Abstract
Background
Maintaining a gluten free diet (GFD) without an underlying diagnosis of celiac disease has enjoyed widespread acceptance in the USA.
Methods
We performed a cross-sectional study utilizing a GFD questionnaire in 1647 patients with inflammatory bowel diseases (IBD) participating in the CCFA Partners longitudinal, Internet-based cohort.
Results
A diagnosis of celiac disease (CD) and non-celiac gluten sensitivity (NCGS) were reported by 10 (0.6%) and 81 (4.9%) respondents, respectively. Three hundred fourteen (19.1%) participants reported having previously tried a GFD and 135 (8.2%) reported current use of GFD. Overall 65.6% of all patients, who attempted a GFD described an improvement of their GI-symptoms and 38.3% reported fewer or less severe IBD flares. In patients currently attempting a GFD, excellent adherence was associated with significant improvement of fatigue (p<0.03).
Conclusion
In this large group of patients with IBD, a substantial number had attempted a GFD, of whom the majority had some form of improvement in GI-symptoms. Testing a GFD in clinical practice in patients with significant intestinal symptoms, which are not solely explained by the degree of intestinal inflammation, has the potential to be a safe and highly efficient therapeutic approach. Further prospective studies into mechanisms of gluten sensitivity in IBD are warranted.
Keywords: IBD, gluten, Crohn’s disease, ulcerative colitis, celiac disease
INTRODUCTION
The pathogenesis of inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), has only been partly elucidated. The most likely factors involved in promoting the onset and continuation of intestinal inflammation in IBD are an individual genetic predisposition, influence of the host microbiome and still largely undefined environmental triggers. Diet has long been implicated as one of the contributing factors for disease flare-ups of IBD.1, 2 Accordingly, an elemental diet is a well-established treatment modality in children with IBD. Nevertheless, up to now, clinical trials investigating various diets have failed to yield significant clinical improvement in adult IBD.3, 4
Adoption of a gluten free diet (GFD) without an underlying diagnosis of celiac disease has experienced a rapid and widespread increase in the US in recent years. Currently at least 0.5% of the US population follow a GFD without having a confirmed diagnosis of celiac disease.5 Even in the absence of celiac disease, gluten is thought to be associated with bloating, diarrhea, abdominal pain, fatigue and nausea, leading to the definition of a new entity designated as non-celiac gluten sensitivity (NCGS).6 Many of the symptoms associated with gluten exposure in the general population are also common in IBD patients and may partially be responsible for a diminished quality of life.
A better understanding of patient-reported outcomes is essential to developing new insights into the effectiveness of IBD therapies. Investigating the effects of diet on disease course could open potentially new research avenues.
With this study we aimed to determine the experience with a GFD in patients with IBD. Specifically we evaluated the current prevalence of self-reported celiac disease, NCGS and use of GFD, symptomatic improvement while being on GFD and the degree of adherence to a GFD in a cross-sectional study within the CCFA Partners cohort.
METHODS
CCFA Partners is an ongoing Internet-based cohort study of patients with IBD7. Participants complete a baseline survey and follow-up surveys occur every 6 months. As part of this cohort, we administered a 12-question survey on GFD from July 2013 through August 2013. Participants were asked if they 1) were ever on a GFD and if yes if they were still on a GFD, 2) had been diagnosed with celiac disease or gluten sensitivity by a health care provider, 3) if GFD improved each of the following symptoms: bloating, diarrhea, abdominal pain, fatigue, nausea 4) if GFD led to less severe or fewer flares of their IBD and 5) if while following a GFD fewer medications were needed to control disease activity. Respondents, who still were on a GFD at the time of the survey, were asked to complete a validated seven-item GFD adherence survey.8
Statistical analyses
Descriptive statistics were used to characterize the population, including proportions and 95% confidence intervals, medians and interquartile ranges, and means and standard deviations (SD) as appropriate. Bivariate statistics were used to compare reduction in flares by IBD subtype (CD versus UC) and by other factors. These statistics included Pearson's chi-square test statistic, Fisher's exact, Wilcoxon rank sum, and Student's t-test as appropriate. STATA version 10.0 (College Station, TX) was used for all analyses and p < 0.05 was considered statistically significant. The Institutional Review Board at the University of North Carolina at Chapel Hill approved the study protocol.
