Abstract
The goal of the present study was to modulate the phenotype expression of hepatocytes in vitro on surfaces imprinted with growth factors (GFs). Hepatocyte growth factor (HGF) or transforming-growth factor-β1 (TGF-β1) were mixed with collagen (I) and robotically printed onto standard glass slides to create arrays of 300 μm or 500 μm diameter spots. Primary rat hepatocytes were seeded on top of the arrays, forming clusters corresponding in size to the underlying protein spots. The TGF-β1 spots appeared to downregulate markers of hepatic (epithelial) phenotype while upregulating expression of mesenchymal markers. Conversely, hepatocytes cultured on HGF spots maintained high level of epithelial markers. When hepatocytes were seeded onto alternating spots of HGF and TGF-β1, their phenotype was found to depend on center-to-center distance between the spots. At shorter distances cross-expression of epithelial and mesenchymal markers was observed while at distances exceeding 1.25 mm divergence of phenotypes, epithelial on HGF and mesenchymal on TGF-β was seen. Overall, our results demonstrate that GF-encoded surfaces can modulate phenotype within groups of cells cultured on the same surface. Given the importance of phenotype switching in development, fibrosis and cancer, this platform may be used to gain useful insights into the mechanisms of processes such as epithelial-to-mesenchymal transition or stem cell fate selections.
Introduction
Local signaling is at the core of multiple biological processes related to embryogenesis, regeneration and tissue injury.1–5 For example, in the liver, stem cell niche is purported to be located in the canals of Hering – an anatomical structure where biliary epithelial cell, stem cell and hepatocyte populations are juxtaposed.6,7 It is presumed that perturbation to the microenvironment, during injury for example, triggers local production of paracrine factors which in turn guide stem cell fate selection to biliary or hepatic phenotype.7,8 Another example of location-specific paracrine signaling is related to liver injury. Because injurious agents are carried into the liver through portal vein, their effects are more pronounced in periportal regions. These regions are associated with aberrant matrix deposition, higher levels of pro-fibrogenic growth factors, presence of activated mesenchymal cells that produce such growth factors.9,10 These are but two of many examples highlighting the importance of local paracrine signaling in tissue development and injury.
Transforming growth factor (TGF)-β1 and hepatocyte growth factor (HGF) are two key liver-related morphogens. TGF-β, a pro-fibrogenic paracrine factor produced in the injured liver by activated mesenchymal cells, is associated with de-differentiation and epithelial-to-mesenchymal transition (EMT) of hepatocytes.11–13 HGF on the other hand has hepatoprotective properties,14–17 has been shown to antagonize TGF-β1 and help maintain epithelial phenotype of hepatocytes during injury.18
Hepatocytes are epithelial cells that favor cell-cell contact, low motility and express only cytokeratin as intermediate filaments (IF).19 Typically, change from epithelial to mesenchymal phenotypes in hepatocytes is associated with loss of cell–cell contact and acquisition of mesenchymal features including switch from E- to N-cadherin and increase in cellular motility.12,13 There is also progressive replacement of cytokeratin by vimentin IFs.20 As noted in the previous paragraph, this transformation is promoted by pro-fibrogenic cytokines such as TGF-β and is prevented by anti-fibrogenic signals such as HGF.18
In the present work, we wanted to deliver contradictory signals, HGF and TGF-β, to primary hepatocytes residing in the same culture dish in order to study phenotype plasticity and communication between the cells. There are a number of strategies for defining local cell microenvironment, including scaffolds,21–23 micropatterned surfaces24–27 and microfluidic devices.28–30 We chose to build on the strategy for GF patterning reported by us previously,31,32 whereby GF molecules are mixed with carrier ECM protein in solution and are printed on the culture surface. These GF molecules were not only retained on the surface for several days under physiological conditions but were also highly functional, helping to maintain phenotype of primary hepatocytes31,32 and inducing hepatic phenotype in stem cells.26,33–35 In the set of experiments described in the present paper, HGF and TGF-β1 spots were printed side by side and were used for cultivation of primary rat hepatocytes. These GF encoded surfaces where used to characterize how epithelial vs. mesenchymal phenotype of cells varied as a function of local delivery of signals. Additional studies were conducted to control the distance between the GF spots in order to investigate paracrine cross-talk between groups of hepatocytes receiving divergent signals.
Materials and methods
Chemicals and materials
Glass slides (75 × 25 mm2) were obtained from VWR (West Chester, PA). (3-acryloxypropyl) trichlorosilane was purchased from Gelest, Inc. (Morrisville, PA). Collagenase, collagen from rat tail (type I), AlexaFluor 488 anti-goat IgG, AlexaFluor 546 anti-mouse IgG were purchased from Invitrogen (Carlsbad, CA). Hepatocyte growth factor (HGF) and transforming growth factor-β1 (TGF-β1) were obtained from Sigma– Aldrich (St. Louis, MO). Phosphate-buffered saline (1× PBS), minimal essential medium (MEM), sodium pyruvate, nonessential amino acids, and fetal bovine serum (FBS) were purchased from Invitrogen. 384-well polypropylene microarray plates were obtained from Genetix (New Milton, Hampshire). Albumin ELISA kits and goat anti-rat cross-adsorbed albumin antibody were obtained from Bethyl Laboratories (Montgomery, TX). Paraformaldehyde was purchased from Electron Microscopy Sciences (Hatfiled, PA). DAPI stain mounting media was purchased from Vectorshield (Burlingame, CA). SU11274 and SB431542 were purchased from Selleckchem.
