Abstract
Objective
To understand examined the relationship between postural response latencies obtained during postural perturbations and representative measures of balance during standing (sway variables) and during walking (trunk motion).
Design
Cross-sectional
Setting
University medical center balance disorders laboratory
Participants
Forty persons with MS were compared with 20 similar aged control subjects. Twenty subjects with MS had normal walking velocity group and 20 had slow walking velocity based on the 25-foot walk time greater than 5 seconds.
Interventions
None
Main Outcome Measures
Postural response latency, sway variables, trunk motion variables Results: We found that subjects with MS with either slow or normal walking velocities had significantly longer postural response latencies than the healthy control group. Postural response latency was not correlated with the 25-ft walk time. Postural response latency was significantly correlated with center of pressure sway variables during quiet standing: root mean square (ρ = 0.334, p=0.040), range (ρ=0.385, p=0.017), mean velocity (ρ=0.337, p=0.038), and total sway area (ρ=0.393, p=0.015). Postural response latency was also significantly correlated with motion of the trunk during walking: sagittal plane range of motion (ρ=0.316, p=0.050) and standard deviation of transverse plane range of motion (ρ=-0.430, p=0.006).
Conclusions
These findings clearly indicate that slow postural responses to external perturbations in patients with MS contribute to disturbances in balance control, both during standing and walking.
Keywords: multiple sclerosis, somatosensory, sway, walking, EMG, inertial sensor
Introduction
Multiple sclerosis (MS) is the most common disabling neurological disease of young adults and results in reduced mobility in 400,000 Americans.1 Almost half of people with MS fall every year and impaired balance is an important contributor to falls.2 Balance control during standing, as reflected by increased postural sway during stance, is larger than normal in many people with MS.3-6 Balance control during walking, as reflected by excessive and more variable trunk motion is abnormal in people with MS.7, 8 Trunk motion during walking is a measure of dynamic balance control since excessive lateral trunk oscillations reflect poor control of the body center of mass which may be addressed by adjusting lateral foot placement during gait.9-11 However, the neurophysiological mechanisms that contribute to balance problems during standing and walking in people MS are not well understood.
MS mobility dysfunction occurs early in MS, often at onset, and can be detected in people with MS who have normal walking speeds.8 Factors contributing to mobility disorders in MS may include slowed spinal somatosensory conduction and abnormal sensorimotor control. 12, 13 MS causes spotty loss of myelin, the fatty sheath insulating nerve fibers, along with axonal transaction throughout the central nervous system. This results in slowing, distortion, and loss of conduction of electrical activity along nerve fibers. Balance control depends upon fast conduction up the cord from somatosensory receptors in muscles, skin and joints of the lower extremities for closed loop feedback.14-16 Thus, disruption of the electrical conduction along nerve fibers in persons with MS would contribute to slowed conduction along the spinal cord. A previous study in our laboratory showed that 10 subjects with MS with mild to moderate levels of disability had long latencies of postural responses measured in response to surface translation. These latencies correlated with their slowed somatosensory evoked conduction up the spinal cord, but not with motor conduction delays from the motor cortex to the muscles.13 The same study showed that subjects with MS compensate for the longer latencies of postural responses by increasing the magnitude and predictive scaling of their postural responses.13 However, it is not clear how slowed somatosensory conduction specifically affects balance control during standing and during walking. Furthermore, we do not know if postural response latencies change as mobility disability level increases. To recommend the most efficacious therapy, a better understanding of the causes of balance dysfunction in patients with MS is needed.
The purpose of this study was to examine the relationship between postural response latencies and balance dysfunction during standing and walking in patients with MS. We hypothesized that patients with longer postural response latencies would exhibit more severe balance dysfunction during both walking and quiet standings tasks. We also hypothesized that subjects with MS with slower walking velocity would have longer postural response latencies indicating their slower walk was to compensate for their poor balance control. Establishing a link between delays in somatosensory-triggered postural responses and the resulting balance deficits will improve our understanding of the physiological mechanisms underlying mobility disability in patients with MS.
Methods
2.1 Participants
A total of 40 subjects with MS (45.6±11.7 years; 166.4±18.4 cm; 78.1±19.9 kg) and 20 healthy controls subjects (41.8±10.7 years, 167.9±15.5 cm, 78.7±17.7 kg) participated in the study.
Patients with MS (n=40), recruited through the University's Multiple Sclerosis Clinic, and healthy control subjects (n=20), recruited through the community, provided informed consent. The research protocol was approved by the University's Institutional Review Board. Inclusion criteria for all subjects with MS were: 1) diagnosis of MS made by a neurologist, 2) ability to perform the T25FW test without a walking aid, 3) no clinical relapses within the previous 60 days, 4) free from any other problems which may affect gait such as vestibular issues, orthopaedic problems, and diabetic neuropathy. All subjects were recruited through the MS clinic at Oregon Health and Science University. Adherence to the inclusion criteria was based on subject screening performed by the neurologists in clinic. Healthy control subjects were also free of any conditions that could affect their walking. On the day of testing, all subjects with MS completed the self-reported Expanded Disability Status Scale (EDSS) as a general classification of global MS-related disability level. The EDSS is a standard and heavily used disability classification scale for patients with MS.17 The self-reported EDSS18 correlates strongly with the clinician administered version indicating strong clinical validity.19 The self-report EDSS was utilized in this study to avoid requiring subjects with MS to visit multiple study locations since the laboratory testing location and participating neurologist were located on different campuses.
