Abstract
Ischemic brain injury inflicted by stroke and cardiac arrest ranks among the leading causes of death and long-term disability in the United States. The brain consumes large amounts of metabolic substrates and oxygen to sustain its energy requirements. Consequently, the brain is exquisitely sensitive to interruptions in its blood supply, and suffers irreversible damage after 10–15 minutes of severe ischemia. Effective treatments to protect the brain from stroke and cardiac arrest have proven elusive, due to the complexities of the injury cascades ignited by ischemia and reperfusion. Although recombinant tissue plasminogen activator and therapeutic hypothermia have proven efficacious for stroke and cardiac arrest, respectively, these treatments are constrained by narrow therapeutic windows, potentially detrimental side effects and the limited availability of hypothermia equipment. Mounting evidence demonstrates the cytokine hormone erythropoietin (EPO) to be a powerful neuroprotective agent and a potential adjuvant to established therapies. Classically, EPO originating primarily in the kidneys promotes erythrocyte production by suppressing apoptosis of proerythroid progenitors in bone marrow. However, the brain is capable of producing EPO, and EPO’s membrane receptors and signaling components also are expressed in neurons and astrocytes. EPO activates signaling cascades that increase the brain’s resistance to ischemia-reperfusion stress by stabilizing mitochondrial membranes, limiting formation of reactive oxygen and nitrogen intermediates, and suppressing pro-inflammatory cytokine production and neutrophil infiltration. Collectively, these mechanisms preserve functional brain tissue and, thus, improve neurocognitive recovery from brain ischemia. This article reviews the mechanisms mediating EPO-induced brain protection, critiques the clinical utility of exogenous EPO to preserve brain threatened by ischemic stroke and cardiac arrest, and discusses the prospects for induction of EPO production within the brain by the intermediary metabolite, pyruvate.
Keywords: apoptosis, blood brain barrier, hypoxia-inducible factor, nitric oxide synthase, peroxynitrite, pyruvate
Introduction
Ischemic syndromes of the central nervous system (CNS) are devastating to the victims and exact an enormous cost on society. Each year nearly 800,000 Americans experience a new or recurrent stroke, of which 87% are ischemic strokes.1 The fourth leading cause of death and the leading cause of long-term disability in the United States, ischemic stroke kills approximately 130,000 Americans annually,1,2 and many survivors experience persistent neurocognitive deficits that profoundly impact their quality of life. Nearly 7 million living American adults have suffered a stroke.2
Cardiac arrest, i.e. sudden cardiac death, which interrupts blood flow to the entire body including the CNS, kills approximately 350,000–400,000 Americans per year, many succumbing to massive brain injury inflicted by the ischemic insult.3,4 Of the 70,000 cardiac arrest victims initially resuscitated each year in the U.S., approximately 70% of these victims die in the hospital, due primarily to extensive brain damage.4–6 40% of initial survivors of cardiac arrest enter a permanent vegetative state, and 80% of them die within 1 year of the event.7 Only 5–14% of resuscitated victims of cardiac arrest survive without significant cerebral impairment.8,9 As the American Heart Association’s 2008 consensus statement on cardiac arrest laments, “…little evidence exists to suggest that the in-hospital mortality rate of patients who achieve recovery of spontaneous circulation (ROSC) after cardiac arrest has changed significantly in the past half-century.”10
In 2000, White et al. commented “There are as yet no clinically effective therapeutic protocols for amelioration of brain damage by ischemia and reperfusion.”11 Regrettably this statement still holds true 14 years later. Aside from early restoration of cerebral perfusion, few interventions have been found to prevent ischemic brain injury, despite enormous investments in preclinical and clinical research. Indeed, recombinant tissue plasminogen activator (rtPA) and therapeutic hypothermia are the only interventions with proven clinical efficacy for ischemic stroke and cardiac arrest, respectively. The challenge to any prospective treatment for CNS ischemia is the sheer complexity of the injury cascade triggered by ischemia-reperfusion. This article summarizes research conducted in the last two decades that has demonstrated the natural cytokine erythropoietin to be a potentially powerful neuroprotectant capable of intervening at multiple points in the injury cascade.
Mechanisms of injury in ischemic and post-ischemic brain
Ischemia and reperfusion ignite a complex cascade of brain injury (Figure 1) mediated by glutamate, intracellular Ca2+ overload, and reactive oxygen and nitrogen intermediates (RONS). The brain requires continuous delivery of oxygen and energy substrates via the cerebral circulation to sustain its high rate of ATP turnover. Occlusion of cerebral arteries or cardiac arrest interrupts oxidative metabolism, precipitating an abrupt decrease in the cytosolic Gibbs free energy of ATP hydrolysis (ΔGATP), the immediate energy source for the ion pumps that manage cytosolic free Ca2+ and repolarize the cell membrane. Depolarization of ischemic neurons causes excessive release of the excitatory amino acid neurotransmitter, glutamate.12–14 Astrocytes normally protect neurons from glutamate toxicity by ATP-dependent sequestration of the neurotransmitter.15 Loss of ΔGATP can cause reversal of glutamate transport, so astrocytes release glutamate. Moreover, RONS attack and disable glutamate transporters.
Figure 1. Cascade of injury in ischemic and post-ischemic brain.
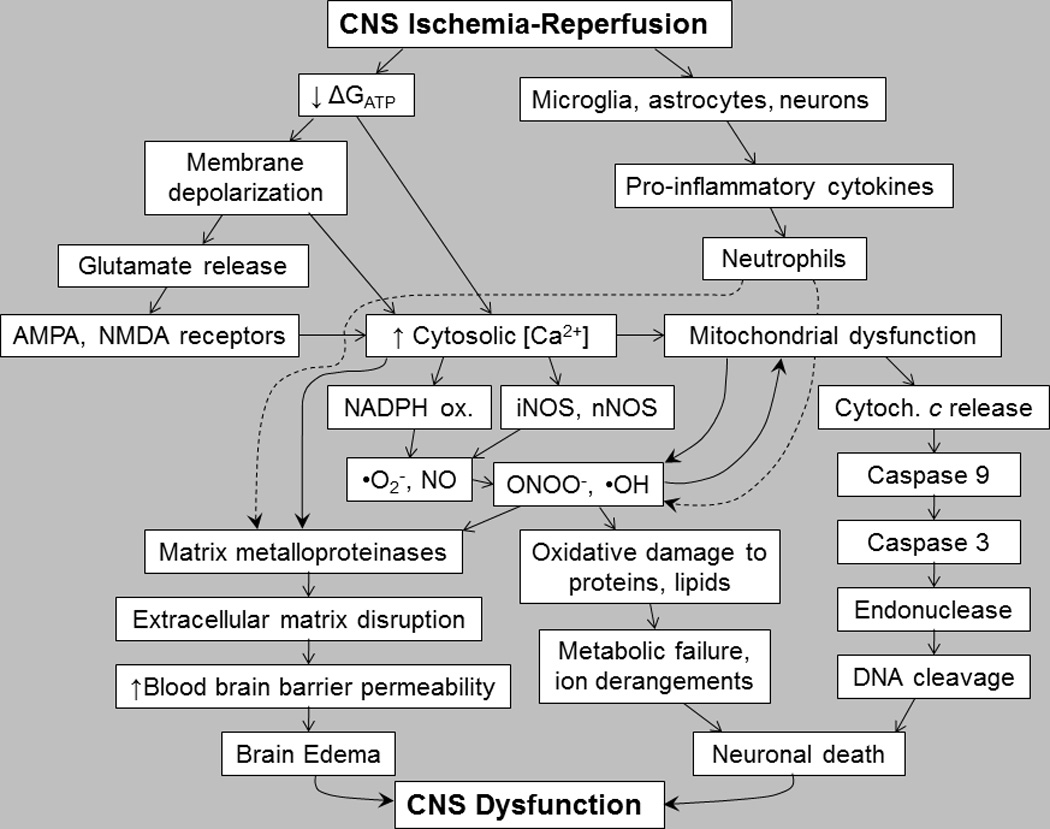
By interrupting cerebrovascular delivery of energy substrates and O2, CNS ischemia depletes Gibbs free energy of ATP hydrolysis (ΔGATP), thus impairing neuronal Ca2+ management and provoking excitotoxic glutamate signaling. Subsequent reperfusion triggers intense formation of reactive oxygen and nitrogen species. These compounds and Ca2+ overload combine to trigger mitochondrial permeability transition, cytochrome c release and energetic collapse, and activate matrix metalloproteinases that degrade the extracellular matrix, allowing neutrophil infiltration in response to pro-inflammatory cytokines and provoking brain edema. See text for details.
Glutamate binding to α-amino-3-hydroxy-5-methyl-4-isoazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors located on neurons, glia and cerebrovascular endothelium3 provokes additional depolarization and intense Ca2+ entry, sufficient to activate destructive Ca2+-dependent proteases and phospholipases, culminating in cellular injury and death.11,13,14 Among the Ca2+-activated proteins is calcineurin, which activates the pro-apoptotic protein, Bad, a promoter of mitochondrial permeability transition, and the inducible nitric oxide synthase (NOS) isoform, iNOS, which catalyzes cytotoxic peroxynitrite (ONOO−) formation.11 Intracellular Ca2+ overload also damages neurons by precipitating mitochondrial dysfunction. A spike in cytosolic Ca2+ concentration above 0.5 µM increases mitochondrial Ca2+ uptake, which provokes sequential opening of the mitochondrial permeability transition pores, collapse of the inner mitochondrial membrane potential, failure of oxidative phosphorylation, and generation of RONS.14
By binding to NMDA receptors, glutamate activates NOS16,17 to produce excessive amounts of NO which condense with superoxide (•O2−), yielding a cytotoxic product, ONOO−.18 At the onset of reperfusion there is a burst of RONS formation in the brain,19 with microglia as a major source of NO.20,21 In addition, ischemia-reperfusion can induce iNOS in astrocytes, causing these cells to release toxic amounts of NO. ONOO− initiates peroxidation of membrane phospholipids, nitrosylates tyrosine and cysteine residues in proteins, and depletes the intracellular antioxidant, glutathione.18,22 Moreover, •O2− reacts with heme, liberating Fe2+ which catalyzes lipid peroxidation.11 Hypothermic circulatory arrest in dogs activated cerebrocortical neuronal NOS (nNOS), which peaked at five times the pre-ischemic activity at 20 h post-arrest.23 In a rat model of status epilepticus, bilateral microinjection of kainate induced hippocampal NO, •O2− and ONOO− formation, which led, sequentially, to inactivation of mitochondrial respiratory complex I, cytochrome c release, initiation and propagation of caspase activity and, finally, DNA fragmentation.24
Calcium25 and RONS26,27 induce astrocytes,25,26,28 microglia25 and cerebrovascular endothelium29–31 to secrete matrix metalloproteinases (MMPs), a class of enzymes that degrade protein components of the extracellular matrix and of the tight junctions within the capillary endothelium that comprise the blood-brain barrier (BBB).32–35 By oxidizing cysteine residues in the autoinhibitory domain of proMMPs, RONS activate MMPs by the ‘cysteine switch’ mechanism.36 MMPs have been implicated in BBB disruption and brain edema and inflammation.37,38 Interstitial brain edema, which develops within 1 hour after cardiac arrest or stroke3 is associated with poor neurological outcome. Brain edema increases intracranial pressure, which compresses the brain, lowers cerebral perfusion pressure and decreases cerebral blood flow. Moreover, BBB disruption allows neutrophils to infiltrate the brain parenchyma, where they release RONS and MMPs that further compromise the BBB. In rats subjected to cardiac arrest – CPR, neutrophils were detected in the susceptible brain regions within 6 h ROSC.9
Neuronal apoptosis after brain ischemia and reperfusion
Brain ischemia triggers two general processes of neuronal death: necrosis and apoptosis.39,40 Which process predominates depends on the duration and intensity of the ischemic insult. In focal ischemia, necrosis is the major cause of cell death in the intensely ischemic core.41 The core is surrounded by the less severely ischemic penumbra, where neurons primarily die by apoptosis, a highly regulated mechanism of cell death.39,40,42,43 Because apoptosis is orchestrated by specific signaling elements, and because its measured pace affords time to initiate treatment, there are opportunities to salvage penumbral cells threatened by ischemic stroke.
Two distinct apoptotic cascades operate in the CNS (Figure 2).39,40,44 In the extrinsic pathway, Fas ligand secreted by neurons, glia and inflammatory leukocytes binds its receptor, Fas, which, via its Fas-activated death domain, activates caspase 8, a protease that mediates apoptosis by activating caspase 3, the major ‘executioner’ caspase, and cleaves Bid to truncated Bid (tBid), which combines with Bad in the mitochondrial membrane forming a channel. The release of cytochrome c through this channel initiates the intrinsic apoptotic pathway. In the cytosol, cytochrome c combines with Apaf-1, dATP and procaspase 9, forming the apoptosome which activates caspase 9 by cleavage of its procaspase. In a similar manner, caspase 9 activates caspase 3, which cleaves numerous targets culminating in the cell’s destruction.
