Abstract
Objective
To describe the 5-year cumulative incidence of cardiac dysfunction in human immunodeficiency virus (HIV)-infected children.
Study design
We used a prospective cohort design, enrolling children at 10 hospitals. Group I included 205 vertically HIV-infected children enrolled at a median age of 1.9 years. Group II consisted of 600 HIV-exposed children enrolled prenatally or as neonates, of whom 93 were ultimately HIV-infected. The main outcome measures were echocardiographic indexes of left ventricular dysfunction.
Results
In group I, the 5-year cumulative incidence of left ventricular fractional shortening ≤25% was 28.0%. The 5-year incidence of left ventricular end-diastolic dilatation was 21.7%, and heart failure and/or the use of cardiac medications 28.8%. The mortality rate 1 year after the diagnosis of heart failure was 52.5% [95% CI, 30.5-74.5]. Within group II, the 5-year cumulative incidence of decreased fractional shortening was 10.7% in the HIV-infected compared with 3.1% in the HIV-uninfected children (P = .01). Left ventricular dilation, heart failure, and/or the use of cardiac medications were more common in infected compared with uninfected children.
Conclusions
During 5 years of follow-up, cardiac dysfunction occurred in 18% to 39% of HIV-infected children and was associated with an increased risk of death. We recommend that HIV-infected children undergo routine echocardiographic surveillance for cardiac abnormalities.
Human immunodeficiency virus (HIV) infection continues to be an important cause of morbidity and mortality in children. We designed the Pediatric pulmonary and cardiovascular complications of vertically transmitted human immunodeficiency virus infection (P2C2) HIV study to determine the incidence of heart and lung abnormalities in HIV-infected children. This study began in 1990 and data collection continued through January 1997. Our group previously reported that over the first 2 years of observation, 10% of HIV-infected children either had congestive heart failure (CHF) or required treatment with cardiac medications or both.1 In addition, 20% of HIV-infected children had cardiac dilation or dysfunction.1 We also reported that over the entire 6-year observation period of our study, cardiac abnormalities were the underlying cause of HIV-related death in 11 (11.8%) of 93 children and were a contributing cause in an additional 22%.2 Furthermore, baseline cardiac dysfunction was a risk factor for death independent of CD4 count, wasting, and other predictors of death.3
Our previous report detailing the incidence of cardiac complications in HIV-infected children was limited to the first 2 years of observation in the P2C2 HIV study.1 The majority of other published studies of cardiac dysfunction in HIV-infected children have been cross-sectional or short-term descriptions.4-8 Because HIV infection often becomes a chronic condition, information regarding the long-term outcomes of HIV-infected children is needed. The purpose of this report is to provide the 5-year incidence of cardiac dysfunction in HIV-infected children enrolled in the P2C2 HIV study and the effect of cardiac abnormalities on their survival.
Methods
Study Design
A total of 805 children born to HIV-infected mothers were studied at 10 hospitals in 5 centers (see Appendix). Each center's institutional review board approved the study, and patients were enrolled after informed consent was obtained.9
Study Cohorts
Group I included 205 vertically HIV-infected children. They entered the study between 1990 and 1993 at a median age of 1.9 years (range, 0.1-14 years), and 89% had symptomatic HIV infection by Centers for Disease Control and Prevention (CDC) classification10 at enrollment. Group II (neonatal inception cohort) subjects were enrolled during fetal life (n = 432) or before 28 days of age (n = 168) and entered the study between 1990 and 1994. In contrast to group I children, the final HIV-infection status of group II children was unknown at the time of enrollment in the study.
Documentation of HIV Status
In group I children, HIV status was documented by a positive HIV antibody test or a positive HIV blood culture. In group II, HIV infection was defined as 2 positive HIV blood cultures. Approximately half of the group II HIV-uninfected cohort was randomly selected to remain in the study as a control group.9 Infants who died (n = 9) or were lost to follow-up (n = 35) before their HIV status could be determined were classified as “indeterminate,” and their data were not used in this report.