Patients with MS were divided into two groups based specifically on their 25-foot walking times. The 25 foot walk time (T25FW) was used to separate groups because this outcome measure is frequently used as a clinical assessment tool of mobility and as an outcome measure in clinical trials with clinically meaningful differences found in changes of greater than 20% of the baseline score.20-24 The T25FW test has been shown to display strong test-retest reliability (ICC = 0.991).25 Twenty subjects with MS who performed the T25FW in less than 5 seconds were classified as the Normal Walking Velocity (NWV) MS. Twenty subjects with MS with a T25FW greater than 5 seconds were classified as Slow Walking Velocity (SWV) MS group (Table 1). There were no statistical differences in age, height, or mass between the NWV MS group, the SWV MS group, and healthy controls.
Table 1.
Demographic information for Mild MS (25 foot walk < 5 sec), Moderate MS (25 foot walk > 5 seconds) and healthy controls.
| Mild MS (n = 20) | Moderate MS (n = 20) | Healthy Controls (n = 20) | |
|---|---|---|---|
| Age (yrs) | 41.4 ± 10.5 | 50.3 ± 11.8 | 41.8 ± 10.7 |
| Sex (F/M) | 15 / 5 | 17 / 3 | 17 / 3 |
| Height (cm) | 164.9 ± 23.9 | 168.1 ± 8.6 | 167.9 ± 15.5 |
| Mass (kg) | 74.5 ± 18.6 | 82.3 ± 21.0 | 78.7 ± 27.7 |
| self-reported EDSS | 3.9 ± 1.2 | 5.0 ± 1.3 | - |
| 25 foot walk (sec) | 4.50 ± 0.39 range: 4.11 – 4.98 |
6.86 ± 1.31 range: 5.15 – 9.42 |
- |
Self-reported EDSS = Self-reported Expanded Disability Status Scale
2.2 Outcome measures
Postural Response Latency Protocol and Data Analysis
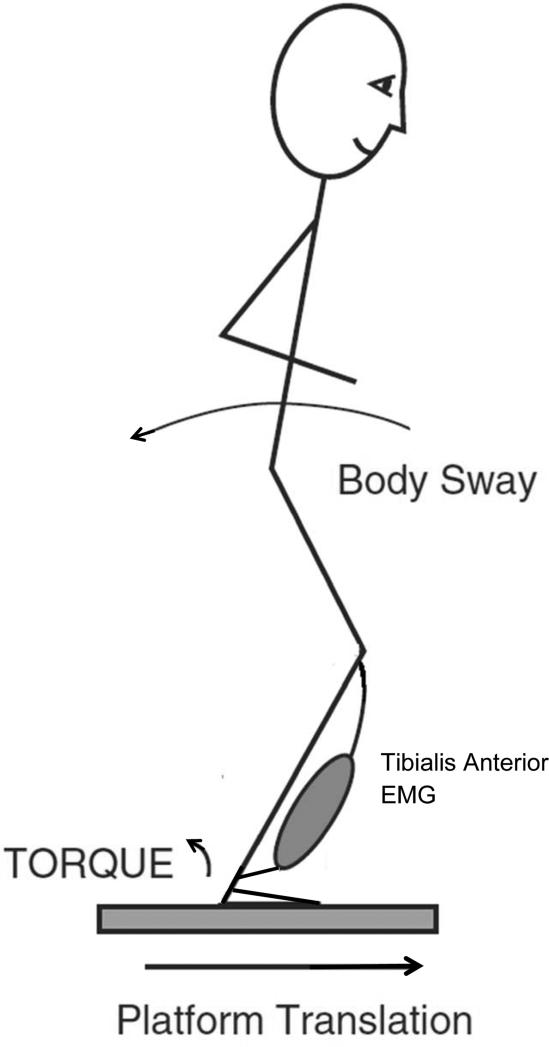
To measure postural response latency, subjects stood on two computer-servo controlled, custom-made, hydraulic platforms that translated forward together causing backward body sway and activation the tibialis anterior bilaterally (Figure 1).26 Subjects stood with arms folded across the chest, eyes open, with their feet at a fixed heel-to-heel distance of 10 cm. Foot placement at the beginning of each trial was controlled by marking the outlines of their feet with tape. Subjects stood on a platform that translated 4 cm forward at a rate of 15 cm/s, which required an in-place response with no stepping (Figure 1).
Figure 1.
Illustration of experimental set up for postural perturbations using the translating force platform.
Surface EMG was recorded in all subjects in the dominant, right tibialis anterior using two 2.5 cm2 surface electrodes place approximately 2 cm apart with a ground electrode on the lateral condyle. Amplified EMG signals were band-pass filtered (70–2000 Hz), rectified, and stored for off-line analysis.13, 15, 27, 28 Although no attempt was made to calibrate EMGs on an absolute scale, amplifier gains were fixed throughout all experimental sessions for all subjects.