Figure 2. Anti-apoptotic mechanisms of erythropoietin.
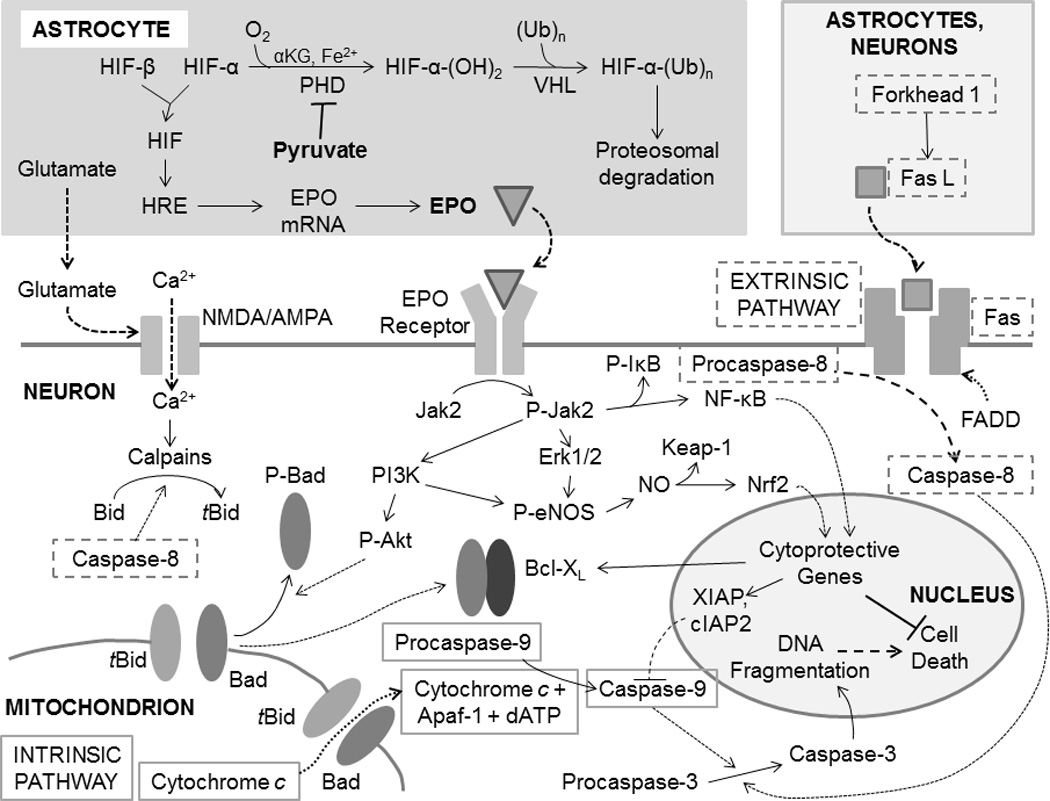
Ischemia-reperfusion activates intrinsic and extrinsic apoptotic cascades, the elements of which are indicated by solid and broken gray outlines, respectively, which converge on caspase-3 as the common effector. Erythropoietin (EPO) activates anti-apoptotic signaling in neurons by binding its membrane receptors. This event initiates a complex cascade of intracellular signaling events, mediated by protein kinases, that (1) prevent formation of Bad-tBid channels that release cytochrome c from mitochondria; (2) blunt the activation of pro-apoptotic caspases; and (3) evoke Nrf2- and NF-κB driven expression of cytoprotective genes that increase neuronal resistance to ischemia-reperfusion stress. Collectively, these mechanisms suppress the intrinsic and extrinsic apoptotic pathways. EPO expression, primarily in astrocytes, is driven by hypoxia-inducible factors (HIF) interacting on hypoxia response elements (HRE) in the promoter regions of EPO and other genes. HIF, in turn, is activated by stabilization of its O2-regulated α subunit. Pyruvate interferes with HIF-α hydroxylation by prolyl hydroxylase (PHD), thereby preventing proteosomal degradation of the subunit and promoting EPO expression.
Neuronal apoptosis is well documented in animal models of cardiac arrest. For example, in rabbits placed on cardiopulmonary bypass and subjected to 2 h hypothermic circulatory arrest, 4 h reperfusion, hippocampal CA1 neurons exhibited caspase-3 activation and DNA fragmentation detectable by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL).45 Böttiger, Teschendorf et al.46,47 examined the progression of apoptotic cell death in rat brain over the first 7 d recovery from cardiac arrest – CPR. Post-arrest caspase activity followed different time-courses in different brain regions. In nucleus reticularis thalami, cortex and striatum, caspase activity and DNA fragmentation detected by TUNEL were already maximal at 6 h ROSC. In the hippocampal CA1 subregion, TUNEL-positive cells were first detected at 3 d, and increased further at 7 d. Thus, cardiac arrest activates caspases and apoptosis in vulnerable brain regions. A strong correlation emerged, both in extent and time-course, between caspase activation and DNA fragmentation.
Nitric oxide generated by the neuronal and inducible NOS isoforms has been implicated in CNS apoptosis following cardiac arrest. Incubation of hippocampal neurons with the NO donor sodium nitroprusside lowered Bcl-2 content and increased Bax content, and activated caspase-3.48 In astrocyte-neuron cocultures, NOS inhibition by L-NMMA increased neuronal survival and prevented the decrease in Bcl-2 and increase in Bax initiated by hypoxia-reoxygenation.49
Erythropoietin: cerebroprotective cytokine
Erythropoietin, a 165 amino acid, 30.4 kDa glycoprotein with four oligosaccharide chains, was identified over 30 years ago as the hormone responsible for inducing erythropoiesis.50 The liver is the major source of EPO during the prenatal period. Postpartum, 90% of EPO production shifts to the kidneys,51 where peritubular interstitial fibroblasts near the corticomedullary border synthesize and secrete EPO in response to hypoxemia.52–54 EPO circulates to the bone marrow, where it suppresses apoptosis of colony-forming unit erythroid cells, promoting the proliferation and development of these cells into mature erythrocytes.50,55 EPO’s anti-apoptotic protection of erythroid precursors was an early indication that the cytokine might similarly protect cells in other tissues, including brain.
Studies in a variety of animal models of CNS ischemia-reperfusion56,57 have defined EPO’s robust neuroprotective properties in brain.58–61 In stroke-prone spontaneously hypertensive rats, cerebroventricular infusion of EPO salvaged cerebral cortex and motor function following permanent middle cerebral artery (MCA) occlusion.62 The abundance of mRNA encoding the EPO receptor was elevated in the ischemic penumbra, potentially enhancing the neuroprotective capabilities of EPO and preventing infarct expansion. Injection of EPO (5,000 IU/kg, ip) at the start of 60 min MCA occlusion in rats decreased infarct size by 75% and suppressed apoptosis in the ischemic penumbra.63 Erythropoietin (1,000 IU/kg, ip) decreased ethanol-induced apoptosis in cerebellum, prefrontal cortex, and hippocampus of mice given subcutaneous ethanol injections.64 In gerbils subjected to 5 min bilateral carotid artery occlusion,65 recombinant human EPO, when injected (50 or 100 IU, ip) at the time of reperfusion, attenuated hippocampal edema, lipid peroxidation and neuronal death, and suppressed NO formation. Thus, EPO treatment may protect sensitive brain regions, at least in part by suppressing NOS.
Transgenic human EPO expression in mouse brain doubled cerebrocortical and striatal EPO content vs. wild type, and decreased infarct volume by 84% following 90 min middle cerebral artery occlusion and 72 h reperfusion.66 In this study, TUNEL-positive and caspase-3-positive neurons were decreased by ∼50 and ∼75%, respectively, in transgenic vs. wild-type striatum. EPO expression sharply increased phosphor-activation of Erk-1, Erk-2 and Akt; the Erk inhibitor PD98059 and the PI3K/Akt inhibitor Wortmannin both prevented the reduction in TUNEL- and caspase-3-positive neurons, implicating both kinases in the neuroprotective cascade.
EPO has been found to be cerebroprotective even when its administration is delayed. In rats, exogenous EPO decreased infarct volume even when given 6 h after MCA occlusion-reperfusion.67 In a rat model of traumatic brain injury, EPO (5,000 IU/kg, ip) given 24 h post-injury produced significant improvement in neurological function and decreased neuronal loss in the hippocampal CA3 subregion, and increased neurogenesis in the injured cortex and dentate gyrus.68 Erythropoietin, injected ip in rats subjected to MCA occlusion, reduced infarct volume by 70–75% whether given 24 h before, during or 3 h after occlusion.63 EPO also sharply lowered TUNEL-positive cells in the ischemic penumbra of these rats. Importantly, some protection was still seen when EPO was administered as late as 6 h post-occlusion, although not at 9 h post-occlusion. EPO’s neuroprotective efficacy for at least the first several h after the ischemic insult expands opportunities for its therapeutic application for acute CNS ischemia.
Although the preponderance of preclinical evidence shows EPO to be neuroprotective, a study in rats subjected to 6 min pre-treatment ventricular fibrillation, 2 min CPR, defibrillatory countershocks and up to 7 d recovery yielded less favorable outcomes.69 EPO (5000 IU/kg), given iv 5 min before cardiac arrest, then injected ip at 24 and 72 h post-arrest, failed to suppress total caspase or caspase-3 activities, prevent DNA fragmentation and neuronal degeneration in the hippocampal CA1 subregion, or improve neurological deficit score at 1, 3 or 7 d recovery. These negative findings merit attention in light of the equivocal results of clinical trials of EPO for CNS ischemia described below.
Mechanisms of erythropoietin neuroprotection
Erythropoietin is an especially promising neuroprotectant because it potentially intervenes at several points in the apoptotic pathway (Figure 2). Brain neurons express homodimeric EPO receptors; EPO binding triggers reciprocal auto-phosphorylation of the two monomers, which in turn phosphorylate and activate the signaling kinase, Jak-2.70 Multiple protein kinases are recruited to the EPO receptor and phosphorylated by activated Jak2, initiating a complex antiapoptotic signaling cascade (Figure 2). Several cytoprotective mechanisms activated by EPO signaling are summarized in the following subsections.
Increased anti-apoptotic proteins and Bcl-XL/Bax ratio
The relative cellular contents of anti- vs. pro-apoptotic members of the Bcl protein family exert a profound effect on cell survival vs. apoptosis.71,72 EPO enhancement of neuronal Bcl-XL content plays a pivotal role in EPO’s anti-apoptotic neuroprotection.60 In cultured rat cortical microglia and astrocytes, EPO shifted the Bcl/Bax ratio in favor of anti-apoptotic Bcl.73 In gerbils subjected to CNS ischemia, EPO up-regulated Bcl-XL mRNA and protein in hippocampal CA1 neurons, and prevented learning disability.74 Transgenic over-expression of human EPO in murine striatum enhanced ischemic induction of Bcl-XL.66 Activated Akt phosphorylates the proapoptotic protein, Bad, preventing the latter’s insertion into the mitochondrial membrane.75 Phosphorylated STAT5 activates nuclear factor κB (NF-κB), which promotes expression of the anti-apoptotic proteins X-linked inhibitor of apoptosis (XIAP) and c-inhibitor of apoptosis-2 (cIAP2) in cultured cerebrocortical neurons.76 c-IAP2 suppresses caspases 3, 8 and 9;77 XIAP binds and suppresses caspases 3 and 9,78 and inhibits activation of procaspase 9 within the apoptosome.79
Enhancement of the brain’s antioxidant defenses
Preclinical studies have demonstrated EPO induction of key components of the brain’s antioxidant armamentarium. In rats, ip injection of 1,000 IU/kg EPO at 8 h intervals beginning 5 min after induction of subarachnoid hemorrhage increased gene expression and content of the antioxidant enzymes glutathione S-transferase, NAD(P)H:quinone oxidoreductase-1 and heme oxygenase-1, and blunted cerebrocortical apoptosis, brain edema and BBB disruption 48 h later.80 EPO (1,000 IU/kg, ip) increased glutathione peroxidase activity and decreased lipid peroxidation in the brains of ethanol-intoxicated mice.64 In brains of rats subjected to hyperoxia-imposed oxidative stress, EPO (20,000 IU/kg, ip) upregulated heme oxygenase-1, dampened lipid peroxidation, and prevented the decline in glutathione redox state.81
Recent studies implicate the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) in EPO’s induction of antioxidant enzymes. Nrf2 activates expression of a gene program encoding several phase II defense enzymes that afford antioxidant and anti-inflammatory cytoprotection,82,83 including heme oxygenase-1, peroxiredoxin, superoxide dismutase, glutathione peroxidase, NAD(P)H:quinone oxidoreductase-1, and the glutathione synthesizing enzyme glutamate-cysteine ligase.80,84,85 Binding of a regulatory protein, Keap1, sequesters Nrf2 in the cytoplasm, targeting Nrf2 for polyubiquitinylation and proteasomal degration and, thus, silencing the Nrf2 gene program.86–88 RONS oxidize Keap1 sulfhydryls,83 liberating Nrf2 which translocates to the nucleus and binds the antioxidant response element in the promoter of phase II response genes. EPO is proposed89 to activate Nrf2 by activating Akt and Erk, which in turn phosphor-activate eNOS, thereby increasing NO formation in the neuronal cytosol (Figure 2). NO or its derivative ONOO− release Nrf2 by nitrosylating Keap1’s regulatory sulfhydryls.90 Accordingly, pharmacological inhibition of Akt and Erk blunted EPO-induced nuclear translocation of Nrf2 and heme oxygenase-1 expression in cultured human neural cells.84
Suppression of matrix metalloproteinases and inflammation
Li et al.91 studied mice subjected to intracerebral hemorrhage, a pro-inflammatory event. EPO (ip injection), given during the first 3 d post-hemorrhage, preserved the BBB, prevented tissue edema, preserved collagen, restrained increases in MMP-2 content and enhanced content of the endogenous MMP inhibitor, tissue inhibitor of metalloproteinase-2 (TIMP-2). In human erythroid progenitor cells, EPO suppressed MMP-9 secretion and induced TIMP-1 expression and secretion.92 ERK1/2 inhibitors PD98059 and U0126 and PI3K inhibitor LY294002 blocked EPO suppression of MMP-9 and induction of TIMP-1. These findings are empirical evidence that EPO preserves the extracellular matrix and prevents CNS injury by inducing TIMPs and suppressing MMPs. In rats undergoing MCA occlusion, EPO (5000 IU/kg body wt, ip) decreased astrocyte activation and recruitment of leukocytes and microglia into the infarct, and suppressed formation of the pro-inflammatory cytokines IL-6, TNF and monocyte chemoattractant protein-1 by >50%.93
Erythropoietin dampens glutamate excitotoxicity
The excitatory amino acid glutamate provokes neuronal Ca2+ entry via NMDA and AMPA channels. Excessive glutamatergic activity in ischemic and post-ischemic brain provokes cytotoxic Ca2+ overload. EPO suppressed glutamate release from hippocampal and cerebellar neurons exposed to ‘chemical ischemia’ produced by excess Ca2+ or ionomycin,94 in spinal neurons exposed to excitotoxic kainic acid95 and in electrically stimulated hippocampal slices.96 By dampening glutamate release, EPO may ameliorate NMDA- and AMPA-channel-mediated Ca2+ entry, thereby preventing excitoxicity and minimizing ATP demands for Ca2+ extrusion by the energy-depleted neurons.
