Abstract
Objectives
Market integration is an important source of cultural change exposing indigenous populations to epidemiologic and nutrition transitions. As children and adolescents are biologically sensitive to the health effects of market integration, we examine community variation of anthropometric indicators of nutritional status and growth among a cross-cultural sample of Kichwa, Shuar, Huaorani and Cofán indigenous groups in the northern Ecuadorian Amazon.
Methods
We measured height, weight, body mass index (BMI), upper arm circumference and triceps skinfolds of 186 children and adolescents aged two to 18 years from seven communities. Anthropometric z-scores were calculated based on the National Health and Nutritional Examination Survey. Comparisons were made to this US reference group, along with between community differences to contextually explore the impacts of varying degrees of market integration.
Results
We found a high prevalence of stunting in both boys (40%) and girls (34%). Adiposity increased with age and 40% of girls between 15 and 18 years old were overweight. There were large sex differences in body composition with higher BMI, arm circumference and triceps skinfolds in adolescent girls. The Kichwa demonstrated the poorest growth outcomes and nutritional stress followed by the Huaorani and Shuar; yet distinctions in under- and over-nutrition were evident within groups.
Conclusion
Market integration is a major factor influencing the developmental and lifestyle mismatch associated with the epidemiologic and nutrition transition in general, and the dual burden pattern of high rates of stunting yet adequate to above average short-term nutritional status indicators found among indigenous Amazonian populations.
Keywords: indigenous health, stunting, overweight, nutrition transition
INTRODUCTION
The Ecuadorian Amazon is experiencing dramatic environmental, ecological and demographic transformations. Intensifying resource extraction, including oil production and logging, has led to the development of transportation networks, igniting urbanization, migration, and colonization of the jungle with important health consequences (San Sebastián and Karin Hurtig, 2004). Although some indigenous Amazon communities are now accessible by roads and have markets and small medical clinics, many lack clean drinking water and sanitation infrastructures. The development of the physical environment along with economic growth is rapidly changing disease patterns among people living in the Amazon (Kuang-Yao Pan et al., 2010). There is increasing evidence of epidemiologic polarization characterized by a high incidence of cardiovascular and metabolic diseases in adults coupled with a heavy burden of chronic undernutrition and communicable disease in children (Waters, 2006). At the same time, Ecuador is undergoing a nutrition transition with a shift from low-calorie plant-based foods to a diet high in processed foods, refined sugars, and saturated fats, resulting in increasing rates of obesity and cardiometabolic disorders in adults (Bernstein, 2008; Popkin, 1998). These complex patterns of disease, accompanied by simultaneous under- and over-nutrition, are shaped by social and economic forces influencing lifestyle changes and integration into market economies.
Chronic childhood undernutrition continues to be a major health concern in Ecuador and is strongly associated with economic disparities, access to water and sanitation infrastructures, and lifestyle patterns of indigenous groups (Larrea and Freire, 2002; PAHO, 2007; Walker, 2007). In 2004, 46.6% of indigenous Ecuadorian children under five years old experienced chronic malnutrition resulting in growth faltering and stunting (Walker, 2007). Indigenous populations experience higher rates of respiratory infections, gastrointestinal illnesses and vector-borne diseases compared to their non-indigenous counterparts living in the Ecuadorian Amazon (Kuang-Yao Pan et al. , 2010). The degree of market integration and acculturation significantly impacted nutritional and growth status among indigenous children living in the Southern Ecuadorian Amazon (Blackwell et al., 2009). These factors are similar to the social and economic effects influencing childhood dietary and growth patterns in other regions of Ecuador (Leonard et al., 2000; Leonard et al., 1990) and in other indigenous groups in the Amazon (Benefice et al., 2007; Byron, 2003; Foster et al., 2005; Godoy et al., 2010; Godoy et al., 2005; Piperata et al., 2011a). This paper contributes to the literature on growth and nutrition of populations experiencing epidemiological and nutrition transitions through a cross-cultural comparison of indigenous groups in the Northern Ecuadorian Amazon, and examination of variation between ethnicities and communities with different degrees of market integration.
Market integration is an important pathway linking the effects of the broader social, economic and environmental changes associated with the epidemiological and nutrition transitions occurring in the Amazon, to impacts on childhood health and nutritional status. Previous studies have documented the complexity of evaluating market integration and its mixed beneficial and deleterious effects cross-culturally (Godoy et al. , 2005), emphasizing the need to include multiple measures of both household production and consumption activities (Lu, 2007), along with accounting for other related confounders such as acculturation (Godoy and Cardenas, 2000). To better understand how these factors impact indigenous health and well-being, this paper provides an ecological analysis of variation in growth and nutritional status of children and adolescents from a cross-cultural sample of seven communities representing four indigenous populations living in the Ecuadorian Amazon. These communities and ethnicities have distinct characteristics, such as location, access to roads, historical trajectories and cultural differences, which provide meaningful variation in market opportunities and strategies that may affect childhood health and nutrition (Lu, 2007). The strength of a group-level analysis is that it allows us to explore the variability between ethnic groups and communities, examining market integration as a contextual factor.
As children are particularly biologically sensitive to the health effects of social and economic change, we examine anthropometric measures of body size and physical status as indicators of chronic mild-to-moderate undernutrition and burden of infection. The objective of this study is to explore variation in growth and nutritional status among the entire indigenous sample and between communities and ethnicities to examine the possible effects of different degrees of market integration. We propose that market integration influences disease loads through increased sedentism and sanitation issues, leading to poor nutritional status and growth outcomes. Concurrently, market integration influences short-term nutritional status and dietary changes with decreases in traditional foods and an increasing reliance on market foods with lower diversity, poor nutritional quality and higher caloric and fat intake. This causes a dual burden of both under- and over-nutrition, resulting in stunted stature yet normal to above normal measures of weight and adiposity, characteristics of populations undergoing nutrition transitions (Popkin et al., 1996; Sawaya et al., 1998). Lastly, we explore potential biological pathways linking early life under- and over-nutrition and risk for obesity in early adulthood.
DATA AND METHODS
Study sample
This paper draws on a sample of anthropometric assessments and market surveys from an NSF-funded project (BCS 0822967) investigating the health effects of market integration in a cross-cultural study of indigenous groups in the Northern Ecuadorian Amazon. This is a region of high biodiversity and endemism currently undergoing rapid rates of deforestation (FAO, 2005). It is also culturally diverse, home to the Shuar, Achuar, lowland Kichwa, Cofán, Siona, Secoya, Záparo, Shiwiar, Andoa, and Huaorani. The Kichwa and Shuar together represent about 85% of the total indigenous Amazonian population (Hicks et al., 1990). We collected data from four groups: the lowland Kichwa (also known as Quichua or Runa), Shuar, Cofán (also known as Kofán or A'i), and Huaorani (also known as Waorani or Waodani) living in the provinces of Sucumbios, Napo, and Orellana in the Northern Ecuadorian Amazon. These groups vary in population size and density, linguistic affiliation, history of contact, economic activities, and socio-cultural patterns (Lu et al., 2012). The communities of Gareno and Quehuereono (also spelled Quehueiri-ono) belong to the Huaorani indigenous group. Zábalo is a Cofán community and Tiguano is a Shuar community. Pachacutik, Pastaza, and Pilchi are Kichwa communities. A total of 186 participants ranging in age from two to 18 years were analyzed for this paper. Table 1 displays participant counts and coverage of households and children and adolescents in the market and anthropometric surveys by community. The study was approved by the Office of Human Research Ethics at the University of North Carolina at Chapel Hill, as well as by the indigenous federations and study communities.
