Abstract
Background
Isolated tumor cells (ITC) are more likely to be identified when serial sectioning and immunohistochemical staining are used to evaluate sentinel lymph nodes (SLN). Our goal was to identify clinicopathologic features associated with ITC in patients undergoing sentinel lymph node dissection (SLND).
Methods
We reviewed clinicopathologic data for 3557 patients with no clinical evidence of lymph node metastases undergoing SLND between November 1993 and March 2007. Patients were staged according to the 6th edition of the American Joint Committee on Cancer staging system, with metastasis ≤.2 mm classified as ITC.
Results
A SLN was identified in 3475 patients (97.7%), including 2518 (72.4%) with negative nodes and 169 (4.9%) with ITC. A statistically significant association existed between lobular histology and the identification of ITC; 13.6% of patients with ITC had lobular histology versus 7.3% of patients with a negative SLN (P = .003). The presence of lymphovascular invasion (LVI) was also associated with ITC; 18.3% of patients with ITC had LVI in the primary tumor versus 8.5% of patients with a negative SLN (P < .001). No difference existed between patients with and without ITC with respect to T stage, grade, estrogen receptor, progesterone receptor, HER2/neu status, or biopsy method.
Conclusion
The association between ITC and LVI, a known predictor of poor outcome, suggests ITC may have clinical relevance. The relationship between lobular histology and ITC is consistent with the known pattern of lobular metastases, which frequently present as small foci requiring immunohistochemistry for detection. Longer follow-up is needed to determine whether ITC have prognostic significance.
Sentinel lymph node dissection (SLND) is now routinely used for axillary staging in patients with early-stage breast cancer. The status of the sentinel lymph node (SLN) has been demonstrated to accurately reflect the status of the regional nodal basin, thereby allowing for accurate staging of the axilla.1–3 In fact, SLND has been shown to increase staging accuracy4 and reduce false-negative rates5 by allowing for a more detailed pathologic evaluation of a smaller number of lymph nodes that are at the highest risk of harboring metastatic disease.
During SLND, an average of three lymph nodes are recovered.6,7 Therefore, pathologists are able perform a more comprehensive examination of these SLNs than would be feasible for the larger number of lymph nodes recovered from a complete axillary lymph node dissection (CALND). This detailed examination includes the use of serial sectioning with hematoxylin and eosin (H&E) staining and may also include immunohistochemical (IHC) staining for cytokeratin. By means of these techniques, pathologists can detect microscopic deposits down to the level of isolated tumor cells (ITC). In response to the increased use of SLND and the identification of microscopic foci of metastasis in lymph nodes, the most recent version of the American Joint Committee on Cancer (AJCC) staging system established definitions for lymph node involvement on the basis of the size of metastasis.8 Definitions of macrometastasis (>2.0 mm), micrometastasis (>.2 to 2.0 mm), and ITC (≤.2 mm) were established, and the pathologic categories pN1mi and pN0(i+) were added to indicate the presence of micrometastases or ITC. Lymph nodes that are negative for metastasis by both H&E staining and IHC are designated as pN0(i–).8
Even though uniform criteria now distinguish ITC and micrometastases from macrometastases, debate remains over the clinical relevance of these small-volume deposits in lymph nodes. Given the overall favorable prognosis of patients with early-stage breast cancer, the answer to this question will likely require multicenter trials that enroll large numbers of patients. Information from the American College of Surgeons Oncology Group (ACOSOG) Z0010 trial and the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial may help resolve questions regarding the impact of small-volume metastases in the SLN.9–11 Both ACOSOG Z0010 and NSABP B-32 completed accrual in 2004. Longterm follow-up of these multicenter trials will be required to assess the prognostic significance of small-volume metastases.
Recognizing that long-term follow-up will be required to determine the effect of ITC on diseasefree survival (DFS) or overall survival (OS), we took a different approach in the current study to investigate the potential relevance of small-volume metastases. Our goal in this retrospective review was to determine if specific pathologic features known to have prognostic significance in breast cancer were associated with the presence of ITC in the SLNs of patients with early-stage disease undergoing SLND.
Methods
The Institutional Review Board at the University of Texas M. D. Anderson Cancer Center approved this study. The study population was identified by a prospective database of patients undergoing intraoperative lymphatic mapping and SLND between November 1, 1993, and March 31, 2007. There were 3557 patients with invasive breast cancer and a clinically negative axilla by physical examination who had undergone SLND. A SLN was successfully identified in 3475 patients (97.7%) who made up the study population. Demographic data to include patient age and sex were noted. The following clinicopathologic data were recorded: biopsy method, primary tumor size (T stage), histologic subtype of the primary tumor, nuclear grade, presence or absence of lymphovascular invasion (LVI), estrogen receptor status, progesterone receptor status, and HER2/neu status. Biopsy methods included fine-needle aspiration biopsy, core biopsy, or excisional biopsy, which were either palpation guided or image guided. For hormone receptor status, we considered >10% positive staining of the cells by IHC to be positive. Tumors overexpressed HER2/neu if they were 3+ by IHC or positive by fluorescence in-situ hybridization.