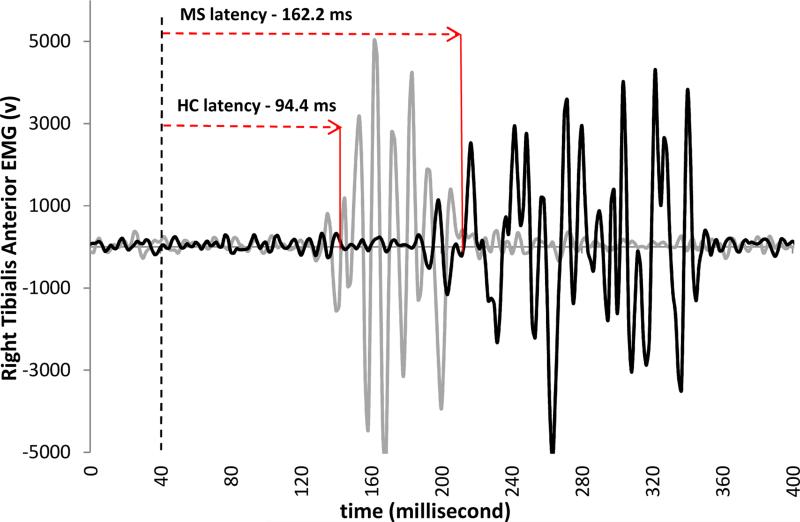
The postural response latency was defined as the time between the onset of surface translation to the first measurable increase in activity of the tibialis anterior muscle greater than 2 SD from baseline that was sustained for at least 50 ms.13, 27 Three translation trials were completed and the average postural response latency for each subject was used for analysis. Figure 2 illustrates the delayed onset of tibialis muscle firing after the onset of the translation in the person with MS compared to the healthy control subject. The time between the translation onset and the muscle firing is the postural response latency. Within the NWV MS Group (n=7) and the SWV Group (n=9), there were subjects with postural response latency values greater than 2 standard deviations above the control mean.
Figure 2.
EMG response to forward surface translation, resulting in backward dysequilibrium. Dashed vertical line indicates the onset of the translation. The time between the dashed line and the onset of EMG firing (Gray trace for Healthy Controls (HC); Black trace for persons with MS) is the postural response latency for this representative trial.
Standing protocol and data analysis
Subjects stood on the split force plate with one foot on each plate with a fixed heel-to-heel distance of 10 cm. Subjects stood for three, thirty-second trials of quiet standing while ground reaction forces were sampled at 100 Hz. Ground reaction forces were filtered at 20 Hz and used to calculated the following center of pressure (CoP) variables: Root mean square (RMS) - to quantify the dispersion of the CoP traces; range - to quantify the peak-to-peak amplitude of CoP traces; mean velocity - to quantify the mean velocity of CoP sway along the entire sway path. Center of pressure variables were calculated according to the methods of Prieto et al29 and have been used previously to evaluate balance in persons with MS.3-6, 30 Mean values for each subject across three trials for each variable were used for analysis.
Walking protocol and data analysis
Subjects walked at a self-selected pace for two minutes up-and-down a 100-foot hallway, while wearing 6 MTX Xsens inertial sensors (49A33G15, Xsens, Culver City, CA) sampling at 50 Hz.31 The sensors contained 3D accelerometers (± 1.7 g) and 3D gyroscopes (± 300°/s range) mounted on: sternum, posterior trunk approximately at L5 level (lumbar), on the anterior surface of the right and left wrist, and on the anterior surface of the right and left lower shank. Turns that occurred at the ends of the hallway were removed from the analysis. The variables of interest were related to trunk motion during walking and included mean sagittal (pitch), lateral (roll), and transverse (yaw) range of motion of the trunk, mean peak horizontal and sagittal angular velocity, standard deviation of sagittal, lateral, and transverse range of motion across all strides, and standard deviation of peak horizontal and angular velocity of the trunk across all strides.7, 8, 32
2.3 Statistical Analysis
To examine the relationship between postural response latency and trunk motion and CoP sway variables, Pearson product-moment correlations were performed. To examine postural response latency, CoP sway, and trunk motion during walking across the MS groups and healthy controls, One-way ANOVAs were performed with independent t-tests used to examine individual group differences post hoc. Alpha value was set at 0.05. All statistics were performed with SPSS software (IBM SPSS Statistic 19).
Results
Relationship between Postural response latency and CoP sway variables
Within the individual NWV MS and SWV MS Groups, there were no significant correlations between postural response latency and CoP displacement RMS, range, mean velocity, or total sway area. Across all subjects with MS, postural response latency was significantly correlated with CoP displacement RMS (r=0.363, p=0.025), range (r=0.370, p=0.022), mean velocity (ρ=0.349, p=0.032), and total sway area (r=0.353, p=0.030). Within the healthy control group, there were no significant correlations between postural response latency and any CoP variables (Table 2).
Table 2.