TABLE 1.
Counts and coverage of households, household-days and children and adolescents in the market and anthropometric surveys
| Community | Gareno | Quehuereono | Zabalo | Pachacutik | Pastaza | Pilchi | Tiguano | Total |
|---|---|---|---|---|---|---|---|---|
| Total Households in Community | 43 | 15 | 33 | 19 | 14 | 25 | 25 | 174 |
| Households in Market Survey | 16 | 10 | 21 | 15 | 12 | 17 | 22 | 113 |
| Coverage of Households in Market Survey | 37% | 67% | 64% | 79% | 86% | 68% | 88% | 65% |
| Mean Household-Days per Household in Market Survey | 33 | 31 | 79 | 67 | 114 | 45 | 88 | 67 |
| Sum of Household-Days in Market Survey | 529 | 314 | 1652 | 1007 | 1364 | 768 | 1943 | 7577 |
| Number of Children and Adolescents in the Anthropometric Survey | 29 | 20 | 38 | 21 | 28 | 26 | 24 | 186 |
| Coverage of Children and Adolescents in Market Survey | 66% | 50% | 92% | 86% | 86% | 65% | 83% | 77% |
The Kichwa are the most numerous of Ecuador's Native Amazonian peoples, with an estimated 60,000 people in Sucumbios, Orellana, Napo, and Pastaza provinces (Irvine, 2000). Like the Shuar, they have a long history of contact with outsiders; indeed, the lowland Amazonian Kichwa first emerged as a distinct ethnic group when pre-existing indigenous societies were decimated by disease, violence, and social disruption during the Spanish conquest. Under the violence and repression suffered at the hands of the Spanish, survivors from these different ethnic groups decided or were obliged to live in mission villages where Quichua, an Andean language, served as a lingua franca. A shared Quichua ethnic identity is thought to have emerged around 1800. The Kichwa communities of Pachacutik and Pastaza in this study have road access and bus travel connecting them to major markets in Lago Agrio, yet Pilchi remains only accessible by boat through the port of Coca. At the time of the study for the Kichwa communities, limited electricity was available by community-owned solar panels.
The Shuar are members of the Jivaroan language group concentrated near the Peru/Ecuadorian border. Numbering about 40,000 persons, they are the second largest indigenous population in the Ecuadorian Amazon. The Shuar history of contact with outsiders began with Catholic priests in the early 20th century. Partly as an attempt to protect their lands against colonist incursions, they have adopted cattle production, which requires clearing large areas of forest, to secure land claims. The Shuar have also reorganized themselves from living in dispersed households to forming nucleated centros or communities on 3,000-6,000 hectare tracts (Rudel et al., 2002). Our study includes such a migrant Shuar community, Tiguano, in Orellana province, about three hours by vehicle south of Coca. This group is not necessarily representative of the larger Shuar population, but is probably similar to other Shuar population groups in the study region.
The Cofán traditionally occupied the area between the San Miguel River, the Guamuez River, the Bajo Putumayo River and the Aguarico in southern Colombia and northern Ecuador. Their origin is unknown, some believing the A’i language to be unique, while others think it belongs to the linguistic Chibcha family of Colombia (Califano and Gonzalo, 1995; Cerón, 1995). Negatively impacted by outsiders at the end of 19th century during the rubber and quinine booms, the Cofán again suffered beginning about 1970 when they were displaced by petroleum extraction. They were forced to move from the region around Lago Agrio (where significant oil deposits were first discovered in the Ecuadorian Amazon in 1967) to form scattered settlements farther east, deeper in the forest. Currently, the Cofán of Ecuador number approximately 500 persons in five communities. The Cofán community of Zábalo is distant from market towns and only accessible by a four-hour boat ride down the river Aguarico outside of Shushufindi, near the Cuyabeno Reserve. During the time of the study, the community was involved in ecotourism and other wildlife monitoring programs.
From a population numbering only about 500 at the time of missionary contact, the Huaorani now number around 2,000 persons (Beckerman et al., 2009). Their language, Huao Tededo, is a linguistic isolate, and their reputation for warfare and spearing raids allowed them to occupy and claim a large territory bordered on the north by the Napo River and on the south by the Curaray River (approximately 20,000 square kilometers) before sustained contact. Currently, they are distributed among five dozen communities in Huaorani Territory (6,786 km2) and Yasuní National Park (6,797 km2). Many of these villages lie along navigable rivers, some are found alongside roads including Gareno, and others, like Quehuereono are more distant and accessible only by foot or landing strip. The Huaorani communities in this study maintain access to relatively abundant land and resources, compared to the other indigenous communities with the exception of Zábalo. During the time of this study, Quehuereono was actively involved in ecotourism and oil companies near Gareno offered residents unskilled wage labor employment.
Market integration
Previous fieldwork in 2001 (Lu, 2007), indicated that Shuar were the most integrated into market activities, relying on wage labor and cash cropping, followed by the Kichwa, which were predominately involved in producing agricultural products for market sale. The Cofán and Huaorani were the least market oriented in terms of participating in wage labor or cash cropping, instead focusing on the sale of wild game and forest products as handicrafts. Unfortunately, market integration data from 2001 is not available for all of the communities in our study.
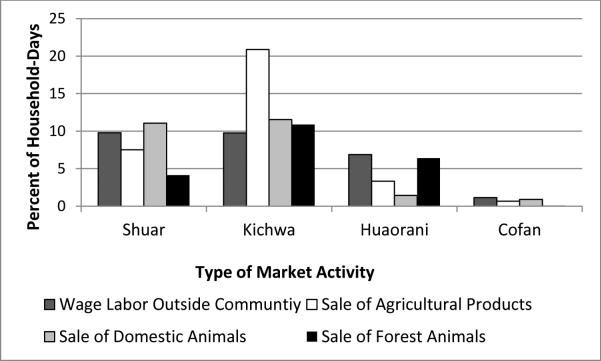
To contextually explore differences in nutritional status and growth between groups, we constructed four market integration measures from questions in the household input-output diaries collected as part of the market survey. Household input-output diaries were collected simultaneously in all communities from January to August of 2009. One hundred and thirteen households participated in the market survey, with total coverage at 65% (Table 1). Households completed input-output diaries that recorded their daily subsistence activities including the purchase and sale of market products. On average, households submitted 67 daily input-output diaries resulting in a total of 7,577 household-days recorded (Table 1). We calculated the percent of household-days reporting participation in the following activities by ethnicity: wage labor outside of the community, sale of agricultural products, sale of domestic animals and the sale of forest animals (Fig. 1).
Fig. 1.
Percent of household-days participating in market activities by ethnicity.
The Shuar continue to be strongly oriented in market activities, with 9.8% of household-days participating in wage labor, followed closely by the Kichwa with 9.7% of household-days participating in wage labor. For the Kichwa, cash cropping continues to be an important economic activity with 20.9% of household-days participating in the sale of agriculture products compared to only 7.5% of household-days among the Shuar. The Huaorani reported 6.9% of household-days participating in wage labor followed by only 1.2% among the Cofán. Sale of forest products remains a central source of income for the Huaorani, with 6.4% of household-days participating in the sale of wild game. The Cofán remain the least orientated towards market activities report less that 1% of household-days participating in sale of agricultural or animal products. Based on the previous fieldwork and preliminary examination of production measures of market activities, we hypothesize that community-level differences will vary by degree of market integration, with the Shuar and Kichwa communities experiencing undernutrition and poor growth outcomes compared to the Huaorani and Cofán communities.