Printing collagen and GF spots on glass substrates
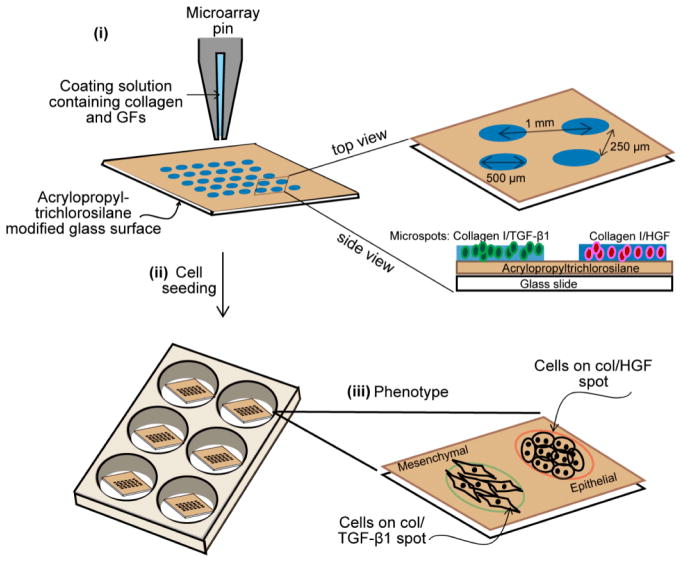
Glass slides were modified with acryloxypropyl trichlorosilane using protocols described previously.31 Collagen was dissolved in 1× PBS + 0.005% Tween-20 at 0.2 mg/mL concentration. GFs were mixed with the ECM solution to the desired final concentration of 500 ng/mL for HGF and 250 ng/mL TGF-β and allowed to bind to the ECM protein for 30 min at room temperature prior to printing. Protein microarrays were contact- printed under ambient conditions on silane-modified 75 × 25 mm2 glass slides using a hand-held MicroCaster. The pins collected protein from a 384-well plate, dispensing 20–70 nL of solution onto the glass slide and forming circular spots ~500 μm in diameter. Spots of this size were used for majority of experiments; however, smaller 150 μm diameter spots were printed in some instances to show multiple spots and divergent cell phenotypes in the same field of view. Arrays of alternating collagen control, HGF, TGF-β and a mixture of HGF and TGF-β (HGF+TGF-β) were created as shown in Fig. 1A. Pins were cleaned in acetone via sonication for 5 min and subsequently washed with pin-cleaning solution (DI water and isopropanol) between each change in growth factor to avoid growth factor cross-contamination. Pins were dried with nitrogen before protein printing. A spotbot arrayer was used to generate patterns with varying distances between GFs. Protein arrays were kept in a refrigerator overnight prior to cell cultivation.
Fig 1. Outline of microarray fabrication.
Collagen I was mixed with HGF or TGF-β1 before being patterned on a glass slide coated with acrylopropyl trichlorosilane. Each spot is 150 μm, 300 μm or 500 μm in diameter and spacing between spots is 1.0 mm (center-to-center) (i). Hepatocytes were seeded onto the glass slides to form a monolayer of cells atop the collagen I spots containing two different growth factors (ii). Schematic diagram showing the morphology of rat primary hepatocytes cultured on HGF and TGF-β1 spots (iii). Abbreviation: GFs, growth factors; col, collagen type I; HGF, hepatocyte growth factor; TGF-β1, transforming growth factor-β1.
Cultivation of primary hepatocytes on GF microarrays
Adult female Lewis rats weighing 125–200g were purchased from Charles River Laboratories (Boston, MA) and fed with a commercial diet and water. All animal experiments were performed according to the National Institutes of Health (NIH) guidelines for the ethical care and use of laboratory animals and the experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California, Davis.
Primary hepatocytes were isolated from adult female Lewis rats using a standard two-step collagenase perfusion procedure.36 Typically, 100 to 200 million hepatocytes were obtained with viability >90% as determined by trypan blue exclusion. Primary hepatocytes were maintained in DMEM supplemented with epidermal growth factor (EGF), glucagon, hydrocortisone sodium succinate, recombinant human insulin, 200 units/mL penicillin, 200 μg/mL streptomycin and 10% FBS. For cell seeding experiments, a glass slide containing printed arrays of ECM/GF was cut into (1 × 1) squares to fit into a 6-well plate. Typically, GFs were printed into arrays of 500 μm diameter spots. The distance between rows was 250 μm and the center-to-center spacing of the spots was 1 mm. Hepatocytes were seeded to form cellular arrays using protocols described elsewhere.25 In brief, glass slides containing printed ECM/GF spots were first exposed to 3 mL of hepatocytes suspended in culture medium at a concentration of 1 × 106 cells/mL. After 1 h of incubation at 37 °C, hepatocytes bound on ECM/GF domains, but did not attach on the surrounding silane-modified surface. The samples were then washed twice in PBS to remove unbound hepatocytes and fresh media was added to the sample well.
Inhibition of HGF signaling
In order to study the roles of HGF and TGF-β1 in distance-dependent modulation of EMT in rat primary hepatocytes, we administered c-met inhibitor (SU11274, 5 μM), a potent inhibitor of HGF induced signaling pathway and TGF-β1 blockers (SB431542, 5 μM). Following initial adhesion, the hepatocytes were cultured for five days in presence of inhibitors. Hepatocyte cultures without inhibitors were always grown in parallel as controls. Media was changed every second day.
Analysis of hepatic phenotype
Expression of hepatic phenotype was assessed by ELISA to measure the albumin and urea secretion. Western blotting and immunostaining was also performed to measure epithelial and mesenchymal markers in protein level. For ELISA, cell culture supernatant was collected everyday and assayed according to manufacturer’s instructions (R&D systems).
For immunostaining, cells were fixed in 4% paraformaldehyde + 0.3% Triton-X100 in PBS for 15 min. The cells were then incubated in blocking solution (1% bovine serum albumin (BSA) in PBS) for 1 h and exposed to primary antibody for 90 min. The samples were washed for three times, and incubated with appropriate secondary antibodies conjugated with Alexa Fluorophore (Invitrogen) for 1 h. After three times washing, slides were mounted onto cover slips using DAPI stain mounting media. All incubations were performed at room temperature unless otherwise mentioned. Primary antibodies used in this study were mouse anti-E-cadherin (BD Transduction Laboratories, 1:50 dilutions), mouse anti-N-cadherin (Sigma, 1:100 dilutions) and goat anti-vimentin (Santa Cruz, 1:100 dilutions). Secondary antibodies were anti-mouse IgG conjugated with alexa-546 (diluted 1:1000) and anti-goat IgG conjugated with alexa-488 (diluted 1:1000). The fluorescence intensity was assessed with imageJ software (rsbweb.nih.gov/ij/) to provide semi-quantitative measure of the expression level of target proteins. Fluorescent images of approximately 5 spots in at least three independent experiments of each condition were captured by confocal microscope (Zeiss LSM Pascal). The expression level was normalized with DAPI intensity.