Erythropoietin modulation of nitric oxide synthase
Erythropoietin exerts divergent effects on the three NOS isoforms. EPO dampened expression of iNOS in oligodendrocytes exposed to inflammatory stimuli.89 Transgenic expression of human EPO in murine brain suppressed nNOS and iNOS expression in striatal neurons.66 In gerbils subjected to bilateral carotid occlusion, post-ischemic EPO injection (c. 800–1500 100 IU/kg, ip) 60 min after reperfusion lowered NO formation in the hippocampus, in parallel with EPO’s suppression of lipid peroxidation and tissue edema.65 Neuronal NOS is Ca2+-activated, so EPO’s suppression of glutamatergic signaling and the resultant Ca2+ overload may contribute to the decreased NOS activity. In contrast, EPO has been shown to activate the endothelial NOS isoform (eNOS), which generates the moderate amounts of NO which activate Nrf2.84,89,90
Clinical trials: exogenous erythropoietin for brain ischemia
As Pytte and Steen97 noted, “…the last three decades have been filled with disappointments regarding pharmacological treatment of cardiac arrest patients.” Indeed, an array of potential treatments has failed to impart significant clinical benefit, including treatments which afforded substantial neuroprotection in animal models. Clinical trials of EPO for brain ischemia have yielded mixed outcomes. Ehrenreich et al.98 conducted a pioneering clinical trial in which iv injections of 33,000 IU EPO, daily for the first 3 days after stroke, improved recovery of neurocognitive function and decreased the persistent neurological deficit evident 18–30 d after stroke. EPO was efficacious when the first dose was given up to 8 h after the onset of stroke symptoms, but massive doses of EPO were required for clinical benefit.
Cariou et al.99 conducted a clinical trial of EPO for brain protection following cardiac arrest. Five intravenous injections of 40,000 IU EPO at 12 h intervals, beginning 42–72 min after out-of-hospital cardiac arrest, failed to improve neurological recovery assessed at day 28 post-arrest. EPO did produce modest increases in hematocrit and hemoglobin content at 14 d post-arrest vs. non-EPO controls. A small trial by Grmec et al.100 showed that a single, massive iv bolus of EPO (90,000 IU), given by emergency responders within 1–2 min of initiating CPR, did increase rates of initial defibrillation, survival to ICU admission, 24 h survival and survival to hospital discharge. Despite these promising short-term outcomes, EPO treatment did not improve neurological outcome.
Ehrenreich et al.101 studied 460 patients with stroke in the MCA perfusion territory. Patients received three iv injections of 40,000 IU EPO, at 6, 24 and 48 h after onset of symptoms. EPO increased death rate (16.4%; 42/256) vs. placebo (9.0%; 24/266) and incidence of cerebrovascular hemorrhage. These adverse effects were seen almost entirely in patients receiving recombinant tissue plasminogen activator (rtPA) beyond its therapeutic window, which is limited to the first 4.5 h after stroke onset.102,103
A recent preclinical study by Jia et al.104 provided valuable insights regarding the detrimental interaction of rtPA and EPO. Rats were subjected to embolic MCA occlusion, followed by EPO (5000 IU/kg, ip injection) and rtPA treatment (10 mg/kg, iv injection) at 2 or 6 h MCA occlusion. When administered at 2 h MCA occlusion, EPO and rtPA were similarly effective at reducing infarct size, but the combination of the two afforded no additional protection over the separate treatments. When administered at 6 h MCA occlusion, although EPO alone decreased infarct size, neither rtPA alone or combined with EPO afforded protection. Indeed, rtPA increased intracerebral hemorrhage at 6 h MCA occlusion vs. saline-injected control rats, and the combined EPO + rtPA treatment increased intracerebral hemorrhage even more than rtPA alone. The combined treatments, but not EPO or rtPA alone, activated MMP-9 via nuclear factor κB (NF-κB) signaling in cerebral microvessels at 6 h MCA occlusion. Thus, when EPO and rtPA are coadministered beyond rtPA’s therapeutic window, the result is activation of MMP-9, culminating in cerebral hemorrhage and infarct expansion.
How readily does erythropoietin traverse the blood-brain barrier?
The transfer of systemically administered EPO from the cerebral circulation across the BBB into the brain parenchyma is less than 1% efficient;67,105,106 consequently, high doses are required to achieve therapeutically effective EPO concentrations within the brain.60 In mice a tiny fraction of intravenously injected EPO, 0.05–0.1% of the injected dose, entered the brain parenchyma, an efficiency that approximated that of albumin.105 In fetal sheep and monkeys injected with high doses of EPO, the EPO activity in the cerebrospinal fluid was only about 2% of the circulating activity.106 Similar results were reported in humans;107 indeed, the dosages of recombinant EPO required to produce neuroprotection (1,000–30,000 IU/kg) are well above those (<500 IU/kg) used to treat anemia.108 Other studies showed that circulating EPO can only enter the brain if the BBB has been compromised. In patients with traumatic brain injury, the appearance of EPO in the ventricular cerebrospinal fluid correlated with the extent of BBB disruption.109 In a patient undergoing resection of a brain tumor, a single iv injection of 6000 IU recombinant human EPO increased serum EPO activity from c. 13 to >6500 IU/l for at least 60 min, but there was no increase in EPO activity in the cerebrospinal fluid.110 Collectively, these studies demonstrate that circulating EPO does not efficiently cross the intact BBB, but can pass from blood to brain if the BBB is disrupted. The high doses of exogenous EPO necessary to surmount the intact BBB may increase blood coagulability enough to precipitate thrombotic events111 and, when combined with tPA therapy, produce deadly hemorrhagic transformation.104,112
Erythropoietin expression within the brain
Noguchi et al.75 stated “EPO production in neural cells can increase the local bioavailability of EPO independent of transit through the blood-brain barrier.” The brain possesses the molecular machinery to manufacture EPO intrinsically, on the “leeward” side of the blood-brain barrier.59,113–115 Indeed, EPO mRNA abundance in the cerebellum, pituitary gland and cerebrocortex rivaled that of the conventionally EPO-expressing liver and kidneys.116 Substantial EPO expression was detected in several brain regions116 and spinal cord117 in preterm human fetuses. Nagai et al.118 examined expression of EPO and its receptors in cultured human astrocytes, neurons, microglia and oligodendrocytes. Only the astrocytes expressed EPO mRNA. Neurons, astrocytes and microglia possessed EPO receptors; the oligodendrocytes did not. In gerbils, sequestration of intrinsic EPO by injection of soluble EPO receptors into the cerebral ventricles intensified neuronal death in the hippocampus following a moderate, ordinarily non-injurious ischemic challenge,119 suggesting that EPO production within the brain contributed to a basal level of neuroprotection.
As in kidney,120,121 hypoxia is a powerful inducer of EPO expression in brain.94,122 This induction is mediated by hypoxia inducible factor-1 (HIF-1), an O2-regulated transcription factor that activates the expression of an extensive gene program encoding proteins that increase cellular resistance to hypoxia and ischemia.51,123 HIF-1 is a heterodimer containing two subunits: a constitutive β subunit and an α subunit which is also constitutively expressed but, in well-oxygenated tissues, rapidly undergoes prolyl hydroxylase-catalyzed, Fe2+- and α-ketoglutarate-dependent hydroxylation of two prolyl residues, earmarking the subunit for poly-ubiquitinylation and proteosomal degradation (Figure 2).124 Hypoxia stabilizes HIF-1α in two ways:114 it deprives prolyl hydroxylase of the O2 required for HIF-1α hydroxylation, and it causes the mitochondrial electron transport chain to generate RONS which convert Fe2+ to Fe3+, removing the source of electrons for the prolyl hydroxylase reaction. Thus stabilized, HIF-1α diffuses from the cytosol to the nucleus and combines with the β subunit, forming the active HIF-1 transcription factor. HIF-1 then binds the hypoxia response element in the promoter regions of an extensive array of genes, including EPO, vascular endothelial growth factor, the entire glycolytic enzyme sequence, and a host of other proteins which, collectively, increase cellular resistance to hypoxia and ischemia.114 Thus, embryonic mouse neocortical neurons and astrocytes expressed EPO mRNA and protein when exposed to hypoxia or the hypoxia-mimetic chemicals desferrioxamine or cobalt chloride.125 While EPO is intensely expressed by astrocytes, its membrane receptors are predominantly located in neurons and cerebrovascular endothelium. EPO secreted by astrocytes may function in a paracrine manner (Figure 2).
By effectively surmounting the BBB, while potentially avoiding the untoward effects of massive systemic EPO dosages, intrinsic EPO expression within the brain parenchyma addresses the important limitations of exogenous EPO. However, a strategy of subjecting critically ill patients to systemic hypoxia in the midst of an acute CNS ischemic event would be dangerous and clinically unacceptable. Is there a safe, simple means of inducing EPO expression in the brain for treatment of acute CNS ischemia?
Neuroprotection by exogenous pyruvate
The neuroprotective capabilities of pyruvate, a natural intermediary metabolite and energy substrate, have been demonstrated in a variety of brain preparations. Although an exhaustive review of these studies is beyond the scope of this article, several reports exemplifying the neuroprotection afforded by pyruvate are summarized here. Lee et al.126 subjected rats to 12 min forebrain ischemia by bilateral occlusion of the carotid arteries. Sodium pyruvate (250, 500 or 1000 mg/kg) sharply lowered mortality to 1 of 26 rats vs. 18 of 31 NaCl-injected control rats when injected ip at 30 min or 1 h reperfusion, but was ineffective when given at 2 or 3 h reperfusion. In the NaCl-injected rats, extensive cell death was detected in the post-ischemic brain 72 h after ischemia-reperfusion; pyruvate (500 mg/kg) prevented cell death. Thus, pyruvate injected ip protected brain from ischemia, even when given 30 or 60 min after reperfusion. In a swine model of hemorrhagic shock, Mongan et al.127 showed that intravenous resuscitation with sodium pyruvate suppressed excitotoxic glutamate release within the cerebral cortex and slowed the post-hemorrhage decline in cortical electrical activity. Kim et al.128 studied kainate-induced epileptic seizures in rats. Sodium pyruvate (500 mg/kg, ip) was injected 30 or 150 min after kainate (10 mg/kg, ip). Pyruvate sharply lowered, by 60–85%, cell death in hippocampal CA1, CA3 and dentate gyrus. Zinc injures neurons by activating metallothioneins, interfering with mitochondrial respiration, inducing ROS formation by the respiratory chain, and activating NADPH oxidase to produce ·O2−. Pyruvate prevented intracellular zinc accumulation in the studies of Lee et al.126 and Kim et al.128
In a study by Sharma et al.,129 pyruvate prevented simulated ischemia-induced damage and death of cultured rat astrocytes subjected to simulated ischemia-reperfusion. Cells were challenged by 6 h profound, substrate-free hypoxia, then reoxygenated for another 6 h in presence of pyruvate or glucose. Pyruvate maintained cellular morphology, prevented lactate dehydrogenase leakage, a measure of membrane rupture and cell death, and suppressed early apoptotic events including mitochondrial cytochrome c release, caspase-3 cleavage and activation, and poly(ADP-ribose) polymerase (PARP) cleavage, in a manner superior to glucose.