Anthropometric measures and analyses
Anthropometric assessments were conducted in all the communities between April and June of 2009, except in Gareno, which was a late addition to the study and where data were collected in November 2009. It is possible that nutritional status in Gareno may have been impacted by seasonal variation; however, rainfall in the lowlands follows a bimodal distribution with peaks in march-April and also in October, thus data collection occurred at the end of the wet and through early dry seasons in all communities. One hundred and eighty-six children and adolescents participated in the anthropometric assessment, with 77% coming from households that participated in the market survey (Table 1). Anthropometric measurements were taken using standard techniques (Lohman et al., 1988; WHO, 1995). Height was measured to the nearest 1 mm using a Seca 214 portable stadiometer or Seca 417 infantometer (Seca Corporation, Hanover, MD) and weight was measured to the nearest 100 g using a Seca standing spring scale. Upper-arm circumference was measured to the nearest 1 mm using a Teflon measuring tape and triceps skinfolds were measured to the nearest 0.5 mm using a Lange skinfold caliper (Beta Technology, Santa Cruz, CA). The body mass index (BMI) was calculated from height and weight measures using the formula of mass(kg)/height(m2). We use the United States’ National Health and Nutrition Examination Survey (NHANES) reference data to create sex- and age-specific anthropometric z-scores for all measures and plot growth curves for height, weight and BMI using calculations presented by Frisancho (2008). A height-for-age z-score at or below −2 indicates stunting, or chronic malnutrition (Frisancho, 2008). Underweight is indicated by a weight-for-age z-score at or below −2. Overweight is represented by a BMI-for-age z-score at or above +1 (de Onis and Lobstein, 2010). Low upper arm circumference and low triceps skinfold are indicated by their respective z-score at or below −2 (Frisancho, 2008).
The raw mean z-score and prevalence for each nutritional status indicator was summarized by sex for the entire sample and by community. Since these indicators of nutritional status are based on a standard reference population, comparisons are possible and variations should reflect differences in market integration as indicators of local change in nutritional and disease conditions. We performed student t-tests on z-scores by sex to assess whether mean measures were significantly different from the US median. Analysis of covariance (ANCOVA) of mean anthropometric z-scores were used to identify independent effects of age and sex, as well as their interaction.
RESULTS
Growth and body composition in children and adolescents
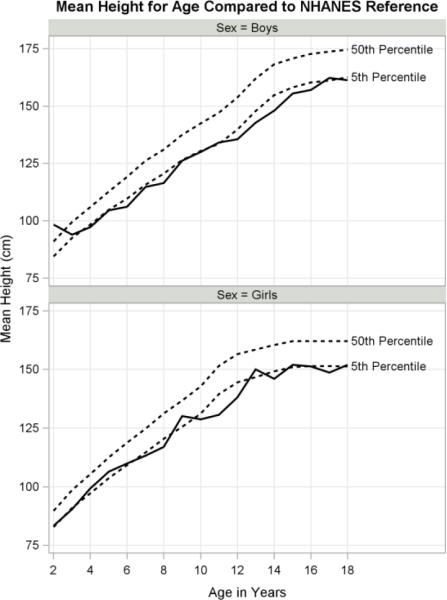
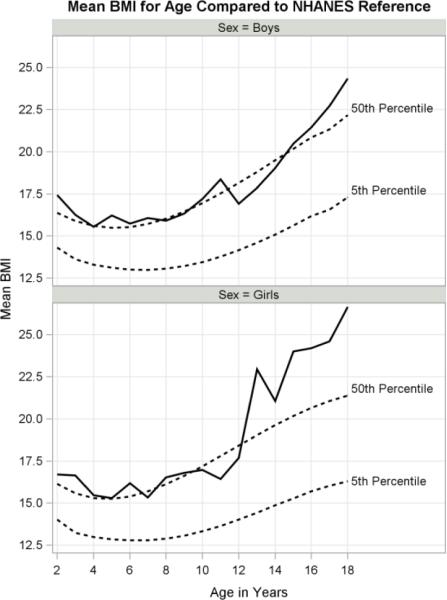
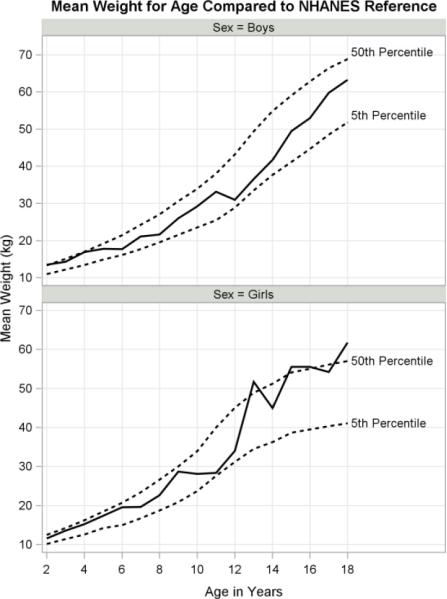
The main findings are summarized by age categories in Table 2. Growth curves for height, weight and BMI are presented in Figures 2 to 4 along with the 5th and 50th US percentiles (Frisancho, 2008). Height roughly follows the 5th percentile falling below in girls between ages 10 to 13 and below in boys between ages 12 to 16 (Fig. 2). Weight falls between the 5th and 50th percentiles for both boys and girls prior to age 12, with weight for girls exceeding the 50th percentile at ages 13, 15 and 18 (Fig. 3). BMI approximates the US median through age 12, after which girls show a striking increase (Fig. 4). Boys steadily continue to rise above the 50th percentile after age 14. Although both girls and boys make substantial gains in BMI starting around age 12, girls far exceed the medians of their US counterparts.
TABLE 2.
Mean anthropometric measures by sex and age group for the entire sample
| Height (cm) | Weight (kg) | BMI (kg/m2) | Arm Circumference (mm) | Triceps Skinfold (mm) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) |
| Girls | 2 to 4 | 24 | 92.6 (8.5) | 24 | 13.9 (2.3) | 24 | 16.2 (1.5) | 20 | 16.5 (1.6) | 13 | 7.7 (2.6) |
| 5 to 9 | 33 | 115.7 (9.6) | 33 | 21.8 (5.0) | 33 | 16.1 (1.3) | 32 | 18.3 (2.2) | 24 | 8.8 (3.0) | |
| 10 to 14 | 25 | 136.9 (9.6) | 25 | 35.3 (9.6) | 25 | 18.5 (2.6) | 25 | 22.0 (2.8) | 21 | 10.5 (4.5) | |
| 15 to 18 | 15 | 150.9 (4.4) | 15 | 57.3 (8.7) | 15 | 25.1 (3.4) | 15 | 27.9 (3.0) | 15 | 15.6 (4.1) | |
| All Girls | 97 | 120.9 (22.2) | 97 | 28.8 (15.9) | 97 | 18.1 (3.8) | 92 | 20.5 (4.5) | 73 | 10.5 (4.6) | |
| Boys | 2 to 4 | 17 | 96.0 (6.0) | 17 | 15.4 (4.2) | 15 | 16 .0 (1.3) | 17 | 16.2 (1.1) | 12 | 6.8 (2.1) |
| 5 to 9 | 34 | 115.5 (8.5) | 34 | 21.6 (3.7) | 34 | 16.1 (1.2) | 34 | 18.1 (1.7) | 27 | 5.9 (1.5) | |
| 10 to 14 | 26 | 136.5 (7.8) | 26 | 33.4 (5.6) | 26 | 17.8 (1.3) | 26 | 20.9 (1.9) | 23 | 6.5 (1.8) | |
| 15 to 18 | 12 | 159.6 (4.7) | 12 | 57.2 (6.3) | 12 | 22.4 (1.9) | 12 | 27.4 (1.7) | 12 | 7.8 (2.6) | |
| All Boys | 89 | 123.9 (21.3) | 89 | 28.7 (13.9) | 87 | 17.5 (2.5) | 89 | 19.8 (3.8) | 74 | 6.6 (2.0) | |
Fig 2.