We recorded the results of the SLND, including the number of SLNs removed, the number of positive SLNs, and the size of the metastasis for those SLNs that were positive. For patients who had undergone CALND, we also recorded the number of additional positive lymph nodes. We determined the status of all patients according to the 6th edition of the AJCC tumor, node, metastasis staging system.
Technique of Intraoperative Lymphatic Mapping and SLND
SLND was performed as previously described.12,13 Briefly, intraoperative lymphatic mapping was performed with a peritumoral injection of blue dye alone, 99mTc-labeled sulfur colloid alone, or a combination of the two. When 99mTc-labeled sulfur colloid was used, patients received filtered 99mTc-labeled sulfur colloid injected peritumorally or into the parenchyma surrounding a biopsy cavity on the day before surgery (2.5 mCi) or on the day of surgery (.5 mCi). When blue dye was used, 5 mL of 1% isosulfan blue dye (Lymphazurin; US Surgical, Norwalk, CT) was injected into the breast parenchyma surrounding the tumor or biopsy cavity. For tumors that were not palpable, the injection was performed according to mammographic or sonographic guidance. In some cases, the surgeon injected the mapping agents into the subdermal or subareolar location. For patients who received 99mTc-labeled sulfur colloid, a handheld gamma detection probe (NeoProbe 2000; US Surgical) was used intraoperatively to scan the axilla transcutaneously to identify the most radioactive area. An axillary incision was made over this hot spot, and SLNs were identified as nodes with uptake of blue dye, radioactive tracer, or both.
Pathologic Evaluation of the SLN
Pathologic evaluation of SLNs at our institution has evolved since we began performing the procedure in 1993. Before April 2000, SLNs were serially sectioned along the short axis at 2- to 3-mm intervals; sections were embedded in paraffin blocks, and one level from each block was stained with H&E. Beginning in April 2000, SLNs were grossly processed in the same manner, and each paraffin block was then serially sectioned at 5-lm intervals with two levels evaluated by routine H&E staining and one level analyzed for cytokeratin by IHC.14 For this study, we identified all patients who had undergone SLND before April 2000 and had a histologically negative SLN. SLNs from these patients were reanalyzed by a senior breast pathologist (A.S.), who resectioned the paraffin blocks at 5-lm intervals. In addition to H&E staining, IHC was used to analyze one level for cytokeratin.
Statistical Analysis
Clinicopathologic factors were compared between groups by χ2 square analysis. Multivariate analysis was performed by ordered logistic regression. All analyses were performed by Stata software, release 10 (StataCorp, College Station, TX). A P value of <.05 was considered statistically significant.
Results
Table 1 details the clinicopathologic characteristics of 3475 patients with early-stage breast cancer and clinically negative axillary lymph nodes who underwent successful SLND. The median number of SLNs identified was 3 (range, 1–14). The SLN had evidence of metastasis in 957 patients (27.5%), including 169 with ITC, 308 with micrometastasis, and 480 with macrometastasis.
Table 1. Characteristics of patients with clinically node-negative, early-stage breast cancer who underwent successful sentinel lymph node dissection (n = 3475).
| Characteristic | Value |
|---|---|
| Age (y), median (range) | 57 (22–92) |
| Sex, n (%) | |
| Male | 35 (1%) |
| Female | 3440 (99%) |
| Biopsy method, n (%) | |
| FNA | 151 (4.3%) |
| Core | 2285 (65.8%) |
| Excisional | 1039 (29.9%) |
| Palpation | 844 |
| Image guided | 195 |
| Histology, n (%) | |
| DCIS with microinvasion | 94 (2.7%) |
| IDC | 2582 (74.3%) |
| ILC | 300 (8.6%) |
| Mixed IDC/ILC | 266 (7.7%) |
| Other | 233 (6.7%) |
| Tubular | 73 |
| Mucinous | 39 |
| Papillary | 26 |
| Sentinel lymph node status, n (%) | |
| Negative | 2518 (72.4%) |
| Isolated tumor cells | 169 (4.9%) |
| Micrometastasis | 308 (8.9%) |
| Macrometastasis | 480 (13.8%) |
| T stage, n (%) | |
| T1mic | 109 (3.1%) |
| T1 | 2661 (76.6%) |
| T2 | 643 (18.5%) |
| T3 | 55 (1.6%) |
| T4 | 7 (.2%) |
| Final N stage, n (%) | |
| pN0 | 2518 (72.5%) |
| pN0(i+) | 165 (4.7%) |
| PN1mi | 277 (8.0%) |
| PN1 | 424 (12.2%) |
| pN2 | 71 (2.0%) |
| pN3 | 20 (.6%) |
| AJCC stage, n (%) | |
| I | 2246 (64.6%) |
| IIA | 893 (25.7%) |
| IIB | 208 (6.0%) |
| IIIA | 101 (2.9%) |
| IIIB | 7 (.2%) |
| IIIC | 20 (.6%) |
| Grade, n (%) | |
| 1 | 518 (14.9%) |
| 2 | 1830 (52.7%) |
| 3 | 1124 (32.3%) |
| Not reported | 3 (.1%) |
| LVI, n (%) | |
| Present | 487 (14.1%) |
| Absent | 2701 (77.7%) |
| Not reported | 287 (8.2%) |
| ER, n (%) | |
| Positive | 2633 (75.7%) |
| Negative | 680 (19.6%) |
| Not reported | 162 (4.7%) |
| PR, n (%) | |
| Positive | 2125 (61.1%) |
| Negative | 1181 (34.0%) |
| Not reported | 169 (4.9%) |
| HER2, n (%) | |
| Positive | 371 (10.7%) |
| Negative | 2696 (77.6%) |
| Not reported | 408 (11.7%) |
FNA, fine-needle aspiration; DCIS, ductal carcinoma-in-situ; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; AJCC, American Joint Committee on Cancer; LVI, lymphovascular invasion; ER, estrogen receptor; PR, progesterone receptor.