In anesthetized dogs, Sharma et al.130 evaluated pyruvate protection of the brain threatened by cardiac arrest and resuscitation. The heart was arrested by epicardial shock, then, after 5 min arrest, cardiac massage was performed for 5 min before defibrillation by epicardial countershocks. Sodium pyruvate or NaCl were infused iv (0.125 mmol • kg−1 • min−1) during cardiac massage and the first 60 min recovery, and then the dogs were recovered for 3 days. The pyruvate infusion increased arterial plasma pyruvate concentration from 0.22 ± 0.02 to 3.6 ± 0.2 mM; pyruvate concentration subsided within 30 min post-infusion.131 Pyruvate sharply lowered neurological deficit 24 and 48 h post-arrest, particularly the deficits in motor function, vs. the NaCl-infused dogs. Pyruvate also lowered neuronal death and caspase-3 activity in the hippocampal CA1 subregion and prevented degeneration of cerebellar Purkinje cells.
Fukushima et al.132 demonstrated pyruvate protection of brain in a rat model of cortical contusion injury. Sodium pyruvate was injected (500 or 1000 mg/kg, ip) 5 min after contusion. Intracerebral pyruvate detected by microdialysis plateaued at 30–75 min after pyruvate injection, confirming that pyruvate traversed the BBB in this model. Both doses of pyruvate sharply lowered the intensity of cortical cell death at 6 h post-contusion.
Recently, Ryou et al.133 examined pyruvate’s neuroprotective capabilities in a rat model of ischemic stroke, in which the left MCA was occluded by advancing a suture into the artery for 120 min; suture withdrawal abruptly reperfused the ischemic tissue. Sodium pyruvate or NaCl control were infused iv from 60 min occlusion until 30 min reperfusion. Analyses of brains harvested at 24 h reperfusion revealed that pyruvate infusion produced an 84% reduction in infarct volume and 80% reduction in apoptotic nuclei vs. the respective control values. Indeed, the reduction in infarct volume afforded by pyruvate was nearly identical to that produced by transgenic human EPO expression in Kilic et al.’s studies in mice subjected to MCA occlusion-reperfusion.66 Collectively, these and other reports demonstrate that timely administration of pyruvate can minimize brain injury from ischemia-reperfusion and other stresses.
Pyruvate traverses the blood brain barrier
Many potentially cerebroprotective compounds have proven ineffective due to their inability to surmount the BBB. In contrast, pyruvate is readily transferred across the BBB by a high-affinity, proton-linked monocarboxylate transport mechanism in the vascular endothelium (Figure 3).134,135 Monocarboxylate transporters also are abundant in the plasma membranes of neurons and astrocytes,136 affording pyruvate uptake by the brain parenchyma. Using cerebrocortical microdialysis in a pig model of hemorrhagic shock, Mongan et al.127 showed that intravenous pyruvate (0.9 mmol • kg−1 bolus followed by 0.08 mmol • kg−1 • min−1 infusion), producing a sustained arterial plasma pyruvate concentration of 5–6 mM, increased pyruvate concentration in cerebrocortical microdialysate from 0.09 to 0.43 mM. Although the fractional recovery of pyruvate in the microdialysate wasn’t reported, the results suggest pyruvate does indeed cross the blood-brain barrier, but doesn’t equilibrate. On the other hand, the neurons and astroglia may have avidly taken up the pyruvate, keeping the interstitial concentration low. Cerebrocortical microdialysis studies in rats by Fukushima et al.132 confirmed that pyruvate, injected ip appeared in the brain parenchyma over a period of several minutes. Additional evidence that pyruvate cerebroprotection requires pyruvate transport was reported by Wang et al.,137 who showed ip injections of 500 mg/kg sodium pyruvate decreased infarct size nearly 50% in rats subjected to 65 min MCA occlusion, and that this cerebroprotective effect was blunted by the monocarboxylate transporter antagonist α-cyano-4-hydroxycinnamate.
Figure 3. Metabolism and cytoprotective mechanisms of pyruvate in brain.
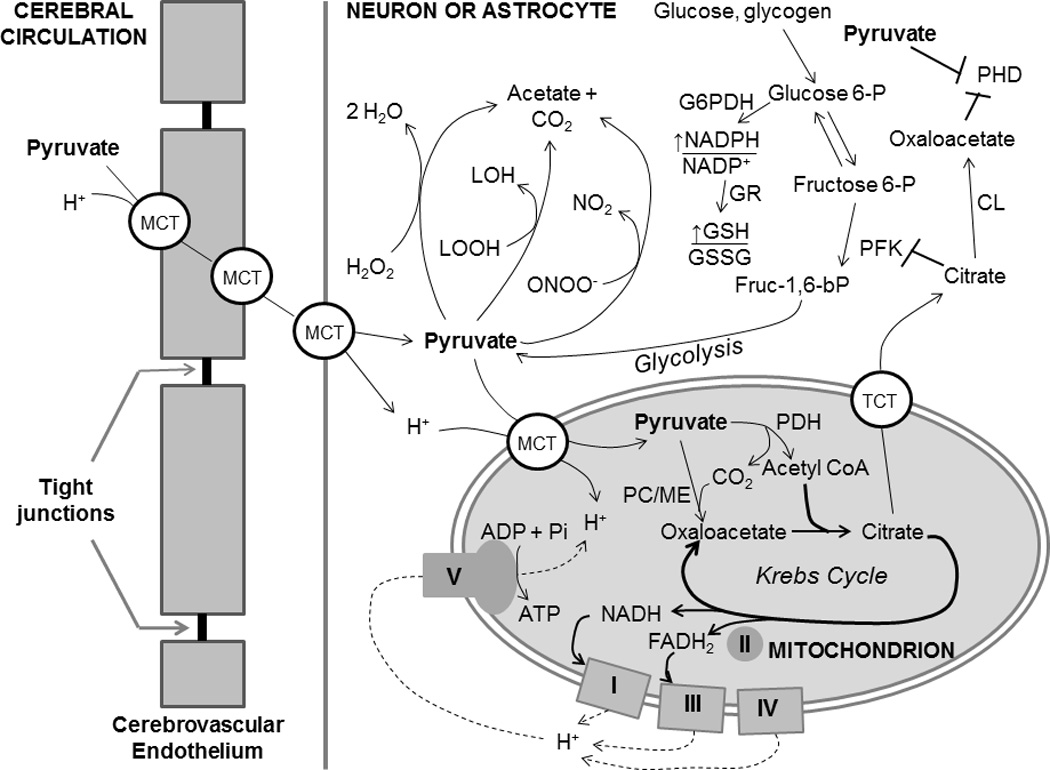
Pyruvate is carried across the cerebrovascular endothelium and cell and mitochondrial membranes within the brain parenchyma my monocarboxylate transporters (MCT). In addition to its induction of EPO expression (Figure 2), pyruvate affords cytoprotection by (1) supporting oxidative metabolism and mitochondrial ATP production; (2) directly detoxifying hydrogen peroxide, lipid peroxides (LOOH) and peroxynitrite; (3) increasing mitochondrial citrate formation, which, when exported to the cytosol by the tricarboxylate transporter (TCT), suppresses phosphofructokinase (PFK) activity, thereby diverting glycolytic flux into the hexose monophosphate shunt, the source of NADPH reducing power by glucose 6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase; (4) cytosolic citrate lyase degrades citrate to acetate and oxaloacetate, which, like pyruvate, competitively inhibits prolyl hydroxylase.
Cerebroprotective mechanisms of pyruvate
Pyruvate may preserve post-ischemic brain by several mechanisms. An energy-yielding, oxidizable fuel,138,139 pyruvate augments oxidative metabolism, thereby generating ATP and phosphocreatine127 and, thus, increasing ΔGATP, the thermodynamic driving force for cellular function. Pyruvate also affords three general antioxidant mechanisms:139,144 (1) as an alpha-keto carboxylate, pyruvate can react with and directly detoxify H2O2, lipid peroxides and ONOO−;140–142 (2) pyruvate oxidizes the cytosolic NADH/NAD+ redox couple, thereby decreasing availability of NADH to NADH oxidase, which generates •O2−;143 (3) pyruvate bolsters intracellular antioxidant defenses by increasing NADPH/NADP+ and, thus, glutathione redox state, the major intracellular antioxidant system.131,145 Pyruvate suppressed DNA fragmentation, a critical event in the progression of apoptosis (Figure 2) in a cultured renal tubular epithelial cell line subjected to antimycin A-induced chemical hypoxia,146 as well as in H2O2-challenged mouse thymocytes147 and post-ischemic rat liver.148 Pyruvate suppression of H2O2-induced glutathione depletion, caspase activation and death of cultured human umbilical vein endothelial cells149,150 paralleled intense Erk1/2 phosphorylation150 as well as increased Bcl-2 and decreased Bax contents and, thus, increased anti-apoptotic Bcl-2/Bax ratio.149 Although pyruvate’s actions in cerebrovascular endothelium are not yet known, effects such as these could stabilize integrity of the cerebrovascular endothelium and blood brain barrier in the face of ischemia-reperfusion.
Several reports over the past decade have demonstrated pyruvate’s antioxidant and anti-apoptotic actions in brain preparations. Wang et al.151 showed that cultured astrocytes released pyruvate which protected co-cultured neurons from copper-catalyzed cysteine autoxidation, a source of hydroxyl radicals. In rat primary neurons, 2.5 mM pyruvate suppressed β-amyloid-induced dichlorofluorescein fluorescence, a measure of ROS formation.152 In another study153 pyruvate protected murine neuroblastoma cells from cell death triggered by H2O2 and 6-hydroxydopamine, an inducer of H2O2 formation. Wang et al.154 exposed cultured human neuroblastoma SK-N-SH cells to 150 µM H2O2, which provoked mitochondrial superoxide formation, collapsed the mitochondrial membrane potential, and killed 85% of the cells. Pyruvate concentration-dependently suppressed cell death; 1–4 mM pyruvate completely prevented H2O2-induced cell death, even when its administration was delayed until 1 h after H2O2 exposure. Pyruvate also suppressed H2O2-induced intracellular and mitochondrial RONS formation, with 2 mM pyruvate exerting near-complete prevention of RONS. Massive mitochondrial depolarization by 3 mM H2O2 was prevented by 1 mM pyruvate.
Pyruvate’s anti-inflammatory actions have been demonstrated in several organs, including brain. Cardiopulmonary bypass provokes a systemic inflammatory response that damages internal organs and compromises post-surgical recovery.155,156 In pigs subjected to cardioplegia-induced cardiac arrest and maintained on-pump, pyruvate-fortified cardioplegia suppressed the pro-inflammatory C-reactive protein, enhanced anti-inflammatory cytokine IL-10, prevented activation of MMP-9, suppressed neutrophil infiltration into the myocardial parenchyma, and blunted nitrotyrosine formation, a measure of nitrosative stress.157 These effects were seen 4 h after pyruvate treatment. In dogs, cardiac arrest and cardiopulmonary resuscitation produced a striking increase in hippocampal MMP activity 3 d later; pyruvate infusion during cardiac massage and the first 60 min recovery suppressed this MMP activation by 80%.130 Sharma and Mongan158 examined the anti-inflammatory capabilities of low-volume, hypertonic sodium pyruvate resuscitation in a rat model of hemorrhagic shock. The pyruvate treatment ameliorated liver injury, suppressed serum and hepatic pro-inflammatory cytokines, NOS and cyclooxygenase-2 activities, caspase-3 activation and poly(ADP ribose) polymerase cleavage and lipid peroxidation, and attenuated liver injury. Thus, pyruvate can supply energy substrate, detoxify RONS and suppress inflammation and apoptosis in CNS threatened by acute ischemia-reperfusion.
Induction of erythropoietin and neuroprotection by pyruvate
Studies in a cultured human glioma cell line revealed a novel action of pyruvate: the stabilization of HIF-1α despite the presence of abundant O2.159,160 Here, pyruvate and oxaloacetate, an α-keto carboxylate structural analogue and product of mitochondrial pyruvate carboxylation (Figure 3),139 suppressed prolyl hydroxylase activity, apparently by competing with the enzyme’s natural substrate, α-ketoglutarate, for access to the enzyme’s catalytic domain.161 These findings raised the possibility that pyruvate could suppress prolyl hydroxylation and subsequent polyubiquitination and degradation of HIF-1α and, thus, augment expression of HIF-1-activated genes, including EPO, in normal tissue.