RESULTS
A total of 1647 patients, who completed both a baseline survey and the specific GFD questionnaire, were included in the study. Ten (0.6%) and 81 (4.9%) patients had been diagnosed with celiac disease and gluten sensitivity by their health care provider, respectively. Three hundred fourteen (19.1%) participants reported ever having tried a GFD and 135 (8.2%) reported current use of GFD. No differences were found between the GFD and non-GFD groups with regard to disease type, duration of disease, concomitant medication, educational status and other baseline characteristics (table 1). In a subanalysis, clinical base line characteristics of individuals who completed the GFD questionnaire were compared to individuals within the CCFA Partners cohort who were not asked to complete a GFD questionnaire. There were no clinical significant differences in disease type or baseline characteristics between both groups (data not shown).
Table 1.
Baseline characteristics of the inflammatory bowel disease population in CCFA Partners, stratified by ever or current attempted GFD and never on GFD for symptom control
| Characteristic | GFD n=314 |
Non-GFD n=1333 |
p-value |
|---|---|---|---|
| Mean (SD) or % |
Mean (SD) or % |
||
| Age, years | 46.5 (15.4) | 46.2 (15.4) | 0.88 |
| Sex | |||
| Female (%) | 74 | 74 | 0.99 |
| Type of IBD (%) | |||
| Crohn’s disease | 61 | 63 | 0.62 |
| Ulcerative/indeterminate colitis | 39 | 37 | |
| Duration of disease years | 17.4 (13.4) | 16.5 (12.8) | 0.25 |
| Ostomy (% yes) | 6.9 | 7.8 | 0.60 |
| Medications (%) | |||
| 5-ASA | 74.5 | 71.4 | 0.27 |
| Corticosteroids | 27.7 | 23.6 | 0.12 |
| Immunomodulators | 45.9 | 47.0 | 0.70 |
| Biologic anti-TNF | 55.4 | 54.7 | 0.82 |
| Education (%) | |||
| High school or less | 7.4 | 6.4 | 0.51 |
| College + | 92.6 | 93.6 | |
| Disease activity | |||
| sCDAI** | 148.8 (97.3) | 142.5 (90.0) | 0.35 |
| SCCAI# | 3.2 (2.5) | 3.1 (2.6) | 0.73 |
| Quality of life SIBDQ^ | 5.0 (1.2) | 5.1 (1.1) | 0.70 |
| BMI | 25.7 (6.7) | 26.0 (7.2) | 0.60 |
by wilcoxon rank sum, t-test, chi square or fisher’s exact as appropriate
short Crohn’s disease activity index
simple clinical colitis activity index
short inflammatory bowel disease questionnaire
Improvement of symptoms on a GFD
Of the 314 patients, who have ever followed or were still following a GFD, 206 (65.6%) reported that they experienced an improvement of at least one specific clinical symptom which has been associated with gluten exposure (see figure 1). Furthermore 38.3% also recounted fewer and less severe flares while being on a GFD and 23.6% stated that they required fewer medications to control the disease. There were no significant differences pertaining to baseline characteristics between patients with or without improvement of clinical symptoms on a GFD (data not shown).
Figure 1.
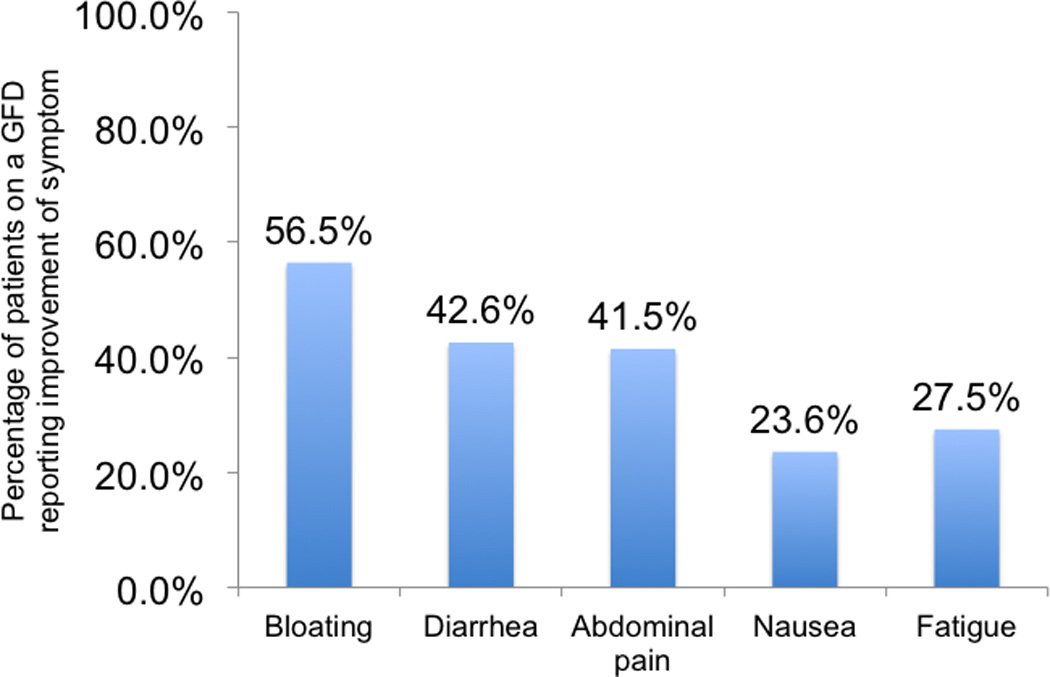
Reported symptom improvements in IBD patients attempting a GFD (n=314)
Adherence to GFD and correlation with symptom improvement
In those patients who were still following a gluten free diet (n=135), adherence was found to be excellent in 41.5%, average in 34.1%, and fair/poor in 24.4%. Excellent adherence to a GFD was associated with reduced fatigue, as compared to fair/poor adherence (p<0.03). Adherence was not associated with significant differences in other clinical symptoms.
