Abstract
Over half of T-cell acute lymphoblastic leukemia (T-ALL) patients have activating mutations in the Notch gene. Moreover, the contaminant 2,3,7,8 Tetrachlorodibenzo-p-dioxin (TCDD) is a known carcinogen that mediates its toxicity through the aryl hydrocarbon receptor (AHR), and crosstalk between activated AHR and Notch signaling pathways has previously been observed. Given the importance of Notch signaling in thymocyte development and T-ALL disease progression, we hypothesized that the activated AHR potentiates disease initiation and progression in an in vivo model of Notch1-induced thymoma. This hypothesis was tested utilizing adult and developmental exposure paradigms to TCDD in mice expressing a constitutively active Notch1 transgene (NotchICN-TG). Following exposure of adult NotchICN-TG mice to a single high dose of TCDD, we observed a significant increase in the efficiency of CD8 thymocyte generation. We next exposed pregnant mice to 3μg/kg of TCDD throughout gestation and lactation to elucidate effects of developmental AHR activation on later-life T cell development and T-ALL-like thymoma susceptibility induced by Notch1. We found that the vehicle-exposed NotchICN-TG offspring have a peripheral T-cell pool heavily biased toward the CD4 lineage, while TCDD-exposed NotchICN-TG offspring were biased toward the CD8 lineage. Furthermore, while the vehicle-exposed NotchICN-TG mice showed increased splenomegaly and B to T cell ratios indicative of disease, mice developmentally exposed to TCDD were largely protected from disease. These studies support a model where developmental AHR activation attenuates later-life Notch1-dependent impacts on thymocyte development and disease progression.
Keywords: 2,3,7,8-tetrachlorodibenzo-p-dioxin; Developmental immunotoxicology; Leukemogenesis; Gene-environment interaction; Notch1; Aryl hydrocarbon receptor
INTRODUCTION
In humans, rates of chronic immune diseases and disorders have progressively increased over the last forty years (Bach, 2002; Dietert et al., 2010; Howlader et al., 2013; Jacobson et al., 1997). Some have suggested the increase in immunodeficiencies is due to the interaction of the environment with genetic susceptibility factors leading to altered immune state or immune cell development (Fazekas de St Groth, 2007; Perl, 2010). Environmentally initiated alterations in developmental pathways may lead to latent disease risk that can emerge at any time later in life following secondary exposures (Barker, 2007; Gluckman, Peter D. et al., 2005; Schug et al., 2012). Therefore, children are more vulnerable than adults to such exposures, as they have the entire life course ahead of them, resulting in more opportunities to impact sensitive time points (Perera and Herbstman, 2011). Environmental factors that are present during early life developmental stages may have particularly long-ranging impact by developmentally reprogramming progenitor cells through epigenetic changes at the chromatin level that potentially impact gene expression later in life. The impacts of progenitor cell reprogramming on later-life disease susceptibility are dependent on the interaction between the maternal intrauterine environment, the timing of exposure to potential insults, and the genetic susceptibility of the child. For example, early mutations in hematopoietic stem cells can increase the risk for development of leukemia (Corces-Zimmerman and Majeti, 2014). Moreover, 60% of all cases of T cell acute lymphoblastic leukemia (T-ALL) are associated with activating genetic mutations in Notch1 (Van Vlierberghe and Ferrando, 2012; Weng et al., 2004), a transmembrane receptor required for T cell development and function and regulation of CD4 vs. CD8 lineage commitment (Fowlkes and Robey, 2002).
Pinpointing environmental exposures as risk factors linked directly to disease outcomes is often complicated because of the latency associated with chronic, low level exposures. However, developmental exposure to the ubiquitous, persistent contaminant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), acting through the aryl hydrocarbon receptor (AHR), has been connected to immune dysfunction later in life (Dietert, 2009; Vorderstrasse et al., 2004), and epidemiological studies that have followed the population in Seveso, Italy accidentally exposed to TCDD in 1976, show an increase in both lymphatic and hematological cancers in adults (Consonni et al., 2008). Furthermore, children whose parents had elevated TCDD in their serum exhibited a decrease in thyroid function (Eskenazi et al., 2010). These results suggest that the adverse effects of TCDD can occur following both acute adult exposure and chronic low level developmental exposure and that the developing immune system is a sensitive target of TCDD in humans.
A potential connection between the AHR and Notch was elucidated in human acute lymphoblastic leukemia (ALL) where hypermethylation of the AHR promoter region was observed (Mulero-Navarro et al., 2006). Additionally, AHR responsive elements (AHREs) have been found in Notch promoters (Bock, 2013), and Notch signaling induces stimulation of the AHR in activated T cells (Alam et al., 2010). However, whether Notch1 and developmental AHR activation interact to promote disease has yet to be determined. This potential of developmental Notch1-AHR crosstalk during hematopoietic system disease initiation warrants further consideration given the genetic link to Notch1 mutations in T-ALL and continued production and persistence of TCDD and other environmental combustion by-products that act on the AHR, particularly in many developing countries undergoing rapid industrialization (Tanabe and Minh, 2010; Terauchi et al., 2009; Tue et al., 2013).
Previously we have demonstrated that developmental exposure to TCDD decreases the ability of hematopoietic progenitor cells to differentiate into mature lymphocytes (Ahrenhoerster et al., 2014). To determine if this phenomenon could have later-life disease implications, we examined the effect of prenatal AHR activation by transplacental exposure to TCDD on development and progression of a Notch1-induced thymoma in mice. We utilized a Notch1 transgenic mouse prone to developing T cell thymoma in order to probe a potential Notch1-AHR gene-environment interaction in disease. We hypothesized that developmental activation of AHR would produce a more severe form of Notch1- influenced thymoma in transgenic mice prone to disease than in unexposed control transgenic mice. To test this hypothesis, timed pregnant dams were exposed to 3μg/kg TCDD or vehicle control throughout pregnancy and at two days past parturition (PPD2). We found that while there was no significant difference in disease onset, severity or incidence between the groups, there was a significant difference in the ratios of circulating T-cells in the adult mice developmentally exposed to TCDD, ultimately impacting the lineage of the thymomas identified in the peripheral immune organs. Specifically, whereas unexposed Notch1 transgenic mice had a higher proportion of circulating CD4+ cells in the blood, Notch1 transgenic mice exposed to TCDD in utero had an increased proportion of circulating CD8+ cells as adults. This T cell lineage switch suggests an AHR-dependent reprogramming of a hematopoietic precursor during development that impacts the later-life intrinsic Notch signal transduction occurring in the CD4 versus CD8 T-cell lineage choice. These data have implications for disease susceptibility in vulnerable populations that may possess genetic instability in the Notch locus and/or have been exposed to environmental AHR agonists developmentally.
Materials and methods
Experimental animals
All animal procedures were conducted according to NIH’s Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and with the approval of the Institutional Animal Care and Use Committee (IACUC) at the University of Wisconsin-Milwaukee. C57BL/6J mice used were offspring of original pups obtained from the Jackson Laboratory (Bar Harbor, ME). C57BL/6-Tg(LckNotch1)9E mice, hereafter referred to as Notch1ICN-TG mice, were offspring of original pups that were a generous gift from B.J. Fowlkes, PhD, at the National Institute of Allergy and Infectious Disease (NIAID), Bethesda, MD. These mice were maintained as homozygous stock prior to breeding to C57BL/6 to generate heterozygous Notch1ICN-TG used for all experimental procedures. After overnight pairings, presence of a vaginal plug was designated gestational day (GD) 0.5. All mice were housed in micro-isolator cages in a specified pathogen-free facility at the University of Wisconsin-Milwaukee, were provided food and water ad libitum and maintained on a 12:12-h light cycle.
TCDD preparation and treatment protocol
TCDD (Cambridge Isotopes, Andover, MA) was prepared as previously described (Ahrenhoerster et al., 2014). Pregnant mice in the treatment group were given 3μg TCDD/kg body weight by oral gavage on gestational days 0.5, 7.5, 14.5, and post-partum day (PPD) 2.5, while control mice received an equivalent volume of olive oil vehicle (0.1ml per 10g) on the same days. Doses were given 7 days apart to insure a relatively constant level of TCDD throughout because the half life of TCDD in a C57BL/6 mouse is approximately one week (Gasiewicz et al., 1983; Hogaboam et al., 2007; Miniero et al., 2001; Weber and Birnbaum, 1985).
For acute exposure experiments, four-week old naïve mice were exposed to a single dose of 10μg or 30μg TCDD/kg body weight or an equal volume of vehicle control by oral gavage and tissues were analyzed 10 days after exposure.