For western blotting, the total cellular protein was extracted with lysis buffer (10 mM Tris-HCl, 150 mM NaCl, 1% Nonidet P-40, 10 mM EDTA, and protease inhibitor cocktail; PH 7.4), and cell lysates were centrifuged at 15000 × g for 15 min at 4 °C. Samples were separated by electrophoresis on mini-protein precast gels (Bio-Rad) and electrophoretically transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore). The primary antibodies were as follows: mouse anti-E-cadherin (BD Transduction Laboratories), rabbit anti-alpha-smooth muscle actin (Abcam), and mouse anti-β-actin (Sigma). The membranes were then reacted with horseradish peroxidase (HRP)-conjugated secondary antibody (1: 10000 dilution; Jackson ImmunoResearch Laboratories) for 1 h. HRP activity was assayed using chemiluminescent substrate (Thermo Scientific) according to the manufacturer’s instruction.
Statistical analyses
Data are presented as the mean ± standard deviations (SD). Statistical analyses were performed with Student’s t-test for paired samples. A p–value <0.05 was considered statistically significant.
Results and discussion
The goal of this study was to investigate how phenotype of hepatocytes is affected by local presentation of GFs. Our experiments demonstrate that phenotype of cells bathed in the same medium and cultured on the same surface may be regulated in a spatially-defined manner using imprinted GFs.
Location-specific differences in cell morphology on GF-containing surfaces
Examples of hepatocytes residing on printed ECM/GF spots are provided in Fig. 2. After 5 days of culture, cells residing on top of HGF started to rolled up into tissue-like spheroids, forming three-dimensional clusters (arrows pointing in Fig. 2), as has been observed previously by our group.31 To monitor the effect of different GFs on the morphology of cells on the same surface, we patterned arrays of alternating col/HGF and col/TGF-β1 spots. The center-to-center distance between spots was 1 mm. In our previous report we observed that hepatocytes cultured on printed HGF spots transitioned form monolayer to spheroids at ~day 5 in culture and that these spheroids expressed high levels of hepatic markers.31 Fig 2A shows an array of 150 μm diameter GF/ECM spots 24 h after seeding where one can see formation of an array of cell clusters corresponding in size to printed protein spots. As seen from Fig 2B, dramatic differences in morphology emerge by day 5 in culture, with hepatocytes on HGF forming spheroids while hepatocytes on neighboring TGF-β spots remained as monolayer. Fig. 2C and 2D provides higher magnification view of the spheroids on HGF as well as cells on TGF-β. In the latter case, some of the cells exhibited spindle like morphology common to mesenchymal cells after 5 days in culture. We also prepared collagen type I spots containing a mixture of HGF and TGF-β1. Interestingly, a divergent population of epithelial-like cells and mesenchymal-like cells was observed from a single population of primary rat hepatocytes cultured on a spot containing a mixture of both GFs (Suppl. Fig. S1A). Overall, micropatterning of HGF and TGF-β on the same surface led to a distinct, location-specific difference in morphology of primary hepatocytes. Given that in hepatocytes HGF and TGF-β promote epithelial and mesenchymal phenotypes respectively, we carried out additional experiments looking at specific markers associated with each phenotype.
Fig 2. Morphology of rat primary hepatocytes cultured on printed arrays of HGF (500 ng/mL) and TGF-β1 (250 ng/mL).
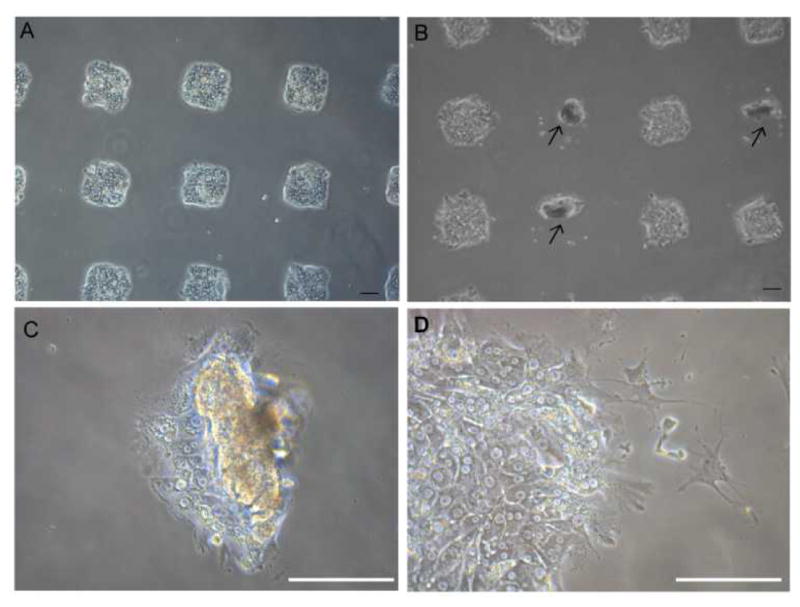
(A) Brignt field microspcopic image of hepatocytes cultured on an array of 150 μm diameter GF/ECM spots 24h after seeding. 150 μm diameter spots were used to have more spots fit into field of view. (B) Hepatocytes showing divergent phenotypes on the same surface. Presented left column to right, alternating col/TGF-β1 and col/HGF spots. Both spheroids (showing in arrow) and spindle-shaped morphology are observed after 5 days in culture. Higher magnification view of the spheroids on HGF (C) as well as cells on TGF-β1 (D). Scale bar: 100 μm.