Height growth curves for boys and girls with NHANES reference of 5th and 50th percentiles.
Fig. 4.
Body mass index curves for boys and girls with NHANES reference of 5th and 50th percentiles.
Fig. 3.
Weight growth curves for boys and girls with NHANES reference of 5th and 50th percentiles.
Overall, indigenous children and adolescents were shorter, lighter and had lower triceps skinfolds than the US reference (Table 3). We found higher than average BMI z-scores in girls, compared to boys and their US counterparts. Indigenous girls and boys fell significantly below the US median in height (both t-tests p<.001) and had mean height-for-age z-scores of −1.55 and −1.59 (SE 0.10, 0.11), respectively. Age had a significant effect on height-for-age z-scores (ANCOVA p<.05), yet sex and the interaction term did not. The prevalence of stunting (height-for-age z-score <=−2SD) was 40% among boys and 34% among girls. Weight-for-age z-scores were significantly below the US median (both t-tests p<.0001) for both girls and boys (Table 3). ANCOVA indicated that age, sex and their interaction were not significant predictors of weight-for-age z-scores. The prevalence of underweight (weight-for-age z-score <=−2 SD) was 2% for girls and boys.
TABLE 3.
Mean anthropometric z-scores and nutritional indicators by sex
| Height-for-Age Z-score | Weight-for-Age Z-score | BMI Z-score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sex | n | Mean (SE) | % <=−2 | n | Mean (SE) | % <=−2 | n | Mean (SE) | % >=1 |
| Girls | 97 | −1.55* (0.10) | 34.02 | 97 | −0.37* (0.08) | 2.06 | 97 | 0.17* (0.07) | 10.31 |
| Boys | 89 | −1.59* (0.11) | 40.45 | 89 | −0.74* (0.08) | 2.25 | 87 | 0.15* (0.06) | 5.75 |
| Total | 186 | −1.57 (0.07) | 37.10 | 186 | −0.54 (0.06) | 2.15 | 184 | 0.16 (0.05) | 8.15 |
| Arm Circumference Z-score | Triceps Skinfold Z-score | |||||
|---|---|---|---|---|---|---|
| Sex | n | Mean (SE) | % <=−2 | n | Mean (SE) | % <=−2 |
| Girls | 92 | 0.26* (0.08) | 0 | 73 | −0.50* (0.10) | 0.00 |
| Boys | 89 | −0.60* (0.07) | 1.12 | 74 | −0.88* (0.10) | 10.81 |
| Total | 181 | −0.16 (0.06) | 0.55 | 147 | −0.69 (0.07) | 5.44 |
One sample T-test of mean z-score s for girls and boys significantly different from zero (p<.05).
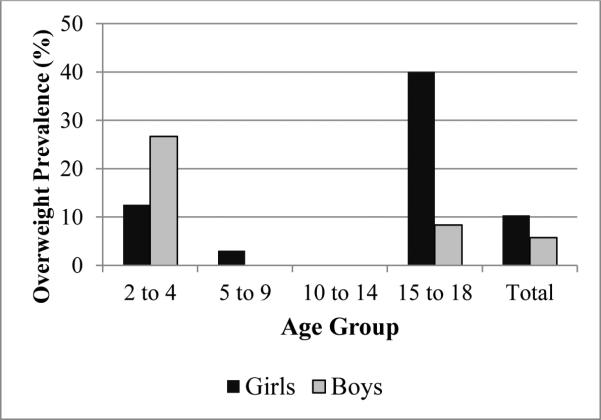
The prevalence of overweight (BMI-for-age z-score >=1) was 10% for girls and 6% for boys (Table 3). ANCOVA indicated both sex and the interaction of sex and age had significant effects on BMI z-scores (ANCOVA p<.05). Younger boys experienced a higher prevalence of overweight than younger girls, and older girls experienced a higher prevalence than older boys (Fig. 5). The prevalence of overweight was highest among girls aged 15 to 18 at 40% (6 of 15), compared to 8% (1 of 12) for boys of that age range. However, 27% of boys (4 of 15) two to five years old were overweight compared to only 13% of girls (3 of 24) in that age range.
Fig 5.
Prevalence of overweight children and adolescents by sex and age group.
Upper arm circumference z-score for girls was 0.26 (SE 0.08), significantly above the US children (t-test p<.05); however boys had a significantly lower average z-score (t-test p<.0001) of −0.60 (SE 0.07) compared to US children (Table 3). Both girls and boys had significantly lower subcutaneous fat depots, as measured by triceps skinfolds z-scores, than their US counterparts (all t-test p<.05). The average triceps skinfold z-scores were −0.50 (SE 0.10) for girls and −0.88 (SE 0.10) for boys. Sex and age were both significant independent predictors of triceps skinfold z-scores (ANCOVA p<.05). Approximately 11% of boys had low triceps skinfold z-score (<=−2).
Community Comparisons
The mean z-scores for height-for-age, weight-for-age, BMI-for-age, arm circumference, and triceps skinfolds are summarized by community in Table 4, along with the prevalence of stunting, low weight, overweight and low arm circumference and triceps skinfolds. The Kichwa communities had the lowest height-for-age z-scores, followed closely by the Huaorani, and then the Shuar and Cofán. The Kichwa communities of Pachacutik, Pastaza, and Pilchi, along with the Huaorani community of Gareno had the highest prevalence of stunting, ranging from 42% to 60% (Table 4). Tiguano, the Shuar community, and boys from the Huaorani community of Quehuereono had a moderate prevalence of stunting ranging from 25% to 38%. The lowest prevalence of stunting was zero among girls from Quehuereono, followed by both sexes in Zábalo ranging between 15% and 17%.
TABLE 4.