Blood and tissue harvest and analysis
For analysis of immune cell ratios, mice were weighed and blood was harvested weekly, from 5 to 12 weeks of age. For blood collection, mice were restrained by hand, and the right cheek was nicked with a 5 mm Goldenrod animal lancet (Medipoint, Inc., Mineola, NY). Approximately 100μL of blood was collected from the maxillary vein and deposited into a 2000μL tube (Eppendorf, USA) containing 50 μL of heparin (Sagent Pharmaceuticals, Schaumburg, IL).
100μL of blood/heparin were washed in 1 ml Hank’s buffered saline solution (HBSS; Corning CellGro, Herndon, VA), supplemented with 0.5% FBS (Invitrogen, Grand Island, NY) and 0.1% sodium azide (J.T. Baker/Avantor, Center Valley, PA). Prior to analysis, Fc receptors were blocked with 2.4G2, red blood cells were lysed with BD Pharm Lyse (BD Biosciences, San Jose, USA), and then cells were stained and analyzed for surface expression of CD4, CD8, CD19, CD11b, CD-3, Gr-1 and CD45 (see section below for details). Because CD4+CD8+ cells are normally found only in the thymus, any blood samples with populations of CD4+CD8+ were eliminated from our analyses (Supp. Fig. 1).
Tissue samples were harvested from mice throughout the experiment. Mice were observed daily, and if a mouse appeared visibly ill (slow-moving, apparent tumor growth), it was euthanized by CO2 inhalation followed by cervical dislocation according to the American Veterinary Medical Association guidelines (AVMA, 2013). For some experiments, half of the mice were euthanized at week 8, and all remaining mice were euthanized at completion of the experiment on week 12. After euthanasia, spleen and thymus were removed from individual mice, mechanically dispersed in HBSS supplemented with 0.5% fetal bovine serum (Invitrogen, Grand Island, NY) and 0.1% sodium azide (J.T. Baker/Avantor, Center Valley, PA), and passed through sterile Nitex nylon mesh (80μm; Wildco, Yulee, FL) to form single cell suspensions. The red blood cells were then depleted with lysis buffer (155 mM NH4Cl + 12 mM NaHCO3 + 0.1 mM EDTA), Fc blocked with 2.4G2, then stained and analyzed for surface expression of CD3, CD4, CD8, CD11b, and CD5.
Antibodies and Fluorescence Activated Cell Sorting
Primary fluorochrome-conjugated monoclonal antibodies were used in flow cytometry analysis on a BD FACSAria III, DIVA version 6.1.3. Weekly blood and tissue cells were identified based on expression of BD-V450-conjugated CD19 (clone 1D3); Alexa488-conjugated CD11b (clone M1/70); PE-conjugated CD45 (clone 30-F11); PE-CF594-conjugated CD3 (clone 145-2C11); PECy5-conjugated CD4 (clone RM4-5); Alexa647-conjugated CD8 (clone 5H10); and APC-Cy7-conjugated Gr-1 (clone RB6-8C5). CD5+ tumor cells were identified by first gating on CD3+ populations, then CD4+ and CD8+. Within CD4+ and CD8+ populations, tumor cells were identified by the presence of Biotin-conjugated CD5 (clone 53-7.3) coupled with Streptavidin FITC. For thymocyte and splenocyte cellular analysis, viable cells were identified with the vital dye Sytox Blue (Life Technologies, Carlsbad, CA) to label nuclei from dead or dying cells with permeable membranes. Sytox Blue negative cells were then identified by the presence of PECy5-conjugated CD4 (clone 30-F11) and/or Alexa647-conjugated CD8 (clone 5H10). All antibodies were used at titrated concentrations and were purchased from BD Biosciences (San Jose, CA). All flow cytometry data were analyzed using FlowJo software (Version 9.5, Treestar, Ashland, OR).
Statistical analysis
Our calculations are based on a compilation of data from at least three replicates of each experiment; n-values are listed in each figure legend. Graphpad Prism 6 (Graphpad Software, LaJolla, CA) was utilized for Student’s t-test, Log-rank (Mantel-Cox) test of survival curve comparison, and graphical presentation of all data.
RESULTS
Notch1ICN-TG-dependent maturation of CD8+ thymocytes is potentiated by TCDD activation of the AHR
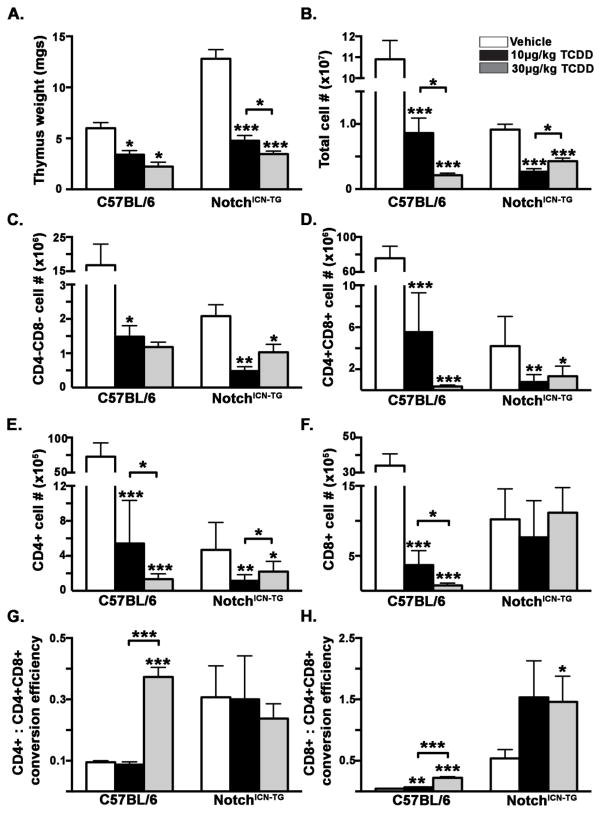
In order to elucidate the potential interaction between Notch1 and the AHR during thymocyte development, we exposed four-week old Notch1ICN-TG and C57BL/6 mice to a single dose of 10 or 30μg/kg TCDD or vehicle control, and analyzed thymus size, cell number, and composition 10 days after exposure. The Notch1ICN-TG transgenic mice utilized for these studies expresses the constitutively active intracellular Notch1 under control of the lck-proximal promoter, restricting its expression to developing thymocytes. These mice have previously been shown to produce a larger percentage of CD8+ thymocytes than CD4+ thymocytes, in contrast to proportions found in wild type C57BL/6 mice (Robey et al., 1996). This increased population of CD8+ cells found in the Notch1ICN-TG thymus is similar to observations made in mice exposed to the AHR ligand TCDD (Gill et al., 2008; Kremer et al., 1995). Following exposure to TCDD, both C57BL/6 and Notch1ICN-TG mice showed a significant decrease in thymic weight and cellularity (Fig. 1A–B). Notably, we found that vehicle-exposed adult Notch1ICN-TG mice had larger thymuses by weight (Fig. 1A) but lower cellularity (Fig. 1B) than C57BL/6 mice. Examination of flow cytometry FSC-A histograms for cell size distribution show a bi-modal peak in Notch1ICN-TG mice compared to the C57BL/6 indicating there is a greater population of large blast-like thymocytes (Supp. Fig. 2).
Figure 1. Thymus cellularity and efficiency of thymocyte conversion in adult male C57BL/6 or Notch1ICN-TG mice after administration of TCDD or vehicle control.
