Light-independent isomerization of bacteriochlorophyll g to chlorophyll a catalyzed by weak acid in vitro. Anal. Chim. Acta. 1998;365:199–203. [Google Scholar]
- 41.Tamiaki H., Xu M., Tanaka T., Mizoguchi T. Photoreduction of zinc 8-vinylated chlorophyll derivative to bacteriochlorophyll-b/g analog possessing an 8-ethylidene group. Bioorg. Med. Chem. Lett. 2013;23:2377–2379. doi: 10.1016/j.bmcl.2013.02.050. [DOI] [PubMed] [Google Scholar]
- 42.Michalski T.J., Hunt J.E., Bowman M.K., Smith U., Bardeen K., Gest H., Norris J.R., Katz J.J. Bacteriopheophytin g: Properties and some speculations on a possible primary role for bacteriochlorophylls b and g in the biosynthesis of chlorophylls. Proc. Natl. Acad. Sci. 1897;84:2570–2574. doi: 10.1073/pnas.84.9.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsukatani Y., Yamamoto H., Mizoguchi T., Fujita Y., Tamiaki H. Completion of biosynthetic pathways for bacteriochlorophyll g in Heliobacterium modesticaldum: The C8-ethylidene group formation. Biochim. Biophys. Acta. 2013;1827:1200–1204. doi: 10.1016/j.bbabio.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Gong L., Lee J.K., Kaplan S. The Q gene of Rhodobacter sphaeroides: its role in puf operon expression and spectral complex assembly. J. Bacteriol. 1994;176:2946–2961. doi: 10.1128/jb.176.10.2946-2961.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sener M.K., Olsen J.D., Hunter C.N., Schulten K. Atomic-level structural and functional model of a bacterial photosynthetic membrane vesicle. Proc. Natl. Acad. Sci. 2007;104:15723–15728. doi: 10.1073/pnas.0706861104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sener M., Strümpfer J., Timney J.A., Freiberg A., Hunter C.N., Schulten K. Photosynthetic vesicle architecture and constraints on efficient energy harvesting. Biophys. J. 2010;99:67–75. doi: 10.1016/j.bpj.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cartron M.L., Olsen J.D., Sener M., Jackson P.J., Brindley A.A., Qian P., Dickman M.J., Leggett G.J., Schulten K., Hunter C.N. Integration of energy and electron transfer processes in the photosynthetic membrane of Rhodobacter sphaeroides. Biochim. Biophys. Acta. 2014 doi: 10.1016/j.bbabio.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujii R., Shimonaka S., Uchida N., Gardiner A.T., Cogdell R.J., Sugisaki M., Hashimoto H. Construction of hybrid photosynthetic units using peripheral and core antennae from two different species of photosynthetic bacteria: detection of the energy transfer from bacteriochlorophyll a in LH2 to bacteriochlorophyll b in LH1. Photosynth. Res. 2008;95:327–337. doi: 10.1007/s11120-007-9260-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.