For the 169 patients with ITC, the median number of SLNs removed was 3 (range, 1–9), and the median number of SLNs with ITC present was one. Twenty-four patients had ITC identified in two SLNs, three patients had ITC identified in three SLNs, and one patient each had ITC identified in four and five SLNs. ITCs were identified by IHC only in 127 patients. We compared the group with a negative SLN and the group with ITC identified in the SLNs, and found no significant difference with respect to the method used to sample the primary tumor—fine-needle aspiration biopsy, core biopsy, or excisional biopsy (P = .10). Significant differences were seen with respect to specific tumor histologies (P = .003), with invasive lobular histology being significantly associated with ITC. A significant difference also existed between negative SLNs and SLNs with ITC with respect to the presence of LVI (P < .001) (Table 2, Fig. 1). Multivariate ordered logistic regression analysis determined that lobular histology and LVI were independently associated with an increased incidence of ITC (P < .001 for each factor).
Table 2. Comparison of clinicopathologic data for patients with negative sentinel lymph node (SLN) dissection vs. those with isolated tumor cells (ITC) or micrometastasis identified in SLN.
| Variable | SLN negative (n = 2518) | SLN with ITC (n = 169) | SLN with micrometastasis (n = 308) | P value (negative vs. ITC) | P value (negative vs. micrometastasis) |
|---|---|---|---|---|---|
| T stage | .13 | <.001 | |||
| T1mic | 98 | 7 | 4 | ||
| T1 | 2017 | 125 | 208 | ||
| ≥T2 | 403 | 37 | 96 | ||
| Histology | .003 | .007 | |||
| Ductala | 2128 | 139 | 267 | ||
| Lobular | 185 | 23 | 30 | ||
| Other | 205 | 7 | 11 | ||
| Grade | .10 | .20 | |||
| 1 | 409 | 17 | 38 | ||
| 2 | 1308 | 87 | 159 | ||
| 3 | 798 | 65 | 111 | ||
| Unknown | 3 | 0 | 0 | ||
| ER status | .55 | .08 | |||
| Negative | 531 | 39 | 54 | ||
| Positive | 1847 | 121 | 248 | ||
| Unknown | 140 | 9 | 6 | ||
| PR status | .13 | .24 | |||
| Negative | 882 | 50 | 102 | ||
| Positive | 1488 | 110 | 200 | ||
| Unknown | 148 | 9 | 6 | ||
| HER2 status | .054 | .08 | |||
| Negative | 1941 | 128 | 245 | ||
| Positive | 245 | 25 | 42 | ||
| Unknown | 332 | 16 | 21 | ||
| LVI | <.001 | <.001 | |||
| Absent | 2148 | 116 | 178 | ||
| Present | 213 | 31 | 81 | ||
| Unknown | 157 | 22 | 49 |
SLN, sentinel lymph node; ITC, isolated tumor cell; ER, estrogen receptor; PR, progesterone receptor; LVI, lymphovascular invasion.
Ductal pathology includes ductal carcinoma with microinvasion, pure ductal carcinoma, and mixed ductal/lobular carcinoma.
Fig. 1.