Wild type C57BL/6 or Notch1ICN-TG mice were administered 10μg/kg TCDD, 30μg/kg TCDD or vehicle control via oral gavage at 4 weeks of age. Ten days later, mice were euthanized, thymuses removed, single cell suspensions made, and cells stained with fluorescent-conjugated antibodies directed against CD4 and CD8. Cells were acquired on a BD FACS Aria III flow cytometer and data analyzed in FlowJo. A) Thymus weight ± SEM; B) Thymocyte cellularity ± SEM; C–F) Absolute cell number (mean ± SEM) in each thymocyte cell population; G–H) Efficiency of SP (CD4+ or CD8+) generation, calculated by dividing the number of CD4+ or CD8+ thymocytes in by the number of CD4+CD8+ precursors; Vehicle mice are represented with white bars, 10μg/kg TCDD mice with black bars, and 30μg/kg TCDD-exposed mice with gray bars. A single * indicates p ≤0.05; ** indicates p ≤0.01; *** indicates p ≤0.001. All compared to vehicle control within genotype unless otherwise indicated by bracket. (n=at least 4 B6 mice in each group; n=at least 8 Notch1ICN-TG mice in each group)
Analysis of thymocyte sub-populations revealed effects dependent on either Notch1ICN-TG, TCDD activation of the AHR, or on the combination of Notch1ICN-TG and TCDD activation of the AHR. Specifically, we found that in both C57BL/6 and Notch1ICN-TG mice, TCDD-activation of the AHR led to a significant decrease in the absolute number of the most immature population of thymocytes identified by the absence of both CD4 and CD8 (Fig 1C). The largest population of thymocytes by cell number, the CD4+CD8+, is reduced in number by more than 10 fold in the C57BL/6 mice after exposure to TCDD as previously reported (Fig 1D; p ≤ 0.001; Laiosa et al., 2003, 2002). Moreover, CD4+CD8+ thymocyte numbers in Notch1ICN-TG are also reduced by approximately 10 fold (p ≤ 0.05), however, the number of CD4+CD8+ thymocytes in the vehicle-exposed Notch1ICN-TG is 10 fold lower than vehicle-exposed C57BL/6, suggesting that the intracellular Notch1 transgene alters the basal thymocyte cell number. In comparison to the reduction observed in CD4+CD8+ thymocytes, the production of CD4+ and CD8+ progeny that result from positive selection are differentially sensitive to the combined effects of Notch1ICN-TG and the TCDD-activated AHR. Specifically, while the number of CD4 lineage thymocytes is significantly reduced by exposure to TCDD in both C57BL/6 and Notch1ICN-TG mice (Fig 1E), CD8+ cells are present in equal number in the vehicle- and TCDD-exposed Notch1ICN-TG mice (Fig 1F). Given the differences in thymocyte cell number between C57BL/6 and Notch1ICN-TG mice, we used a ratio of single positive CD4+ or CD8+ to CD4+CD8+ as a way to normalize the efficiency of thymocyte conversion. Typically, 95–99% of CD4+CD8+ thymocytes fail selection, which is reflected by ratios of less than 0.2 in the C57BL/6 mice. In comparison, the thymocyte conversion efficiency in Notch1ICN-TG mice is 3 to 5 fold higher in the CD4 and CD8 lineages, respectively. Moreover, 30μg/kg TCDD produces an excess of CD8 cells compared to DP cells thus yielding a ratio of CD8+ to CD4+CD8+ cells that is greater than one, indicating an increase in CD8 conversion efficiency in Notch1ICN-TG mice (Fig 1H; p ≤ 0.05). These results suggest a potential additive interaction between the thymocyte-restricted constitutively active intracellular Notch1 and the ligand-activated AHR during thymocyte development.
Developmental Exposure to TCDD Does Not Affect Survival Into Adulthood of Notch1ICN-TG transgenic mice
Based on our recent findings linking developmental TCDD exposure to attenuation of Notch1-dependent lymphocyte differentiation (Ahrenhoerster et al., 2014), and previous work demonstrating that developmental AHR activation adversely impacts later life immunity (Hogaboam et al., 2007), we hypothesized that developmental AHR activation by TCDD reprograms hematopoietic progenitor cells such that susceptibility to later-life genetically-based T-cell leukemia-like disease is increased. We tested this hypothesis in T-cell thymoma prone NotchICN-TG mice by initially following the survival of Notch1ICN-TG mice exposed to either vehicle or 3μg/kg of TCDD throughout gestation (GD 0.5, 7.5, 14.5 and PPD 2.5). The in utero and lactationally-exposed pups were followed until death or 12 weeks of age. Although approximately 40% of Notch1ICN-TG mice died during the experiment, there was no significant difference in overall survival between TCDD- or vehicle-exposed Notch1ICN-TG mice (Fig. 2).
Figure 2. Survival of C57BL/6 or Notch1ICN-TG mice following developmental exposure to 3μg/kg TCDD or vehicle control.
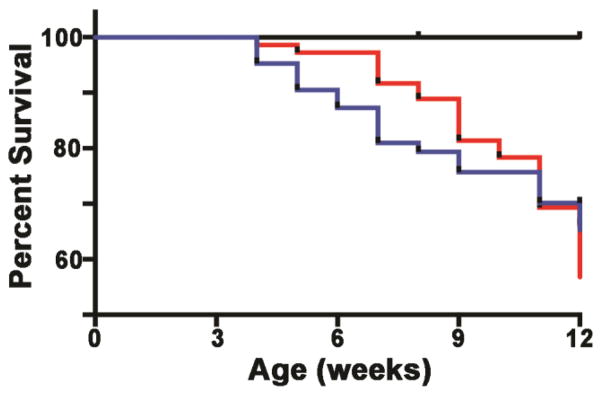
Pregnant C57BL/6 dams (paired with either C57BL/6 or Notch1ICN-TG males) were exposed to 3μg/kg TCDD or vehicle control throughout gestation (GD0.5, GD7.5, GD14.5, PPD2.5). Resulting offspring were followed through twelve weeks of age to determine age of death. C57BL/6 mice are represented with a black line (vehicle and TCDD; n=35). Notch1ICN-TG mice are represented with a red line (vehicle; n=72) or blue line (TCDD; n=63). Survival fractions were calculated using Kaplan-Meier analysis, and survival curves were compared using the log-rank (Mantel-Cox) test (C57BL/6 p=1.00; Notch1ICN-TG p=0.62).
Developmental TCDD exposure in Notch1ICN-TG mice skews blood B cell to T cell and CD4 to CD8 ratios in adult mice
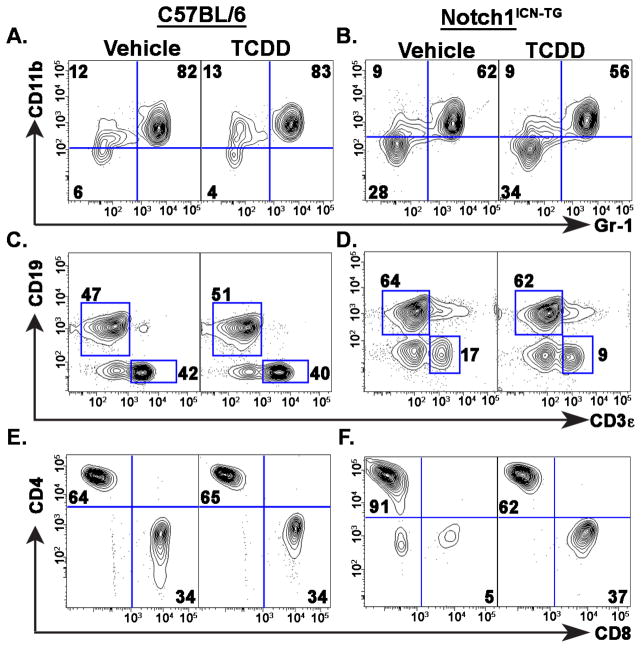
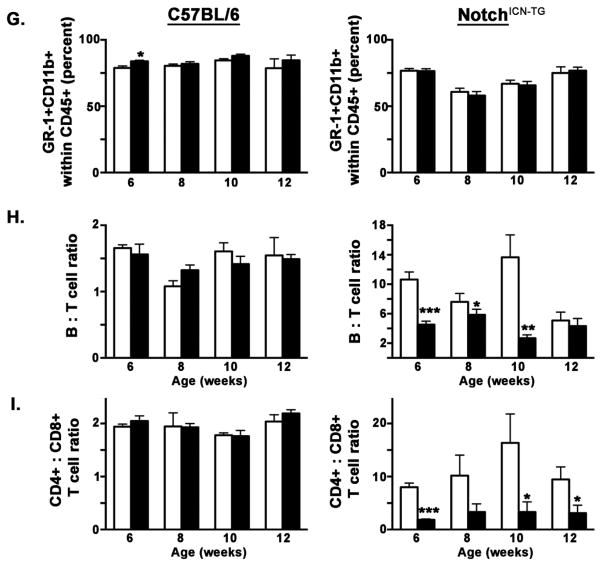
It is well established that Notch1ICN-TG mice have an altered ratio of CD4+ to CD8+ T cells compared to non-transgenic mice (Fowlkes and Robey, 2002; Robey et al., 1996), but the combined effect of developmental exposure to AHR agonists such as TCDD had not been examined in these mice. In order to more specifically quantify differences in thymoma initiation and progression following developmental exposure to TCDD in Notch1ICN-TG mice, we collected blood samples each week between 5 and 12 weeks of age, and quantified the proportions of circulating lymphocytes (Fig. 3A–C). No significant difference was found between treatments at any time point in either C57BL/6 or Notch1ICN-TG mice in the percentage of macrophages and granulocytes, as evidenced by percent of CD11b+ or Gr1+CD11b+ cells, respectively (Fig. 3A, B & G). Similarly, we found no significant difference between vehicle or TCDD exposure in the ratio of B cells to T cells or in CD4+ T cells to CD8+ T cells in the C57BL/6 mice (Fig. 3C & H). However, in Notch1ICN-TG mice, we saw a significantly lower ratio of B cells to T cells following developmental exposure to TCDD (Fig. 3D & H). Additionally, we saw a significantly lower ratio of CD4+ T cells to CD8+ T cells in mice developmentally exposed to TCDD (Fig 3F & I). Notably, in Notch1ICN-TG mice, the ratio of B cells to T cells, and the ratio of CD4+ to CD8+ T cells in mice developmentally exposed to TCDD decreased almost to the level found in C57BL/6 mice. These data suggest that developmental exposure to TCDD provides protection from the alterations caused by a constitutively active Notch1 in thymocytes.