Pearson product-moment correlations between postural response latency and the listed sway variables during quiet standing.
| Correlation Coefficient (p-value) | |||||
|---|---|---|---|---|---|
| NWV | SWV | All MS subjects | Healthy Controls | ||
| Sway Variables | RMS | 0.033 (0.89) | 0.412 (0.07) | 0.363 (0.03)* | 0.100 (0.68) |
| Range | 0.037 (0.88) | 0.424 (0.06) | 0.370 (0.02)* | 0.211 (0.39) | |
| Velocity | 0.015 (0.95) | 0.367 (0.11) | 0.349 (0.03)* | 0.283 (0.24) | |
| Sway Area | 0.053 (0.84) | 0.354 (0.13) | 0.353 (0.03)* | 0.308 (0.20) | |
| Trunk Variables | Transverse ROM | −0.176 (0.45) | −0.204 (0.43) | −0.109 (0.516) | 0.322 (0.18) |
| STD | 0.332 (0.14) | −0.569 (0.03)* | −0.443 (0.005)* | −0.083 (0.73) | |
| Sagittal ROM | 0.075 (0.75) | 0.345 (0.18) | 0.311 (0.05)* | −0.068 (0.78) | |
| STD | −0.204 (0.37) | 0.101 (0.70) | −0.160 (0.34) | 0.261 (0.28) | |
| Lateral ROM | 0.107 (0.66) | 0.050 (0.87) | 0.070 (0.70) | 0.054 (0.83) | |
| STD | −0.005 (0.99) | 0.095 (0.75) | 0.109 (0.54) | 0.360 (0.13) | |
| Peak Horizontal angular velocity | 0.105 (0.98) | −0.064 (0.81) | 0.080 (0.63) | −0.211 (0.39) | |
| STD | −0.332 (0.14) | −0.254 (0.33) | −0.282 (0.08) | −0.086 (0.73) | |
| Peak Sagittal angular velocity | 0.077 (0.74) | 0.447 (0.07) | 0.283 (0.08) | 0.130 (0.60) | |
| STD | −0.094 (0.68) | 0.317 (0.22) | 0.088 (0.59) | 0.105 (0.67) | |
Significant correlation
NWV – Normal walking velocity multiple sclerosis group (n = 20)
SWV – Slow walking velocity multiple sclerosis group (n = 20)
All MS subjects (n = 40)
Relationship between Postural response latency and Trunk motion variables
Within the NWV MS group only, there were no significant correlations between postural response latency and any trunk motion variables. Within the SWV MS group only, postural response latency significantly correlated with standard deviation of the transverse range of motion (r= -0.569, p=0.025). Across all subjects with MS, postural response latency was significantly correlated with sagittal plane range of motion (r=0.311, p=0.050) and with the standard deviation of the transverse range of motion of the trunk (r=-0.443, p=0.005).
Relationship between Postural response latency and T25FW
There was no significant correlation between postural response latency and T25FW within the NWV MS group (r=0.250, p=0.487) or within the SWV MS group (r=0.087, p=0.730). Across all MS subjects, there was no significant correlation between postural latency and T25FW (r=0.292, p=0.131).
Group Effects of Walking Speed on Latencies and Balance
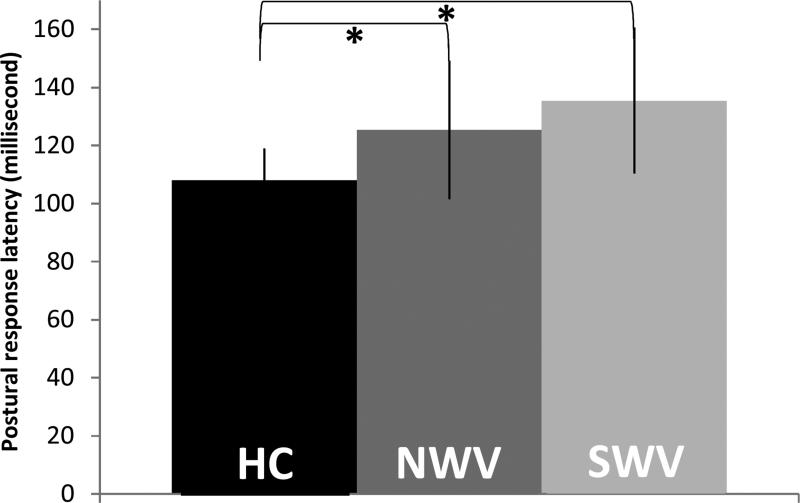
There was a significant group effect for Group (F2, 58 =10.19; p<0.001), where postural response latency was significantly longer in both NWV and SWV MS subjects (p=0.024, p= 0.000 respectively) compared to healthy controls. No significant difference in postural response latency was found between the NWV and SWV MS groups (p=0.159) (Figure 4).
Figure 4.
Postural response latency values for Healthy control, NWV MS Group, and SWV MS Group. The median postural response was 117.8 milliseconds for the NWV MS Group, 129.0 milliseconds for the SWV MS Group, and 111.1 milliseconds for the healthy control group.
*Significant difference (p < 0.05) between groups.