Mean anthropometric z-scores and nutritional indicators by community
| Height-for-age Z-score | Weight-for-age Z-score | BMI Z-score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Community | Sex | n | Mean (SE) | % <=−2 | n | Mean (SE) | % <=−2 | n | Mean (SE) | % >=1 |
| Gareno | Girls | 19 | −1.61 (0.19) | 42.11 | 19 | −0.16 (0.15) | 0 | 19 | 0.48 (0.17) | 15.79 |
| Boys | 10 | −1.99 (0.17) | 60.00 | 10 | −0.17 (0.45) | 0 | 9 | 0.49 (0.13) | 11.11 | |
| Quehuereono | Girls | 9 | −0.96 (0.20) | 0 | 9 | −0.09 (0.20) | 0 | 9 | 0.10 (0.21) | 0 |
| Boys | 11 | −1.56 (0.17) | 27.27 | 11 | −0.62 (0.20) | 9.09 | 11 | 0.12 (0.20) | 0 | |
| Zabalo | Girls | 18 | −1.20 (0.25) | 16.67 | 18 | −0.07 (0.19) | 0 | 18 | 0.38 (0.14) | 22.22 |
| Boys | 20 | −0.85 (0.31) | 15.00 | 20 | −0.54 (0.15) | 0 | 19 | 0.22 (0.09) | 0 | |
| Pachacutik | Girls | 9 | −2.17 (0.22) | 44.44 | 9 | −0.75 (0.26) | 11.11 | 9 | 0.02 (0.21) | 11.11 |
| Boys | 12 | −2.05 (0.20) | 58.33 | 12 | −1.01 (0.13) | 0 | 12 | 0.16 (0.11) | 8.33 | |
| Pastaza | Girls | 15 | −1.82 (0.43) | 60.00 | 15 | −0.72 (0.28) | 6.67 | 15 | −0.23 (0.17) | 0 |
| Boys | 13 | −1.74 (0.21) | 46.15 | 13 | −0.98 (0.18) | 0 | 13 | 0.03 (0.19) | 15.38 | |
| Pilchi | Girls | 11 | −1.87 (0.17) | 45.45 | 11 | −0.67 (0.19) | 0 | 11 | 0.01 (0.17) | 9.09 |
| Boys | 15 | −1.94 (0.27) | 53.33 | 15 | −0.94 (0.18) | 6.67 | 15 | 0.04 (0.16) | 6.67 | |
| Tiguano | Girls | 16 | −1.40 (0.20) | 25.00 | 16 | −0.34 (0.17) | 0 | 16 | 0.20 (0.13) | 6.25 |
| Boys | 8 | −1.41 (0.29) | 37.50 | 8 | −0.90 (0.17) | 0 | 8 | 0.00 (0.28) | 0 | |
| Arm Circumference Z-score | Triceps Skinfold Z-score | ||||||
|---|---|---|---|---|---|---|---|
| Community | Sex | n | Mean (SE) | % <=−2 | n | Mean (SE) | % <=−2 |
| Gareno | Girls | 17 | 0.52 (0.19) | 0 | 4 | −0.30 (0.40) | 0 |
| Boys | 10 | −0.17 (0.16) | 0 | 2 | −0.26 (0.74) | 0 | |
| Quehuereono | Girls | 9 | 0.49 (0.26) | 0 | 4 | −1.21 (0.28) | 0 |
| Boys | 11 | −0.28 (0.15) | 0 | 9 | −1.59 (0.18) | 33.33 | |
| Zabalo | Girls | 17 | 0.57 (0.16) | 0 | 18 | −0.11 (0.25) | 0 |
| Boys | 20 | −0.69 (0.14) | 0 | 19 | −1.31 (0.19) | 21.05 | |
| Pachacutik | Girls | 9 | −0.22 (0.30) | 0 | 9 | −0.72 (0.30) | 0 |
| Boys | 12 | −1.06 (0.18) | 8.33 | 12 | −0.95 (0.21) | 8.33 | |
| Pastaza | Girls | 14 | −0.25 (0.15) | 0 | 12 | −0.70 (0.19) | 0 |
| Boys | 13 | −0.95 (0.16) | 0 | 9 | −0.21 (0.27) | 0 | |
| Pilchi | Girls | 10 | −0.10 (0.30) | 0 | 10 | −0.94 (0.16) | 0 |
| Boys | 15 | −0.41 (0.21) | 0 | 15 | −0.40 (0.17) | 0 | |
| Tiguano | Girls | 16 | 0.47 (0.14) | 0 | 16 | −0.25 (0.14) | 0 |
| Boys | 8 | −0.45 (0.18) | 0 | 8 | −0.79 (0.16) | 0 | |
The mean weight-for-age z-scores of all the communities fell significantly below the US medians (Table 4). Kichwa communities had the lowest mean z-scores along with boys from Tiguano. Pachacutik girls and Quehuereono boys had the highest prevalence of low-weight ranging from 9% to 11%, while no participants from Gareno, Zábalo or Tiguano were of low-weight (Table 4).
All communities had mean BMI-for-age z-scores greater than the US reference, except for Kichwa girls from Pastaza (Table 4). Gareno had the highest means z-scores and Pastaza had the lowest. Girls from Zábalo experienced the highest prevalence of overweight at 22%, followed by Gareno girls at 16% and Pastaza boys at 15% (Table 4). Quehuereono was the only community that did not have any overweight girls or boys.
All boys and only the Kichwa girls had upper arm circumference means lower than the US reference (Table 4). Boys from Pachacutik, Pastaza, and Zábalo experienced the lowest mean z-scores. Huaorani, Cofán, and Shuar girls had similar higher mean z-scores. Pachacutik boys were the only group who experienced low arm circumference indicating malnutrition, at approximately 8% (Table 4).
Boys from Quehuereono and Zábalo experienced the lowest mean triceps skinfold z-scores, although all groups were below their US counterparts (Table 4). The prevalence of low triceps z-scores indicating deficient fat stores were found in 33% of Quehuereono boys, 21% of Zábalo boys, and 8% of Pachacutik boys (Table 4).
DISCUSSION
In this study we found large sex differences in body composition with adolescent girls having higher BMI than boys, with a 40% prevalence of overweight in girls 15 to 18 (Fig. 5). Girls also had larger arm circumference and triceps skinfold than boys (Table 3). However, in the youngest age range of two to five year olds, boys had a higher prevalence of overweight than girls. High rates of stunting indicating chronic malnutrition were documented at 34% for girls and 40% for boys; however there were no sex differences in height. We found little evidence of underweight or low arm circumference signifying that protein energy malnutrition is not a widespread nutritional issue. In contrast, rates of overweight indicate overnutrition is becoming a problem in some communities, particularly among adolescent girls.
Nutritional status in this study follows a similar pattern to that found in other Amazonian and indigenous groups characterized by high rates of stunting, average BMI or height-for-weight scores compared to US standards and low rates of wasting (Dufour, 1992; Foster et al. , 2005; Orr et al., 2001; Piperata et al., 2011b; Zonta et al., 2010; Zonta et al., 2011). The high prevalence of stunting in indigenous Amazonian populations results from an interaction between chronic undernutrition and heavy burden of infection (Hodge and Dufour, 1991; Santos and Coimbra, 1991). Blackwell and colleagues (2009) found similar rates of stunting among Shuar children and adolescents under 18 years old in the Southern Ecuadorian Amazon, with the prevalence of 41% in boys and 38% in girls. BMI was higher among the Shuar than the non-indigenous children living in the area and even the US reference population, with an obesity prevalence of 2% of both boys and girls (Blackwell et al. , 2009). Previously, the highest prevalence of overweight among adolescent girls from indigenous Amazonian populations was from the Ribeirinhos of Brazil, where 13% of girls aged 11 to 18 years were overweight (Piperata et al. , 2011b); however, Zonta and colleagues (2011) report an overweight prevalence of 22% among Mbyá-Guaraní adolescents in Argentina.
We found considerable between-ethnicity differences with the Kichwa demonstrating the poorest growth status followed by the Huaorani and Shuar communities. Kichwa communities had the highest rates of stunting ranging from 44% to 60%, the lowest mean weight-for-age z-scores, the only group falling below the US median for BMI, and the lowest mean arm circumference z-scores with 8% of boys from Pachacutik experiencing low arm circumference (<=−2 z-score) indicating malnutrition (Table 4). The Kichwa are most involved in market activities, relying heavily on cash cropping, along with the sale of domestic and forest animals, and some wage labor, which might have a negative impact on childhood health and growth. We found large differences in the sale of agricultural products, domestic animals and forest game among the Kichwa communities. The community of Pilchi, located two hours below the city of Coca on the Napo River, had substantially more market activities than Pachacutik and Pastaza, which are distant from main roads. In Pilchi, 25.9% of household-days reported the sale of domestic animals and 23.4% reported the sale of forest animals, compared to fewer than 7.0% for those measures in both Pachacutik and Pastaza.