Figure 3. Proportion circulating lymphocytes in the blood following developmental exposure to 3μg/kg TCDD or vehicle control.
Pregnant C57BL/6 dams (after pairing with either C57BL/6 or Notch1ICN-TG males) were exposed to 3μg/kg TCDD or vehicle control throughout gestation (GD0.5, GD7.5, GD14.5, PPD2.5). A–F) Representative flow cytometry plots from week 8 blood samples, illustrating distribution and analysis of CD11b and Gr-1 positive cells (A), CD3+ T cells and CD19+B cells (C & D), and CD8+ and CD4+ T cells, gated on CD3+ (E & F). Frequency of Gr1+CD11b+ blood cells (G), the ratio of B cells to T cells in blood (H), or the ratio of CD4+ T cells to CD8+ T cells in blood (I) in both C57BL/6 mice (graphs on left) and Notch1ICN-TG (graphs on right). Vehicle treated mice are represented by a white bar and mice treated with TCDD are represented by a filled bar in G–I. Bars graph mean with SE. A single * indicates p ≤0.05; ** indicates p ≤0.01; *** indicates p ≤0.001.
To determine if the change in the CD4+ to CD8+ ratio following developmental TCDD exposure could be explained by preferential survival of the CD8+ cells, we used the TUNEL assay to compare apoptosis in the thymus of 8 week old mice. We found no significant difference in the survival of CD4+ cells, CD8+ cells, or CD4+CD8+ cells following developmental TCDD exposure in either gender of mice (Supp. Fig. 3).
Adult thymocytes are unchanged by developmental TCDD exposure in NotchICN-TG mice
Given that the blood T cells and CD4:CD8 T cell ratios were altered in Notch1ICN-TG mice following developmental exposure to TCDD, and the variation was not explained by apoptosis, we examined CD4:CD8 thymocyte ratios in mice developmentally exposed to vehicle or 3μg/kg TCDD at 8 weeks and at 12 weeks of age. Although the distribution of viable thymocytes (as defined by sytox blue negative cells) differed in Notch1ICN-TG mice following developmental exposure to TCDD (Supp. Fig. 4A), we found no significant difference in CD4 to CD8 ratios or percent of CD4+CD8+ cells at either 8 weeks or 12 weeks in C57BL/6 or in Notch1ICN-TG thymi (Supp. Fig. 4B–C). This unchanged distribution of thymocytes from developmentally exposed NotchICN-TG mice contrasts with what was observed in the periphery, suggesting that differential emigration of thymocytes into the bloodstream or differential survival once reaching the periphery is the explanation for the profound differences in CD4:CD8 ratios in the blood.
Peripheral thymomas in Notch1ICN-TG mice following developmental TCDD exposure exhibit altered B cell:T cell ratios and differential expression of CD5
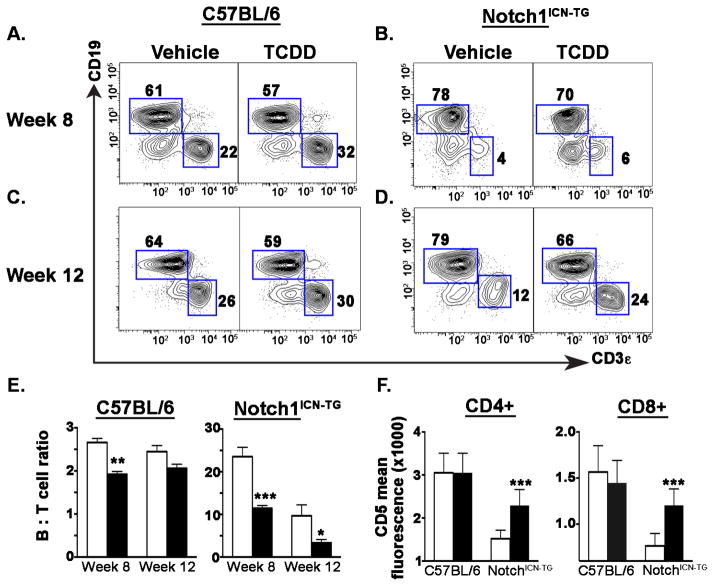
The spleen is almost always involved in murine leukemia and neoplasms (Yang et al., 2013) thus we further examined splenic tissue to search for metastatic thymomas. We observed alterations in the B cell to T cell ratio in spleens (Fig. 4), similar to that found in blood (Fig. 3). Specifically, TCDD-exposed C57BL/6 mice showed a significantly lower B cell to T cell ratio difference at 8 weeks (Fig. 4A & E; p ≤ 0.01). Notch1ICN-TG mice also showed a significant decrease in the ratio of B cells to T cells following developmental exposure at both 8 weeks (p ≤0.001) and 12 weeks (p ≤0.05) of age (Fig. 4B, D & E). Although both week 8 C57BL/6 and weeks 8 and 12 Notch1ICN-TG mice showed significant differences in B cell to T cell ratios, the magnitude of difference in Notch1ICN-TG mice was much greater. Whereas in C57BL/6 mice, the ratio decreased by 30 percent (2.7 in vehicle-exposed to 1.9 in TCDD-exposed), in Notch1ICN-TG mice, the ratio decreased over 50 percent at week 8 (23.6 in vehicle-exposed to 11.4 in TCDD-exposed) and over 60 percent at week 12 (9.7 in vehicle-exposed to 3.3 in TCDD-exposed).
Figure 4. Cell proportions in spleen are altered following developmental exposure to 3μg/kg TCDD or vehicle control.
Pregnant C57BL/6 dams (paired with either C57BL/6 or Notch1ICN-TG males) were exposed to 3μg/kg TCDD or vehicle control throughout gestation (GD0.5, GD7.5, GD14.5, PPD2.5). A and C) Representative flow cytometry plots delineating B and T cells from weeks 8 and 12 C57BL/6 spleen samples; B and D) Representative splenic B versus T cell flow cytometry plots from weeks 8 and12 in Notch1ICN-TG; E) Ratios of B cells to T cells at 8 weeks and 12 weeks, with C57BL/6 mice on the left and Notch1ICN-TG mice on the right; F) Mean fluorescence intensity of CD5 in T-cells from 6–8 week old mice, within the CD4 population on the left and the CD8 population on the right; Vehicle treated mice are represented by a white bar and mice treated with TCDD are represented by a filled bar in D–E. Bars graph mean with SE. A single * indicates p ≤0.05; ** indicates p ≤0.01; *** indicates p ≤0.001. (n=3 C57BL/6 vehicle; n=3 C57BL/6 TCDD; n=8 Notch1ICN-TG vehicle; n=7 Notch1ICN-TG TCDD at week 8; n=4 C57BL/6 vehicle; n=5 C57BL/6 TCDD; n=6 Notch1ICN-TG vehicle; n=8 Notch1ICN-TG TCDD at week 12).
We further measured CD5 expression in CD4+ and CD8+ splenic T cells as a proxy for T cell activation (Fig. 4F). We found the mean fluorescence intensity (MFI), an indication of the density of CD5 on the cell surface, was nearly two-fold lower in the CD8+ population. However, in both the CD4+ and CD8+ populations, cells from mice developmentally exposed to vehicle control had greatly decreased MFI. Additionally, in both cases, MFI in cells isolated from spleens of Notch1ICN-TG mice developmentally exposed to TCDD returned to near the MFI levels seen in C57BL/6 mice. These data show that Notch1ICN-TG mice developmentally exposed to TCDD approach a B cell to T cell ratio and a CD5 MFI similar to the wild type C57BL/6 vehicle-exposed mice.