HC – Healthy Controls
NWV – Normal walking velocity MS group
SWV – Slow walking velocity MS group
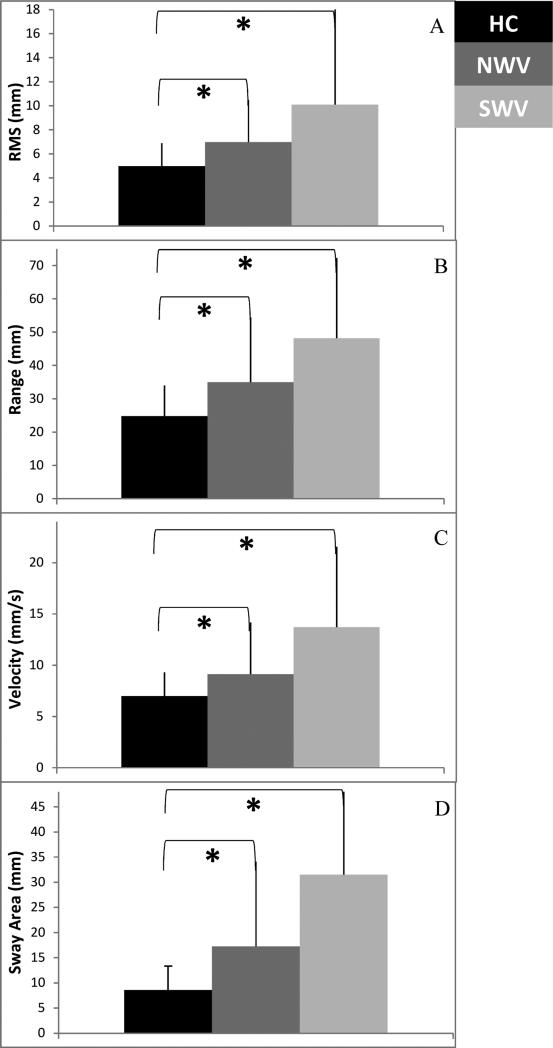
There was a significant Group effect for CoP sway root mean square (F2, 58 =4.933, p=0.011), range (F2, 58 =5.032, p=0.010), velocity (F2, 58 =7.672, p=0.001), and area (F2, 58 =8.268, p=0.001) (Table 3; Figure 4).
Table 3.
Group main effects for one-way ANOVA for sway variables
| Sway Variables | NWV mean (S.D.) | SWV mean (S.D.) | HC mean (S.D.) | ANOVA | NWV/HC paired test | SWV/HC paired test | NWV/SWV paired test | |
|---|---|---|---|---|---|---|---|---|
| F-value | p-value | p-value | p-value | p-value | ||||
| RMS | 6.99 (3.452) | 10.10 (8.01) | 4.99 (1.88) | 4.933 | 0.011** | 0.030* | 0.011* | 0.128 |
| Range | 34.96 (19.47) | 48.15 (34.13) | 24.77 (9.22) | 5.032 | 0.010** | 0.048* | 0.007* | 0.150 |
| Velocity | 9.12 (5.05) | 13.72 (7.84) | 6.97 (2.33) | 7.672 | 0.001** | 0.094 | 0.001* | 0.037* |
| Sway Area | 17.25 (16.86) | 31.49 (25.75) | 8.56 (4.78) | 8.268 | 0.001** | 0.042* | 0.001* | 0.048* |
Significant Group effect
Significant difference between groups
NWV – Normal walking velocity MS group
SWV – Slow walking velocity MS group
HC – Healthy controls
There was a significant Group effect for standard deviation of transverse (yaw) range of motion (F2, 58 =4.431, p=0.016). Paired tests showed that standard deviation of transverse range of motion was significantly greater in the SWV MS group compared to healthy controls (p=0.005) but there was no difference between the NWV MS group and healthy controls (p=0.086) or between the NWV and SWV MS groups (p=0.243). There were no other significant Group effects for any other trunk motion variables (Table 4).
Table 4.
Group main effects for one-way ANOVA for trunk motion variables.
| Trunk Variables | ANOVA | |
|---|---|---|
| F-value | p-value | |
| Transverse ROM | 1.228 | 0.30 |
| STD Transverse ROM | 4.431 | 0.01* |
| Sagittal ROM | 0.871 | 0.42 |
| STD Sagittal ROM | 0.250 | 0.78 |
| Lateral ROM | 0.468 | 0.63 |
| STD Lateral ROM | 0.815 | 0.45 |
| Peak Horizontal angular velocity | 3.062 | 0.06 |
| STD Horizontal angular velocity | 2.518 | 0.09 |
| Peak Sagittal angular velocity | 0.473 | 0.63 |
| STD Sagittal angular velocity | 0.138 | 0.87 |
Significant Group effect
Discussion
The purpose of this study was to examine the relationship between postural response latencies and balance dysfunction during standing and walking in patients with MS. Postural response latency was measured in subjects with MS who had no clinical gait deficits (NWV MS group) and in subjects with MS with slow gait velocity (SWV MS group), as well as in similar aged healthy control subjects. Healthy control subject's automatic postural responses to these moderate perturbations were 107.9 ± 11.0 milliseconds, consistent with the literature33 but latencies in our subjects with MS ranges from 101.6 to 188.9 milliseconds. Since there was no difference in postural latencies between the subjects with MS with normal and slow gait, slowing of gait does not appear to be a compensatory strategy used by those with delayed postural responses. In fact, 7 out of 20 MS subjects in the NWV MS group had postural response latencies greater than two standard deviations above the control mean. Thus, it's possible that postural response latency is a better indicator of disability than walking speed in persons with MS.