Cardiac Studies
Children underwent 2-dimensional echocardiographic and Doppler studies every 4 to 6 months. They also had a chest radiograph every 12 months. Echocardiograms were performed according to a protocol in 10 pediatric cardiology laboratories at the 5 centers as previously described.1,11,12 A total of 5026 echocardiograms were performed and 4732 were measured at the central echocardiography laboratory at the Boston Children's Hospital. We excluded left ventricular (LV) measurements of 109 echocardiograms from 72 children who had segmental wall abnormalities or abnormal interventricular septal motion because these conditions can affect the LV function measurements. We excluded function studies of 3 children with congenital heart abnormalities. LV measurements were converted to Z scores.11,13 Tachycardia on the echocardiogram was defined as a heart rate on the 2-D echocardiogram with a Z score >3.13 The diagnosis of CHF was determined by a pediatric cardiologist at each center and was based on the patient's clinical findings. A study protocol modification was made in March 1994, requiring that children undergo a standardized P2C2 HIV study physical examination by a pediatric cardiologist when a noninvasive test showed signs of an abnormality or if the examination was clinically indicated. Some children had echocardiographic abnormalities of LV size and function without a clinical diagnosis of CHF and were treated with cardiac medications (digoxin, furosemide, spironolactone, captopril, enalapril, dobutamine, or nifedipine). For the purposes of this study, a child was considered to have a cardiac impairment if he or she had LV fractional shortening (LVFS) ≤25% after 6 months of age, CHF, or if the child was being treated with cardiac medications. Chronic cardiac impairment was defined as evidence of cardiac abnormality found on testing separated by at least 3 months. The cutoff of LVFS ≤25% was chosen because this is below the lower limits of normal for children (36 ± 4%; mean ± SD)14 and because this level was associated with clinical signs of CHF in a separate group of HIV-infected children.15 The presence or absence of cardiomegaly on chest radiography was noted by a pediatric radiologist.16 Cardiomegaly was not formally defined by the study radiologists, who followed standard terminology defining cardiomegaly as a cardiac width >50% of the cardiothoracic ratio on frontal examination17 and slightly more in a neonate on a supine film.18
Definitions of HIV-1 Disease Progression
We defined a rapid progressor as an infant who, during the first year of life, had an AIDS-defining condition (other than lymphoid interstitial pneumonitis/pulmonary lymphoid hyperplasia), severe immunosuppression (CDC immunologic category 3),10 or both.19 A child who did not have either of these conditions was considered a nonrapid progressor. For those who died (n = 11) or were lost to follow-up before 1 year of age (n = 3), the group assignment was made with available data.
Statistical Analysis
The prevalence of complications at the initial cardiac study among the group I children was summarized through the use of proportions. After excluding the prevalent cases, the 5-year cumulative incidence rates for each complication were obtained from Kaplan-Meier analyses. Similarly, the cumulative incidence rates for cardiac complications at 5 years of age were calculated for HIV-infected and uninfected infants from the neonatal cohort (group II). Complication rates between HIV-infected and uninfected children were compared by means of log-rank tests. Incidence rates were also calculated by disease progression category (rapid and nonrapid progressors) for both cohorts and compared with log-rank tests. Statistical tests were 2-sided. A P value ≤ .05 was considered statistically significant.
Kaplan-Meier analyses were used to estimate group I mortality rates after the diagnosis of CHF, CHF and/or treatment with cardiac medications, and cardiac impairment. To estimate the relative risk of death and to examine the temporal relation between cardiac abnormalities and death, we included each measure of cardiac abnormality as a time-dependent covariate (yes or no) in a Cox regression model of time to death. This model was fit separately for each of the 3 measures of cardiac abnormality. Estimates of risk were also adjusted for disease severity using CD4 T-cell counts as a time-dependent covariate in the Cox model. Additionally, Kaplan-Meier estimates of cumulative mortality rates for children diagnosed with CHF and for children without a diagnosis of CHF were compared by means of the log-rank test. Cumulative mortality rates were also compared between children with, and those without, cardiac impairment.
Complication rates per 100 child-years and 95% CIs were estimated with an exact method based on the Poisson distribution when fewer than 15 events occurred per group.20 When more than 15 events occurred per group, a Poisson approximation was used.21 Rates were compared by using an exact test for comparing 2 Poisson-distributed events.20
Results
Demographics
Group I consisted of 205 HIV-infected children of whom 89% had symptomatic HIV infection at enrollment.1,9 Of the 600 live-born infants in group II, 93 were HIV infected, 463 remained uninfected, and 44 had indeterminate HIV status.
Cardiac Size and Function (Group I, Older Children)
After excluding prevalent cases, the cumulative incidence of LV dysfunction (LVFS, 19%-25%) after 5 years in the study was 28.0% (Table I). Cardiomegaly (LV end-diastolic [LVED] dimension Z score >2) was seen in 21.7% after 5 years. Cardiomegaly on chest radiography was noted in 39.0% after 5 years of follow-up.
Table I. Baseline prevalence and cumulative 5-year incidence of cardiac complications in the cohort of children known to be HIV-infected at enrollment (group I).