Characterization of hepatocellular phenotype on homogeneous GF containing surfaces
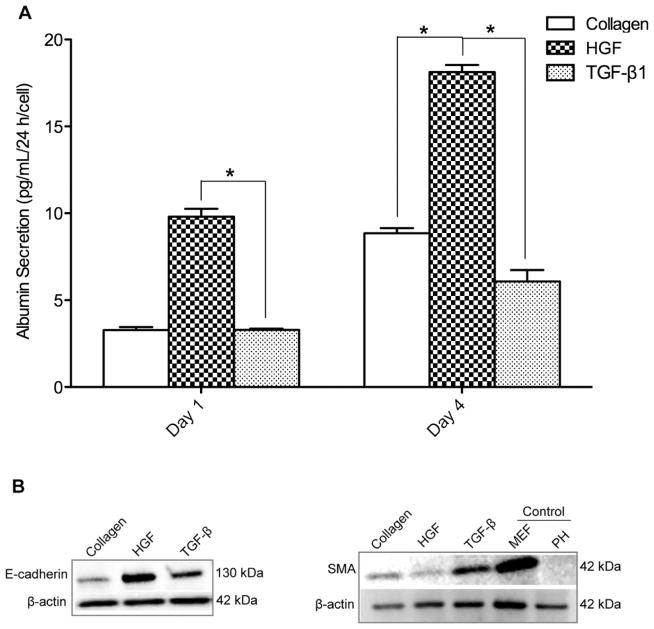
In these experiments we sought to characterize phenotype of hepatocytes cultured on surfaces containing only one type of morphogen. Synthesis of albumin – serum protein produced by the liver – was monitored to assess expression of hepatic (epithelial) phenotype on printed GF spots. Primary rat hepatocytes were cultured for four days on four surface types: collagen type I (col) as a control, col/HGF, col/TGF-β, and col/HGF + col/TGF-β. As shown in Fig. 3A, hepatocytes cultivated on col/HGF exhibited high levels of albumin. The upregulation of albumin production by solid-phase presentation of HGF has been shown by us previously.23,31 In contrast the hepatocytes residing on TGF-β spots exhibited more than 3 fold lower level of albumin at day 4 in culture compared to cells on HGF spots (Fig. 3A). Interestingly, for spots containing a mixture of HGF and TGF-β, we observed intermediate levels of albumin production, higher than on TGF-β alone but lower than on HGF alone (Suppl. Fig. S1B). Moreover, we performed experiments where different concentrations of HGF were printed and the rate of albumin secretion was analyzed (Suppl. Fig. S2). We observed HGF concentration dependent responses in production of albumin in hepatocytes. We would also like to point out our previous work demonstrating GF concentration dependent effects of HGF and BMP7 in protecting hepatocytes against injury.32 Furthermore, urea production rates were also affected by presence of HGF or TGF-β1 on the culture surface (Suppl. Fig. S3). While hepatocytes on TGF-β undergo apoptosis at a higher rate than cells on HGF (17% vs. 7%),32 this cannot explain 2 or 3 fold changes in albumin and urea synthesis. Thus we attribute them to phenotype expression and not cell death.
Fig 3. Functional tests of primary hepatocytes cultured on HGF and TGF-β1 microarrays.
(A) ELISA results for hepatocytes cultured on collagen type I spots with HGF and TGF-β1. HGF/collagen spots induce significantly higher levels of albumin secretion compared to collagen spots. TGF-β1/collagen spots significantly reduced levels of albumin secretion compared to collagen spots at day 4. *p-value < 0.05. (B) Western blot was performed to assess total cellular E-cadherin and alpha-smooth muscle actin (SMA) proteins in rat primary hepatocytes cultured for 4 days on collagen I, HGF, and TGF-β1 spots. Freshly isolated rat primary hepatocytes (PH) in suspension immediately after isolation and mouse embryonic fibroblast (MEF) cells were used as controls. The protein expression level was normalized using β-actin.
To further verify the effects of HGF and TGF-β1 on hepatocellular phenotype, western blot analysis was performed to assess total E-cadherin and α-smooth muscle actin (SMA) in hepatocytes cultured for four days on three surface types, HGF/col I spots, TGF-β1/col I spots and col I spots. E-cadherin is an epithelial marker that plays a key role in the maintenance of an epithelial phenotype, whereas the expression of SMA was used as a marker of mesenchymal phenotype. Increased expression of E-cadherin and downregulation of SMA was found to occur concurrently in hepatocytes cultured on HGF spots (Fig. 3B). In contrast, SMA expression level was higher on TGF-β1 spots. The data in Fig. 3B show that, similar to albumin, cells atop HGF spots showed strong epithelial markers while cells on TGF-β showed weak expression of epithelial marker but higher levels of mesenchymal marker.