In the present study, subjects with MS demonstrated prolonged postural response latencies, which involve both the somatosensory and motor pathways contributing to responses to perturbations.34 However, it is not possible to determine whether the long postural latencies were the result of delayed sensory or motor part of the loop, but it is likely a combination. It has been shown previously that persons with MS have slowed spinal somatosensory conduction, as measured with SSEPs, that is highly correlated with their postural response latencies13, so we assume that this is the primary source of the long postural latencies. In contrast, vestibular or visual loss does not alter postural response latencies, consistent with postural responses triggered by somatosensory inputs.28, 35
Consistent with our hypothesis, postural response latencies in subjects with MS were significantly related to balance control where subjects with the longer postural response latencies showed larger postural sway. The significant relationships between CoP sway measures and postural latencies suggests that delayed nerve conduction speeds due to demyelination compromises standing balance control. Postural sway is maintained through the integration of somatosensory, visual, and vestibular information, all of which can be impaired by MS.36 However, somatosensory input has the greatest contribution to control of balance during standing.16 Delays in the ascending somatosensory tracts such as the dorsal horn and dorsal spinal cerebellar tracts, as reflected by prolonged postural response latencies, would therefore be expected to have a profound effect upon postural sway. The group effects on sway variables found in this study agree with the previous literature, which reports increased sway area in persons with MS.4, 30, 6, 37
Postural response latency was also significantly related to trunk motion during gait (Table 2). Patients with longer latencies had greater sagittal (anterior-posterior rotation, pitch motion) range of trunk motion and less variability in transverse plane (medial-lateral rotation, yaw motion) range of trunk motion during gait. Trunk motion was examined because excessive trunk motion during walking has been associated with falls in the elderly.38 As in quiet standing, the body also uses somatosensory feedback during gait to control balance during forward motion.39 The significant relationships between postural response latencies and trunk motion during gait support our hypothesis that disruption of somatosensory feedback would affect trunk stability during gait in persons with MS. Subjects with MS, especially those with slow walking speed (SWV), showed less than normal variability of trunk motion in the transverse plane (horizontal, axial yaw motion). These findings are in agreement with previous work from our laboratory that also showed decreased variability of trunk motion during gait.31
The significant correlations found between postural latencies and CoP sway and trunk motion variables in subjects with MS support the importance of continuous somatosensory feedback to maintain postural control during standing and during walking.16, 40 In the present study, however, all of these significant correlations between postural response latencies and postural sway during standing were weak to moderate (ρ = 0.334 – 0.528), which indicates that postural response latency is not the only factor that contributes to abnormal postural control during standing and walking in persons with MS. Greater levels of spasticity results in greater CoP sway area and sway velocity.4 Weakness and fatigue, which is reported by up to 85% of persons with MS41, could also affect postural control. It is likely that a combination of disease mechanisms affect balance in persons with MS, but unlike fatigue reports and manual tests to assess spasticity, postural response latency can be quantified directly for each subject, making it a reliable and more sensitive indicator of balance dysfunction.
There was no relationship between gait speed and postural response latencies. This lack of a significant correlation between postural response latencies and speed of the T25FW indicates that walking speed has accommodated to slowed somatosensory feedback or does not rely upon it. Despite lack of effect on walking speed, walking strategies were likely affected by slow postural loops as trunk control during gait (sagittal range of motion) was directly related to postural latencies.
Study Limitations
The findings of this study shed insight into postural control deficits during both standing and walking in persons with MS, but this study also has some limitations. In future studies, obtaining the clinician scored EDSS would also allow for examination of the relationship between EDSS subscores (pyramidal, cerebellar, etc.) and postural response latency in subjects with MS. Another limitation was measurements of postural latencies from only the right tibialis muscle since the effects of MS may be asymmetrical and affect some muscles more than others. It will be of interest to examine postural responses to multi-directional postural displacements and to determine if postural response magnitude is related to balance during stance or gait.
Conclusions
Persons with MS have delayed responses to postural perturbation and these responses contribute to disturbances in postural control during both standing and walking. However, it is not clear how other factors, such as loss of strength, spasticity, and fatigue, each impact gait and postural control. It will be necessary to examine postural response latency and gait and balance variables across a larger and broader spectrum of disability levels in subjects with MS to better understand whether postural response latencies are a biomarker of disability and disease progression in persons with MS.
Suppliers.
Xsens North America Inc. 10557 Jefferson Blvd, Suite C, Culver City, CA 90232
IBM SPSS Statistics, 1 New Orchard Road, Armonk, NY 10504
Matlab, Mathworks, 3 Apple Hill Drive, Natick, MA 01760
Microsoft, One Microsoft Way, Redmond, WA 98052
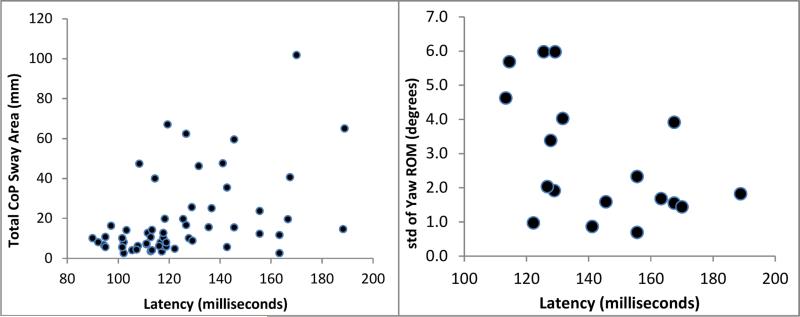
Figure 3.
Correlations plots for total CoP area (left) and standard deviation of yaw range of motion (right) correlated with postural response latency. These plots represent the strongest significant correlations for a CoP variable (ρ = 0.393) across all MS subjects (n = 40) and trunk motion variable (ρ = -0.528) in the slow walking velocity MS group only (n = 20).