| Complication | Prevalence | Incidence | |||
|---|---|---|---|---|---|
|
|
|
||||
| Frequency | % | No. of events | 5-year incidence | 95% CI | |
| ↓Fractional shortening (≤25%) | (11/193) | 5.7 | 38 | 27.6 | 19.8, 35.4 |
| ↓Fractional shortening (19% – 25%) | (8/193) | 4.1 | 39 | 28.0 | 20.2, 35.8 |
| ↓Fractional shortening (<19%) | (3/193) | 1.6 | 9 | 7.4 | 2.4, 12.4 |
| ↑LVED dimension (Z score >2) | (16/192) | 8.3 | 29 | 21.7 | 14.4, 29.0 |
| ↑LVED dimension (Z score >3) | (2/192) | 1.0 | 11 | 7.3 | 3.0, 11.5 |
| ↑LVES dimension (Z score >2) | (34/192) | 17.7 | 53 | 42.7 | 33.5, 51.9 |
| ↑LVES dimension (Z score >3) | (10/192) | 5.2 | 29 | 20.0 | 13.3, 26.7 |
| Cardiomegaly (radiography) | (28/201) | 13.9 | 46 | 39.0 | 28.9, 49.2 |
| Pericardial effusion (≥5 mm maximal diameter) | (0/201) | 0.0 | 4 | 2.8 | 0.1, 5.5 |
| ↑Heart rate (echocardiogram) (Z score >2) | (41/196) | 20.9 | 103 | 77.6 | 70.1, 85.1 |
| ↑Heart rate (echocardiogram) (Z score >3) | (14/196) | 7.1 | 62 | 42.2 | 33.9, 50.5 |
| CHF | (2/201) | 1.0 | 19 | 14.0 | 7.9, 20.1 |
| CHF and/or cardiac medications | (9/201) | 4.5 | 40 | 28.8 | 20.8, 36.7 |
| Cardiac impairment | (14/201) | 7.0 | 56 | 39.1 | 30.7, 47.5 |
Cardiac Size and Function (Group II, Neonatal Groups)
The 5-year cumulative incidence rate of LVFS between 19% and 25% was 9.3% in the HIV-infected neonatal group compared with 2.9% in the uninfected children (P = .02; Table II). The 5-year incidence rates of LVED and LV end-systolic (LVES) dilation were higher in HIV-infected children compared with HIV-uninfected children. The 5-year cumulative incidence rate of cardiomegaly on chest radiography was 51.3%.
Table II. Cumulative 5-year incidence of cardiac complications in the neonatal cohort (group II).
| Complication | 5-Year incidence | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| HIV-infected (n = 93) | HIV-uninfected (n = 463) | ||||||
|
|
|
||||||
| No. of events | % | 95% CI | No. of events | % | 95% CI | P value (from log-rank test) | |
| ↓Fractional shortening (≤25%) | 8 | 10.7 | 3.7, 17.8 | 13 | 3.1 | 1.4, 4.7 | .01 |
| ↓Fractional shortening (19%–25%) | 7 | 9.3 | 2.7, 15.9 | 12 | 2.9 | 1.3, 4.5 | .02 |
| ↓Fractional shortening (<19%) | 1 | 1.4 | 0.0, 4.1 | 1 | 0.2 | 0.0, 0.6 | .23 |
| ↑LVED dimension (Z score >2) | 7 | 10.3 | 3.0, 17.6 | 8 | 2.1 | 0.6, 3.5 | .007 |
| ↑LVED dimension (Z score >3) | 0 | 0.0 | — | 2 | 0.6 | 0.0, 1.4 | .51 |
| ↑LVES dimension (Z score >2) | 30 | 41.7 | 28.8, 54.7 | 52 | 17.1 | 12.0, 22.2 | .001 |
| ↑LVES dimension (Z score >3) | 10 | 14.1 | 5.9, 22.3 | 7 | 1.8 | 0.5, 3.1 | .001 |
| Cardiomegaly (radiography) | 29 | 51.3 | 33.5, 69.1 | — | — | — | — |
| Pericardial effusion (≥5 mm maximal diameter) | 3 | 5.4 | 0.0, 7.1 | 2 | 0.9 | 0.0, 2.1 | .05 |
| ↑Heart rate (echocardiogram) (Z score >2) | 57 | 78.7 | 67.7, 89.7 | 78 | 32.2 | 23.6, 40.7 | .001 |
| ↑Heart rate (echocardiogram) (Z score >3) | 23 | 35.5 | 22.2, 48.7 | 17 | 8.3 | 2.5, 14.2 | .001 |
| CHF | 4 | 5.1 | 0.2, 10.0 | 1 | 0.2 | 0.0, 0.7 | < .001 |
| CHF and/or cardiac medications | 9 | 13.6 | 4.8, 22.3 | 2 | 0.5 | 0.0, 1.2 | .001 |
| Cardiac impairment | 12 | 17.7 | 8.1, 27.3 | 2 | 0.5 | 0.0, 1.2 | < .001 |
Other Cardiac Abnormalities
In group I, the 5-year cumulative incidence of a pericardial effusion (≥5-mm diameter) was 2.8%. The 5-year cumulative incidence of an effusion (≥5 mm) was 5.4% in the group II HIV-infected children versus 0.9% in the uninfected children (P = .05). No episodes of tamponade occurred in either group.
In group I, the 5-year cumulative incidence of Doppler evidence of mitral valve regurgitation was 8.3% and of tricuspid regurgitation was 18.6%. Valve regurgitation was rare in group II, and differences in rates between the infected and uninfected group II children were not statistically significant.
Congestive Heart Failure
Two group I children had CHF at enrollment and 19 additional children had CHF diagnosed during the first 5 years of follow-up. The 5-year cumulative incidence rate of CHF in group I children was 14.0%. The median age at diagnosis of CHF in group I was 53 months (range, 8-168 months). In group I, an additional 28 children received cardiac medications for a diagnosis of cardiomyopathy or LV dysfunction (n = 24) or hypertension (n = 4), without a specific diagnosis of CHF. The 5-year cumulative incidence of CHF and/or treatment with cardiac medications was 28.8% (Table I). The most commonly used cardiac drugs were digoxin (n = 35) and furosemide (n = 27).