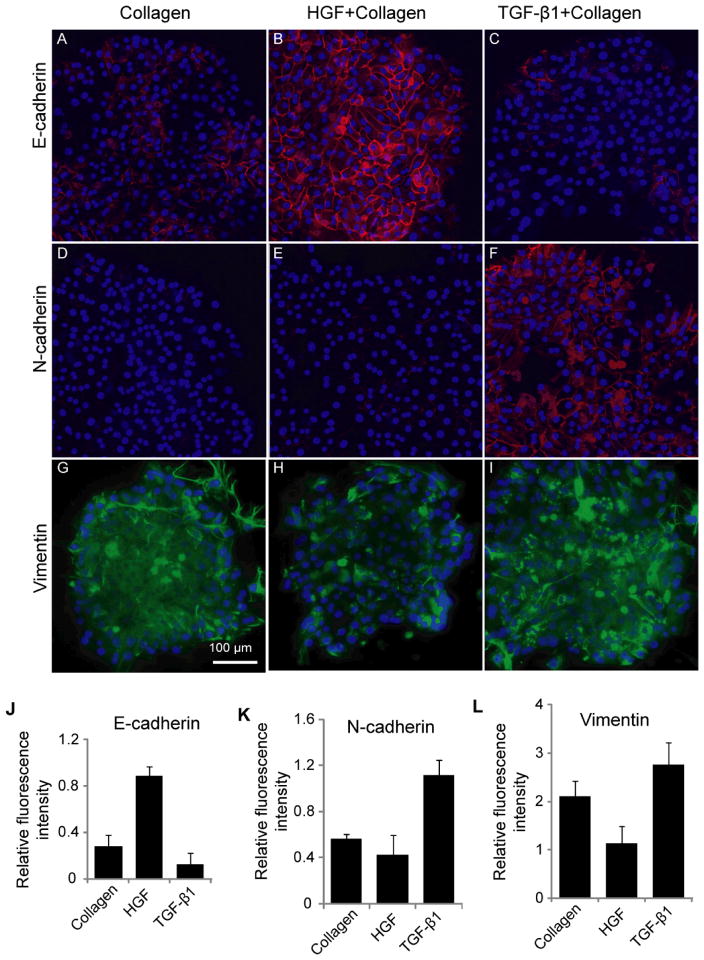
Additional phenotype analysis was carried out by immunofluorescent staining for cell adhesion markers (E- and N-cadherin). N-cadherin is another mesenchymal marker characteristic of fibroblastic cells. The first EMT event during development is the cadherin switch whereby there is repression in E-cadherin expression and an upregulation in N-cadherin expression.37 Expression of E- and N-cadherin in hepatocytes residing on GF spots was assessed in order to ascertain induction of mesenchymal or epithelial phenotype with surface-immobilized GFs. These data presented in Fig. 4A–C reveal several interesting observations. Similar to albumin ELISA and western blot analysis, the highest level of E-cadherin expression was observed on HGF spots. In contrast, E-cadherin expression level was significantly lower on TGF-β1 spots. On the other hand, N-cadherin was sharply upregulated in cells on TGF-β1 spots whereas its expression was undetectable in cells cultured on HGF spots (Fig. 4D–F). Immunofluorescent staining for vimentin, an intermediate filament characteristic of mesenchymal cells and epithelial-to-mesenchymal transition,38 revealed high levels of expression in cells cultured on TGF-β1 spots (Fig. 4G–I). The expression of mesenchymal markers was intermediate in cells on collagen spots without growth factors. The fluorescence intensity associated with intracellular E-cadherin, N-cadherin, and vimentin was assessed with imageJ software (Fig. 4J–L) to provide semi-quantitative measure of the immunofluorescence results.
Fig 4. Immunohistochemical examination of cadherins and vimentin expression in hepatocytes.
(A–C) E-cadherin expression was upregulated by HGF and downregulated TGF- β1. (D–F) N-cadherin expression was upregulated by TGF- β1. (G–I) Immunostaining images showing higher expression of vimentin in cells cultured on spots TGF- β1, while least expression was found on HGF spot. The fluorescence intensity of E-cadherin (J), N-cadhrein (K) and vimentin (L) was quantified using imageJ software and normalized with their DAPI intensity. Data are mean ± SD, n=3.
Phenotype expression on heterogeneous GF-containing surfaces
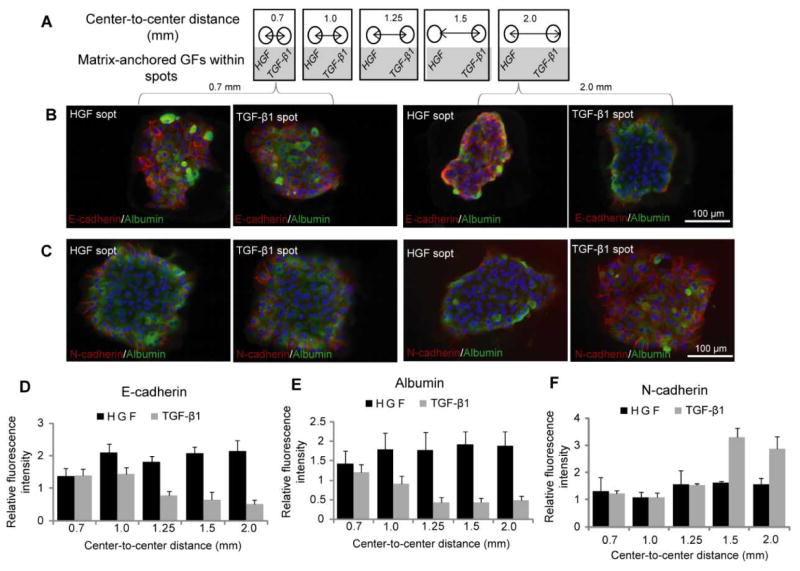
After demonstrating that phenotype of hepatocytes could be modulated by surfaces containing either HGF or TGF-β spots, we wanted to observe how hepatic phenotype would be affected when both types of spots are present on the same culture surface. As described in Fig. 5A, we printed alternating col/HGF and col/TGF-β1 300 μm diameter spots while varying center to center distances as follows: 0.7 mm, 1.0 mm, 1.25 mm, 1.5 mm and 2.0 mm. Immunofluorescent staining for epithelial (E-cadherin and albumin) and mesenchymal (N-cadherin) was used to characterize hepatic phenotype (Fig. 5B and 5C). In the course of these experiments it was observed that cells cultured on HGF and TGF-β1 spots at shorter distance of 0.7 mm had similar levels of E-cadherin, albumin and N-cadherin expression. However, cells cultured on HGF spots at longer distance (2.0 mm) expressed high levels of E-cadherin and abumin, while cells on TGF-β1 strongly expressed mesenchymal marker N-cadherin (Fig. 5B and 5C). Quantification of fluorescence intensity of epithelial markers shown in Fig. 5D and 5E highlights distance-dependent differences in phenotype. Distance-dependent trend was also observed for N-cadherin (Fig. 5F). This mesenchymal marker was expressed at a similar level in cells residing on HGF and TGF-β at shorter center-to-center distances, but as the distance increased to 1.5 mm and beyond a clear difference was observed, with N-cadherin levels in hepatocytes on TGF-β1 spots significantly higher than in cells on HGF spots.