DISCUSSION
This is the largest survey analyzing patient-reported data about the prevalence of a gluten free diet in a Western IBD population. In this Internet based cohort nearly 20% of all patients reported having tried a GFD and 8% were currently attempting a GFD. This is a significantly higher percentage than the current GFD prevalence of 0.5% among individuals without celiac disease in the USA.5 More than half of the patients reported symptom improvement and nearly 40% fewer flare-ups of IBD while being on a GFD. This observation suggests that in a subgroup of IBD patients, gluten may cause intestinal (diarrhea, bloating, abdominal pain) and extraintestinal (fatigue, nausea) symptoms. Similar effects of a GFD have been described in patients with irritable bowel syndrome (IBS) indicating a potential trigger effect of gluten containing foods in gluten susceptible patients.9–11
NCGS is defined by the exclusion of celiac disease including negative celiac serologies and/or normal intestinal architecture and negative immunoglobulin (Ig)E-mediated allergy tests to wheat. Additionally, to meet criteria for NCGS, the clinical symptomatology of IBS type of symptoms has to improve after gluten withdrawal and worsen after the ingestion of gluten. A specific reaction to gluten in patients with NCGS is currently debated.12 Biesiekierski et al recently showed that gluten by itself might be not the culprit for the IBS type symptoms in patients with previously diagnosed NCGS, but rather the intake of low-fermentable, poorly absorbed, short-chain carbohydrates (fermentable, oligo-, di-, monosaccharides, and polyols; FODMAPs) may be responsible for these effects.13 Also, gluten does not elicit an inflammatory response in the duodenum in patients with NCGS.14 The diagnosis of NCGS was reported by nearly 5% of the respondents in our survey. Thus far studies in patients with IBD investigating inflammatory responses to gluten in duodenal or colonic biopsies have not been performed. Theoretically gluten could create a pro-inflammatory environment in the intestine, leading to more frequent disease flares and the need for more intensified therapies, similar to patients with IBD and concurrent celiac disease.15 However, we cannot exclude that a GFD leads to a significant reduction of dietary FODMAPs, which as shown by Biesiekierski et al., leads to an improvement of the GI symptoms of the patients.13 Of note, an exploratory study has demonstrated that dietary reduction of FODMAPs leads to significant amelioration of symptoms including abdominal pain, bloating, gas and diarrhea in patients with IBD.16
Those patients maintaining a GFD at the time period of the survey were asked to fill out a recently validated 7-item Celiac Dietary Adherence Test.8 The additive scores of this test reflect the adherence to a GFD and correlate highly with a standardized dietician evaluation and appear to outperform serological testing. However, the test does not quantify the amount of gluten intake, but rather points to the likelihood of gluten contamination. More than 40% of the respondents were strictly maintaining to a GFD, whereas roughly 25% of the patients were found to be fair or poorly adherent. Intriguingly, of all clinical symptoms, only fatigue improved significantly with good adherence. Fatigue in the absence of iron deficiency anemia is a leading symptom in many patients with IBD.17 It is possible that fatigue conversely influenced adherence in our cohort, but coincidently, the worsening of fatigue was also the most significant finding in a gluten challenge study conducted in patients with NCGS.9