In the group II infected children, 4 cases of CHF occurred and the 5-year cumulative incidence was 5.1%. In group II, an additional 5 HIV-infected children received cardiac medications without a specific diagnosis of CHF: 4 had cardiomyopathy or LV dysfunction and 1 had hypertension.
Cardiac Abnormalities and Death
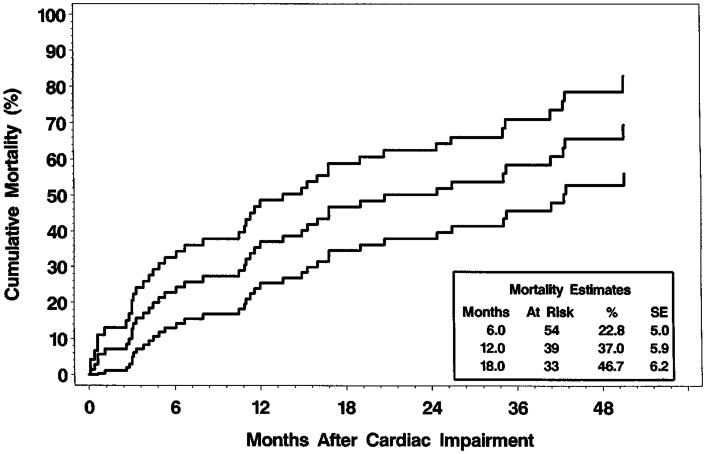
In group I children, the 5-year cumulative death rate from all causes was 35.4% (95% CI, 28.3-42.6). Of the 22 group I children with CHF, 17 died, and cardiomyopathy was the underlying or contributing cause of death in 8. The cumulative mortality rate 1 year after the initial diagnosis of CHF was 52.5% (Fig 1). After excluding the 14 prevalent cases (7.0%), the 5-year cumulative incidence of cardiac impairment was 39.1% among group I children. The cumulative mortality rate 18 months after the identification of cardiac impairment was 46.7% (Fig 2).
Fig 1.
Cumulative mortality rates and 95% CI after the initial diagnosis of CHF (17 of 22 died).
Fig 2.
Cumulative mortality rates and 95% CI after identification of cardiac impairment (41 of 71 died).
The relative risk of death after cardiac impairment in group I children was 8.5 (95% CI, 5.2-13.9, Table III). These relative risks increase to 12.6 for children with the diagnosis of CHF or the use of cardiac medications to 14.6 for patients with CHF alone. The risk of death after CHF remained elevated even after adjustment for severity of HIV disease with the use of CD4 T-cell counts (adjusted relative risk = 5.7 [95% CI, 3.1-10.3]; P < .001). In the group I children with CHF, the 4-year mortality rate was 59.1% compared with 25.3% in the children without CHF (P < .001; log-rank test). The 5-year cumulative mortality rate in group I children with cardiac impairment was 56.9% compared with 22.8% in those children without cardiac impairment (P < .001; log-rank test).
Table III. Effects of cardiac impairment, CHF and/or medications, and CHF on risk of death among group I cohort (Cox regression analysis).
| Covariate | n (deaths) | Estimated β | SE | P value | RR | 95% CI |
|---|---|---|---|---|---|---|
| Cardiac impairment | 201 (69) | 2.137 | 0.167 | < .001 | 8.5 | 5.2, 13.9 |
| CHF and/or cardiac medications | 201 (69) | 2.532 | 0.250 | < .001 | 12.6 | 7.8, 20.4 |
| CHF alone* | 201 (69) | 2.682 | 0.525 | < .001 | 14.6 | 8.2, 26.0 |
Risk of death after CHF remained elevated after adjustment for disease severity with CD4 T-cell counts (adjusted relative risk [RR] = 5.7 [95% CI, 3.1-10.3, P < .001]).
In the group II cohort, the 5-year cumulative incidence of cardiac impairment was 17.7%. The 5-year cumulative death rate from all causes was 32.7% [95% CI, 22.3-43.1] in the HIV-infected children compared with 1 death in the 463 uninfected children. Three of the 4 group II children with CHF died during the 5-year observation period. Cardiomyopathy was the underlying or contributing cause of death in 1 child.
Chronic or Recurrent Cardiac Abnormalities
Many children with cardiac impairment had >1 notation of cardiac dysfunction. A total of 83 children (71 in group I and 12 of the infected in group II) had 267 notations of cardiac impairment. Chronic cardiac impairment was noted in 43 of 83 children; 19 had 1 and 24 had ≥2 notations of chronic impairment. Twelve of 43 children died within 3 months of the notation of chronic cardiac impairment. These data suggest that cardiac impairment was not merely a transient finding and identifies a subset of children at increased risk for death.
Incidence of Cardiac Abnormalities in Rapid Progressors
In group I, the 5-year incidence of LVFS ≤25% was 35.3% in the rapid progressor group (n = 71) and 23.8% in the nonrapid progressor group (n = 134; P = .11). The incidence of increased LVED dimension was 31.6% (95% CI, 17.4-45.8) in group I rapid progressors compared with 17.0% (95% CI, 8.9-25.1) (P = .01) in group I nonrapid progressors. Group I rapid progressors had increased mortality rates as well as increased rates of CHF and cardiac impairment compared with nonrapid progressors (Table IV).