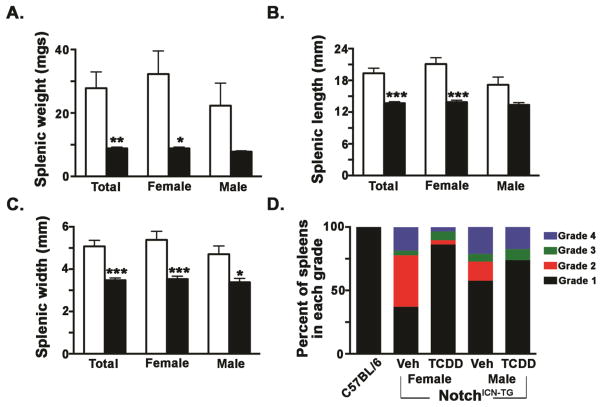
Spleen size is significantly smaller in Notch1ICN-TG mice following developmental TCDD exposure than in vehicle controls
Upon necropsy, many of the vehicle control Notch1ICN-TG mice had enlarged spleens--a sign of leukemic infiltration (Zhang et al., 2013) and this enlargement was much more common and severe in vehicle mice than in developmentally TCDD-exposed Notch1ICN-TG mice. Specifically, we found a statistically significant difference in spleen weight, size and length between the exposed and control groups (p ≤ 0.01; Fig. 5A–C). Furthermore, we used a numerical grading system in order to insure that spleens exhibiting severe splenomegaly did not bias the mean values shown in Fig. 5A–C. As previously published (Duggan et al., 2012), the grading system categorized spleens into grades 1–4 based on the following weights: normal (<0.2 g; Grade 1), moderate enlargement (≥0.2 – <0.5g; Grade 2), severe enlargement (≥0.05 – <1.0g; Grade 3), or morbid enlargement (≥1.0g; Grade 4). Whereas over 80% of spleens in the gender-combined TCDD-treated group fell into the “normal spleen” range, less than half of those in the vehicle group could be categorized as normal (Fig. 5D). Furthermore, this effect was even more pronounced in the female Notch1ICN-TG mice. None of the C57BL/6 wild type mice used in this experiment exhibited any spleen enlargement, supporting presence of metastatic thymomas as the cause of splenomegaly.
Figure 5. TCDD-exposed Notch1ICN-TG mice fail to develop Notch1-mediated splenomegaly.
Developmental exposure to TCDD in Notch1ICN-TG mice results in significantly smaller adult spleen size. Pregnant C57BL/6 dams were paired with Notch1ICN-TG males and exposed to 3μg/kg TCDD or vehicle control throughout gestation (GD0.5, GD7.5, GD14.5, PPD2.5). Vehicle treated mice are represented by a white bar and mice treated with TCDD are represented by a filled bar. Bars graph mean with SE. A single * indicates p ≤0.05; ** indicates p ≤0.01; *** indicates p ≤0.001. Comparisons of spleen weight (A), length (B), and width (C) were all made on 8 week old mice; spleen grading was done across all mice between 8–12 weeks of age at any age of necropsy. (n= 38 vehicle and 23 TCDD mice in A–C; n=61 vehicle and 52 TCDD mice in D)
DISCUSSION
In the present study, we demonstrate that developmental exposure to the environmental contaminant and AHR agonist TCDD during development in Notch1-dependent thymoma-prone mice reprograms long-term HSCs such that the adult thymic progeny of these cells have an attenuated response to the Notch1-dependent CD4 versus CD8 lineage decision. Specifically, the unusually large CD4:CD8 ratio observed in Notch1ICN-TG mice is restored to nearly C57BL/6 wild-type levels in the Notch1ICN-TG TCDD-exposed offspring. These data are therefore consistent with the conclusion that developmental AHR activation provides a means of disease protection to mice possessing a Notch1-mediated propensity to develop leukemic thymomas.
We have previously shown that developmental exposures to TCDD reprogram long-term reconstituting HSCs, impacting their ability to complete lymphocyte lineage commitment (Ahrenhoerster et al., 2014). These studies extend the impact of AHR-induced HSC reprogramming by demonstrating a long-term attenuated response to Notch1- mediated T-cell differentiation evidenced by the CD4 to CD8 lineage switch observed in peripheral T-cells from offspring exposed to TCDD during development. Taken together, these data are consistent with the conclusion that in a progenitor cell population in the fetus there are epigenetic or stable changes to the chromatin influenced by AHR activation that have later-life impacts on gene expression and cellular differentiation. Notably, the impact of this reprogramming on hematopoietic differentiation is not readily apparent in wild type C57BL/6 mice. Rather, for the later-life alterations to produce a measurable change in cellular function, the progeny of the long-term progenitor cells need to differentiate and be challenged by a secondary stressor such as the constitutively active intracellular Notch transgene in the thymus.
The acute exposure experiment demonstrated an increase in CD8 conversion efficiency following TCDD exposure in both C57Bl/6 and Notch1ICN-TG adult mice in the thymus, though not in the periphery (data not shown), providing initial evidence of potential AHR-Notch crosstalk. However, the substantial thymic atrophy resulting from adult TCDD exposure complicated interpretation of the mechanism for the increase in CD8+ cell conversion efficiency. Thus the switch to a developmental exposure model combined with a 10-fold decrease in dose throughout gestation allowed us to more clearly elucidate the effect that AHR activation had on Notch-mediated T cell differentiation throughout the lifecourse. Using this developmental model, any TCDD-induced thymic atrophy that occurred in fetal or neonatal mice was resolved by adulthood as indicated by similar thymic CD4+ and CD8+ populations as adults. Thus, the long-term developmental studies identified more sensitive and longer lasting outcomes than the adult exposure experiments. Specifically, developmental TCDD-exposure affected peripheral T-cell homeostasis demonstrated by a significant change in the efficiency of generating post-selection CD8+ T cells.
One interpretation of this study could be that early life AHR activation by TCDD is actually beneficial for Notch-mediated hematopoietic disease. However, Notch signaling is crucial for fetal HSC development and proliferation, for T lineage choice (Bigas and Espinosa, 2012), and for normal T cell development in both the fetus and adult (Osborne and Minter, 2007; Schmitt and Zúñiga-Pflücker, 2002). Therefore, impairment of physiological Notch signaling in HSCs and T cells has the potential to alter normal development and the consequences of this attenuation could impact a spectrum of hematological-based diseases.
Physiological Notch1-dependent signal transduction is characterized by engagement of the extracellular portion of Notch with Jagged or Delta-like ligands on a neighboring cell. This protein-protein engagement results in the gamma secretase-mediated cleavage of the intracellular Notch1 (ICN) domain. The ICN translocates into the nucleus and associates with the CBF1/Su(H)/Lag-1 (CSL) transcription factor complex where it acts as a co-activator driving transcription of a broad array of genes, including those responsible for T cell lineage commitment. A potential consequence of ICN over-expression in Notch1ICN-TG mice could be that the transgene stoichiometrically saturates the CSL binding site in the Notch signaling pathway leading to aberrant gene regulation attenuating physiological Notch activity (Beres et al., 2006). The importance of the transgenic ICN saturation of the CSL binding site in our studies follows the knowledge that physiological Notch1 is an AHR-regulated gene (Stevens et al., 2009). Though it is not known whether Notch transcription is directly controlled by the AHR (Kiss and Diefenbach, 2012), our data suggests that AHR activation during development leads to a change in responsiveness to ICN-mediated T cell development that persists throughout the life course.
Despite equivalent survival rates between Notch1ICN-TG mice developmentally exposed to TCDD or vehicle (p=0.62), the fatality rate in vehicle-exposed Notch1ICN-TG mice was higher than the 20% tumor rate previously reported at 5 months (Beverly and Capobianco, 2003). It is unknown whether the propensity for thymoma development increased in our sub-strain of Notch1ICN-TG mice prior to arriving in our vivarium, or was selected for inadvertently from our original breeder pair. However, this increased spontaneous thymoma rate made it difficult to form a conclusion about the original hypothesis that developmental TCDD exposure would increase later-life disease susceptibility. Nevertheless, the altered T cell ratios in the blood and splenomegaly differences are consistent with the conclusion that developmental TCDD exposure impacts Notch1-dependent disease initiation.
In terms of the splenomegaly, the expected result that tumor-resistant C57BL/6 mice survived for the duration of the 12-week experiment without splenomegaly, indicates that the enlarged spleens are unique to the transgenic strain we used and not due to the developmental TCDD exposure. The moderate protection against splenomegaly by developmental TCDD is similar to autoimmune and inflammatory amelioration following AHR activation (Busbee et al., 2013; Di Meglio et al., 2014; Kerkvliet, 2009). These data raise the intriguing possibility that AHR modulators could have efficacy as chemopreventatives or chemotherapeutics in certain types of leukemia, particularly if combined with existing treatments allowing for the decreasing of current doses. For example, gamma-secretase inhibitors (GSIs) that block Notch activation are a promising recent treatment for T-ALL, however, the negative side-effects include dose-limiting diarrhea (Hernandez Tejada et al., 2014; Takebe et al., 2014).