The class II MHC haplotype HLA-DQ2 and HLA-DQ8 are present in almost all CD patients and interestingly can be also found in 50% of patients, who are improving on a gluten free diet, which is higher than can be expected in the general population.6 Studies in patients with irritable bowel syndrome with predominantly diarrhea (IBS-D) have also shown, that carrier of HLA-DQ2 respond favorably to a gluten free diet. In fact in 60% of the patients with IBS-D HLA-DQ2 positivity, but no signs of overt celiac disease (negative TTG antibodies and no signs of active celiac disease on biopsies obtained in the duodenum), symptoms such as diarrhea and bloating improved on a 6 months gluten free diet compared to only 12% in patients without HLA-DQ2 positivity.18 As it is speculated in patients with NCGS, gluten might have a direct impact on intestinal barrier function and the mucosal immune system in IBD patients with the HLA-DQ-2 or DQ 8 genotype.19 In a recent study by Vazquez-Roque et al the small intestinal permeability was significantly increased in IBS-D patients with HLA-DQ2 or 8 positivity on a gluten containing diet (GCD), but this was not the case in HLA-DQ 2 and 8 negative patients.11 Also RNA expressions of several proteins associated with the epithelial barrier in the in colonic mucosa (zonula occludens-1, occludin and claudin) were generally lower in participants on a GCD compared to participants on a GFD. However, diet-associated changes of RNA expression reached only statistical significance in study participants, who were found to have a HLA-DQ 2 or 8 positive status. HLA-DQ2 or DQ8 is not found in higher frequencies in IBD patients, but it would be fascinating to evaluate the associations of these haplotypes with the response to a gluten free diet in IBD patients in prospective studies.20 Moreover the degree of intestinal inflammation in non-celiac IBD patients could be influenced by the recently identified non-gluten α-amylase / tryptase inhibitors (ATIs), which can be found in wheat and related cereals. These ATI are strong activators of the innate immune response via the Toll-like receptor 4, leading to the upregulation of pro-inflammatory cytokines in vitro and in vivo.21
Patient-reported data based from the CCFA partners cohort have several limitations as outlined recently.22 CCFA-Partners is a volunteer sample of patients and thus the above-described findings may not reflect similar diet habits in all IBD patients. To address the possibility of selection bias within the sample completing the GFD questionnaire, we compared the characteristics of those who completed the questionnaire to those of the CCFA Partners cohort in general and found no clinical significant differences. Since the study was based on a single questionnaire without collecting blood samples, we did not rule out occult celiac disease with serologic tissue-transglutaminase testing neither could we determine the HLA DQ2 or DQ 8 status. Previous studies have shown that the prevalence of celiac disease in patients with IBD is comparable to the prevalence in the non-IBD population.15, 23 The finding that 0.6% of patients reported to be diagnosed with celiac disease is comparable to the currently reported 0.7% prevalence of celiac disease (including diagnosed and undiagnosed cases) in the United States.24 Currently the majority of celiac disease patients in the USA are undiagnosed, but since IBD patients suffer from similar GI-symptoms as many celiac disease patients, it is very likely that in the setting of the diagnostic work-up for IBD, concurrent celiac disease is diagnosed either by serologic testing or by endoscopy.
In conclusion, the high prevalence of a GFD in the CCFA Partners cohort strongly suggests a potential role of this diet in the adjunctive therapeutic management of subgroups of IBD patients. Testing GFD in clinical practice in patients with significant intestinal symptoms, which are not solely explained by the degree of intestinal inflammation, has the potential to be a safe and highly efficient therapeutic approach following appropriate testing for celiac disease. Further research into investigating possible mechanisms of gluten-mediated worsening of intestinal inflammation in susceptible IBD patients is also warranted.
Acknowledgments
The Crohn’s and Colitis Foundation of America Partners cohort is supported by grants from the Crohn’s and Colitis Foundation of America and the National Institutes of Health (P30 DK34987).