Table IV. Rates of mortality, CHF, and cardiac impairment comparing rapid and nonrapid progressors in HIV-infected children.
| Cohort of children known to be HIV-infected at enrollment (group I) | |||||
|---|---|---|---|---|---|
|
| |||||
| Event | Rapid progressors (n = 71) | Nonrapid progressors (n = 134) | P value | ||
|
|
|
||||
| Rate/100 child-years | 95% CI | Rate/100 child-years | 95% CI | ||
| Death | 12.9 | 9.1, 18.2 | 6.1 | 4.4, 8.5 | .004 |
| CHF | 5.7 | 2.9, 10.3 | 2.1 | 1.0, 3.9 | .04 |
| Cardiac impairment | 16.2 | 11.1, 23.5 | 7.6 | 5.4, 10.7 | .007 |
| Repeat cardiac impairment | 50.9 | 41.5, 62.4 | 29.6 | 25.0, 35.2 | <.001 |
| Neonatal cohort (group II) | |||||
|
| |||||
| Rapid progressors (n = 45) | Nonrapid progressors (n = 48) | ||||
|
|
|
||||
| Event | Rate/100 child-years | 95% CI | Rate/100 child-years | 95% CI | P value |
|
| |||||
| Death | 18.9 | 12.7, 28.1 | 2.1 | 0.6, 5.3 | <.001 |
| CHF | 2.8 | 0.6, 8.2 | 0.6 | 0.0, 3.6 | .37 |
| Cardiac impairment | 9.5 | 4.4, 18.0 | 2.0 | 0.4, 5.7 | .02 |
| Repeat cardiac impairment | 27.1 | 18.6, 39.4 | 3.3 | 1.1, 7.6 | <.001 |
In group II rapid progressors (n = 45), the 5-year incidence of LVFS ≤25% was 20.6% (95% CI, 6.8-34.4) compared with 2.4% (95% CI, 0-7.2) (P = .01) in nonrapid progressors (n = 48). The incidence of increased LVED dimensions was 20.7% (95% CI, 5.7-35.7) in the group II rapid progressors and 2.5% (95% CI, 0-7.3) (P = .02) in the nonrapid progressors. The incidence rates for death and repeated episodes of cardiac impairment were also higher in the rapid progressors compared with the nonrapid progressors (Table IV).
Physical Examination
Data were available from 61 group I and 18 group II HIV-infected children who had a cardiac examination under the P2C2 HIV Study protocol. Conditions noted at the initial examination in group I children included fatigue (31%), inappropriate weight gain (21%), exertional dyspnea (20%), feeding difficulties (16%), and excessive perspiration (12%). Physical findings included hepatomegaly (30%), systolic murmur (30%), and rales (10%). Among 13 group I children who were referred specifically because of CHF, fatigue was noted in 67% and exertional dyspnea in 33%. Physical findings in the CHF group included hepatomegaly (62%), systolic murmur (46%), and rales (23%).
Discussion
Our study shows that cardiac dysfunction occurs frequently in children with HIV infection and that these children have cardiac abnormalities ranging from asymptomatic cardiac dilation to severe CHF. The relative risk of death during the 5-year follow-up period in children who had cardiac impairment or CHF was 8.5 to 14.6 times higher than in the children without these complications. Our study also shows that CHF and cardiac impairment are important risk factors for death in HIV-infected children, extending our previous work1-3 and confirming an association noted by other investigators.5,7 The rates of death and cardiac complications were highest in the rapid progressor group, which suggests that this group is at increased risk.
We expected the overall rates of cardiac abnormalities to be higher in group I children who were older and sicker than the newborn cohort. On enrollment in the study, most group I children were immunodeficient and cardiac impairment was already present in 14 (7%) of 201 children. Heart failure developed in 20 children, and an additional 37 were treated with cardiac medications or had low LVFS. The overall rate of cardiac dysfunction in group I may have been overestimated because of possible selection bias at enrollment. In comparison, group II infants were enrolled because of their in utero exposure to HIV and before their HIV status was determined. In this neonatal cohort, the 5-year incidence of CHF was 5.1% and the 5-year incidence of LV dysfunction was 10.7%, values similar to the prevalence rates in the group I cohort. The data from this unbiased sample (group II neonatal cohort) suggest that our study provides reasonable estimates of the rates of cardiac dysfunction in HIV-infected children.
Other estimates of rates of cardiac dysfunction in HIV-infected children, which are mostly reports from single institutions and cross-sectional in nature, range from 1% to 40%.5,22,23 In our prospective multicenter study, the 5-year cumulative incidence of cardiac dysfunction ranged from 18% to 39% of HIV-infected children.