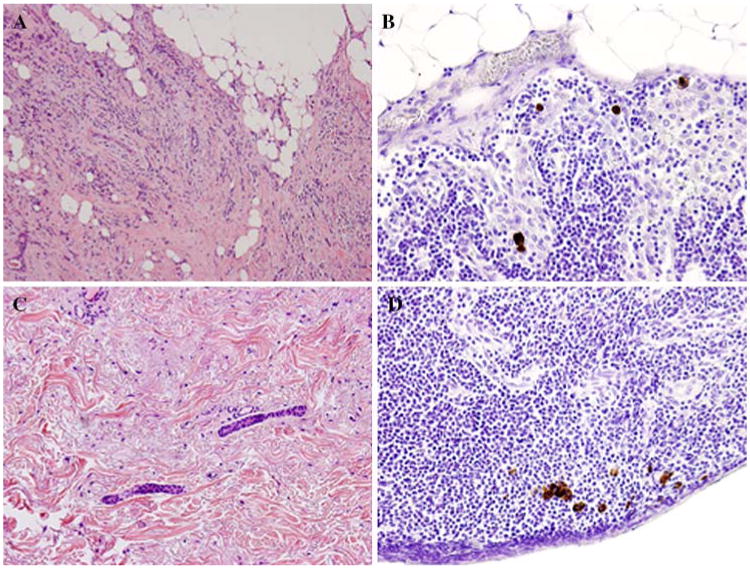
Lobular histology and lymphovascular invasion (LVI) in the primary tumor are associated with the detection of isolated tumor cells (ITC) in patients with early-stage breast cancer. (A) Hematoxylin and eosin (H&E) staining of a tumor from a patient with early-stage breast cancer demonstrating lobular histology. (B) Immunohistochemical (IHC) staining for cytokeratin of a sentinel lymph node (SLN) from the same patient demonstrates scattered foci of ITC staining brown. (C) H&E staining of a second patient's primary tumor demonstrating LVI. (D) IHC performed on the SLN from the second patient showing ITC.
CALND was performed in 890 patients, including 24 patients (14.2%) with ITC identified in their SLNs. Thirteen of these patients (54.2%) had ITC identified in their SLNs before implementation of the 6th edition of the AJCC staging system on January 1, 2003. After the AJCC staging system was revised, our institution's multidisciplinary group determined that patients with ITC identified in a SLN would be treated as having node-negative disease, consistent with their pN0(i+) designation. Therefore, a CAL-ND was not routinely performed. The 11 patients who underwent CALND after January 1, 2003, did so at the discretion of the attending surgeon, often because of other biologic factors such as primary tumor size (i.e., T2 or larger in seven patients). Additional nodal metastases were identified in four patients (16.7%) undergoing CALND after ITC were found in their SLNs. In all four patients, the additional metastases were macrometastases, resulting in upstaging from node-negative to node-positive disease.
Of 169 patients with ITC, 3 (1.8%) have experienced recurrence of disease. Median follow-up time for the whole group was 38 months. Median time to recurrence in the three patients mentioned above was 16 months (distant disease in two and locoregional [infraclavicular] in one). The patient with an infraclavicular recurrence was one of the patients who underwent CALND and was upstaged to pN1 disease. This patient had a 6-cm tumor that was negative for estrogen receptor, progesterone receptor, and HER2/neu, and she declined adjuvant chemotherapy. The three other patients found to have additional metastases at the time of CALND have not had evidence of recurrent disease.
Current AJCC criteria classify ITC (pN0[i+]) as node-negative disease and micrometastases (pN1mi) as node-positive disease. We were therefore interested in determining whether the same clinicopathologic features that were associated with ITC, which currently are considered node negative, would be associated with micrometastasis that are also considered small-volume disease but designated as node positive. We compared the group with negative SLN (pN0[i–]) and the group with micrometastasis (pN1mi) and found that there was no difference (P = .19) on the basis of the biopsy method used. Significant differences were again seen with respect to lobular histology (P = .007) and the presence of LVI (P<.001) (Table 2). There was also a significant difference with respect to T stage, with micrometastasis more commonly seen in patients with T2 primary lesions (≥2 cm) (P < .001).
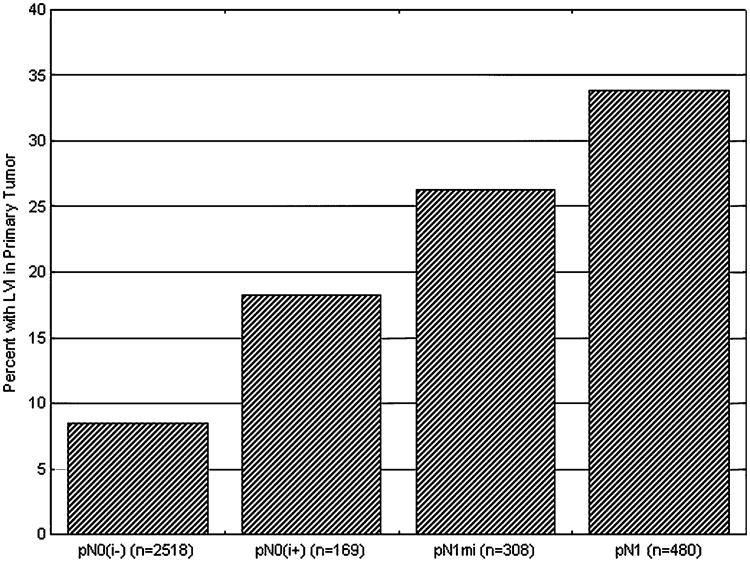
Having demonstrated that the presence of LVI was associated with an increased incidence of both ITC and micrometastasis in the SLNs of early-stage breast cancer patients, we investigated whether the presence of LVI affected SLN tumor burden, defined as the size of the metastasis. We found that an increasing SLN burden was associated with a greater likelihood of finding LVI in the primary tumor (Fig. 2). LVI was found in the primary tumor of 8.5% (n = 213) of patients who were SLN negative, in 18.3% (n = 31) of patients with ITC, 26.3% (n = 81) with micrometastasis, and 33.8% (n = 162) with ma-crometastasis (P < .001).