Ryou et al.’s studies in a porcine cardiopulmonary bypass model revealed, for the first time, pyruvate induction of EPO synthesis in a mammalian organ, the heart.162 Here, pyruvate-enriched cardioplegia stabilized HIF-1α content, which paralleled robust myocardial mRNA expression and synthesis of EPO. Elements of EPO’s intracellular signaling cascades, Erk and eNOS, were activated following pyruvate cardioplegia. Thus, temporary (60 min) pyruvate treatment evoked EPO expression and its cytoprotective signaling cascades that persisted several h after treatment. Indeed, the myocardium released EPO into the coronary venous effluent for at least 4 h after crossclamp release and washout of the pyruvate-enriched cardioplegia.
In Ryou et al.’s rat model of ischemic stroke,133 pyruvate treatment increased cerebral EPO content severalfold, in the ischemic tissue as well as the contralateral, non-ischemic hemisphere. Additional experiments were conducted in glioma and neuronal cell lines subjected to oxygen-glucose deprivation and reoxygenation, a cell culture model of ischemia-reperfusion, to assess the roles of HIF-1α, EPO and the downstream signaling in pyruvate’s neuroprotection.133 Five and 10 mM pyruvate afforded significant cytoprotection, paralleled by marked increases in HIF-1α and EPO contents and phosphor-activation of Akt but not Erk. Incubation with soluble EPO receptor, and siRNA suppression of HIF-1α expression, blunted pyruvate’s cytoprotection. Collectively, these results support the hypothesis that pyruvate prevents ischemic injury of brain, at least in part by stabilizing HIF-1α, thereby increasing EPO synthesis and activating the cytoprotective Akt signaling cascade.
Recently Ryou et al. tested pyruvate’s ability to limit rtPA toxicity in a cultured neuronal cell line and primary microvascular endothelial cells.163 Six and 10 h of oxygen-glucose deprivation produced marked neuronal cell death which was exacerbated by rtPA. Pyruvate (8 mM) prevented cell death in the absence of rtPA, dampened cell death in the rtPA-exposed cells, suppressed rtPA-induced RONS formation, and sharply lowered basal and rtPA-induced MMP-2 content, while inducing Akt and Erk phosphorylation. Interestingly, pyruvate alone or combined with rtPA increased cellular content of monocarboxylate transporter-2 vs. the respective pyruvate-free conditions. These results suggested that pyruvate might extend rtPA’s therapeutic window by dampening rtPA-induced cytotoxicity; it is essential to test this interaction in intact animals.
Conclusion and perspectives
Cardiac arrest and stroke, two of the leading causes of death and long-term disability in the United States and Europe, heretofore have proven refractory to pharmacological interventions. Extensive preclinical research has identified EPO as a potentially powerful treatment to limit the ischemic damage to the CNS inflicted by these scourges. Unlike agents that failed to protect the CNS in clinical trials, EPO is not a “one trick pony;” it activates several intracellular mechanisms that intervene at multiple steps in the cascade of ischemia-reperfusion injury (Figure 2). However, despite favorable outcomes in early clinical trials, two factors threaten to limit EPO’s clinical utility for stroke and cardiac arrest: its potentially dangerous interaction with rtPA inducing hemorrhagic transformation within the cerebral circulation, and the high dosages of EPO required to surmount the BBB.
The brain’s intrinsic ability to express and synthesize EPO may afford an alternative strategy: the administration of compounds that promote EPO gene expression within the brain by stabilizing the transcription factor HIF-1, the principal activator of EPO gene expression. Pyruvate offers several advantages as an enhancer of HIF-1-driven EPO expression in the CNS: a natural intermediary metabolite, pyruvate is nontoxic at cerebroprotective dosages; aside from its EPO induction, pyruvate is a physiological antioxidant and energy-yielding, oxidizable fuel; pyruvate is efficiently transferred from the circulation to the brain parenchyma by monocarboxylate transporters within the cerebrovascular endothelium and in the plasma membranes of neurons and glia, delivering it to the sites of ischemia-reperfusion injury and of EPO synthesis; pyruvate is highly water soluble, so that aqueous solutions of concentrated sodium pyruvate suitable for intravenous infusion164 are readily prepared. Thus, pyruvate therapy may offer a facile means of evoking EPO expression and cytoprotection within the CNS. It should be noted that pyruvate has been shown to be safe and efficacious as an intracoronary intervention in patients with congestive heart failure165,166 and cardiogenic shock,167 and as a component of cardioplegia in patients undergoing coronary revascularization on cardiopulmonary bypass.168
Potential limitations of pyruvate therapy must be acknowledged. Given HIF-1’s fundamental role in promoting survival and growth of solid tumors,159 protracted pyruvate treatment might impose unacceptable risks in cancer patients. However, this concern would not apply to a single pyruvate treatment for acute CNS ischemia. It has been argued169,170 that pyruvate may be unsuitable for protracted storage due to its chemical instability. However, pyruvate can be kept indefinitely in powder form and, as noted above, dissolved to high concentrations immediately before its administration. Esterified derivatives of pyruvate, most notably ethyl pyruvate, have been found to be highly stable in aqueous solution, although these compounds are somewhat less soluble than authentic pyruvate,139 and to suppress systemic inflammation in rat models of endotoxemia171 and hemorrhagic shock.172 However, it has been reported that ethyl-pyruvate resuscitation affords no short-term energetic and hemodynamic advantages over standard lactated Ringer’s.173 Moreover, the ability of these pyruvate derivatives to traverse the BBB has not yet been established.
Acknowledgements
This work was supported by research grant R01 NS076975 from the National Institute of Neurological Disorders and Stroke. AQN and BHC were supported by predoctoral fellowships from the UNTHSC Physician Scientist Program and the UNTHSC Neurobiology of Aging Program, respectively. GFS was supported by a Postdoctoral Fellowship from the National Institute of Neurological Disorders and Stroke.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoazolepropionic acid
- BBB
blood brain barrier
- cIAP2
c-inhibitor of apoptosis-2
- CNS
central nervous system
- CPR
cardiopulmonary resuscitation
- EPO
erythropoietin
- ΔGATP
Gibbs free energy of ATP hydrolysis
- HIF
hypoxia-inducible factor
- Keap1
Kelch-like ECH-associated protein 1
- MCA
middle cerebral artery
- MMP
matrix metalloproteinase
- NF-κB
nuclear factor κB
- NMDA
N-methyl-D-aspartate
- NOS
nitric oxide synthase (eNOS, endothelial NOS
- iNOS
inducible NOS
- nNOS
neuronal NOS)
- Nrf2
nuclear factor erythroid 2-related factor 2
- RONS
reactive oxygen and nitrogen species
- ROSC
recovery of spontaneous circulation
- rtPA
recombinant tissue plasminogen activator
- TIMP
tissue inhibitor of metalloproteinase
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- XIAP
X-linked inhibitor of apoptosis
Footnotes
Authors’ contributions: AQN, BHC, GFS, MGR and RTM researched the literature and wrote the manuscript; AQN and RTM created the figures; RTM edited the manuscript.
References
- 1.Go AS, Mozaffarian D, Roger VL. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics - 2013 update. Circulation. 2013;127:e6–245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM. American Heart Association Statistics Committee and Stroke Statistics Committee. Heart disease and stroke statistics - 2012 update. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao F. Bench to bedside: brain edema and cerebral resuscitation: the present and future. Acad Emerg Med. 2002;9:933–46. doi: 10.1111/j.1553-2712.2002.tb02196.x. [DOI] [PubMed] [Google Scholar]
- 4.Idris AH, Roberts LJII, Caruso L, Showstark M, Layon AJ, Becker LB, Vanden Hoek T, Gabrielli A. Oxidant injury occurs rapidly after cardiac arrest, cardiopulmonary resuscitation, and reperfusion. Crit Care Med. 2005;33:2043–2048. doi: 10.1097/01.ccm.0000174104.50799.bd. [DOI] [PubMed] [Google Scholar]
- 5.Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, Nichol G, Lane-Truitt T, Potts J, Ornato JP, Berg RA. National Registry of Cardiopulmonary Resuscitation Investigators. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295:50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 6.Nolan JP, Laver SR, Welch CA, Harrison DA, Gupta V, Rowan K. Outcome following admission to UK intensive care units after cardiac arrest: a secondary analysis of the ICNARC Case Mix Programme Database. Anesthesia. 2007;62:1207–1216. doi: 10.1111/j.1365-2044.2007.05232.x. [DOI] [PubMed] [Google Scholar]
- 7.Madl C, Holzer M. Brain function after resuscitation from cardiac arrest. Curr Opin Crit Care. 2004;10:213–217. doi: 10.1097/01.ccx.0000127542.32890.fa. [DOI] [PubMed] [Google Scholar]
- 8.Westfal RE, Reissman S, Doering G. Out-of-hospital cardiac arrests: an 8-year New York City experience. Am J Emerg Med. 1996;14:364–368. doi: 10.1016/S0735-6757(96)90050-9. [DOI] [PubMed] [Google Scholar]
- 9.Böttiger BW, Grabner C, Bauer H, Bode C, Weber T, Motsch J, Martin E. Long term outcome after out-of-hospital cardiac arrest with physician staffed emergency medical services: the Utstein style applied to a midsized urban/suburban area. Heart. 1999;82:674–679. doi: 10.1136/hrt.82.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neumar RW, Nolan JP, Adrie C, Aibiki M, Berg RA, Böttiger BW, Callaway C, Clark RSB, Geocadin RG, Jauch EC, Kern KB, Laurent I, Longstreth WT, Jr, Merchant RM, Morley P, Morrison LJ, Nadkarni V, Peberdy MA, Rivers EP, Rodriguez-Nunez A, Sellke FW, Spaulding C, Sunde K, Vanden Hoek T. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. Circulation. 2008;118:2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- 11.White BC, Sullivan JM, DeGracia DJ, O’Neil BJ, Neumar RW, Grossman LI, Rafols JA, Krause GS. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- 12.Guyot LL, Diaz FG, O-Regan MH, Song D, Phillis JW. The effect of streptozotocin-induced diabetes on the release of excitotoxic and other amino acids from the ischemic rat cerebral cortex. Neurosurgery. 2011;48:385–390. doi: 10.1097/00006123-200102000-00030. [DOI] [PubMed] [Google Scholar]
- 13.Belousov AB. Novel model for the mechanisms of glutamate-dependent excitotoxicity: role of neuronal gap junctions. Neurosci Lett. 2012;524:16–19. doi: 10.1016/j.brainres.2012.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konstady BB. The role of glutamate in neuronal ischemic injury: the role of spark in fire. Neurol Sci. 2012;33:223–237. doi: 10.1007/s10072-011-0828-5. [DOI] [PubMed] [Google Scholar]
- 15.Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. Curr Molec Med. 2004;4:193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- 16.Mayhan WG, Didion SP. Glutamate-induced disruption of the blood-brain barrier in rats. Role of nitric oxide. Stroke. 1996;27:965–969. doi: 10.1161/01.str.27.5.965. [DOI] [PubMed] [Google Scholar]
- 17.Nicotera P, Lipton SA. Excitotoxins in neuronal apoptosis and necrosis. J Cereb Blood Flow Metab. 1999;19:583–591. doi: 10.1097/00004647-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Manukhina EB, Downey HF, Mallet RT. Role of nitric oxide in cardiovascular adaptation to intermittent hypoxia. Exp Biol Med. 2006;231:343–365. doi: 10.1177/153537020623100401. [DOI] [PubMed] [Google Scholar]
- 19.Basu S, Liu X, Nozari A, Rubertsson S, Miclescu A, Wiklund L. Evidence for time-dependent maximum increase of free radical damage and eicosanoid formation in the brain as related to duration of cardiac arrest and cardio-pulmonary resuscitation. Free Radic Res. 2003;37:251–256. doi: 10.1080/1071576021000043058. [DOI] [PubMed] [Google Scholar]
- 20.Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992;149:2736–2741. [PubMed] [Google Scholar]