On the basis of our study design of frequent cardiac testing, we probably identified all children with chronic cardiac dysfunction. We may have missed children with cardiac decompensation that developed shortly before death or children whose families withdrew active medical support before the child's death. Although the clinical diagnosis of CHF was not defined by the study protocol, detailed studies on a small subset of children with CHF suggests that they had physical signs that are typical of heart failure in children. A potential limitation of the study is the fact that we excluded echocardiograms from children with interventricular septal and LV segmental wall abnormalities because of the inaccuracy of M-mode measurements in children with those conditions. Because it is possible that HIV infection may cause segmental wall abnormalities, future studies using other diagnostic techniques will be needed to assess the degree of cardiac dysfunction in this subset of children.
The majority of patients in this study were treated with a wide variety of antiretroviral agents available between 1990 and 1996 or intravenous immunoglobulin.2 Few received the newer antiretroviral agents such as protease inhibitors. The effect of these new agents on the progression of HIV disease has been encouraging, and many children's symptoms decrease while they are taking these new medications. However, some HIV-infected children continue to have evidence of cardiac dysfunction even with an undetectable HIV viral load. The applicability of our data to patients on new regimens with protease inhibitors is unknown, and it remains to be seen if the incidence of cardiac dysfunction will change with new therapies.
The cause of HIV cardiomyopathy is unknown and probably is not due to any one single mechanism.24 Several isolated reports have described the HIV virus in myocardial or pericardial tissue,25,26 whereas others have proposed that HIV cardiomyopathy may be related to antiretroviral medications.23,27 Cardiomyopathy in HIV infection may be due to a coinfection from a second virus or pathogen and may not always be a direct effect of the HIV virus.28,29
On the basis of our findings, we recommend that all HIV-infected children have a 2-D echocardiogram at the time of diagnosis to determine intracardiac anatomy and baseline cardiac function. Children who have symptoms of heart failure or unexplained or prolonged respiratory disease should undergo cardiac evaluation and ultrasonography. Children with heart dysfunction should be followed as clinically indicated. Asymptomatic HIV-infected children should undergo routine echocardiograms every several years. Children who are symptomatic from their HIV disease should have surveillance echocardiograms on a yearly basis. Because of the higher rates of cardiac abnormalities in rapid progressors, this group may need more frequent testing.
The efficacy of cardiac treatment has not been formally studied in HIV-infected children. Because of the ominous prognosis after diagnosis of CHF in our series, we suggest that heart failure should be aggressively treated and that protocols should be developed to determine the optimal care for these patients. HIV-associated cardiomyopathy in adults has a much worse prognosis than other causes of cardiomyopathy.30 With optimal treatment of the HIV infection and attention to the treatment of CHF, we may be able to lower the high mortality rates seen in these children.
Acknowledgments
Supported by the National Heart, Lung, and Blood Institute (NO1-HR-96037, NO1-HR-96038, NO1-HR-96039, NO1-HR-96040, NO1-HR-96041, NO1-HR-96042, and NO1-HR-96043) and in part by the National Institutes of Health General Clinical Research Center Grants (RR-00865, RR-00188, RR-02172, RR-00533, RR-00071, RR-00645, RR-00685, and RR-00043).
Glossary
- CDC
Centers for Disease Control and Prevention
- CHF
Congestive heart failure
- FS
Fractional shortening
- HIV
Human immunodeficiency virus
- LV
Left ventricular
- LVED
Left ventricular end-diastolic
- LVES
Left ventricular end-systolic
- P2C2 HIV
Pediatric pulmonary and cardiovascular complications of vertically transmitted human immunodeficiency virus infection
Appendix
A partial list of participants in the P2C2HIV study is listed below, with Principal Investigators identified with an asterisk. A complete list of study participants can be found in reference 9.