Fig. 2.
Increasing sentinel lymph node (SLN) tumor burden is associated with a greater likelihood of lymphovascular invasion (LVI) in the primary tumor. Within each pathologic category for SLNs obtained from patients with early-stage breast cancer, we determined the percentage of patients with LVI present in the primary tumor. The percentage of patients with LVI in their primary tumors increased significantly with increasing tumor burden in the SLN (P < .001).
Discussion
This study showed that the presence of LVI and lobular histology are greatly associated with detection of ITC and micrometastasis in SLNs in a large cohort of patients with early-stage breast cancer undergoing SLND. Importantly, with increasing SLN tumor burden, there was an increase in the percentage of patients with LVI in their primary tumor. Taken together, these data suggest that small-volume metastasis may have biologic relevance in this patient population.
Most data available on the prognostic value of ITC and micrometastases come from older retrospective studies. In several early studies, patients with metastases 2.0 mm or smaller identified in complete axillary lymph node specimens by routine sectioning and H&E staining had no survival disadvantage when compared with patients with node-negative disease.15–17 Additional studies have examined the impact of more rigorous pathologic examination, including serial sectioning and IHC staining for cytokeratin (Table 3). In those studies, some investigators found that the presence of small-volume occult metastases negatively affected outcome, whereas others demonstrated no effect.18–20 In a study that looked specifically at the relevance of ITC, Querzoli et al.21 reviewed 702 patients from a single institution. Whole axillary dissection specimens were analyzed, and 377 cases identified as being pN0 were reevaluated by means of serial sectioning and IHC. After a median follow-up of 8 years, these authors found that ITC had a marked impact on event-free survival, with an unadjusted hazard ratio of 2.51 for pN0(i+) disease versus pN0(i–) disease (P < .001).
Table 3. Summary of studies investigating the effect of occult axillary lymph node metastases detected by serial sectioning and immunohistochemical staining for cytokeratin on disease-free and overall survival.
| Study | No. of patients with negative lymph nodes | Patients with occult disease (%) | Multivariate analysis of disease-free survival (RR and 95% CI) | Multivariate analysis of overall survival (RR and 95% CI) |
|---|---|---|---|---|
| Tan et al. (2008)20 | 368 | 23 | Node negative 1.0 Met. ≤0.2 mm 1.7 (1.0 to 2.9) Met. 0.3–2.0 mm 4.2 (2.1 to 8.5)a | Node negative 1.0 Met. ≤0.2 mm 1.4 (0.9 to 2.2) Met. 0.3–2.0 mm 2.5 (1.3 to 4.6)b |
| Cummings et al. (2002)18 | 208 | 25 | Node Negative 1.0 Occult metastasis 2.17 (1.16 to 4.05)c | NS |
| Nasser et al. (1993)19 | 159 | 31 | NS | NS |
RR, relative risk; 95% CI, 95% confidence interval; NS, not significant.
P < 0.001.
P = 0.02.
P = 0.015.
These earlier studies all analyzed CALND specimens. Few studies have addressed the prognostic significance of ITC or micrometastases identified in SLNs. Recently, two large single-institution series looked at the clinical significance of small-volume metastases. Hansen et al.22 reported on 790 patients undergoing SLND at the John Wayne Cancer Center. Four hundred eighty-six patients (61.5%) were node negative, 84 (10.6%) had ITC, 54 (6.8%) had micrometastasis, and 166 (21.0%) had macrometastasis. After a median follow-up of 72.5 months, there was no statistically significant difference in DFS or OS for patients with pN0(i–) disease versus pN0(i+) disease or pN1mi disease. In a report from the H. Lee Moffitt Cancer Center, Cox et al.23 reviewed 2381 patients found to have pN0(i–) (n = 2108), pN0(i+) (n = 151), or pN1mi (n = 122) disease on SLND. Patients with pN1mi disease had significantly worse DFS and OS than patients with pN0(i–) disease (P = .006 and P < .001, respectively). Disease-free and OS of patients with pN0(i+) disease were not markedly different than that of patients with pN0(i–) disease.23
It is likely that several factors—including the use of adjuvant therapy, sample size, and length of follow-up—are responsible for the differences seen in these highlighted studies. For example, in the study from the John Wayne Cancer Institute, no difference in DFS or OS was demonstrated after a median follow-up of only 6 years. Although this follow-up would seem adequate for many breast cancer studies, we think it may not have been long enough to differentiate outcomes between patients with pN0(i–), pN0(i+), and pN1mi disease, who have very low rates of disease recurrence after administration of multimodality therapy.