In addition to the CD4 to CD8 ratio changes mediated by developmental TCDD exposure, we also observed alterations in the CD5 MFI in both CD4+ and CD8+ splenic T cells. Specifically, CD5 levels, as determined by MFI, were significantly decreased in vehicle-exposed Notch1ICN-TG mice when compared to C57BL/6 mice (Fig. 5E). However, following developmental exposure to TCDD, the MFI of CD5 in both CD4+ and CD8+ T cells is partially restored to C57BL/6 levels. The consequences of the lower CD5 expression is consistent with a model of higher TCR signal transduction in T cells from Notch1ICN-TG mice (Germain, 2002), leading to a potential increase in activation-induced cell death in the periphery. While we were not able to detect any increases in apoptosis in vivo (supplemental figure 3), we and others have previously reported the difficulty in measuring apoptosis in vivo due to rapid clearance by macrophages (Laiosa et al, 2007). Thus we relied on indirect observations, such as splenomegaly to support the hypothesis of increased activation-induced cell death given that spleen enlargement is consistent with the removal of dead and dying cells. Moreover, elevated removal of dead and dying cells as occurs in a number of hematological disorders, including leukemia and lymphoma, is a hallmark of disease. Taken together, the developmental TCDD-dependent increase in CD5 expression in T cells from Notch1ICN-TG mice and the reduction in splenomegaly in TCDD-exposed Notch1ICN-TG mice supports a model of inhibition of T cell activation later in life.
While developmental AHR activation offers protection from later-life CD4+ to CD8+ T cell ratio imbalance and splenomegaly caused by constitutively-active ICN, the increase in CD8+ T cells that restores the ratio may not be beneficial in a more physiological model. Specifically, a decrease in the ratio of CD4+ T cells to CD8+ T cells in blood has been linked to poorer disease outcomes following diagnosis and treatment in several immunological diseases, including childhood leukemia (Lustfeld et al., 2014), hepatitis (Ikeda et al., 2012), and HIV (Serrano-Villar et al., 2014). We found that Notch1ICN-TG mice developmentally exposed to TCDD showed a persistently lower CD4+ to CD8+ ratio in peripheral blood, suggesting a less favorable response to disease treatment.
In summary, our results indicate that prenatal exposure to the environmental toxicant and AHR agonist TCDD reprograms HSCs and significantly changing the course of disease in a Notch1-induced murine thymoma model, as measured by ratios of B cells to T cells, and CD4+ T cells to CD8+ T cells. Future research to identify epigenetic changes influenced by exogenous stimulation of the AHR during development may facilitate identification of secondary stressors beyond Notch1 that vulnerable populations are burdened with that increase their propensity for later-life hematological and other immune deficiencies.
Supplementary Material
Supplementary Figure 1. Presence of DP (CD4+CD8+) cells in peripheral blood is an indicator of thymoma metastasis and rationale for specimen exclusion. Flow cytometry plot of blood illustrating normal DP population shown on the left and flow cytometry plot of blood illustrating DP population in excess of 2.5% on the right; Mice possessing blood cell phenotype similar to the right were eliminated from our analysis of based an advanced disease with DP cells indicating presence of a metastatic thymoma.
Supplementary Figure 2. Thymocytes from Notch1ICN-TG mice exhibit a bimodal forward scatter histogram. Thymocytes were isolated from Notch1ICN-TG and C57BL/6 mice, then analyzed on a BD FACS Aria III flow cytometer for forward scatter, a measure of cell size A) representative plot of thymocytes in female mice B) representative plot of thymocytes in male mice; Black histogram represents C57BL/6 mice and red histogram represents Notch1ICN-TG mice.
Supplementary Figure 3. Percentage of apoptotic thymocytes in Notch1ICN-TG mice are not significantly different following developmental exposure to vehicle control or 3μg/kg TCDD. Notch1ICN-TG mice were exposed to 3μg/kg TCDD or vehicle control on GD 0.5, 7.5, 14.5 and PPD 2.5. Thymuses were harvested from 8 week old mice and a TUNEL assay was used to determine percentage of cells undergoing apoptosis, as described below. A) CD4+ thymocytes; B) CD8+ thymocytes; C) Double positive (CD4+CD8+) thymocytes
Apoptosis of Thymocytes via APO-BRDU™ TUNEL Assay. Thymocytes were harvested from Notch1ICN-TG mice developmentally exposed to vehicle control or 3μg/kg TCDD. One million thymocytes per well were incubated overnight in 24 well plates (Corning, NY) at 37°C and 5% CO2 in RPMI (Life Technologies) supplemented with 10 mg/mL gentamycin (Gibco, Grand Island, NY), 10% FBS (Gibco), and 0.5M 2-Mercaptoethanol (EMD Chemicals, Gibbstown, NJ). Thymocytes were then stained for surface markers PE-conjugated CD4 (clone RN4-5) and Alexa647-conjugated CD8 (clone MCD0821) as described above. After a 15 minute fixation with 1% paraformaldehyde, thymocytes were resuspended in 70% EtOH, and frozen at −20°C overnight. A Terminal deoxynucleotide transferase dUTP Nick End Labeling (TUNEL) assay was performed following manufacturer’s protocol outlined in the Phoenix Flow Systems, Inc. APO-BRDU™ Kit (San Diego, CA), eliminating the addition of Propidium Iodide/RNase A Solution. Cells were analyzed on BD FACSAria III as previously described, using FSC-Area by SSC-Area for doublet exclusion, followed by identification of thymocytes by presence of both CD4 and CD8. Anti-BrdU cells were identified within the thymocyte population by FITC fluorescence.
Supplementary Figure 4: CD4 and CD8 cell proportions in thymus are not significantly different following developmental exposure to 3μg/kg TCDD or vehicle control. Pregnant C57BL/6 dams (paired with either C57BL/6 or Notch1ICN-TG males) were exposed to 3μg/kg TCDD or vehicle control throughout gestation (GD0.5, GD7.5, GD14.5, PPD2.5).
A) Representative flow cytometry plots from week 8 thymus samples; B) Ratios of B cells to T cells at 8 weeks and 12 weeks, with C57BL/6 mice on the left and Notch1ICN-TG mice on the right; C) Frequency of viable cells (defined by absence of sytox blue stain) in double positive (CD4+CD8+) quadrant of flow cytometry plots. Vehicle treated mice are represented by a white bar and mice treated with TCDD are represented by a filled bar in B-C. Bars graph mean with SE. (n=3 C57BL/6 vehicle; n=3 C57BL/6 TCDD; n=8 Notch1ICN-TG vehicle; n=7 Notch1ICN-TG TCDD at week 8; n=4 C57BL/6 vehicle; n=5 C57BL/6 TCDD; n=6 Notch1ICN-TG vehicle; n=8 Notch1ICN-TG TCDD at week 12); C57BL/6 mice are pictured on the left. Notch1ICN-TG mice are pictured on the right.
Highlights.
Adult mice exposed to 30μg/Kg TCDD have higher efficiency of CD8 thymocyte generation.
Mice carrying a constitutively active Notch transgene were exposed to 3μg/Kg TCDD throughout development.
Progression of Notch-induced thymoma was different in offspring exposed to TCDD developmentally.
Developmental AHR activation attenuates later-life Notch1 impacts T cell differentiation
Acknowledgments
The authors gratefully acknowledge Dr. Doug Steeber for his critical review of this manuscript; Dr. Berri Forman, Jenny Nemke, and the staff of the Animal Resource Center at the University of Wisconsin-Milwaukee for outstanding care and attention to humane treatment of animals; Darren Almagro for technical assistance with flow cytometry analysis and sorting, and Dr. B.J. Fowlkes at the NIAID for her generous gift of the C57BL/6-Tg(LckNotch1)9E mice used in this study.
FUNDING INFORMATION:
This work was supported by the National Institute of Environmental Health Sciences at the National Institutes of Health [grant number R00ES016585], with partial support from the UW-Milwaukee Children’s Environmental Health Science Center [grant number P30ES004184].