NATIONAL HEART, LUNG, AND BLOOD INSTITUTE: Hannah Peavy, MD (Project Officer), Anthony Kalica, PhD, Elaine Sloand, MD, George Sopko, MD, MPH, Margaret Wu, PhD; CHAIRMAN, THE STEERING COMMIT-TEE: Robert Mellins, MD; CLINICAL CEN-TERS: Baylor College of Medicine, Houston, TX: William Shearer, MD, PhD,* Nancy Ayres, MD, J. Timothy Bricker, MD, Arthur Garson, MD, Linda Davis, RN, BSN, Paula Feinman, Mary Beth Mauer, RN, BSN; University of Texas: Debra Mooneyham, RN; Teresa Tonsberg, RN; Children's Hospital/Harvard Medical School, Boston, MA: Steven Lipshultz, MD,* Steven Colan, MD, Lisa Hornberger, MD, Steven Sanders, MD, Marcy Schwartz, MD, Helen Donovan, Janice Hunter, MS, RN, Ellen McAuliffe, BSN, Nandini Moorthy, Patricia Ray, BS, Sonia Sharma, BS; Boston Medical Center: Suzanne Steinbach, MD, Karen Lewis, RN, BSN; Mount Sinai School of Medicine, New York, NY: Meyer Kattan, MD,* Wyman Lai, MD, MPH, Diane Carp, MSN, RN, Donna Lewis, Sue Mone, MS; Beth Israel Medical Center: Mary Anne Worth, RN; Presbyterian Hospital in the City of New York/Columbia University, New York, NY: Robert Mellins, MD,* Fred Bierman, MD* (through 5/91), Welton Gersony, MD, Jane Pitt, MD, Thomas Starc, MD, MPH, Anthony Brown, Margaret Challenger, Kimberly Geromanos, RN, MS, CNS; UCLA School of Medicine, Los Angeles, CA: Samuel Kaplan, MD,* Y. Al-Khatib, MD, Robin Doroshow, MD, Josephine Isabel-Jones, MD, Roberta Williams, MD, Helene Cohen, RN, PNP, Sharon Golden, RDMS, Karen Simandle, RDMS, Ah-Lin Wong, RDMS; Children's Hospital LA, Los Angeles, CA: Arno Hohn, MD, Barry Marcus, MD, Audrey Gardner, BS, Toni Ziolkowski, RN; LAC/USC: Lynn Fukushima, MSN, RN; CLINICAL COORDINATING CENTER: The Cleveland Clinic Foundation, Cleveland, OH: Kirk A. Easley, MS,* Michael Kutner, PhD* (through 12/99), Mark Schluchter, PhD* (through 04/98), Johanna Goldfarb, MD, Douglas Moodie, MD, Cindy Chen, MS, Scott Husak, BS, Victoria Konig, ART, Sunil Rao, PhD, Amrik Shah, ScD, Susan Sunkle, BA, Weihong Zhang, MS; POLICY, DATA, AND SAFETY MONITORING BOARD: Henrique Rigatto, MD (Chairman), Edward B. Clark, MD, Robert B. Cotton, MD, Vijay V. Joshi, MD, Paul S. Levy, ScD, Norman S. Talner, MD, Patricia Taylor, PhD, Robert Tepper, MD, PhD, Janet Wittes, PhD, Robert H. Yolken, MD, Peter E. Vink, MD.
References
- 1.Starc TJ, Lipshultz SE, Kaplan S, Easley KA, Bricker JT, Colan SD, et al. Cardiac complications in children with human immunodeficiency virus infection. Pediatrics. 1999;104(2):e14/1–9. doi: 10.1542/peds.104.2.e14. electronic citation. Available at: http://www.pediatrics.org/cgi/con-tent/full/104/2/e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langston C, Cooper ER, Goldfarb J, Easley K, Husak S, Sunkle S, et al. Human immunodeficiency virus-related mortality in infants and children: data from the Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV (P2C2) Study. Pediatrics. 2001;107:328–38. doi: 10.1542/peds.107.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipshultz SE, Easley KA, Orav EJ, Kaplan S, Starc TJ, Bricker JT, et al. Cardiac dysfunction and mortality in HIV-infected children: the Prospective P2C2 HIV Multicenter Study. Circulation. 2000;102:1542–8. doi: 10.1161/01.cir.102.13.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipshultz SE, Chanock S, Sanders SP, Colan SD, Perez-Atayde A, McIntosh K. Cardiac manifestations of human immunodeficiency virus infection in infants and children. Am J Cardiol. 1989;63:1489–97. doi: 10.1016/0002-9149(89)90014-3. [DOI] [PubMed] [Google Scholar]
- 5.Luginbuhl LM, Orav EJ, McIntosh K, Lipshultz SE. Cardiac morbidity and related mortality in children with HIV infection. JAMA. 1993;269:2869–75. [PubMed] [Google Scholar]
- 6.Steinherz LJ, Brochstein JA, Robins J. Cardiac involvement in congenital acquired immunodeficiency syndrome. Am J Dis Child. 1986;140:1241–4. doi: 10.1001/archpedi.1986.02140260043022. [DOI] [PubMed] [Google Scholar]
- 7.Grenier MA, Karr SS, Rakusan TA, Martin GR. Cardiac disease in children with HIV: relationship of cardiac disease to HIV symptomatology. Pediatr AIDS HIV Infect. 1994;5:174–9. [Google Scholar]
- 8.Johann-Liang R, Cervia JS, Noel GJ. Characteristics of human immunodeficiency virus-infected children at the time of death: an experience in the 1990s. Pediatr Infect Dis J. 1997;16:1145–50. doi: 10.1097/00006454-199712000-00009. [DOI] [PubMed] [Google Scholar]
- 9.P2C2 HIV Study Group. The pediatric pulmonary and cardiovascular complications of vertically transmitted human immunodeficiency virus (P2C2 HIV) infection study: design and methods. J Clin Epidemiol. 1996;49:1285–94. doi: 10.1016/s0895-4356(96)00230-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention 1994 revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR Morb Mortal Wkly Rep CDC Surveill Summ. 1994;43:1–10. [Google Scholar]