References
- 1.Zallot C, Quilliot D, Chevaux JB, et al. Dietary beliefs and behavior among inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19:66–72. doi: 10.1002/ibd.22965. [DOI] [PubMed] [Google Scholar]
- 2.Cohen AB, Lee D, Long MD, et al. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig Dis Sci. 2013;58:1322–1328. doi: 10.1007/s10620-012-2373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zachos M, Tondeur M, Griffiths AM. Enteral nutritional therapy for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2007:CD000542. doi: 10.1002/14651858.CD000542.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto T, Nakahigashi M, Saniabadi AR. Review article: diet and inflammatory bowel disease--epidemiology and treatment. Aliment Pharmacol Ther. 2009;30:99–112. doi: 10.1111/j.1365-2036.2009.04035.x. [DOI] [PubMed] [Google Scholar]
- 5.Digiacomo DV, Tennyson CA, Green PH, et al. Prevalence of gluten-free diet adherence among individuals without celiac disease in the USA: results from the Continuous National Health and Nutrition Examination Survey 2009–2010. Scand J Gastroenterol. 2013;48:921–925. doi: 10.3109/00365521.2013.809598. [DOI] [PubMed] [Google Scholar]
- 6.Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13. doi: 10.1186/1741-7015-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long MD, Kappelman MD, Martin CF, et al. Development of an internet-based cohort of patients with inflammatory bowel diseases (CCFA Partners): methodology and initial results. Inflamm Bowel Dis. 2012;18:2099–2106. doi: 10.1002/ibd.22895. [DOI] [PubMed] [Google Scholar]
- 8.Leffler DA, Dennis M, Edwards George JB, et al. A simple validated gluten-free diet adherence survey for adults with celiac disease. Clin Gastroenterol Hepatol. 2009;7:530–536. doi: 10.1016/j.cgh.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Biesiekierski JR, Newnham ED, Irving PM, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011;106:508–514. doi: 10.1038/ajg.2010.487. [DOI] [PubMed] [Google Scholar]
- 10.Carroccio A, Mansueto P, Iacono G, et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. Am J Gastroenterol. 2012;107:1898–1906. doi: 10.1038/ajg.2012.236. [DOI] [PubMed] [Google Scholar]
- 11.Vazquez-Roque MI, Camilleri M, Smyrk T, et al. A Controlled Trial of Gluten-Free Diet in Patients With Irritable Bowel Syndrome-Diarrhea: Effects on Bowel Frequency and Intestinal Function. Gastroenterology. 2013;144:903–911. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanga R, Leffler DA. Gluten sensitivity: not celiac and not certain. Gastroenterology. 2013;145:276–279. doi: 10.1053/j.gastro.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Biesiekierski JR, Peters SL, Newnham ED, et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145:320–328. doi: 10.1053/j.gastro.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 14.Bucci C, Zingone F, Russo I, et al. Gliadin does not induce mucosal inflammation or basophil activation in patients with nonceliac gluten sensitivity. Clin Gastroenterol Hepatol. 2013;11:1294–1299. doi: 10.1016/j.cgh.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 15.Oxford EC, Nguyen DD, Sauk J, et al. Impact of coexistent celiac disease on phenotype and natural history of inflammatory bowel diseases. Am J Gastroenterol. 2013;108:1123–1129. doi: 10.1038/ajg.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gearry RB, Irving PM, Barrett JS, et al. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease-a pilot study. J Crohns Colitis. 2009;3:8–14. doi: 10.1016/j.crohns.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 17.van Langenberg DR, Gibson PR. Systematic review: fatigue in inflammatory bowel disease. Aliment Pharmacol Ther. 2010;32:131–143. doi: 10.1111/j.1365-2036.2010.04347.x. [DOI] [PubMed] [Google Scholar]
- 18.Wahnschaffe U, Schulzke JD, Zeitz M, et al. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007;5:844–850. doi: 10.1016/j.cgh.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the "no man's land" of gluten sensitivity. Am J Gastroenterol. 2009;104:1587–1594. doi: 10.1038/ajg.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brant SR. Promises, delivery, and challenges of inflammatory bowel disease risk gene discovery. Clin Gastroenterol Hepatol. 2013;11:22–26. doi: 10.1016/j.cgh.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Junker Y, Zeissig S, Kim SJ, et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J Exp Med. 2012;209:2395–2408. doi: 10.1084/jem.20102660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ananthakrishnan AN, Long MD, Martin CF, et al. Sleep disturbance and risk of active disease in patients with Crohn's disease and ulcerative colitis. Clin Gastroenterol Hepatol. 2013;11:965–971. doi: 10.1016/j.cgh.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leeds JS, Horoldt BS, Sidhu R, et al. Is there an association between coeliac disease and inflammatory bowel diseases? A study of relative prevalence in comparison with population controls. Scand J Gastroenterol. 2007;42:1214–1220. doi: 10.1080/00365520701365112. [DOI] [PubMed] [Google Scholar]
- 24.Rubio-Tapia A, Ludvigsson JF, Brantner TL, et al. The prevalence of celiac disease in the United States. Am J Gastroenterol. 2012;107:1538–1544. doi: 10.1038/ajg.2012.219. [DOI] [PubMed] [Google Scholar]