ABBREVIATIONS
- AHR
aryl hydrocarbon receptor
- GSI
gamma-secretase inhibitors
- GD
gestational day
- ICN
intracellular Notch
- MFI
mean fluorescence intensity
- PPD
post-partum day
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
Footnotes
CONFLICT OF INTEREST STATEMENT:
The authors declare that they have no actual or potential competing financial interests.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahrenhoerster LS, Tate ER, Lakatos PA, Wang X, Laiosa MD. Developmental exposure to 2,3,7,8 tetrachlorodibenzo-p-dioxin attenuates capacity of hematopoietic stem cells to undergo lymphocyte differentiation. Toxicol Appl Pharmacol. 2014;277:172–182. doi: 10.1016/j.taap.2014.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MS, Maekawa Y, Kitamura A, Tanigaki K, Yoshimoto T, Kishihara K, Yasutomo K. Notch signaling drives IL-22 secretion in CD4+ T cells by stimulating the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2010;107:5943–5948. doi: 10.1073/pnas.0911755107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AVMA. AVMA Guidelines for the Euthanasia of Animals. American Veterinary Medical Association; Schaumburg, IL: 2013. [Google Scholar]
- Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Beres TM, Masui T, Swift GH, Shi L, Henke RM, MacDonald RJ. PTF1 is an organ-specific and Notch-independent basic helix-loop-helix complex containing the mammalian Suppressor of Hairless (RBP-J) or its paralogue, RBP-L. Mol Cell Biol. 2006;26:117–130. doi: 10.1128/MCB.26.1.117-130.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly LJ, Capobianco AJ. Perturbation of Ikaros isoform selection by MLV integration is a cooperative event in Notch(IC)-induced T cell leukemogenesis. Cancer Cell. 2003;3:551–564. doi: 10.1016/s1535-6108(03)00137-5. [DOI] [PubMed] [Google Scholar]
- Bigas A, Espinosa L. Hematopoietic stem cells: to be or Notch to be. Blood. 2012 doi: 10.1182/blood-2011-10-355826. [DOI] [PubMed] [Google Scholar]
- Bock KW. The human Ah receptor: hints from dioxin toxicities to deregulated target genes and physiologic functions. Biol Chem. 2013 doi: 10.1515/hsz-2012-0340. [DOI] [PubMed] [Google Scholar]
- Busbee PB, Rouse M, Nagarkatti M, Nagarkatti PS. Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutr Rev. 2013;71:353–369. doi: 10.1111/nure.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consonni D, Pesatori AC, Zocchetti C, Sindaco R, D’Oro LC, Rubagotti M, Bertazzi PA. Mortality in a population exposed to dioxin after the Seveso, Italy, accident in 1976: 25 years of follow-up. Am J Epidemiol. 2008;167:847–858. doi: 10.1093/aje/kwm371. [DOI] [PubMed] [Google Scholar]
- Corces-Zimmerman MR, Majeti R. Pre-leukemic evolution of hematopoietic stem cells - the importance of early mutations in leukemogenesis. Leukemia. 2014 doi: 10.1038/leu.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meglio P, Duarte JH, Ahlfors H, Owens NDL, Li Y, Federica Villanova Tosi I, Hirota K, Nestle FO, Mrowietz U, Gilchrist MJ, Stockinger B. Activation of the Aryl Hydrocarbon Receptor Dampens the Severity of Inflammatory Skin Conditions. Immunity. 2014 doi: 10.1016/j.immuni.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietert RR. Developmental immunotoxicology: focus on health risks. Chem Res Toxicol. 2009;22:17–23. doi: 10.1021/tx800198m. [DOI] [PubMed] [Google Scholar]
- Dietert RR, DeWitt JC, Germolec DR, Zelikoff JT. Breaking patterns of environmentally influenced disease for health risk reduction: immune perspectives. Environ Health Perspect. 2010;118:1091–1099. doi: 10.1289/ehp.1001971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan JM, Okonta H, Elnaggar D, French J, West R, Chakraborty J. Retrovirus-induced lymphomagenesis: a correlation between disease pathogenesis and flow cytometric analysis. J Gen Virol. 2012;93:2028–2036. doi: 10.1099/vir.0.043661-0. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Warner M, Marks AR, Samuels S, Needham L, Brambilla P, Mocarelli P. Serum dioxin concentrations and time to pregnancy. Epidemiol Camb Mass. 2010;21:224–231. doi: 10.1097/EDE.0b013e3181cb8b95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas de St Groth B. Regulatory T-cell function: when suppressor cells can’t suppress. Immunol Cell Biol. 2007;85:179–181. doi: 10.1038/sj.icb.7100052. [DOI] [PubMed] [Google Scholar]
- Fowlkes BJ, Robey EA. A reassessment of the effect of activated Notch1 on CD4 and CD8 T cell development. J Immunol. 2002;169:1817–1821. doi: 10.4049/jimmunol.169.4.1817. [DOI] [PubMed] [Google Scholar]
- Gasiewicz TA, Geiger LE, Rucci G, Neal RA. Distribution, excretion, and metabolism of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J, DBA/2J, and B6D2F1/J mice. Drug Metab Dispos Biol Fate Chem. 1983;11:397–403. [PubMed] [Google Scholar]
- Germain RN. T-cell development and the CD4 CD8 lineage decision. Nat Rev Immunol. 2002;2:309–322. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- Gill BC, Jeon CH, Sung HN, Kim HL, Jin DW, Park JH. 2,3,7,8-Tetrachlorodibenzo-p-dioxin modulates the expression of cKrox and Runx3, transcription regulatory factors controlling the lineage commitment of CD4+CD8+ into CD4 and CD8 thymocytes, respectively. Toxicol Lett. 2008;180:189–195. doi: 10.1016/j.toxlet.2008.06.856. [DOI] [PubMed] [Google Scholar]
- Gluckman Peter D, Hanson Mark A, Pinal Catherine. The developmental origins of adult disease. Matern Child Nutr. 2005;1:130–141. doi: 10.1111/j.1740-8709.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez Tejada FN, Galvez Silva JR, Zweidler-McKay PA. The challenge of targeting notch in hematologic malignancies. Front Pediatr. 2014;2:54. doi: 10.3389/fped.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogaboam JP, Moore AJ, Lawrence BP. The aryl hydrocarbon receptor affects distinct tissue compartments during ontogeny of the immune system. Toxicol Sci. 2007;102:160–170. doi: 10.1093/toxsci/kfm283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlader N, Noone A, Krapcho M, Garshell J, Miller D, Altekruse S, Kosary C, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis D, Chen H, Feuer E, Cronin K. SEER Cancer Statistics Review, 1975–2011. National Cancer Institute. National Cancer Institute; Bethesda, MD: 2013. [Google Scholar]
- Ikeda T, Morimoto A, Nakamura S, Yokoyama K, Hayase T, Oh Y, Kashii Y, Yotsumoto S, Okamoto H, Momoi YM. A marked decrease in CD4-positive lymphocytes at the onset of hepatitis in a patient with hepatitis-associated aplastic anemia. J Pediatr Hematol Oncol. 2012;34:375–377. doi: 10.1097/MPH.0b013e31822bf699. [DOI] [PubMed] [Google Scholar]
- Jacobson DL, Gange SJ, Rose NR, Graham NM. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol. 1997;84:223–243. doi: 10.1006/clin.1997.4412. [DOI] [PubMed] [Google Scholar]
- Kerkvliet NI. AHR-mediated immunomodulation: the role of altered gene transcription. Biochem Pharmacol. 2009;77:746–760. doi: 10.1016/j.bcp.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss EA, Diefenbach A. Role of the Aryl Hydrocarbon Receptor in Controlling Maintenance and Functional Programs of RORγt+ Innate Lymphoid Cells and Intraepithelial Lymphocytes. Front Immunol. 2012;3 doi: 10.3389/fimmu.2012.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer J, Lai ZW, Esser C. Evidence for the promotion of positive selection of thymocytes by Ah receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin. Eur J Pharmacol. 1995;293:413–427. doi: 10.1016/0926-6917(95)90062-4. [DOI] [PubMed] [Google Scholar]
- Laiosa MD, Lai ZW, Thurmond TS, Fiore NC, DeRossi C, Holdener BC, Gasiewicz TA, Silverstone AE. 2,3,7,8-tetrachlorodibenzo-p-dioxin causes alterations in lymphocyte development and thymic atrophy in hemopoietic chimeras generated from mice deficient in ARNT2. Toxicol Sci Off J Soc Toxicol. 2002;69:117–124. doi: 10.1093/toxsci/69.1.117. [DOI] [PubMed] [Google Scholar]
- Laiosa MD, Wyman A, Murante FG, Fiore NC, Staples JE, Gasiewicz TA, Silverstone AE. Cell proliferation arrest within intrathymic lymphocyte progenitor cells causes thymic atrophy mediated by the aryl hydrocarbon receptor. J Immunol Baltim Md. 2003;1950–171:4582–4591. doi: 10.4049/jimmunol.171.9.4582. [DOI] [PubMed] [Google Scholar]
- Laiosa MD, Eckles KE, Langdon M, Rosenspire AJ, McCabe MJ. Exposure to inorganic mercury in vivo attenuates extrinsic apoptotic signaling in Staphylococcal aureus Enterotoxin B stimulated T-cells. Toxicol Appl Pharmacol. 2007;225:229–336. doi: 10.1016/j.taap.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustfeld I, Altvater B, Ahlmann M, Ligges S, Brinkrolf P, Rosemann A, Moericke A, Rossig C. High proportions of CD4+ T cells among residual bone marrow T cells in childhood acute lymphoblastic leukemia are associated with favorable early responses. Acta Haematol. 2014;131:28–36. doi: 10.1159/000351429. [DOI] [PubMed] [Google Scholar]