- 21.Boje KM, Arora PK. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992;587:250–256. doi: 10.1016/0006-8993(92)91004-x. [DOI] [PubMed] [Google Scholar]
- 22.Guix FX, Uribesalgo I, Coma M, Muñoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Progr Neurobiol. 2005;76:126–152. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Brock MV, Blue ME, Lowenstein CJ, Northington FA, Lange MS, Johnston MV, Baumgartner WA. Induction of neuronal nitric oxide after hypothermic circulatory arrest. Ann Thorac Surg. 1996;62:1313–1320. doi: 10.1016/0003-4975(96)00775-8. [DOI] [PubMed] [Google Scholar]
- 24.Chuang Y-C, Chen S-D, Liou C-W, Lin T-K, Chang W-N, Chan SHH, Chang AYW. Contribution of nitric oxide, superoxide anion, and peroxynitrite to activation of mitochondrial apoptotic signaling in hippocampal CA3 subfield following experimental temporal lobe status epilepticus. Epilepsia. 2009;50:731–746. doi: 10.1111/j.1528-1167.2008.01778.x. [DOI] [PubMed] [Google Scholar]
- 25.Neria F, del Carmen Serrano-Perez M, Velasco P, Urso K, Tranque P, Cano E. NFATc3 promotes Ca2+-dependent MMP3 expression in astroglial cells. Glia. 2013;61:1052–1066. doi: 10.1002/glia.22494. [DOI] [PubMed] [Google Scholar]
- 26.Ralay Ranaivo H, Hodge JN, Choi N, Wainwright MS. Albumin induces upregulation of matrix metalloproteinase-9 in astrocytes via MAPK and reactive oxygen species-dependent pathways. J Neuroinflammation. 2012;9:68. doi: 10.1186/1742-2094-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh HL, Chi PL, Lin CC, Yang CC, Yang CM. Up-regulation of ROS-dependent matrix metalloproteinase-9 from high-glucose-challenged astrocytes contributes to the neuronal apoptosis. Mol Neurobiol. 2014 doi: 10.1007/s12035-013-8628-y. in press. [DOI] [PubMed] [Google Scholar]
- 28.Gottschall PE, Deb S. Regulation of matrix metalloproteinase expressions in astrocytes, microglia and neurons. Neuroimmunomodulation. 1996;3:69–75. doi: 10.1159/000097229. [DOI] [PubMed] [Google Scholar]
- 29.Harkness KA, Adamson P, Sussman JD, Davies-Jones GA, Greenwood J, Woodroofe MN. Dexamethasone regulation of matrix metalloproteinase expression in CNS vascular endothelium. Brain. 2000;123:698–709. doi: 10.1093/brain/123.4.698. [DOI] [PubMed] [Google Scholar]
- 30.Lee JM, Yin KJ, Hsin I, Chen S, Fryer JD, Holtzman DM, Hsu CY, Xu J. Matrix metalloproteinase-9 and spontaneous hemorrhage in an animal model of cerebral amyloid angiopathy. Ann Neurol. 2003;54:379–382. doi: 10.1002/ana.10671. [DOI] [PubMed] [Google Scholar]
- 31.Wang L, Zhang ZG, Zhang RL, Gregg SR, Hozeska-Solgot A, LeTourneau Y, Wang Y, Chopp M. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, Chan PH, Park TS. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H558–H568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- 33.Lischper M, Beuck S, Thanabalasundaram G, Pieper C, Galla HJ. Metalloproteinase mediated occluding cleavage in the cerebral microcapillary endothelium under pathological conditions. Brain Res. 2010;1326:114–127. doi: 10.1016/j.brainres.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 34.Lehner C, Gehwolf R, Tempfer H, Krizbai I, Hennig B, Bauer HC, Bauer H. Oxidative stress and blood-brain barrier dysfunction under particular consideration of matrix metalloproteinases. Antioxid Redox Signal. 2011;15:1305–1323. doi: 10.1089/ars.2011.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdul Muneer PM, Alikunju S, Szlachetka AM, Haorah J. The mechanism of cerebral vascular dysfunction and neuroinflammation by MMP-mediated degradation of VEGFR-2 in alcohol ingestion. Arterioscler Thromb Vasc Biol. 2012;32:1167–1177. doi: 10.1161/ATVBAHA.112.247668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okamoto T, Akuta T, Tamura F, van der Vliet A, Akaike T. Molecular mechanism for activation and regulation of matrix metalloproteinases during bacterial infections and respiratory inflammation. Biol Chem. 2004;385:997–1006. doi: 10.1515/BC.2004.130. [DOI] [PubMed] [Google Scholar]
- 37.Gasche Y, Soccal PM, Kanemitsu M, Copin JC. Matrix metalloproteinases and diseases of the central nervous system with a special emphasis on ischemic brain. Front Biosci. 2006;11:1289–1301. doi: 10.2741/1883. [DOI] [PubMed] [Google Scholar]
- 38.Planas AM, Solé S, Justicia C. Expression and activation of matrix metalloproteinase-2 and −9 in rat brain after transient focal cerebral ischemia. Neurobiol Dis. 2001;8:834–846. doi: 10.1006/nbdi.2001.0435. [DOI] [PubMed] [Google Scholar]
- 39.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 40.Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta. 2010;1802:80–92. doi: 10.1016/j.bbadis.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Belayev L, Zhao WZ, Busto R, Ginsberg MD. Transient middle cerebral artery occlusion by intraluminal suture 1. Three-dimensional autoradiographic analysis of local cerebral glucose metabolism-blood flow interrelationships during ischemia and early recirculation. J Cereb Blood Flow Metab. 1997;17:1266–1280. doi: 10.1097/00004647-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Ferrer I, Planas AM. Signaling of cell death and cell survival following cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol. 2003;62:329–339. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- 43.Jordan J, de Groot PW, Galindo MF. Mitochondria: the headquarters in ischemis-induced neuronal death. Cent Nerv Syst Agents Med Chem. 2011;11:98–106. doi: 10.2174/187152411796011358. [DOI] [PubMed] [Google Scholar]
- 44.Bredesen DE, Mehlen P, Rabizadeh S. Apoptosis and dependence receptors: a molecular basis for cellular addiction. Physiol Rev. 2004;84:411–430. doi: 10.1152/physrev.00027.2003. [DOI] [PubMed] [Google Scholar]
- 45.Yeh CH, Wang YC, Wu YC, Lin YM, Lin PJ. Ischemic preconditioning or heat shock pretreatment ameliorates neuronal apoptosis following hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2004;128:203–210. doi: 10.1016/j.jtcvs.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Böttiger BW, Schmitz B, Wiessner C, Vogel P, Hossman K-A. Neuronal stress response and neuronal cell damage after cardiocirculatory arrest in rats. J Cereb Blood Flow Metab. 1998;18:1077–1087. doi: 10.1097/00004647-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 47.Teschendorf P, Padosch SA, Spöhr F, Albertsmeier M, Schneider A, Vogel P, Choi Y-H, Böttiger BW, Popp E. Time course of caspase activation in selectively vulnerable brain areas following global cerebral ischemia due to cardiac arrest in rats. Neurosci Lett. 2008;448:194–199. doi: 10.1016/j.neulet.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 48.Tamatani M, Ogawa S, Nunez G, Tohyama M. Growth factors prevent changes in Bcl-2 and Bax expression and neuronal apoptosis induced by nitric oxide. Cell Death Differ. 1998;5:911–919. doi: 10.1038/sj.cdd.4400439. [DOI] [PubMed] [Google Scholar]
- 49.Tamatani M, Ogawa S, Niitsu Y, Tohyama M. Involvement of Bcl-2 family and caspase-3-like protease in NO-mediated neuronal apoptosis. J Neurochem. 1998;71:1588–1596. doi: 10.1046/j.1471-4159.1998.71041588.x. [DOI] [PubMed] [Google Scholar]
- 50.Fisher JW. Landmark advances in the development of erythropoietin. Exp Biol Med. 2010;235:1398–1411. doi: 10.1258/ebm.2010.010137. [DOI] [PubMed] [Google Scholar]
- 51.Semenza GL. Regulation of erythropoietin production. New insights into molecular mechanisms of oxygen homeostasis. Hematol Oncol Clin North Am. 1994;8:863–884. [PubMed] [Google Scholar]
- 52.Ishii Y, Sawada T, Murakami T, Sakuraoka Y, Shiraki T, Shimizu A, Kubota K, Fuchinoue S, Teraoka S. Renoprotective effect of erythropoietin against ischaemia-reperfusion injury in a non-human primate model. Nephrol Dial Transplant. 2011;26:1157–1162. doi: 10.1093/ndt/gfq601. [DOI] [PubMed] [Google Scholar]
- 53.Moore E, Bellomo R. Erythropoietin (EPO) in acute kidney injury. Ann Intensive Care. 2011;1:3. doi: 10.1186/2110-5820-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benderro GF, LaManna JC. Kidney EPO expression during chronic hypoxia in aged mice. Adv Exp Med Biol. 2013;765:9–14. doi: 10.1007/978-1-4614-4989-8_2. [DOI] [PubMed] [Google Scholar]
- 55.Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med. 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- 56.Chong ZZ, Kang J-Q, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Brit J Pharmacol. 2003;138:1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hasselblatt M, Ehrenreich H, Sirén A-L. The brain erythropoietin system and its potential for therapeutic exploitation in brain disease. J Neurosurg Anesthesiol. 2006;18:132–138. doi: 10.1097/00008506-200604000-00007. [DOI] [PubMed] [Google Scholar]
- 59.McPherson RJ, Juul SE. Recent trends in erythropoietin-mediated neuroprotection. Int J Devel Neurosci. 2008;26:103–111. doi: 10.1016/j.ijdevneu.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rabie T, Marti HH. Brain protection by erythropoietin: a manifold task. Physiology. 2008;23:263–274. doi: 10.1152/physiol.00016.2008. [DOI] [PubMed] [Google Scholar]
- 61.Mammis A, McIntosh TK, Maniker AH. Erythropoietin as a neuroprotective agent in traumatic brain injury. Surg Neurol. 2009;71:527–531. doi: 10.1016/j.surneu.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 62.Sadamoto Y, Igase K, Sakanaka M, Sato K, Otsuka H, Sasaki S, Masuda S, Sasaki R. Erythropoietin prevents place navigation disability and cortical infarction in rats with permanent occlusion of the middle cerebral artery. Biochem Biophys Res Commun. 1998;253:26–32. doi: 10.1006/bbrc.1998.9748. [DOI] [PubMed] [Google Scholar]
- 63.Sirén A, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumral A, Tugyan K, Gonenc S, Genc K, Genc S, Sonmez U, Yilmaz O, Duman N, Uysal N, Ozkan H. Protective effects of erythropoietin against ethanol-induced apoptotic neurodegeneration and oxidative stress in the developing C57BL/6 mouse brain. Devel Brain Res. 2005;160:146–156. doi: 10.1016/j.devbrainres.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 65.Calapai G, Marciano MC, Corica F, Allegra A, Parisi A, Frisina N, Caputi AP, Buemi M. Erythropoietin protects against brain ischemic injury by inhibition of nitric oxide formation. Eur J Pharmacol. 2000;401:349–356. doi: 10.1016/s0014-2999(00)00466-0. [DOI] [PubMed] [Google Scholar]
- 66.Kilic E, Kilic Ü, Soliz J, Bassetti CL, Gassmann M, Hermann DM. Brain-derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/−2 and Akt pathways. FASEB J. 2005;19:2026–2028. doi: 10.1096/fj.05-3941fje. [DOI] [PubMed] [Google Scholar]
- 67.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xiong Y, Mahmood A, Meng Y, Zhang Y, Qu C, Schallert T, Chopp M. Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J Neurosurg. 2010;113:598–608. doi: 10.3171/2009.9.JNS09844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Popp E, Vogel P, Teschendorf P, Böttiger BW. Effects of the application of erythropoietin on cerebral recovery after cardiac arrest in rats. Resuscitation. 2007;74:344–351. doi: 10.1016/j.resuscitation.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 70.Witthuhn BA, Quelle FW, Silvennoinen O, Yi T, Tang B, Miura O, Ihle JN. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- 71.Crow MT, Mani K, Nam Y-J, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 72.Baines CP. The cardiac mitochondrion: nexus of stress. Annu Rev Physiol. 2010;72:61–80. doi: 10.1146/annurev-physiol-021909-135929. [DOI] [PubMed] [Google Scholar]
- 73.Vairano M, Russo CD, Pozzoli G, Battaglia A, Scambia G, Tringali G, Aloe-Spiriti MA, Preziosi P, Navara P. Erythropoietin exerts anti-apoptotic effects on rat microglial cells in vitro . Eur J Neurosci. 2002;16:584–592. doi: 10.1046/j.1460-9568.2002.02125.x. [DOI] [PubMed] [Google Scholar]
- 74.Wen TC, Sadamoto Y, Tanaka J, Zhu PX, Nakata K, Ma YJ, Hata R, Sakanaka M. Erythropoietin protects neurons against chemical hypoxia and cerebral ischemic injury by up-regulating Bcl-xL expression. J Neurosci Res. 2002;67:795–803. doi: 10.1002/jnr.10166. [DOI] [PubMed] [Google Scholar]