The findings in the current study provide important information regarding these small-volume metastasis. It has been reported that identification of ITC in the SLN may not be due to lymphatic dissemination from the tumor but due to iatrogenic dislodgment after manipulations such as needling procedures to obtain tissue for diagnosis or pre-SLND breast massage.27–28 In the current series of 169 patients with ITC, we have shown that biopsy method was not associated with the identification of these small-volume tumor deposits. This finding suggests that ITC are true malignant cells. These data emphasize the importance of an experienced breast pathologist interpreting the results of SLND, particularly when IHC is used.
Our finding that LVI is associated with the presence of ITC in the SLN suggests that small-volume metastases may be biologically relevant and therefore may ultimately be found to have prognostic significance. LVI has been demonstrated in many studies to be a poor prognostic factor in breast cancer.27–31 With respect to the effect of LVI on nodal metastasis, the presence of LVI has been shown to predict the presence of disease in four or more axillary lymph nodes.32 In addition, our group previously reported that LVI independently predicts non-SLN involvement in patients with a positive SLN.33 In a publication by Van Zee et al.,34 investigators at the Memorial Sloan Kettering Cancer Center also determined that LVI was predictive of identifying additional, non-SLN metastases in patients with a positive SLN. The presence of LVI was therefore incorporated into a what is now a widely used nomogram developed to predict non-SLN disease in patients with positive SLNs. Recently, a nomogram was published that predicts the likelihood that a patient with breast cancer will have a positive SLN.35 On multivariate analysis used to construct that nomogram, LVI was associated with SLN metastasis.
We identified a relationship between increased SLN tumor burden and an increased likelihood of finding LVI in the primary tumor. Only 8.5% of patients with node-negative (pN0[i–]) disease had LVI in the primary tumor, compared with 18.3% with ITC, 26.3% with micrometastases, and 33.8% with macrometastases. These data provide strong evidence that ITC are true metastases and of the significance of LVI in predicting the spread of tumor to the lymph nodes.
In this study, we also determined that lobular histology is associated with identifying ITC and micrometastasis. This finding is consistent with data reported by Cserni et al.36 In a study reporting on a multi-institutional cohort of 449 patients with invasive lobular cancer staged by SLND, 189 patients (42%) were found to have nodal involvement, including 19 with ITC and 64 with micrometastasis. In most ITC cases (17 of 19, 90%) and micrometastases (40 of 64, 63%), IHC was required to identify disease after routine H&E staining yielded negative results.36 A similar finding was recently published by Tan et al.20 from the Memorial Sloan Kettering Cancer Center. These investigators reviewed records from 368 patients with axillary node-negative invasive breast cancer that had been treated with mastectomy and axillary dissection, but no systemic therapy. The investigators reexamined the axillary tissue blocks by serial sectioning and IHC and found that 83 patients (23%) had evidence of metastasis, including 61 (73%) with ITC and 17 (20%) with micrometastasis. They found a higher rate of occult metastasis in invasive lobular carcinoma (40%) than in invasive ductal carcinoma (20%), overrepresentation of lobular carcinoma among IHC-detected (36%) versus H&E-detected (15%) lesions, and overrepresentation of lobular carcinoma among patients with single-cell (59%) versus clustered metastases (7%).
The relationship between lobular histology and the presence of ITC is likely explained by the biology of invasive lobular cancer metastases, which are often noncohesive cells.36 Metastases from lobular cancer therefore frequently manifest as small foci scattered throughout a lymph node, which can be difficult to detect with H&E staining. These data suggest that IHC staining for cytokeratin may be particularly important in the evaluation of SLNs from patients with lobular carcinoma.20,36 Taken together with data from the current study, these findings suggest that physicians should consider CALND in patients with lobular carcinoma who have ITC in their SLNs because the current pN0(i+) designation may understage disease in these patients if ITC represent true metastases in this histologic subtype.
Review of the literature suggests that there is no consensus as to the appropriate surgical management of the axilla in patients with ITC or micrometastases in the SLN. At the University of Texas M. D. Anderson Cancer Center, we have recommended CALND for patients with micrometastases, in accordance with AJCC criteria classifying these lesions as node-positive disease. Similarly, we have treated patients with only ITC in their SLNs as having node-negative disease, and we have not routinely performed further axillary surgery.37 The results of the current study have caused us to reassess this approach. Despite the small number of patients in this study who had undergone CALND after identification of ITC, additional nodal disease was identified in approximately 17% of them, resulting in disease upstaging for these patients. This finding of additional metastasis in 17% is consistent with a study by Viale et al.,38 which reviewed the experience at the European Institute of Oncology and found additional metastases in 14.7% of patients with ITC in their SLNs. After evaluating these data together with the data from the current study, we now recommend performing CALND selectively in patients with ITC identified in their SLNs.
Metastases from lobular carcinoma frequently present as small foci scattered throughout a lymph node, which may only be seen as ITC. Therefore, we now recommend CALND for patients with lobular carcinoma who have ITC in the SLNs. The relationship between LVI and the presence of ITC is also interesting. LVI has clearly been demonstrated to be a poor prognostic factor in breast cancer, and the presence of LVI has influenced adjuvant systemic therapy decisions.39 It is reasonable to consider the presence of LVI when recommending locoregional treatment. We are therefore considering a registry trial in which all patients with LVI and ITC would undergo CALND. Our goal is to further refine a profile of clinicopathologic features that will determine which patients with ITC identified in their SLNs will benefit from CALND.