Contrary to our predictions that the Shuar would also have poor outcomes based on their comparable levels of wage labor and sale of domestic animals, rates of stunting were lower than in the Kichwa communities. Stunting in Tiguano ranged from 25% to 38% (Table 4), however the community had the second highest mean height-for-age z-score for boys and third highest for girls, falling only below the Cofán community of Zábalo and Quehuereono females. Tiguano boys demonstrated one of the lowest mean weight-for-age z-scores in boys and girls; yet we found no evidence of low (<=−2 z-score) weight, arm circumference or triceps skinfold z-scores (Table 4). The differences between the Shuar and Kichwa may be due to the Shuar’s longer history of involvement in market activities (Lu , 2007). Market participation among the Shuar was stable between 2001 and 2009, whereas in the Kichwa communities, the percent of household-days participating in wage labor increased from 1.0% to 9.7% between 2001 and 2009, and the percentage of household-days selling agricultural products increased from 5.5% to 20.9% in over the same time period (Lu, 2007) (Fig. 1).
We found a distinctive pattern between the Huaorani communities of Gareno and Quehuereono, with Gareno exhibiting elevated rates of chronic malnutrition and Quehuereono exhibiting higher rates of short-term nutritional stress. The prevalence of stunting in Gareno ranged from 42% to 60% overlapping with the Kichwa, compared to the lower range of 0% to 27% in Quehuereono. Boys in Quehuereono had some of the highest rates compared to the other communities of underweight (<=−2 z-score) at 9% and low triceps skinfolds (<=−2 z-score) at 33%, indicating short- term nutritional stress and deficient fat stores. On the other hand Gareno had some of the highest rates of overweight ranging from 11% to 16% and no evidence of overnutrition was documented in Quehuereono. The distinctive patterns of nutritional status and growth outcomes between the Huaorani communities may be explained by employment and intensity of different market orientated practices. Gareno is more actively involved in wage labor at 8.3% of household-days and the sale of forest animals at 7.0%, compared to Quehuereono participation at 4.5% for wage labor and 5.4% for the sale of forest animals. Quehuereono is only accessible by canoe or bush plane, and may have a stronger continued reliance on their traditional livelihoods intermixed with some market activities, whereas Gareno lies on a road and has greater market activity. The pattern of overnutrition in Gareno and short-term undernutrition in Quehuereono could be reflective of seasonal changes since data collection in Gareno occurred approximately six months after the other communities.
As predicted, the Cofán experienced overall better nutritional and growth outcomes comparatively, based on their strong continued reliance on traditional subsistence strategies with little participation in wage labor and sale of market goods. The Cofán had some of the lowest rates of stunting ranging from 15% to 17% and sex differences in short-term nutritional status was evident. Zábalo girls experienced the highest rates of overweight (>=1 BMI z-score) at 22% and one of the highest mean weight-for-age z-score compared to other communities; however, boys showed evidence of deficient fat stores with a high prevalence of low triceps skinfolds (<= −2 z-score) at 21%.
As Native Amazonians become more integrated to the market economy, formerly semi-nomadic populations become increasingly sedentary because they are reduced to smaller portions of their traditional territory (i.e., push factors) and also take advantage of amenities provided by outsiders, such as markets, schools and health clinics (i.e., pull factors). Increased population density and decreased mobility result in health issues when sanitation and other infrastructure are inadequate. Before sustained contact, the Huaorani had practically no intestinal parasites due to drinking from feeder streams with headwaters rarely visited by outsiders; however, within three years of contact their parasite loads were nearly equal to those of neighboring groups (Davis and Yost, 1983). Studies of Naporuna schoolchildren in the Ecuadorian Amazon found that 82% of children were infected with one or more helminth species, with roundworm (Ascaris lumbricoides) and hookworm (Necator americanus) infection common (Buitrón et al., 2004; Quizhpe et al., 2003). Children from Mybá-Guaraní indigenous communities in Argentina had similar high rates of poly-parasitic infections linked to stunting and short-term nutritional stress, which were attributed to poor living conditions and inadequate sanitation (Zonta et al. , 2010). Indigenous households in the Amazon have limited to no access to piped water and sanitation infrastructure, and often raise chickens and other animals around the household (Orr et al. , 2001). Early life infectious disease burdens and peridomestic microbe exposures are significant drivers of childhood stunting and possibly predict future conditions of nutritional stress.
In addition to sedentarism, changes in dietary patterns are also important pathways affecting health outcomes. The synergism between undernutrition and disease during childhood often results in growth faltering (Dewey and Mayers, 2011; Scrimshaw and SanGiovanni, 1997; Scrimshaw et al., 1959; Ulijaszek, 1996), and is highly influenced by dietary quality and quantity, along with weaning practices (Lutter and Rivera, 2003). Traditional diets are generally regarded as balanced and nutritionally adequate (Robson and Wadsworth, 1977) with high diversity among subsistence agricultural populations (DeWalt, 1993; Fleuret and Fleuret, 1980). Although the diet of Amerindian populations is diverse, reliance on bulk foods such as cassava and plantain suggests that the nutrient density may be insufficient for children (Dufour, 1992). As indigenous populations become less reliant on traditional subsistence, health status may be adversely impacted.
Exposure to market foods and a cash economy often leads to preferential consumption of purchased foods over locally produced foods (Dewey, 1989). A shift to market foods and consumption of calorically dense foods with high refined sugars and saturated fats leads to increased obesity and other metabolic diseases typical of the nutrition transition (Popkin, 2004). Among the Ribeirinhos, male participation in wage labor was associated with overweight and obesity due to changes in diet and physical activity (Piperata, 2007). With an increased reliance on market foods after the abandonment of the household manioc gardens, adult females experienced increased consumption of fatty meats, and refined carbohydrates and sugars (Piperata et al. , 2011a). There was also evidence of a dual burden effect, where 60% of males and 70% of females were stunted and 31% of males and 29% of females were overweight or obese (Piperata, 2007). Economic development in the Amazon is increasing market integration of traditional communities and driving nutrition transition, causing increased rates of overweight and obesity in Ecuador, Bolivia (Benefice et al. , 2007) and Brazil (Port Lourenço et al., 2008; Welch et al., 2009).
The dual burden of under- and over-nutrition, signified by stunting and overweight or obesity, is a focus of the Developmental Origins of Adult Health and Disease paradigm that is especially relevant in developing countries where it is increasingly common in the same households and individuals (Popkin et al., 2012; Uauy et al., 2011). Stunting in early life may be leading to increased risk of obesity in later life through changes in energy metabolism causing reduced fat oxidation, as a long-term adaptation to undernutrition (Hoffman et al., 2000). In addition to stunting, reduced fat oxidation is also associated with increased adiposity and higher lipids and leptin levels, which are risk factors of cardiometabolic disorders (Leonard et al., 2009). The interaction between the developmental effects of early life chronic undernutrition and lifestyle changes associated with the nutrition transition produces the dual burden pattern in nutritional status (Frisancho, 2003). Decreased fat oxidation and increased reliance on carbohydrate metabolism, coupled with high dietary fat intake and reduced activity levels, leads to higher risk of obesity among stunted individuals. This developmental and lifestyle mismatch is the likely cause of the pattern of high rates of stunting, low wasting and normal to above normal BMI in the Ecuadorian Amazon.