Fig 5. HGF can rescue hepatocytes residing on neighboring TGF-β1 surfaces.
(A) Schematic representation of the collagen spot containing HGF and TGF-β1 at varying distances. (B) E-cadherin (red) and albumin (green) immunostaining on col/HGF and col/TGF-β1 spots reveal that at smaller distances, EMT is prevented in hepatocytes cultured on TGF-β1 spots. The distances between the spots were varied from 0.7 mm to 2.0 mm. (C) Similar immunofluorescence images of N-cadherin (red) and albumin (green) on col/HGF and col/TGF-β1. A mesenchymal phenotype was more prominent on TGF-β1 as the distance between the two growth factors was increased. The fluorescence intensity of E-cadherin (D), N-cadherin (E) and albumin (F) was quantified using imageJ software and normalized with their DAPI intensity. Data are mean ± SD, n=3.
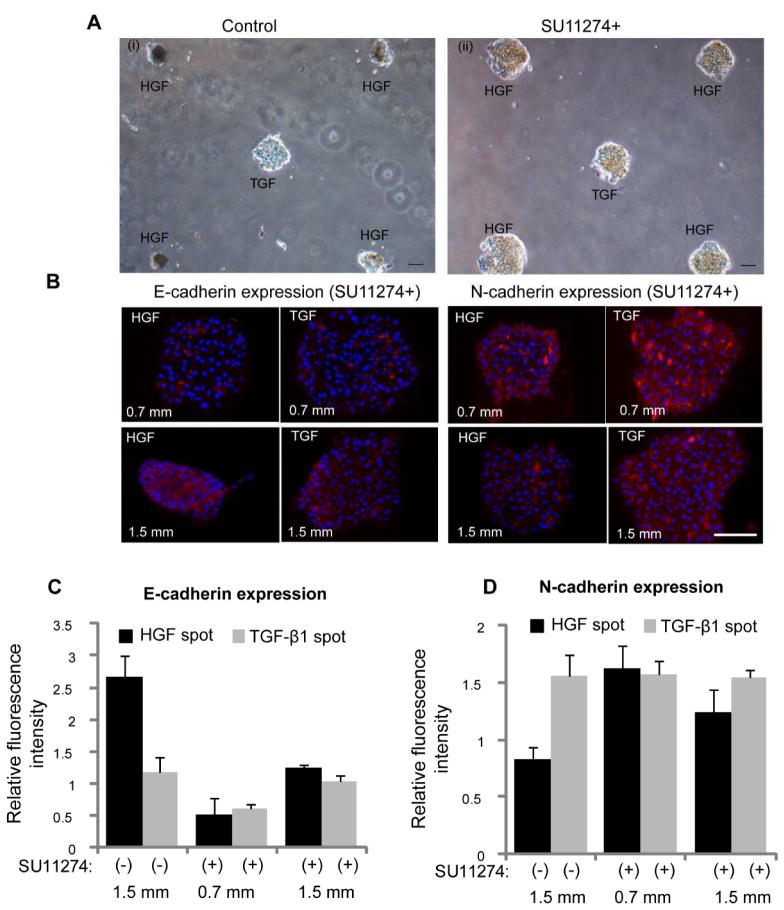
Additional experiments were carried out to investigate the contributions of HGF signaling to the phenotype divergence behavior described in Fig. 5. In these experiments, hepatocytes were cultured in presence of HGF receptor (c-met) inhibitor (SU11274) on micropatterned surfaces containing alternating HGF and TGF-β spots. One striking observation was that hepatocytes did not undergo spheroid formation when pre-incubated with c-met inhibitor. Fig. 6A-i shows images of hepatocytes forming spheroids on HGF spots while remaining as monolayer on TGF-β spots. These data highlight local changes in cellular morphology conferred by imprinted GFs. In contrast, hepatocytes treated with c-met inhibitor (SU11274) did not form spheroids (Fig. 6A-ii). The level of E-cadherin expression in hepatocytes exposed to c-met blocker was lower for cells cultured on HGF and TGF-β spots compared to controls not treated with c-met blocker (Fig. 6B and 6C). Importantly, the same set of data show that c-met inhibition abrogated distance dependent effects in E-cadherin expression on TGF-β spots. Fig. 6B and 6D also shows that in the presence of c-met inhibitor, N-cadherin went up on HGF spots. Similarly, there is no distance dependent effect in N-cadherin expression on TGF-β spots. This is in contrast to Fig. 5 that shows an increase in N-cadherin expression as distance increases and, presumably, the concentration of paracrine signals secreted from the HGF-stimulated spots decreases. Similarly, Suppl. Fig. S4 shows dual E-cadherin/albumin and N-cadherin/albumin immunostaining in the presence of TGF-β1 blockers. In this case, the effects of TGF-β1 are mitigated and both cadherin and albumin expression are dominated by the printed HGF. Unlike in the case with no blockers where there are increased mesenchymal phenotype characteristics on HGF spots, the epithelial phenotype on HGF spots is favored in the presence of TGF-β1 blockers. This experiment helps prove that TGF-β1 is primarily responsible for the changes in cadherin and albumin expression on HGF spots at closer spot-to-spot distances.
Fig 6. Inhibition of c-met is associated with lower level of epithelial marker expression.
(A) Hepatocytes were cultured onto micropatterned surfaces containing alternating HGF and TGF-β spots in absence (i) and presence (ii) of HGF receptor (c-met) inhibitor, SU11274. Spheroid formation on HGF was not observed in hepatocytes cultured for five days in presence of c-met inhibitor. The center-to-center distance between spots was 1.5 mm and spot size was 300 μm diameter. (B) Immunostaining images showing the lower expression of epithelia cell marker (E-cadherin) and higher expression of mesenchymal marker (N-cadherin) in cells cultured for four days on both HGF and TGF-β1 spots in presence of SU11274. Hepatocytes cultured on micropatterened surfaces containing alternating spots of HGF and TGF-β1 spots in absence of c-met inhibitor were used as control. The center-to-center distance between spots was 0.7 mm and 1.5 mm. (C, D) The fluorescence intensity of E-cadherin and N-cadherin was quantified using imageJ software and normalized with their DAPI intensity. Data are mean ± SD, n=3. Scale bar: 100 μm.