- 75.Noguchi CT, Asavaritikrai P, Teng R, Jia Y. Role of erythropoietin in the brain. Crit Rev Oncol Hematol. 2007;64:159–171. doi: 10.1016/j.critrevonc.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaP signaling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 77.Genc S, Koroglu TF, Genc K. Erythropoietin as a novel neuroprotectant. Restor Neurol Neurosci. 2004;22:105–119. [PubMed] [Google Scholar]
- 78.Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed JC. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ, Li P, Srinivasula SM, Alnemri ES, Fairman R, Shi Y. Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell. 2003;11:519–527. doi: 10.1016/s1097-2765(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 80.Zhang J, Zhu Y, Zhou D, Wang Z, Chen G. Recombinant human erythropoietin (rhEPO) alleviates early brain injury following subarachnoid hemorrhage in rats: possible involvement of Nrf2-ARE pathway. Cytokine. 2010;52:252–257. doi: 10.1016/j.cyto.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 81.Sifringer M, Brait D, Weichelt U, Zimmerman G, Endesfelder S, Brehmer F, von Haefen C, Friedman A, Soreq H, Bendix I, Gerstner B, Felderhoff-Mueser U. Erythropoietin attenuates hyperoxia-induced oxidative stress in the developing rat brain. Brain Behav Immun. 2010;24:792–799. doi: 10.1016/j.bbi.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 82.Innamorato NG, Rojo AI, Garcia-Yagüe AJ, Yamamoto M, de Ceballos ML, Cuadrado A. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol. 2008;181:680–689. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- 83.Zhang GL, Wang W, Kang YX, Xue Y, Yang H, Zhou CM, Shi GM. Chronic testosterone propionate supplement activated the Nrf2-ARE pathway in the brain and ameliorated the behaviors of age rats. Behav Brain Res. 2013;252:388–395. doi: 10.1016/j.bbr.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 84.Genc K, Egrilmez MY, Genc S. Erythropoietin induces nuclear translocation of Nrf2 and heme oxygenase-1 expression in SH-SY5Y cells. Cell Biochem Funct. 2010;28:197–201. doi: 10.1002/cbf.1639. [DOI] [PubMed] [Google Scholar]
- 85.Jin R, Song Z, Yu S, Piazza A, Nanda A, Penninger JM, Granger DN, Li G. Phosphatidylinositol-3-kinase gamma plays a central role in blood-brain barrier dysfunction in acute experimental stroke. Stroke. 2011;42:2033–2044. doi: 10.1161/STROKEAHA.110.601369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McMahon M, Itoh K, Yamamoto M, Hayes JD. Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem. 2003;278:21592–21600. doi: 10.1074/jbc.M300931200. [DOI] [PubMed] [Google Scholar]
- 87.Villeneuve NF, Lau A, Zhang DD. Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: an insight into cullin-ring ubiquitin ligases. Antioxid Redox Signal. 2010;13:1699–1712. doi: 10.1089/ars.2010.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Uruno A, Motohashi H. The Keap1-Nrf2 system as an in vivo sensor for electrophiles. Nitric Oxide. 2011;25:153–160. doi: 10.1016/j.niox.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 89.Genc S. Endothelial nitric oxide-mediated Nrf2 activation as a novel mechanism for vascular and neuroprotection by erythropoietin in experimental subarachnoid hemorrhage. Med Hypotheses. 2006;67:424. doi: 10.1016/j.mehy.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 90.Buckley BJ, Li S, Whorton AR. Keap1 modification and nuclear accumulation in response to S-nitrosocysteine. Free Radic Biol Med. 2008;44:692–698. doi: 10.1016/j.freeradbiomed.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Y, Ogle ME, Wallace GC, 4th, Lu ZY, Yu SP, Wei L. Erythropoietin attenuates intracerebral hemorrhage by diminishing matrix metalloproteinases and maintaining blood-brain barrier integrity in mice. Acta Neurochir. 2008;105(suppl):105–112. doi: 10.1007/978-3-211-09469-3_22. [DOI] [PubMed] [Google Scholar]
- 92.Kadri Z, Petitfrère E, Boudot C, Freyssinier J-M, Fichelson S, Mayeux P, Emonard H, Hornebeck W, Haye B, Billat C. Erythropoietin induction of tissue inhibitors of metalloproteinase-1 expression and secretion is mediated by mitogen-activated protein kinase and phsphatidylinositol 3-kinase pathways. Cell Growth Differen. 2000;11:573–580. [PubMed] [Google Scholar]
- 93.Villa P, Bigini P, Mennini T, Agnello D, Laragione T, Cagnotto A, Viviani B, Marinovich M, Cerami A, Coleman TR, Brines M, Ghezzi P. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198:971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kawakami M, Sekiguchi M, Sato K, Kozaki S, Takahashi M. Erythropoietin receptor-mediated inhibition of exocytotic glutamate release confers neuroprotection during chemical ischemia. J Biol Chem. 2001;276:39469–39475. doi: 10.1074/jbc.M105832200. [DOI] [PubMed] [Google Scholar]
- 95.Won YJ, Yoo JY, Lee JH, Hwang SJ, Kim D, Hong HN. Erythropoietin is neuroprotective on GABAergic neurons against kainic acid-excitotoxicity in the rat spinal cell cultures. Brain Res. 2007;1154:31–39. doi: 10.1016/j.brainres.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 96.Kamal A, Al Shaibani T, Ramakers G. Erythropoietin decreases the excitatory neurotransmitter release probability and enhances synaptic plasticity in mice hippocampal slices. Brain Res. 2011;1410:33–37. doi: 10.1016/j.brainres.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 97.Pytte M, Steen PA. Are we closer to a new strategy in the treatment of cardiac arrest? Resuscitation. 2009;80:613–614. doi: 10.1016/j.resuscitation.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 98.Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck H-H, Breiter N, Jacob S, Knerlich F, Bohn M, Poser W, Rüther E, Kochen M, Gefeller O, Gleiter C, Wessel TC, De Ryck M, Itri L, Prange H, Cerami A, Brines M, Sirén A-L. Erythropoietin therapy for acute stroke is both safe and beneficial. Molec Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- 99.Cariou A, Claessens Y-E, Pène F, Marx J-S, Spaulding C, Hababou C, Casadevall N, Mira J-P, Carli P, Hermine O. Early high-dose erythropoietin therapy and hypothermia after out-of-hospital cardiac arrest: a matched control study. Resuscitation. 2008;76:397–404. doi: 10.1016/j.resuscitation.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 100.Grmec Š, Strnad M, Kupnik D, Sinkovič A, Gazmuri RJ. Erythropoietin facilitates the return of spontaneous circulation and survival in victims of out-of-hospital cardiac arrest. Resuscitation. 2009;80:631–637. doi: 10.1016/j.resuscitation.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 101.Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, Schellinger PD, Bohn M, Becker H, Wegrzyn M, Jähnig P, Herrmann M, Knauth M, Bähr M, Heide W, Wagner A, Schwab S, Reichmann H, Schwendemann G, Dengler R, Kastrup A, Bartels C. EPO Stroke Trial Group. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–e656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 102.Green AR. Pharmacological approaches to acute ischaemic stroke: reperfusion certainly, neuroprotection possibly. Br J Pharmacol. 2008;153:S325–S338. doi: 10.1038/sj.bjp.0707594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 h after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 104.Jia L, Chopp M, Zhang L, Lu M, Zhang Z. Erythropoietin in combination of tissue plasminogen activator exacerbates brain hemorrhage when treatment is initiated 6 hours after stroke. Stroke. 2010;41:2071–2076. doi: 10.1161/STROKEAHA.110.586198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Banks WA, Jumbe NL, Farrell CL, Niehoff ML, Heatherington AC. Passage of erythropoietic agents across the blood-brain barrier: a comparison of human and murine erythropoietin and the analog darbepoietin alfa. Eur J Pharmacol. 2004;505:93–101. doi: 10.1016/j.ejphar.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 106.Juul SE, McPherson RJ, Farrell FX, Jolliffe L, Ness DJ, Gleason CA. Erythropoietin concentrations in cerebrospinal fluid of nonhuman primates and fetal sheep following high-dose recombinant erythropoietin. Biol Neonate. 2004;85:138–144. doi: 10.1159/000074970. [DOI] [PubMed] [Google Scholar]
- 107.Dame C, Juul SE, Christensen RD. The biology of erythropoietin in the central nervous system and its neurotrophic and neuroprotective potential. Biol Neonate. 2001;79:228–235. doi: 10.1159/000047097. [DOI] [PubMed] [Google Scholar]
- 108.Haiden N, Klebermass K, Cardona F, Schwindt J, Berger A, Kohlhauser-Vollmuth C, Jilma B, Pollak A. A randomized, controlled trial of the effects of adding vitamin B12 and folate to erythropoietin for the treatment of anemia of prematurity. Pediatrics. 2006;118:180–188. doi: 10.1542/peds.2005-2475. [DOI] [PubMed] [Google Scholar]
- 109.Marti HH, Gassmann M, Wenger RH, Kvietikova I, Morganti-Kossmann MC, Kossmann T, Trentz O, Bauer C. Detection of erythropoietin in human liquor: intrinsic erythropoietin production in the brain. Kidney Int. 1997;51:416–418. doi: 10.1038/ki.1997.55. [DOI] [PubMed] [Google Scholar]
- 110.Buemi M, Allegra A, Corica F, Floccari F, D’Avella D, Aloisi C, Calapai G, Iacopino G, Frisina N. Intravenous recombinant erythropoietin does not lead to an increase in cerebrospinal fluid erythropoietin concentration. Nephrol Dial Transplant. 2000;15:422–423. doi: 10.1093/ndt/15.3.422. [DOI] [PubMed] [Google Scholar]
- 111.McPherson RJ, Juul SE. Recent trends in erythropoietin-mediated neuroprotection. Int J Devel Neurosci. 2008;26:103–111. doi: 10.1016/j.ijdevneu.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.García-Yébenes I, Sobrado M, Zarruk JG, Castellanos M, Pérez de la Ossa N, Dávalos A, Serena J, Lizasoain I, Moro MA. A mouse model of hemorrhagic transformation by delayed tissue plasminogen activator administration after in situ thromboembolic stroke. Stroke. 2011;42:196–203. doi: 10.1161/STROKEAHA.110.600452. [DOI] [PubMed] [Google Scholar]
- 113.Baciu I, Oprisiu C, Deverenco P, Vasile V, Muresan A, Hriscu M, Chris I. The brain and other sites of erythropoietin production. Rom J Physiol. 2000;37:3–14. [PubMed] [Google Scholar]
- 114.Marti HH. Erythropoietin and the hypoxic brain. J Exp Biol. 2004;207:3233–3242. doi: 10.1242/jeb.01049. [DOI] [PubMed] [Google Scholar]
- 115.Benderro GF, Sun X, Kuang Y, LaManna JC. Decreased VEGF expression and microvascular density, but increased HIF-1 and 2α accumulation and EPO expression in chronic moderate hyperoxia in the mouse brain. Brain Res. 2012;1471:46–55. doi: 10.1016/j.brainres.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dame C, Bartmann P, Wolber E-M, Fahnenstich H, Hofmann D, Fandrey J. Erythropoietin gene expression in different areas of the developing human central nervous system. Dev Brain Res. 2000;125:69–74. doi: 10.1016/s0165-3806(00)00118-8. [DOI] [PubMed] [Google Scholar]
- 117.Juul SE, Anderson DK, Li Y, Christensen RD. Erythropoietin and erythropoietin receptor in the developing human central nervous system. Pediatr Res. 1998;43:40–44. doi: 10.1203/00006450-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 118.Nagai A, Nakagawa E, Choi HB, Hatori K, Kobayashi S, Kim SU. Erythropoietin and erythropoietin receptors in human CNS neurons, astrocytes, microglia, and oligodendrocytes grown in culture. J Neuropathol Exp Neurol. 2001;60:386–392. doi: 10.1093/jnen/60.4.386. [DOI] [PubMed] [Google Scholar]
- 119.Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nangaku M, Eckardt KU. Hypoxia and the HIF system in kidney disease. J Mol Med (Berl) 2007;85:1325–1330. doi: 10.1007/s00109-007-0278-y. [DOI] [PubMed] [Google Scholar]
- 121.Haase VH. Regulation of erythropoiesis by hypoxia-inducible factors. Blood Rev. 2013;27:41–53. doi: 10.1016/j.blre.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fandrey J. Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression. Am J Physiol Regul Integr Comp Physiol. 2004;286:R977–R988. doi: 10.1152/ajpregu.00577.2003. [DOI] [PubMed] [Google Scholar]
- 123.Semenza GL. Expression of hypoxia-inducible factor 1: mechanisms and consequences. Biochem Pharmacol. 2000;59:47–53. doi: 10.1016/s0006-2952(99)00292-0. [DOI] [PubMed] [Google Scholar]