CONCLUSION
The findings presented in this paper demonstrate a complex link between community market participation and variations in patterns of childhood nutritional status and growth among the four indigenous groups in our study. However, group-level analyses are limited in testing the causal pathways between specific market strategies and their differential and simultaneous effects on under- and over-nutrition. In future papers we will examine household and individual level measures of health, market integration and lifestyle using multilevel models to further examine the relationships between exposure to nutrition transition and market integration with their effects on childhood health, nutritional status and growth.
Acknowledgements
We are grateful to the study participants and residents of the study communities and to the anonymous reviewers for their helpful comments. This work was supported in part by a grant from the National Science Foundation (NSF #0822967).
Contract Grant Sponsor: National Science Foundation, Contract Grant Number: NSF #0822967
LITERATURE CITED
- Beckerman S, Erickson PI, Yost J, Regalado J, Jaramillo L, Sparks C, Iromenga M, Long K. Life histories, blood revenge, and reproductive success among the Waorani of Ecuador. Proceedings of the National Academy of Sciences. 2009;106(20):8134–8139. doi: 10.1073/pnas.0901431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benefice E, Lopez R, Monroy SL, Rodríguez S. Fatness and overweight in women and children from riverine Amerindian communities of the Beni River (Bolivian Amazon). Am J Hum Biol. 2007;19(1):61–73. doi: 10.1002/ajhb.20580. [DOI] [PubMed] [Google Scholar]
- Bernstein A. Emerging patterns in overweight and obesity in Ecuador. Rev Panam Salud Publica. 2008;24(1):71–74. doi: 10.1590/s1020-49892008000700010. [DOI] [PubMed] [Google Scholar]
- Blackwell AD, Pryor G, III, Pozo J, Tiwia W, Sugiyama LS. Growth and market integration in Amazonia: a comparison of growth indicators between Shuar, Shiwiar, and nonindigenous school children. Am J Hum Biol. 2009;21(2):161–171. doi: 10.1002/ajhb.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitrón D, Hurtig AK, San Sebastián M. Nutritional status of Naporuna children under five in the Amazon region of Ecuador. Rev Panam Salud Publica. 2004;15(3):151–159. doi: 10.1590/s1020-49892004000300003. [DOI] [PubMed] [Google Scholar]
- Byron EM. Market integration and health: the impact of markets and acculturation on the self-perceived morbidity, diet, and nutritional status of the Tsimane'Amerindians of lowland. University of Florida; Bolivia: 2003. [Google Scholar]
- Califano M, Gonzalo JA. Los A'i (Cofán) del río Aguarico, Mito y Cosmovisión. Ediciones Abya-Yala; Quito: 1995. [Google Scholar]
- Cerón MC. Etnobiología de los Cofánes de Dureno. Ediciones Abya-Yala; Quito: 1995. [Google Scholar]
- Davis EW, Yost JA. The ethnomedicine of the Waorani of Amazonian Ecuador. J Ethnopharmacol. 1983;9(2):273–297. doi: 10.1016/0378-8741(83)90036-3. [DOI] [PubMed] [Google Scholar]
- de Onis M, Lobstein T. Defining obesity risk status in the general childhood population: Which cut-offs should we use? Int J Pediatr Obes. 2010;5(6):458–460. doi: 10.3109/17477161003615583. [DOI] [PubMed] [Google Scholar]
- DeWalt KM. Nutrition and the commercialization of agriculture: ten years later. Soc Sci Med. 1993;36(11):1407–1416. doi: 10.1016/0277-9536(93)90383-f. [DOI] [PubMed] [Google Scholar]
- Dewey KG. Nutrition and the commoditation of food systems in Latin America and the Caribbean. Soc Sci Med. 1989;28(5):415–424. doi: 10.1016/0277-9536(89)90097-x. [DOI] [PubMed] [Google Scholar]
- Dewey KG, Mayers DR. Early child growth: how do nutrition and infection interact? Matern Child Nutr. 2011;7(s3):129–142. doi: 10.1111/j.1740-8709.2011.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour DL. Nutritional ecology in the tropical rain forests of Amazonia. Am J Hum Biol. 1992;4(2):197–207. doi: 10.1002/ajhb.1310040205. [DOI] [PubMed] [Google Scholar]
- FAO State of the world's forests, 2005. Rome. 2005 [Google Scholar]
- Fleuret P, Fleuret A. Nutrition, consumption, and agricultural change. Hum Organ. 1980;39(3):250–260. [Google Scholar]
- Foster Z, Byron E, Reyes García V, Huanca T, Vadez V, Apaza L, Perez E, Tanner S, Gutierrez Y, Sandstrom B. Physical growth and nutritional status of Tsimane'Amerindian children of lowland Bolivia. Am J Phys Anthropol. 2005;126(3):343–351. doi: 10.1002/ajpa.20098. [DOI] [PubMed] [Google Scholar]
- Frisancho AR. Reduced rate of fat oxidation: a metabolic pathway to obesity in the developing nations. Am J Hum Biol. 2003;15(4):522–532. doi: 10.1002/ajhb.10191. [DOI] [PubMed] [Google Scholar]
- Frisancho AR. Anthropometric standards: an interactive nutritional reference of body size and body composition for children and adults. University of Michigan Press; 2008. [Google Scholar]
- Godoy R, Cardenas M. Markets and the health of indigenous people: A methodological contribution. Hum Organ. 2000;59(1):117–124. [Google Scholar]
- Godoy R, Nyberg C, Eisenberg D, Magvanjav O, Shinnar E, Leonard W, Gravlee C, Reyes-Garca V, McDade T, Huanca T. Short but catching up: statural growth among native Amazonian Bolivian children. Am J Hum Biol. 2010;22(3):336–347. doi: 10.1002/ajhb.20996. [DOI] [PubMed] [Google Scholar]
- Godoy R, Reyes-García V, Byron E, Leonard WR, Vadez V. The effect of market economies on the well-being of indigenous peoples and on their use of renewable natural resources. Annu Rev Anthropol. 2005;34:121–138. [Google Scholar]
- Hicks JF, Daly HE, Davis SH, de Freitas ML. Ecuador's Amazon Region. Development issues and options. World Bank Discussion Paper 75. 1990 [Google Scholar]
- Hodge LG, Dufour DL. Cross-sectional growth of young Shipibo Indian children in Eastern Peru. Am J Phys Anthropol. 1991;84(1):35–41. doi: 10.1002/ajpa.1330840104. [DOI] [PubMed] [Google Scholar]
- Hoffman DJ, Sawaya AL, Verreschi I, Tucker KL, Roberts SB. Why are nutritionally stunted children at increased risk of obesity? Studies of metabolic rate and fat oxidation in shantytown children from Sao Paulo, Brazil. Am J Clin Nutr. 2000;72(3):702–707. doi: 10.1093/ajcn/72.3.702. [DOI] [PubMed] [Google Scholar]
- Irvine D. Indigenous federations and the market: the Runa of Napo, Ecuador. Indigenous Peoples and Conservation Organizations. 2000:21–46. [Google Scholar]
- Kuang-Yao Pan W, Erlien C, Bilsborrow RE. Morbidity and mortality disparities among colonist and indigenous populations in the Ecuadorian Amazon. Soc Sci Med. 2010;70(3):401–411. doi: 10.1016/j.socscimed.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrea C, Freire W. Social inequality and child malnutrition in four Andean countries. Rev Panam Salud Publica. 2002;11(5-6):356–364. doi: 10.1590/s1020-49892002000500010. [DOI] [PubMed] [Google Scholar]