Figure 5.
Center of pressure (COP) sway variables – (A) RMS, (B) Range, (C) Velocity, (D) Sway Area. All variables showed a significant effect of Group.
*Significant difference (p < 0.05) between individual groups.
HC – Healthy Controls
NWV – Normal walking velocity MS group
SWV – Slow walking velocity MS group
Acknowledgements
Support for this work was provided by the National Multiple Sclerosis Society (MB 0011) and the NIH R37 (AG006457)
Abbreviations
- MS
multiple sclerosis
- T25FW
25 foot walk time
- NWV
normal walking velocity
- SWV
slow walking velocity
- EDSS
expanded disability status scale
- CoP
center of pressure variables
- EMG
electromyography
- SSEP
somatosensory evoked potentials
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. NEnglJMed. 2000;343(13):938. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Peterson EW, Ben Ari E, Asano M, Finlayson ML. Fall attributions among middle-aged and older adults with multiple sclerosis. Arch Phys Med Rehabil. 2013;94(5):890–5. doi: 10.1016/j.apmr.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 3.Van Emmerik RE, Remelius JG, Johnson MB, Chung LH, Kent-Braun JA. Postural control in women with multiple sclerosis: effects of task, vision and symptomatic fatigue. Gait & posture. 2010;32(4):608. doi: 10.1016/j.gaitpost.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Sosnoff JJ, Shin S, Motl RW. Multiple sclerosis and postural control: the role of spasticity. Archives of Physical Medicine and Rehabilitation. 2010;91(1):93. doi: 10.1016/j.apmr.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Karst GM, Venema DM, Roehrs TG, Tyler AE. Center of pressure measures during standing tasks in minimally impaired persons with multiple sclerosis. JNeurolPhysTher. 2005;29(4):170. doi: 10.1097/01.npt.0000282314.40230.40. [DOI] [PubMed] [Google Scholar]
- 6.Huisinga JM, Yentes JM, Filipi ML, Stergiou N. Postural control strategy during standing is altered in patients with multiple sclerosis. Neurosci Lett. 2012;524(2):124–8. doi: 10.1016/j.neulet.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Huisinga JM, Mancini M, St George RJ, Horak FB. Accelerometry reveals differences in gait variability between patients with multiple sclerosis and healthy controls. Ann Biomed Eng. 2013;41(8):1670–9. doi: 10.1007/s10439-012-0697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spain RI, St George RJ, Salarian A, Mancini M, Wagner JM, Horak FB, et al. Body-worn motion sensors detect balance and gait deficits in people with multiple sclerosis who have normal walking speed. Gait Posture. 2012;35(4):573–8. doi: 10.1016/j.gaitpost.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavanagh JJ, Menz HB. Accelerometry: a technique for quantifying movement patterns during walking. Gait & posture. 2008;28(1):1–15. doi: 10.1016/j.gaitpost.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Moe-Nilssen R. A new method for evaluating motor control in gait under real-life environmental conditions. Part 2: Gait analysis. Clin Biomech (Bristol, Avon) 1998;13(4-5):328–35. doi: 10.1016/s0268-0033(98)00090-4. [DOI] [PubMed] [Google Scholar]
- 11.Bauby CE, Kuo AD. Active control of lateral balance in human walking. JBiomech. 2000;33(11):1433. doi: 10.1016/s0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 12.Cowan JM, Rothwell JC, Dick JP, Thompson PD, Day BL, Marsden CD. Abnormalities in central motor pathway conduction in multiple sclerosis. Lancet. 1984;2(8398):304. doi: 10.1016/s0140-6736(84)92683-7. [DOI] [PubMed] [Google Scholar]
- 13.Cameron MH, Horak FB, Herndon RR, Bourdette D. Imbalance in multiple sclerosis: a result of slowed spinal somatosensory conduction. SomatosensMotRes. 2008;25(2):113. doi: 10.1080/08990220802131127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horak FB. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing. 2006;35(Suppl 2):ii7. doi: 10.1093/ageing/afl077. [DOI] [PubMed] [Google Scholar]
- 15.Horak FB, Nashner LM, Diener HC. Postural strategies associated with somatosensory and vestibular loss. ExpBrain Res. 1990;82(1):167. doi: 10.1007/BF00230848. [DOI] [PubMed] [Google Scholar]
- 16.Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88(3):1097. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 17.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 18.Goodin DS. A questionnaire to assess neurological impairment in multiple sclerosis. Mult Scler. 1998;4(5):444–51. doi: 10.1177/135245859800400508. [DOI] [PubMed] [Google Scholar]
- 19.Bowen J, Gibbons L, Gianas A, Kraft GH. Self-administered Expanded Disability Status Scale with functional system scores correlates well with a physician-administered test. Mult Scler. 2001;7(3):201–6. doi: 10.1177/135245850100700311. [DOI] [PubMed] [Google Scholar]