Acknowledgments
The authors acknowledge Shu-wan Kau, RN, for her assistance with database management and the Breast Cancer Management System Database at the University of Texas M. D. Anderson Cancer Center.
References
- 1.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 2.Purushotham AD, Upponi S, Klevesath MB, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol. 2005;23:4312–21. doi: 10.1200/JCO.2005.03.228. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–53. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 4.Giuliano AE, Dale PS, Turner RR, et al. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995;222:394–9. doi: 10.1097/00000658-199509000-00016. discussion 399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liberman L. Pathologic analysis of sentinel lymph nodes in breast carcinoma. Cancer. 2000;88:971–7. [PubMed] [Google Scholar]
- 6.Boughey JC, Bedrosian I, Meric-Bernstam F, et al. Comparative analysis of sentinel lymph node operation in male and female breast cancer patients. J Am Coll Surg. 2006;203:475–80. doi: 10.1016/j.jamcollsurg.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Cox C, White L, Allred N, et al. Survival outcomes in node-negative breast cancer patients evaluated with complete axillary node dissection versus sentinel lymph node biopsy. Ann Surg Oncol. 2006;13:708–11. doi: 10.1245/ASO.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Greene FL, Page DL, Fleming ID, editors. AJCC Cancer Staging Manual. 6th. New York: Springer; 2002. Breast; pp. 223–40. [Google Scholar]
- 9.Krag DN, Julian TB, Harlow SP, et al. NSABP-32: phase III, randomized trial comparing axillary resection with sentinal lymph node dissection: a description of the trial. Ann Surg Oncol. 2004;11:208S–10S. doi: 10.1007/BF02523630. [DOI] [PubMed] [Google Scholar]
- 10.Weaver DL, Krag DN, Ashikaga T, et al. Pathologic analysis of sentinel and nonsentinel lymph nodes in breast carcinoma: a multicenter study. Cancer. 2000;88:1099–107. [PubMed] [Google Scholar]
- 11.American College of Surgeons Oncology Group (ASOCOG) [Accessed September 5 2008]; Available at: www.acosog.org/
- 12.Breslin TM, Cohen L, Sahin A, et al. Sentinel lymph node biopsy is accurate after neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2000;18:3480–6. doi: 10.1200/JCO.2000.18.20.3480. [DOI] [PubMed] [Google Scholar]
- 13.Lambert LA, Ayers GD, Hwang RF, et al. Validation of a breast cancer nomogram for predicting nonsentinel lymph node metastases after a positive sentinel node biopsy. Ann Surg Oncol. 2006;13:310–20. doi: 10.1245/ASO.2006.03.078. [DOI] [PubMed] [Google Scholar]
- 14.Yared MA, Middleton LP, Smith TL, et al. Recommendations for sentinel lymph node processing in breast cancer. Am J Surg Pathol. 2002;26:377–82. doi: 10.1097/00000478-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Fisher ER, Swamidoss S, Lee CH, et al. Detection and significance of occult axillary node metastases in patients with invasive breast cancer. Cancer. 1978;42:2025–31. doi: 10.1002/1097-0142(197810)42:4<2025::aid-cncr2820420452>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 16.Huvos AG, Hutter RV, Berg JW. Significance of axillary macrometastases and micrometastases in mammary cancer. Ann Surg. 1971;173:44–6. doi: 10.1097/00000658-197101000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pickren JW. Significance of occult metastases. A study of breast cancer. Cancer. 1961;14:1266–71. doi: 10.1002/1097-0142(196111/12)14:6<1266::aid-cncr2820140617>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Cummings MC, Walsh MD, Hohn BG, et al. Occult axillary lymph node metastases in breast cancer do matter: results of 10-year survival analysis. Am J Surg Pathol. 2002;26:1286–95. doi: 10.1097/00000478-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Nasser IA, Lee AK, Bosari S, et al. Occult axillary lymph node metastases in “node-negative” breast carcinoma. Hum Pathol. 1993;24:950–7. doi: 10.1016/0046-8177(93)90108-s. [DOI] [PubMed] [Google Scholar]
- 20.Tan LK, Giri D, Hummer AJ, et al. Occult axillary node metastases in breast cancer are prognostically significant: results in 368 node-negative patients with 20-year follow-up. J Clin Oncol. 2008;26:1803–9. doi: 10.1200/JCO.2007.12.6425. [DOI] [PubMed] [Google Scholar]
- 21.Querzoli P, Pedriali M, Rinaldi R, et al. Axillary lymph node nanometastases are prognostic factors for disease-free survival and metastatic relapse in breast cancer patients. Clin Cancer Res. 2006;12:6696–701. doi: 10.1158/1078-0432.CCR-06-0569. [DOI] [PubMed] [Google Scholar]