- Leonard W, DeWalt K, Stansbury J, McCaston M. Influence of dietary quality on the growth of highland and coastal Ecuadorian children. Am J Hum Biol. 2000;12(6):825–837. doi: 10.1002/1520-6300(200011/12)12:6<825::AID-AJHB10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Leonard WR, Leatherman T, Carey J, Thomas R. Contributions of nutrition versus hypoxia to growth in rural Andean populations. Am J Hum Biol. 1990;2(6):613–626. doi: 10.1002/ajhb.1310020605. [DOI] [PubMed] [Google Scholar]
- Leonard WR, Sorensen MV, Mosher M, Spitsyn V, Comuzzie AG. Reduced fat oxidation and obesity risks among the Buryat of Southern Siberia. Am J Hum Biol. 2009;21(5):664–670. doi: 10.1002/ajhb.20903. [DOI] [PubMed] [Google Scholar]
- Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual: Human Kinetics Books Champaign. 1988 [Google Scholar]
- Lu F. Integration into the market among indigenous peoples: a cross-cultural perspective from the Ecuadorian Amazon. Curr Anthropol. 2007;48(4):593–602. [Google Scholar]
- Lu F, Bilsborrow RE, Oña AI. Modos de Vivir y Sobrevivir: Un Estudio Transcultural de Cinco Etnias de la Amazonía Ecuatoriana. Abda Yala; Quito: 2012. [Google Scholar]
- Lutter CK, Rivera JA. Nutritional status of infants and young children and characteristics of their diets. J Nutr. 2003;133(9):2941S–2949S. doi: 10.1093/jn/133.9.2941S. [DOI] [PubMed] [Google Scholar]
- Orr CM, Dufour DL, Patton JQ. A comparison of anthropometric indices of nutritional status in Tukanoan and Achuar Amerindians. Am J Hum Biol. 2001;13(3):301–309. doi: 10.1002/ajhb.1053. [DOI] [PubMed] [Google Scholar]
- PAHO . Regional Office of the World Health Organization; Washington, DC: 2007. Ecuador. [Google Scholar]
- Piperata BA. Nutritional status of Ribeirinhos in Brazil and the nutrition transition. Am J Phys Anthropol. 2007;133(2):868–878. doi: 10.1002/ajpa.20579. [DOI] [PubMed] [Google Scholar]
- Piperata BA, Ivanova SA, Da-gloria P, Gonçalo V, Polsky A, Spence JE, Murrieta RSS. Nutrition in transition: Dietary patterns of rural Amazonian women during a period of economic change. Am J Hum Biol. 2011a;23(4):458–469. doi: 10.1002/ajhb.21147. [DOI] [PubMed] [Google Scholar]
- Piperata BA, Spence JE, Da-Gloria P, Hubbe M. The nutrition transition in amazonia: rapid economic change and its impact on growth and development in Ribeirinhos. Am J Phys Anthropol. 2011b;146(1):1–13. doi: 10.1002/ajpa.21459. [DOI] [PubMed] [Google Scholar]
- Popkin BM. The nutrition transition and its health implications in lower-income countries. Public Health Nutr. 1998;1:5–22. doi: 10.1079/phn19980004. [DOI] [PubMed] [Google Scholar]
- Popkin BM. The nutrition transition: an overview of world patterns of change. Nutr Rev. 2004;62:S140–S143. doi: 10.1111/j.1753-4887.2004.tb00084.x. [DOI] [PubMed] [Google Scholar]
- Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70(1):3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin BM, Richards MK, Montiero CA. Stunting is associated with overweight in children of four nations that are undergoing the nutrition transition. J Nutr. 1996;126(12):3009. doi: 10.1093/jn/126.12.3009. [DOI] [PubMed] [Google Scholar]
- Port Lourenço AE, Ventura Santos R, Orellana JDY, Coimbra JRCEA. Nutrition transition in Amazonia: obesity and socioeconomic change in the Suruí Indians from Brazil. Am J Hum Biol. 2008;20(5):564–571. doi: 10.1002/ajhb.20781. [DOI] [PubMed] [Google Scholar]
- Quizhpe E, San Sebastián M, Hurtig AK, Llamas A. Prevalence of anemia in schoolchildren in the Amazon area of Ecuador. Rev Panam Salud Publica. 2003;13(6):355–361. doi: 10.1590/s1020-49892003000500003. [DOI] [PubMed] [Google Scholar]
- Robson J, Wadsworth G. The health and nutritional status of primitive populations. Ecol Food Nutr. 1977;6(3):187–202. [Google Scholar]
- Rudel TK, Bates D, Machinguiashi R. Ecologically noble Amerindians? Cattle ranching and cash cropping among Shuar and colonists in Ecuador. Latin American Research Review. 2002:144–159. [Google Scholar]
- San Sebastián M, Karin Hurtig A. Oil exploitation in the Amazon basin of Ecuador: a public health emergency. Rev Panam Salud Publica. 2004;15(3):205–211. doi: 10.1590/s1020-49892004000300014. [DOI] [PubMed] [Google Scholar]
- Santos RV, Coimbra CEA., Jr Socioeconomic transition and physical growth of Tupí- Mondê Amerindian children of the Aripuanã Park, Brazilian Amazon. Hum Biol. 1991;63(6):795–819. [PubMed] [Google Scholar]
- Sawaya AL, Grillo LP, Verreschi I, da Silva AC, Roberts SB. Mild stunting is associated with higher susceptibility to the effects of high fat diets: studies in a shantytown population in Sao Paulo, Brazil. J Nutr. 1998;128(2):415S–420S. doi: 10.1093/jn/128.2.415S. [DOI] [PubMed] [Google Scholar]
- Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66(2):464S–S477. doi: 10.1093/ajcn/66.2.464S. [DOI] [PubMed] [Google Scholar]
- Scrimshaw NS, Taylor CE, Gordon JE. Interactions of nutrition and infection. Am J Med Sci. 1959;237(3):367–403. [PubMed] [Google Scholar]
- Uauy R, Kain J, Corvalan C. How can the Developmental Origins of Health and Disease (DOHaD) hypothesis contribute to improving health in developing countries? Am J Clin Nutr. 2011;94(6):1759S–1764S. doi: 10.3945/ajcn.110.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulijaszek S. Relationships between undernutrition, infection, and growth and development. Human evolution. 1996;11(3):233–248. [Google Scholar]
- Walker I. Nutritional failure in Ecuador: causes, consequences, and solutions: World bank. 2007 [Google Scholar]
- Waters WF. Globalization and local response to epidemiological overlap in 21st century Ecuador. Globalization and health. 2006;2:8. doi: 10.1186/1744-8603-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JR, Ferreira AA, Santos RV, Gugelmin SA, Werneck G, Coimbra CEA. Nutrition transition, socioeconomic differentiation, and gender among adult Xavante indians, Brazilian Amazon. Hum Ecol. 2009;37(1):13–26. [Google Scholar]
- WHO . WHO Expert Committee on physical status: the use and interpretation of anthropometry: report of a WHO Expert Committee. World Health Organization; 1995. [PubMed] [Google Scholar]
- Zonta ML, Oyhenart EE, Navone GT. Nutritional status, body composition, and intestinal parasitism among the Mbyá-Guaraní communities of Misiones, Argentina. Am J Hum Biol. 2010;22(2):193–200. doi: 10.1002/ajhb.20977. [DOI] [PubMed] [Google Scholar]
- Zonta ML, Oyhenart EE, Navone GT. Nutritional vulnerability in Mbyá-Guaraní adolescents and adults from Misiones, Argentina. Am J Hum Biol. 2011;23(5):592–600. doi: 10.1002/ajhb.21175. [DOI] [PubMed] [Google Scholar]