- 20.Kragt JJ, van der Linden FA, Nielsen JM, Uitdehaag BM, Polman CH. Clinical impact of 20% worsening on Timed 25-foot Walk and 9-hole Peg Test in multiple sclerosis. MultScler. 2006;12(5):594. doi: 10.1177/1352458506070768. [DOI] [PubMed] [Google Scholar]
- 21.Goodman AD, Brown TR, Krupp LB, Schapiro RT, Schwid SR, Cohen R, et al. Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet. 2009;373(9665):732. doi: 10.1016/S0140-6736(09)60442-6. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman M, Moyer D, Norton J. The significant change for the Timed 25-foot Walk in the multiple sclerosis functional composite. Mult Scler. 2000;6(4):286–90. doi: 10.1177/135245850000600411. [DOI] [PubMed] [Google Scholar]
- 23.Schwid SR, Goodman AD, Apatoff BR, Coyle PK, Jacobs LD, Krupp LB, et al. Are quantitative functional measures more sensitive to worsening MS than traditional measures? Neurology. 2000;55(12):1901–3. doi: 10.1212/wnl.55.12.1901. [DOI] [PubMed] [Google Scholar]
- 24.Bever CT, Judge SI. Sustained-release fampridine for multiple sclerosis. Expert OpinInvestigDrugs. 2009;18(7):1013. doi: 10.1517/13543780903002082. [DOI] [PubMed] [Google Scholar]
- 25.Learmonth YC, Dlugonski DD, Pilutti LA, Sandroff BM, Motl RW. The reliability, precision and clinically meaningful change of walking assessments in multiple sclerosis. Mult Scler. 2013 doi: 10.1177/1352458513483890. In Press. [DOI] [PubMed] [Google Scholar]
- 26.Horak FB, Nashner LM. Central programming of postural movements: adaptation to altered support-surface configurations. J Neurophysiol. 1986;55(6):1369–81. doi: 10.1152/jn.1986.55.6.1369. [DOI] [PubMed] [Google Scholar]
- 27.Horak FB, Diener HC, Nashner LM. Influence of central set on human postural responses. J Neurophysiol. 1989;62(4):841–53. doi: 10.1152/jn.1989.62.4.841. [DOI] [PubMed] [Google Scholar]
- 28.Horak FB, Diener HC. Cerebellar control of postural scaling and central set in stance. J Neurophysiol. 1994;72(2):479. doi: 10.1152/jn.1994.72.2.479. [DOI] [PubMed] [Google Scholar]
- 29.Prieto TE, Myklebust JB, Hoffmann RG, Lovett EG, Myklebust BM. Measures of postural steadiness: differences between healthy young and elderly adults. IEEE TransBiomedEng. 1996;43(9):956. doi: 10.1109/10.532130. [DOI] [PubMed] [Google Scholar]
- 30.Cattaneo D, Jonsdottir J. Sensory impairments in quiet standing in subjects with multiple sclerosis. MultScler. 2009;15(1):59. doi: 10.1177/1352458508096874. [DOI] [PubMed] [Google Scholar]
- 31.Huisinga JM, Mancini M, St George RJ, Horak FB. Accelerometry Reveals Differences in Gait Variability Between Patients with Multiple Sclerosis and Healthy Controls. Ann Biomed Eng. 2012 doi: 10.1007/s10439-012-0697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horak FB, Mancini M. Objective biomarkers of balance and gait for Parkinson's disease using body-worn sensors. Mov Disord. 2013;28(11):1544–51. doi: 10.1002/mds.25684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horak FB, Macpherson JM, editors. Handbook of Physiology, Section 12: Exercise: Regulation and Integration of multiple systems. Oxford University Press; New York: 1996. Postural orientation and equilibrium. [Google Scholar]
- 34.Macpherson JM, Horak F. Posture. In: Kandel E, Schwartz J, Jessell T, Siegelbaum S, Hudspeth AJ, editors. Principles of Neural Science. McGraw-Hill; United Stated: 2012. p. 1060. [Google Scholar]
- 35.Inglis JT, Horak FB, Shupert CL, Jones-Rycewicz C. The importance of somatosensory information in triggering and scaling automatic postural responses in humans. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 1994;101(1):159–64. doi: 10.1007/BF00243226. [DOI] [PubMed] [Google Scholar]
- 36.Nelson SR, Di Fabio RP, Anderson JH. Vestibular and sensory interaction deficits assessed by dynamic platform posturography in patients with multiple sclerosis. AnnOtolRhinolLaryngol. 1995;104(1):62. doi: 10.1177/000348949510400110. [DOI] [PubMed] [Google Scholar]
- 37.Huisinga JM, Filipi M, Stergiou N. Supervised resistance training results in changes in postural control in multiple sclerosis patients. Motor control. 2011;16(1):50–63. doi: 10.1123/mcj.16.1.50. [DOI] [PubMed] [Google Scholar]
- 38.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. JAmGeriatrSoc. 1997;45(3):313. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 39.O'Connor SM, Kuo AD. Direction-dependent control of balance during walking and standing. J Neurophysiol. 2009;102(3):1411. doi: 10.1152/jn.00131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peterka RJ. Simplifying the complexities of maintaining balance. IEEE engineering in medicine and biology magazine : the quarterly magazine of the Engineering in Medicine & Biology Society. 2003;22(2):63–8. doi: 10.1109/memb.2003.1195698. [DOI] [PubMed] [Google Scholar]
- 41.Ford H, Trigwell P, Johnson M. The nature of fatigue in multiple sclerosis. Journal of psychosomatic research. 1998;45(1):33–8. doi: 10.1016/s0022-3999(98)00004-x. [DOI] [PubMed] [Google Scholar]