- 22.Hansen N, Grube B, Ye C, et al. The impact of micrometas-tases in the sentinel nodes of patients with invasive breast cancer. Breast Cancer Res Treat. 2007;106(Suppl 1):S15. [Google Scholar]
- 23.Cox CE, Kiluk JV, Riker AI, et al. Significance of sentinel lymph node micrometastases in human breast cancer. J Am Coll Surg. 2008;206:261–8. doi: 10.1016/j.jamcollsurg.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Bleiweiss IJ, Nagi CS, Jaffer S. Axillary sentinel lymph nodes can be falsely positive due to iatrogenic displacement and transport of benign epithelial cells in patients with breast carcinoma. J Clin Oncol. 2006;24:2013–8. doi: 10.1200/JCO.2005.04.7076. [DOI] [PubMed] [Google Scholar]
- 25.Carter BA, Jensen RA, Simpson JF, et al. Benign transport of breast epithelium into axillary lymph nodes after biopsy. Am J Clin Pathol. 2000;113:259–65. doi: 10.1309/7EF8-F1W7-YVNT-H8H5. [DOI] [PubMed] [Google Scholar]
- 26.Diaz NM, Cox CE, Ebert M, et al. Benign mechanical transport of breast epithelial cells to sentinel lymph nodes. Am J Surg Pathol. 2004;28:1641–5. doi: 10.1097/00000478-200412000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Lee AK, Loda M, Mackarem G, et al. Lymph node negative invasive breast carcinoma 1 centimeter or less in size (T1a,b-NOMO): clinicopathologic features and outcome. Cancer. 1997;79:761–71. [PubMed] [Google Scholar]
- 28.Leitner SP, Swern AS, Weinberger D, et al. Predictors of recurrence for patients with small (one centimeter or less) localized breast cancer (T1a,b N0 M0) Cancer. 1995;76:2266–74. doi: 10.1002/1097-0142(19951201)76:11<2266::aid-cncr2820761114>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 29.Mann GB, Port ER, Rizza C, et al. Six-year follow-up of patients with microinvasive, T1a, and T1b breast carcinoma. Ann Surg Oncol. 1999;6:591–8. doi: 10.1007/s10434-999-0591-5. [DOI] [PubMed] [Google Scholar]
- 30.Quiet CA, Ferguson DJ, Weichselbaum RR, et al. Natural history of node-negative breast cancer: a study of 826 patients with long-term follow-up. J Clin Oncol. 1995;13:1144–51. doi: 10.1200/JCO.1995.13.5.1144. [DOI] [PubMed] [Google Scholar]
- 31.Shen J, Hunt KK, Mirza NQ, et al. Predictors of systemic recurrence and disease-specific survival after ipsilateral breast tumor recurrence. Cancer. 2005;104:479–90. doi: 10.1002/cncr.21224. [DOI] [PubMed] [Google Scholar]
- 32.Rivers AK, Griffith KA, Hunt KK, et al. Clinicopathologic features associated with having four or more metastatic axillary nodes in breast cancer patients with a positive sentinel lymph node. Ann Surg Oncol. 2006;13:36–44. doi: 10.1245/ASO.2006.03.080. [DOI] [PubMed] [Google Scholar]
- 33.Hwang RF, Krishnamurthy S, Hunt KK, et al. Clinicopatho-logic factors predicting involvement of nonsentinel axillary nodes in women with breast cancer. Ann Surg Oncol. 2003;10:248–54. doi: 10.1245/aso.2003.05.020. [DOI] [PubMed] [Google Scholar]
- 34.Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomo-gram for predicting the likelihood of additional nodal metas-tases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140–51. doi: 10.1245/aso.2003.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Bevilacqua JL, Kattan MW, Fey JV, et al. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J Clin Oncol. 2007;25:3670–9. doi: 10.1200/JCO.2006.08.8013. [DOI] [PubMed] [Google Scholar]
- 36.Cserni G, Bianchi S, Vezzosi V, et al. The value of cytokeratin immunohistochemistry in the evaluation of axillary sentinel lymph nodes in patients with lobular breast carcinoma. J Clin Pathol. 2006;59:518–22. doi: 10.1136/jcp.2005.029991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittendorf EA, Hunt KK. Significance and management of micrometastases in patients with breast cancer. Expert Rev Anticancer Ther. 2007;7:1451–61. doi: 10.1586/14737140.7.10.1451. [DOI] [PubMed] [Google Scholar]
- 38.Viale G, Maiorano E, Pruneri G, et al. Predicting the risk for additional axillary metastases in patients with breast carcinoma and positive sentinel lymph node biopsy. Ann Surg. 2005;241:319–25. doi: 10.1097/01.sla.0000150255.30665.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanrahan EO, Valero V, Gonzalez-Angulo AM, et al. Prognosis and management of patients with node-negative invasive breast carcinoma that is 1 cm or smaller in size (stage 1;T1a,bN0M0): a review of the literature. J Clin Oncol. 2006;24:2113–22. doi: 10.1200/JCO.2005.02.8035. [DOI] [PubMed] [Google Scholar]