- Miniero R, De Felip E, Ferri F, di Domenico A. An overview of TCDD half-life in mammals and its correlation to body weight. Chemosphere. 2001;43:839–844. doi: 10.1016/s0045-6535(00)00442-2. [DOI] [PubMed] [Google Scholar]
- Mulero-Navarro S, Carvajal-Gonzalez JM, Herranz M, Ballestar E, Fraga MF, Ropero S, Esteller M, Fernandez-Salguero PM. The dioxin receptor is silenced by promoter hypermethylation in human acute lymphoblastic leukemia through inhibition of Sp1 binding. Carcinogenesis. 2006;27:1099–1104. doi: 10.1093/carcin/bgi344. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. National Academies Press; Washington, D.C: 2011. [PubMed] [Google Scholar]
- Osborne BA, Minter LM. Notch signalling during peripheral T-cell activation and differentiation. Nat Rev Immunol. 2007;7:64–75. doi: 10.1038/nri1998. [DOI] [PubMed] [Google Scholar]
- Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol. 2011;31:363–373. doi: 10.1016/j.reprotox.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl A. Systems biology of lupus: mapping the impact of genomic and environmental factors on gene expression signatures, cellular signaling, metabolic pathways, hormonal and cytokine imbalance, and selecting targets for treatment. Autoimmunity. 2010;43:32–47. doi: 10.3109/08916930903374774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey E, Chang D, Itano A, Cado D, Alexander H, Lans D, Weinmaster G, Salmon P. An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. Cell. 1996;87:483–492. doi: 10.1016/s0092-8674(00)81368-9. [DOI] [PubMed] [Google Scholar]
- Schmitt TM, Zúñiga-Pflücker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- Schug TT, Barouki R, Gluckman P, Grandjean P, Hanson M, Heindel JJ. PPTOX III: Environmental Stressors in the Developmental Origins of Disease: Evidence and Mechanisms. Toxicol Sci Off J Soc Toxicol. 2012 doi: 10.1093/toxsci/kfs267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL, Ferre AL, Hayes TL, Somsouk M, Hsue PY, Van Natta ML, Meinert CL, Lederman MM, Hatano H, Jain V, Huang Y, Hecht FM, Martin JN, McCune JM, Moreno S, Deeks SG. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014;10:e1004078. doi: 10.1371/journal.ppat.1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127:299–311. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe N, Nguyen D, Yang SX. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther. 2014;141:140–149. doi: 10.1016/j.pharmthera.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S, Minh TB. Dioxins and organohalogen contaminants in the Asia-Pacific region. Ecotoxicol Lond Engl. 2010;19:463–478. doi: 10.1007/s10646-009-0445-8. [DOI] [PubMed] [Google Scholar]
- Terauchi H, Takahashi S, Lam PKS, Min B-Y, Tanabe S. Polybrominated, polychlorinated and monobromo-polychlorinated dibenzo-p-dioxins/dibenzofurans and dioxin-like polychlorinated biphenyls in marine surface sediments from Hong Kong and Korea. Environ Pollut Barking Essex. 2009;1987–157:724–730. doi: 10.1016/j.envpol.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Tue NM, Takahashi S, Subramanian A, Sakai S, Tanabe S. Environmental contamination and human exposure to dioxin-related compounds in e-waste recycling sites of developing countries. Environ Sci Process Impacts. 2013;15:1326–1331. doi: 10.1039/c3em00086a. [DOI] [PubMed] [Google Scholar]
- Van Vlierberghe P, Ferrando A. The molecular basis of T cell acute lymphoblastic leukemia. J Clin Invest. 2012;122:3398–3406. doi: 10.1172/JCI61269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorderstrasse BA, Cundiff JA, Lawrence BP. Developmental exposure to the potent aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin Impairs the cell-mediated immune response to infection with influenza a virus, but enhances elements of innate immunity. J Immunotoxicol. 2004;1:103–112. doi: 10.1080/15476910490509244. [DOI] [PubMed] [Google Scholar]
- Weber H, Birnbaum LS. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and 2,3,7,8-tetrachlorodibenzofuran (TCDF) in pregnant C57BL/6N mice: distribution to the embryo and excretion. Arch Toxicol. 1985;57:159–162. doi: 10.1007/BF00290880. [DOI] [PubMed] [Google Scholar]
- Weng AP, Ferrando AA, Lee W, Morris JP, 4th, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- Yang M, Busche G, Ganser A, Li Z. Morphology and quantitative composition of hematopoietic cells in murine bone marrow and spleen of healthy subjects. Ann Hematol. 2013;92:587–594. doi: 10.1007/s00277-012-1653-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hua C, Cheng H, Wang W, Hao S, Xu J, Wang X, Gao Y, Zhu X, Cheng T, Yuan W. Distinct sensitivity of CD8+ CD4- and CD8+ CD4+ leukemic cell subpopulations to cyclophosphamide and rapamycin in Notch1-induced T-ALL mouse model. Leuk Res. 2013;37:1592–1601. doi: 10.1016/j.leukres.2013.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Presence of DP (CD4+CD8+) cells in peripheral blood is an indicator of thymoma metastasis and rationale for specimen exclusion. Flow cytometry plot of blood illustrating normal DP population shown on the left and flow cytometry plot of blood illustrating DP population in excess of 2.5% on the right; Mice possessing blood cell phenotype similar to the right were eliminated from our analysis of based an advanced disease with DP cells indicating presence of a metastatic thymoma.
Supplementary Figure 2. Thymocytes from Notch1ICN-TG mice exhibit a bimodal forward scatter histogram. Thymocytes were isolated from Notch1ICN-TG and C57BL/6 mice, then analyzed on a BD FACS Aria III flow cytometer for forward scatter, a measure of cell size A) representative plot of thymocytes in female mice B) representative plot of thymocytes in male mice; Black histogram represents C57BL/6 mice and red histogram represents Notch1ICN-TG mice.
Supplementary Figure 3. Percentage of apoptotic thymocytes in Notch1ICN-TG mice are not significantly different following developmental exposure to vehicle control or 3μg/kg TCDD. Notch1ICN-TG mice were exposed to 3μg/kg TCDD or vehicle control on GD 0.5, 7.5, 14.5 and PPD 2.5. Thymuses were harvested from 8 week old mice and a TUNEL assay was used to determine percentage of cells undergoing apoptosis, as described below. A) CD4+ thymocytes; B) CD8+ thymocytes; C) Double positive (CD4+CD8+) thymocytes
Apoptosis of Thymocytes via APO-BRDU™ TUNEL Assay. Thymocytes were harvested from Notch1ICN-TG mice developmentally exposed to vehicle control or 3μg/kg TCDD. One million thymocytes per well were incubated overnight in 24 well plates (Corning, NY) at 37°C and 5% CO2 in RPMI (Life Technologies) supplemented with 10 mg/mL gentamycin (Gibco, Grand Island, NY), 10% FBS (Gibco), and 0.5M 2-Mercaptoethanol (EMD Chemicals, Gibbstown, NJ). Thymocytes were then stained for surface markers PE-conjugated CD4 (clone RN4-5) and Alexa647-conjugated CD8 (clone MCD0821) as described above. After a 15 minute fixation with 1% paraformaldehyde, thymocytes were resuspended in 70% EtOH, and frozen at −20°C overnight. A Terminal deoxynucleotide transferase dUTP Nick End Labeling (TUNEL) assay was performed following manufacturer’s protocol outlined in the Phoenix Flow Systems, Inc. APO-BRDU™ Kit (San Diego, CA), eliminating the addition of Propidium Iodide/RNase A Solution. Cells were analyzed on BD FACSAria III as previously described, using FSC-Area by SSC-Area for doublet exclusion, followed by identification of thymocytes by presence of both CD4 and CD8. Anti-BrdU cells were identified within the thymocyte population by FITC fluorescence.
Supplementary Figure 4: CD4 and CD8 cell proportions in thymus are not significantly different following developmental exposure to 3μg/kg TCDD or vehicle control. Pregnant C57BL/6 dams (paired with either C57BL/6 or Notch1ICN-TG males) were exposed to 3μg/kg TCDD or vehicle control throughout gestation (GD0.5, GD7.5, GD14.5, PPD2.5).
A) Representative flow cytometry plots from week 8 thymus samples; B) Ratios of B cells to T cells at 8 weeks and 12 weeks, with C57BL/6 mice on the left and Notch1ICN-TG mice on the right; C) Frequency of viable cells (defined by absence of sytox blue stain) in double positive (CD4+CD8+) quadrant of flow cytometry plots. Vehicle treated mice are represented by a white bar and mice treated with TCDD are represented by a filled bar in B-C. Bars graph mean with SE. (n=3 C57BL/6 vehicle; n=3 C57BL/6 TCDD; n=8 Notch1ICN-TG vehicle; n=7 Notch1ICN-TG TCDD at week 8; n=4 C57BL/6 vehicle; n=5 C57BL/6 TCDD; n=6 Notch1ICN-TG vehicle; n=8 Notch1ICN-TG TCDD at week 12); C57BL/6 mice are pictured on the left. Notch1ICN-TG mice are pictured on the right.