- 11.Lipshultz SE, Easley KA, Orav EJ, Kaplan S, Starc TJ, Bricker JT, et al. Left ventricular structure and function in children infected with human immunodeficiency virus: the prospective P2C2 HIV multicenter study. Circulation. 1998;97:1246–56. doi: 10.1161/01.cir.97.13.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipshultz SE, Easley KA, Orav EJ, Kaplan S, Starc TJ, Bricker JT, et al. Reliability of multicenter pediatric echocardiographic measurements of left ventricular structure and function: the prospective P2C2 HIV Study. Circulation. 2001;104:310–6. doi: 10.1161/01.cir.104.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colan SD, Parness IA, Spevak PJ, Sanders SP. Developmental modulation of myocardial mechanics: age-and-growth-related alterations in afterload and contractility. J Am Coll Cardiol. 1992;19:619–29. doi: 10.1016/s0735-1097(10)80282-7. [DOI] [PubMed] [Google Scholar]
- 14.Silverman NH. Pediatric echocardiography. Baltimore (MD): Williams and Wilkins; 1993. p. 38. [Google Scholar]
- 15.Lipshultz SE, Orav EJ, Sanders SP, McIntosh K, Colan SD. Limitations of fractional shortening as an index of contractility in pediatric patients infected with human immunodeficiency virus. J Pediatr. 1994;125:563–70. doi: 10.1016/s0022-3476(94)70008-7. [DOI] [PubMed] [Google Scholar]
- 16.Cleveland RH, Schluchter M, Wood BP, Berdon WE, Boechat MI, Easley KA, et al. Chest radiographic data acquisition and quality assurance in multicenter studies. Pediatr Radiol. 1997;27:880–7. doi: 10.1007/s002470050262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felson B. Chest roentgenology. Philadelphia (PA): WB Saunders; 1973. p. 496. [Google Scholar]
- 18.Condon VR, Jaffe RB. The heart and great vessels. In: Silverman FN, Kuhn JP, editors. Caffey's pediatric x-ray diagnosis: an integrated imaging approach. 9th. St Louis (MO): Mosby; 1993. p. 705. [Google Scholar]
- 19.Shearer W, Lipshultz SE, Easley KA, McIntosh K, Pitt J, Quinn TC, et al. Alterations in cardiac and pulmonary function in pediatric rapid human immunodeficiency virus type 1 disease progressors. Pediatrics. 2000;105(1):e9/1–8. doi: 10.1542/peds.105.1.e9. electronic citation. Available at: http://www.pediatrics.org/cgi/content/full/105/1/e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brownlee K. Statistical theory and methodology in science and engineering. 2nd. New York: John Wiley; p. 1965.p. 184. [Google Scholar]
- 21.Kahn HA, Sempos CT. Statistical methods in epidemiology. New York: Oxford University Press; 1989. p. 218. [Google Scholar]
- 22.Turner BJ, Denison M, Eppes SC, Houchens R, Fanning T, Markson LE. Survival experience of 789 children with acquired immunodeficiency syndrome. Pediatr Infect Dis J. 1993;12:310–20. doi: 10.1097/00006454-199304000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Domanski MJ, Sloas MM, Follmann DA, Scalise PP, Tucker EE, Egan D, et al. Effect of zidovudine and didanosine treatment on heart function in children infected with human immunodeficiency virus. J Pediatr. 1995;127:137–46. doi: 10.1016/s0022-3476(95)70275-x. [DOI] [PubMed] [Google Scholar]
- 24.Cheitlin MM. Cardiac involvement in the patient with AIDS. In: Merigan TC, Bartlett JG, Bolognesi D, editors. Text-book of AIDS medicine. Baltimore (MD): Williams and Wilkins; 1999. pp. 601–10. [Google Scholar]
- 25.Lipshultz SE, Fox CH, Perez-Atayde AR, Sanders SP, Colan SD, McIntosh K, et al. Identification of human immunodeficiency virus-1 RNA and DNA in the heart of a child with cardiovascular abnormalities and congenital acquired immune deficiency syndrome. Am J Cardiol. 1990;66:246–50. doi: 10.1016/0002-9149(90)90603-x. [DOI] [PubMed] [Google Scholar]
- 26.Kovacs A, Hinton DR, Wright D, Xu J, Li XL, Rasheed S, et al. Human immunodeficiency virus type 1 infection of the heart in three infants with acquired immunodeficiency syndrome and sudden death. Pediatr Infect Dis J. 1996;15:819–24. doi: 10.1097/00006454-199609000-00017. [DOI] [PubMed] [Google Scholar]
- 27.Herskowitz A, Willoughby SB, Baughman KL, Schulman SP, Bartlett JD. Cardiomyopathy associated with antiretroviral therapy in patients with HIV infection: a report of six cases. Ann Intern Med. 1992;116:311–3. doi: 10.7326/0003-4819-116-4-311. [DOI] [PubMed] [Google Scholar]
- 28.Wu TC, Pizzorno MC, Hayward GS, Willoughby S, Neumann DA, Rose NR, et al. In-situ detection of human cytomegalovirus immediate-early gene transcripts within cardiac myocytes of patients with HIV-associated cardiomyopathy. AIDS. 1992;6:777–85. doi: 10.1097/00002030-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Bowles NE, Kearney DL, Ni J, Perez-Atayde AR, Kline MW, Bricker JT, et al. The detection of viral genomes by polymerase chain reaction in myocardium of pediatric patients with advanced HIV disease. J Am Coll Cardiol. 1999;34:857–65. doi: 10.1016/s0735-1097(99)00264-8. [DOI] [PubMed] [Google Scholar]
- 30.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–84. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]