Overall, we demonstrate that hepatic phenotype may depend on both the types of GF signals being delivered and on the distance between cell clusters receiving divergent GF signal. We also demonstrate that signaling through HGF receptor c-met is central to both changes in cellular morphology and communication between the cell clusters. Our observations may point to the presence of paracrine signals exchanged between hepatocytes residing on HGF and TGF-β spots. While this remains to be proven experimentally, we hypothesize that hepatocytes atop HGF-containing protein spots may be stimulated to secrete more HGF in an autocrine fashion and that these secreted HGF molecules diffuses across to rescue epithelial phenotype in hepatocytes atop TGF-β1 spots. As the inter-spot distance increases the rescue effects of HGF diminish and mesenchymal phenotype promoted by TGF-β1 signaling becomes more pronounced.
Our findings on distance dependence of hepatocellular phenotype are consistent with reports of Bhatia and co-workers who pointed to the presence of diffusible signals effective in stimulating hepatic phenotype at distances of up to 1 mm in stimulating hepatic phenotype.27,39 However, unlike these previous report looking at heterotypic signaling in co-cultures, our paper shows cells of the same type engaging in paracrine signaling.
Conclusions
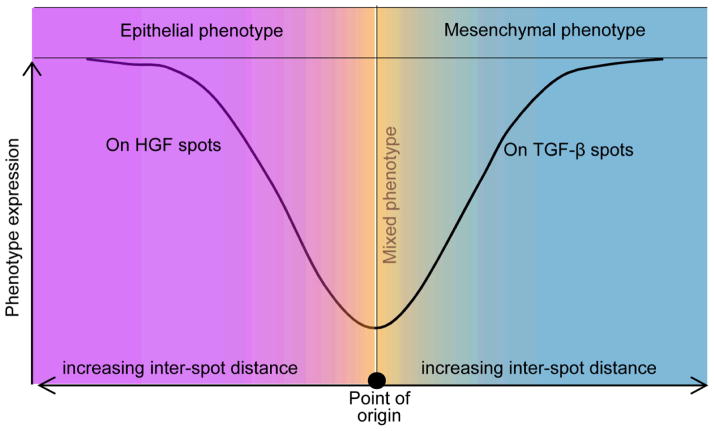
The present study investigated the phenotype of primary rat hepatocytes cultured on printed protein spots containing HGF and TGF-β1 molecules. Cultivation of cells on HGF spots resulted in high levels of hepatic phenotype expression whereas hepatic cultures on TGF-β1 and mixed HGF/TGF spots showed lower levels of liver marker expression. Subsequent characterization revealed that printed TGF-β1 induced hepatocytes to exhibit mesenchymal characteristics, with some cells adopting fibroblast-like morphology, with decreased membrane-bound E-cadherin and increased N-cadherin. Additional set of experiments demonstrated that phenotype of hepatocytes cultured on arrays containing alternating spots of HGF and TGF-β1 spots was dependent on spot to spot distance. When the center to center distance exceeded 1.25 mm, hepatocytes on HGF spots showed high levels of albumin and E-cadherin expression whereas hepatocytes on neighboring TGF-β1 spots exhibited lower levels of these liver markers. This experiment is particularly interesting as it suggests that phenotype expression of cells bathed in the same culture medium may be guided by patterning GFs on culture surfaces. These observations are summarized in a diagram of Fig. 7, with mixed phenotype of hepatocytes (both epithelial and mesenchymal markers expressed) at shorter distances and divergence of phenotypes at longer distances between the spots. While these experiments are being carried out currently, early evidence suggests that it is not the leaching of GF molecules from the printed spots but the signals released by stimulated cells that drive distance dependent effects in cell phenotype. The platform described in this paper may be used in the future for further understanding the underlying mechanisms involved in EMT of the liver. There is also potential for surfaces patterned with growth factors to be used in tissue engineering where divergent cells population are required to recapitulate a tissue or organ of interest.
Fig 7. Schematic diagram showing the generation of divergent phenotypes in primary hepatocytes (point of origin) using growth factor micropatterning.
We demonstrate that hepatocytes residing on HGF spots express epithelial (hepatic) markers such as albumin and E-cadherin whereas hepatocytes on TGF-β1 express N-cadherin and vimentin– mesenchymal markers. Interestingly, we find that the differences in expression of epithelial vs. mesenchymal markers are much more pronounced at longer distances suggesting that the HGF can rescue liver-specific functionality of hepatocytes exposed to TGF-β1 in distance-dependent manner.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Prof. Soichiro Yamada and Dr. Wentig Shih for assistance with cadherin staining. Financial support for this project was provided by NIH (DK073901 and AA017939-01).