- 124.Jelkmann W. Regulation of erythropoietin production. J Physiol. 2011;589:1251–1258. doi: 10.1113/jphysiol.2010.195057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bernaudin M, Bellail A, Marti HH, Yvon A, Vivien D, Duchatelle I, Mackenzie ET, Petit E. Neurons and astrocytes express EPO mRNA: oxygen-sensing mechanisms that involve the redox-state of the brain. Glia. 2000;30:271–278. [PubMed] [Google Scholar]
- 126.Lee J-Y, Kim Y-H, Koh J-Y. Protection by pyruvate against transient forebrain ischemia in rats. J Neurosci. 2001;21 doi: 10.1523/JNEUROSCI.21-20-j0002.2001. RC171(1–6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mongan PD, Capacchione J, Fontana JL, West S, Bünger R. Pyruvate improves cerebral metabolism during hemorrhagic shock. Am J Physiol Heart Circ Physiol. 2001;281:H854–H864. doi: 10.1152/ajpheart.2001.281.2.H854. [DOI] [PubMed] [Google Scholar]
- 128.Kim T-Y, Yi J-S, Chung S-J, Kim D-K, Byun H-R, Lee J-Y, Koh J-Y. Pyruvate protects against kainite-induced epileptic brain damage in rats. Exp Neurol. 2007;208:159–167. doi: 10.1016/j.expneurol.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 129.Sharma P, Karian J, Sharma S, Liu S, Mongan PD. Pyruvate ameliorates post ischemic injury of rat astrocytes and protects them against PARP mediated cell death. Brain Res. 2003;992:104–113. doi: 10.1016/j.brainres.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 130.Sharma AB, Barlow MA, Yang SH, Simpkins JW, Mallet RT. Pyruvate enhances neurological recovery following cardiopulmonary arrest and resuscitation. Resuscitation. 2008;76:108–119. doi: 10.1016/j.resuscitation.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sharma AB, Knott EM, Bi J, Martinez RR, Sun J, Mallet RT. Pyruvate improves cardiac electromechanical and metabolic recovery from cardiopulmonary arrest and resuscitation. Resuscitation. 2005;66:71–81. doi: 10.1016/j.resuscitation.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 132.Fukushima M, Lee SM, Moro N, Hovda DA, Sutton RL. Metabolic and histologic effects of sodium pyruvate treatment in the rat after cortical contusion injury. J Neurotrauma. 2009;26:1095–1110. doi: 10.1089/neu.2008.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ryou MG, Liu R, Ren M, Sun J, Mallet RT, Yang SH. Pyruvate protects the brain against ischemia-reperfusion injury by activating the erythropoietin signaling pathway. Stroke. 2012;43:1101–1107. doi: 10.1161/STROKEAHA.111.620088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miller LP, Oldendorf WH. Regional kinetic constants for blood-brain barrier pyruvic acid transport in conscious rats by the monocarboxylic acid carrier. J Neurochem. 1986;46:1412–1416. doi: 10.1111/j.1471-4159.1986.tb01756.x. [DOI] [PubMed] [Google Scholar]
- 135.Steele RD. Blood-brain barrier transport of the alpha-keto acid analogs of amino acids. Fed Proc. 1986;45:2060–2064. [PubMed] [Google Scholar]
- 136.Lin T, Koustova E, Chen H, Rhee PM, Kirkpatrick J, Alam HB. Energy substrate-supplemented resuscitation affects brain monocarboxylate transporter levels and gliosis in a rat model of hemorrhagic shock. J Trauma. 2005;59:1191–1202. doi: 10.1097/01.ta.0000188646.86995.9d. [DOI] [PubMed] [Google Scholar]
- 137.Wang Y, Guo SZ, Bonen A, Li RC, Kheirandish-Gozal L, Zhang SX, Brittian KR, Gozal D. Monocarboxylate transporter 2 and stroke severity in a rodent model of sleep apnea. J Neurosci. 2011;31:10241–10248. doi: 10.1523/JNEUROSCI.1462-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mallet RT. Pyruvate: metabolic protector of cardiac performance. Proc Soc Exp Biol Med. 2000;223:136–148. doi: 10.1046/j.1525-1373.2000.22319.x. [DOI] [PubMed] [Google Scholar]
- 139.Mallet RT, Sun J, Knott EM, Sharma AB, Olivencia-Yurvati AH. Metabolic cardioprotection by pyruvate: recent progress. Exp Biol Med. 2005;230:435–443. doi: 10.1177/153537020523000701. [DOI] [PubMed] [Google Scholar]
- 140.Constantopoulos G, Barranger JA. Nonenzymatic decarboxylation of pyruvate. Anal Biochem. 1984;139:353–258. doi: 10.1016/0003-2697(84)90016-2. [DOI] [PubMed] [Google Scholar]
- 141.DeBoer LW, Bekx PA, Han L, Steinke L. Pyruvate enhances recovery of rat hearts after ischemia and reperfusion by preventing free radical generation. Am J Physiol Heart Circ Physiol. 1993;265:H1571–H1576. doi: 10.1152/ajpheart.1993.265.5.H1571. [DOI] [PubMed] [Google Scholar]
- 142.Vásquez-Vivar J, Denicola A, Radi R, Augusto O. Peroxynitrite-mediated decarboxylation of pyruvate to both carbon dioxide and carbon dioxide radical anion. Chem Res Toxicol. 1997;10:786–794. doi: 10.1021/tx970031g. [DOI] [PubMed] [Google Scholar]
- 143.Bassenge E, Sommer O, Schwemmer M, Bünger R. Antioxidant pyruvate inhibits cardiac formation of reactive oxygen species through changes in redox state. Am J Physiol Heart Circ Physiol. 2000;279:H2431–H2438. doi: 10.1152/ajpheart.2000.279.5.H2431. [DOI] [PubMed] [Google Scholar]
- 144.Mallet RT, Sun J. Antioxidant properties of myocardial fuels. Mol Cell Biochem. 2003;253:103–111. doi: 10.1023/a:1026009519783. [DOI] [PubMed] [Google Scholar]
- 145.Tejero-Taldo MI, Caffrey JL, Sun J, Mallet RT. Antioxidant properties of pyruvate mediate its potentiation of β-adrenergic inotropism in stunned myocardium. J Mol Cell Cardiol. 1999;31:1863–18672. doi: 10.1006/jmcc.1999.1020. [DOI] [PubMed] [Google Scholar]
- 146.Hagar H, Ueda N, Shah S. Role of reactive oxygen metabolites in DNA damage and cell death in chemical hypoxic injury to LLC-PK1 cells. Am J Physiol Renal Fluid Electrolyte Physiol. 1996;271:F209–F215. doi: 10.1152/ajprenal.1996.271.1.F209. [DOI] [PubMed] [Google Scholar]
- 147.Ramakrishnan N, Chen R, McClain DE, Bünger R. Pyruvate prevents hydrogen peroxide-induced apoptosis. Free Radic Res. 1998;29:283–295. doi: 10.1080/10715769800300321. [DOI] [PubMed] [Google Scholar]
- 148.Sileri P, Schena S, Morini S, Rastellini C, Pham S, Benedetti E, Cicalese L. Pyruvate inhibits hepatic ischemia-reperfusion injury in rats. Transplantation. 2001;72:27–30. doi: 10.1097/00007890-200107150-00008. [DOI] [PubMed] [Google Scholar]
- 149.Lee YJ, Kang IJ, Bünger R, Kang YH. Mechanisms of pyruvate inhibition of oxidant-induced apoptosis in human endotelial cells. Microvasc Res. 2003;66:91–101. doi: 10.1016/s0026-2862(03)00052-9. [DOI] [PubMed] [Google Scholar]
- 150.Lee YJ, Kang IJ, Bünger R, Kang YH. Enhanced survival effect of pyruvate correlates MAPK and NF-κB activation in hydrogen peroxide-treated human endothelial cells. J Appl Physiol. 2004;96:793–801. doi: 10.1152/japplphysiol.00797.2003. [DOI] [PubMed] [Google Scholar]
- 151.Wang XF, Cynader MS. Pyruvate released by astrocytes protects neurons from copper-catalyzed cysteine neurotoxicity. J Neurosci. 2001;21:3322–3331. doi: 10.1523/JNEUROSCI.21-10-03322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Alvarez G, Ramos M, Ruiz F, Satrústegui J, Bogónez E. Pyruvate protection against β-amyloid-induced neuronal death: role of mitochondrial redox state. J Neurosci Res. 2003;73:260–269. doi: 10.1002/jnr.10648. [DOI] [PubMed] [Google Scholar]
- 153.Mazzio EA, Soliman KF. Cytoprotection of pyruvic acid and reduced beta-nicotinamide adenine dinucleotide against hydrogen peroxide toxicity in neuroblastoma cells. Neurochem Res. 2003;28:733–741. doi: 10.1023/a:1022813817743. [DOI] [PubMed] [Google Scholar]
- 154.Wang X, Perez E, Liu R, Yan L-J, Mallet RT, Yang S-H. Pyruvate protects mitochondria from oxidative stress in human neuroblastoma SK-N-SH cells. Brain Res. 2007;1132:1–9. doi: 10.1016/j.brainres.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75(Suppl):715–720. doi: 10.1016/s0003-4975(02)04701-x. [DOI] [PubMed] [Google Scholar]
- 156.Van Harten AE, Scheeren TW, Absalom AR. A review of postoperative cognitive dysfunction and neuroinflammation associated with cardiac surgery and anaesthesia. Anaesthesia. 2012;67:280–293. doi: 10.1111/j.1365-2044.2011.07008.x. [DOI] [PubMed] [Google Scholar]
- 157.Ryou MG, Flaherty DC, Hoxha B, Gurji H, Sun J, Hodge LM, Olivencia-Yurvati AH, Mallet RT. Pyruvate-enriched cardioplegia suppresses cardiopulmonary bypass-induced myocardial inflammation. Ann Thorac Surg. 2010;90:1529–1535. doi: 10.1016/j.athoracsur.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 158.Sharma P, Mongan PD. Hypertonic sodium pyruvate solution is more effective than Ringers ethyl pyruvate in the treatment of hemorrhagic shock. Shock. 2010;33:532–540. doi: 10.1097/SHK.0b013e3181cc02b3. [DOI] [PubMed] [Google Scholar]
- 159.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 160.Dalgard CL, Lu H, Mohyeldin A, Verma A. Endogenous 2-oxoacids differentially regulate expression of oxygen sensors. Biochem J. 2004;380:419–424. doi: 10.1042/BJ20031647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lu H, Dalgard CL, Mohyeldin A, McFate T, Tait AS, Verma A. Reversible inactivation of HIF-1 prolyl hydroxylases allows cell metabolism to control basal HIF-1. J Biol Chem. 2005;280:41928–41939. doi: 10.1074/jbc.M508718200. [DOI] [PubMed] [Google Scholar]
- 162.Ryou MG, Flaherty DC, Hoxha B, Sun J, Gurji H, Rodriguez S, Bell G, Olivencia-Yurvati AH, Mallet RT. Pyruvate-fortified cardioplegia evokes myocardial erythropoietin signaling in swine undergoing cardiopulmonary bypass. Am J Physiol Heart Circ Physiol. 2009;297:H1914–H1922. doi: 10.1152/ajpheart.01213.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ryou MG, Choudhury GR, Winters A, Xie L, Mallet RT, Yang SH. Pyruvate minimizes rtPA toxicity from in vitro oxygen0glucose deprivation. Brain Res. 2013;1530:66–75. doi: 10.1016/j.brainres.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gurji HA, White DW, Hoxha B, Sun J, Harbor JP, Schulz DR, Jr, Williams AG, Olivencia-Yurvati AH, Mallet RT. Pyruvate-enriched resuscitation: metabolic support of post-ischemic hindlimb muscle in hypovolemic goats. Exp Biol Med. 2014 doi: 10.1177/1535370213514329. in press. [DOI] [PubMed] [Google Scholar]
- 165.Hermann HP, Pieske B, Schwarzmüller E, Keul J, Just H, Hasenfuss G. Haemodynamic effects of intracoronary pyruvate in patients with congestive heart failure: an open study. Lancet. 1999;353:1321–1323. doi: 10.1016/s0140-6736(98)06423-x. [DOI] [PubMed] [Google Scholar]
- 166.Hermann HP, Arp J, Pieske B, Kögler H, Baron S, Janssen PM, Hasenfuss G. Improved systolic and diastolic myocardial function with intracoronary pyruvate in patients with congestive heart failure. Eur J Heart Fail. 2004;6:213–218. doi: 10.1016/j.ejheart.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 167.Schillinger W, Hünlich M, Sossalia S, Hermann HP, Hasenfuss G. Intracoronary pyruvate in cardiogenic shock as an adjunctive therapy to catecholamines and intra-aortic balloon pump shows beneficial effects on hemodynamics. Clin Res Cardiol. 2011;100:433–438. doi: 10.1007/s00392-010-0261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Olivencia-Yurvati AH, Blair JL, Baig M, Mallet RT. Pyruvate-enhanced cardioprotection during surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2003;17:715–720. doi: 10.1053/j.jvca.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 169.Fink MP. Ringer’s ethyl pyruvate solution: a novel resuscitation fluid. Minerva Anesthesiol. 2001;67:190–192. [PubMed] [Google Scholar]
- 170.Fink MP. Ethyl pyruvate: a novel anti-inflammatory agent. J Intern Med. 2007;261:349–362. doi: 10.1111/j.1365-2796.2007.01789.x. [DOI] [PubMed] [Google Scholar]
- 171.Venkataraman R, Kellum JA, Song M, Fink MP. Resuscitation with Ringer’s ethyl pyruvate solution prolongs survival and modulates plasma cytokine and nitrite/nitrate concentrations in a rat model of lipopolysaccharide-induced shock. Shock. 2002;18:507–512. doi: 10.1097/00024382-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 172.Yang R, Gallo DJ, Baust JJ, Uchiyama T, Watkins SK, Delude RL, Fink MP. Ethyl pyruvate modulates inflammatory gene expression in mice subjected to hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol. 2002;283:G212–G221. doi: 10.1152/ajpgi.00022.2002. [DOI] [PubMed] [Google Scholar]
- 173.Mulier KE, Beilman GJ, Conroy MJ, Taylor JH, Skarda DE, Hammer BE. Ringer’s ethyl pyruvate in hemorrhagic shock and resuscitation does not improve early hemodynamics or tissue energetics. Shock. 2005;23:248–252. [PubMed] [Google Scholar]