References
- 1.Discher DE, Mooney DJ, Zandstra PW. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peifer M, Polakis P. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 3.Nelson CM, Bissell MJ. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutolf MP, Hubbell JA. Nat biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 5.Lee K, Silva EA, Mooney DJ. J R Soc Interface. 2011;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saxena R, Theise N. Semin Liver Dis. 2004;24:43–48. doi: 10.1055/s-2004-823100. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka M, Itoh T, Tanimizu N, Miyajima A. J Biochem. 2011;149:231–239. doi: 10.1093/jb/mvr001. [DOI] [PubMed] [Google Scholar]
- 8.Sicklick JK, Choi SS, Bustamante M, McCall SJ, Perez EH, Huang J, Li YX, Rojkind M, Diehl AM. Am J Physiol Gastrointest Liver Physiol. 2006;291:G575–583. doi: 10.1152/ajpgi.00102.2006. [DOI] [PubMed] [Google Scholar]
- 9.Bataller R, Brenner DA. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kisseleva T, Brenner DA. J Gastroenterol Hepatol. 2007;22(Suppl 1):S73–78. doi: 10.1111/j.1440-1746.2006.04658.x. [DOI] [PubMed] [Google Scholar]
- 11.Kaimori A, Potter J, Kaimori JY, Wang C, Mezey E, Koteish A. J Biol Chem. 2007;282:22089–22101. doi: 10.1074/jbc.M700998200. [DOI] [PubMed] [Google Scholar]
- 12.Meindl-Beinker NM, Dooley S. J Gastroenterol Hepatol. 2008;23:S122–S127. doi: 10.1111/j.1440-1746.2007.05297.x. [DOI] [PubMed] [Google Scholar]
- 13.Bi WR, Yang CQ, Shi Q. Hepatogastroenterol. 2012;59:1960–1963. doi: 10.5754/hge11750. [DOI] [PubMed] [Google Scholar]
- 14.Jiang DP, Jiang ZT, Han FY, Zhang YB, Li ZZ. Eur J Appl Physiol. 2008;103:489–493. doi: 10.1007/s00421-008-0733-7. [DOI] [PubMed] [Google Scholar]
- 15.Xiao GH, Jeffers M, Bellacosa A, Mitsuuchi Y, Vande Woude GF, Testa JR. Proc Natl Acad Sci U S A. 2001;98:247–252. doi: 10.1073/pnas.011532898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishino M, Iimuro Y, Ueki T, Hirano T, Fujimoto J. Surgery. 2008;144:374–384. doi: 10.1016/j.surg.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Nature. 1995;373:699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- 18.Narmada BC, Chia SM, Tucker-Kellogg L, Yu H. J Cell Physiol. 2013;228:393–401. doi: 10.1002/jcp.24143. [DOI] [PubMed] [Google Scholar]
- 19.Pagan R, Martin I, Llobera M, Vilaro S. Hepatology. 1997;25:598–606. doi: 10.1002/hep.510250318. [DOI] [PubMed] [Google Scholar]
- 20.Pagan R, Sanchez A, Martin I, Llobera M, Fabregat I, Vilaro S. J Hepatol. 1999;31:895–904. doi: 10.1016/s0168-8278(99)80292-x. [DOI] [PubMed] [Google Scholar]
- 21.Wylie RG, Ahsan S, Aizawa Y, Maxwell KL, Morshead CM, Shoichet MS. Nat Mater. 2011;10:799–806. doi: 10.1038/nmat3101. [DOI] [PubMed] [Google Scholar]
- 22.Mann BK, Schmedlen RH, West JL. Biomaterials. 2001;22:439–444. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 23.Kim M, Lee JY, Jones CN, Revzin A, Tae G. Biomaterials. 2010;31:3596–3603. doi: 10.1016/j.biomaterials.2010.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thery M. J Cell Sci. 2010;123:4201–4213. doi: 10.1242/jcs.075150. [DOI] [PubMed] [Google Scholar]
- 25.Revzin A, Rajagopalan P, Tilles AW, Berthiaume FO, Yarmush ML, Toner M. Langmuir. 2004;20:2999–3005. doi: 10.1021/la035827w. [DOI] [PubMed] [Google Scholar]
- 26.Lee JY, Tuleuova N, Jones CN, Ramanculov E, Zern MA, Revzin A. Integr Biol (Camb) 2009;1:460–468. doi: 10.1039/b905757a. [DOI] [PubMed] [Google Scholar]
- 27.Hui EE, Bhatia SN. Proc Natl Acad Sci U S A. 2007;104:5722–5726. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung BG, Flanagan LA, Rhee SW, Schwartz PH, Lee AP, Monuki ES, Jeon NL. Lab Chip. 2005;5:401–406. doi: 10.1039/b417651k. [DOI] [PubMed] [Google Scholar]
- 29.Hubbell JA. Nat Mater. 2008;7:609–610. doi: 10.1038/nmat2238. [DOI] [PubMed] [Google Scholar]
- 30.Przybyla L, Voldman J. Annu Rev Anal Chem (Palo Alto Calif) 2012;5:293–315. doi: 10.1146/annurev-anchem-062011-143122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones CN, Tuleuova N, Lee JY, Ramanculov E, Reddi AH, Zern MA, Revzin A. Biomaterials. 2009;30:3733–3741. doi: 10.1016/j.biomaterials.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 32.Jones CN, Tuleuova N, Lee JY, Ramanculov E, Reddi AH, Zern MA, Revzin A. Biomaterials. 2010;31:5936–5944. doi: 10.1016/j.biomaterials.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuleuova N, Lee JY, Lee J, Ramanculov E, Zern MA, Revzin A. Biomaterials. 2010;31:9221–9231. doi: 10.1016/j.biomaterials.2010.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghaedi M, Tuleuova N, Zern MA, Wu J, Revzin A. Biochem Biophys Res Commun. 2011;407:295–300. doi: 10.1016/j.bbrc.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghaedi M, Duan Y, Zern MA, Revzin A. J Regen Med Tissue Eng. 2012 doi: 10.1002/term.1595. [DOI] [PubMed] [Google Scholar]
- 36.Dunn JCY, Yarmush ML, Koebe HG, Tompkins RG. FASEB J. 1989;3:174–179. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 37.Gravdal K, Halvorsen OJ, Haukaas SA, Akslen LA. Clin Cancer Res. 2007;13:7003–7011. doi: 10.1158/1078-0432.CCR-07-1263. [DOI] [PubMed] [Google Scholar]
- 38.Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C, Tiron C, Lorens JB, Ivaska J. Oncogene. 2011;30:1436–1448. doi: 10.1038/onc.2010.509. [DOI] [PubMed] [Google Scholar]
- 39.March S, Hui EE, Underhill GH, Khetani S, Bhatia SN. Hepatology. 2009;50:920–928. doi: 10.1002/hep.23085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.