Abstract
Objective
To compare loss in sensitivity measured using standard automated perimetry (SAP) with local retinal ganglion cell layer (RGC) thickness measured using frequency-domain optical coherence tomography in the macula of patients with glaucoma.
Methods
To compare corresponding locations of RGC thickness with total deviation (TD) of 10-2 SAP for 14 patients with glaucoma and 19 controls, an experienced operator hand-corrected automatic segmentation of the combined RGC and inner plexiform layer (RGC + IPL) of 128 horizontal B-scans. To account for displacement of the RGC bodies around the fovea, the location of the SAP test points was adjusted to correspond to the location of the RGC bodies rather than to the photoreceptors, based on published histological findings. For analysis, RGC + IPL thickness vs SAP (TD) data were grouped into 5 eccentricities, from 3.4° to 9.7° radius on the retina with respect to the fovea.
Results
The RGC + IPL thickness correlated well with SAP loss within approximately 7.2° of the fovea (Spearman ρ = 0.71–0.74). Agreement was worse (0.53–0.65) beyond 7.2°, where the normal RGC layer is relatively thin. A linear model relating RGC + IPL thickness to linear SAP loss provided a reasonable fit for eccentricities within 7.2°.
Conclusion
In the central 7.2°, local RGC + IPL thickness correlated well with local sensitivity loss in glaucoma when the data were adjusted for RGC displacement.
Various studies have focused on the relationship between structural and functional damage as a way to improve our ability to detect the presence and progression of glaucomatous damage. Measurement of functional loss typically relies on behavioral tests, such as standard automated perimetry (SAP). Structural loss, on the other hand, can be assessed by examining the retinal ganglion cells (RGCs) and their axons, which constitute most of the retinal nerve fiber layer (RNFL), and the shape of the optic nerve head. Early analyses of structure-function relationships in glaucoma relied on optic disc appearance1 or on histological cell counts2–5 as a measure of structural loss. However, advances in noninvasive techniques, such as optical coherence tomography (OCT),6,7 confocal scanning laser ophthalmoscopy, and scanning laser polarimetry, allow for structural measurements in vivo, furthering structure-function analyses. (See other studies8–21 for additional references.) A variety of studies15–20 used peripapillary RNFL measurements obtained from time-domain OCT. One limitation of using the RNFL is the inability to conduct precise local measurements comparing structure with corresponding retinal areas of function because for a given region of the retina, the axons in the RNFL are originating from different regions. Second, and more important, the RNFL of the temporal region of the disc is relatively thin, with a high degree of variability, even in healthy individuals19 and, thus, may not be a good measure of structural damage to the all-important macular region.
A measure of local RGC thickness might mitigate some of these problems and could lead to improved structure vs function relationships, especially in the macular region, where the ganglion cell layer is the thickest and comprises multiple layers of soma. Although studies suggest macular involvement in glaucoma22,23 and macular thickness has been studied using time-domain OCT,24–26 the newer frequency-domain OCT allows higher-resolution structural imaging, making it easier to identify and measure the layers of the inner retina at the macula.27–29 For example, Wang et al29 found that local thickness of the RGC plus inner plexiform layer (RGC + IPL) could be obtained from frequency-domain OCT scans using a manual segmentation procedure30 and that these measures show qualitative agreement with local loss in visual field sensitivity. However, relatively little has been done to compare local sensitivity loss with local RGC + IPL thinning, although a variety of studies compared global measures of the thickness of the inner retinal regions at the macula. (See the study by Tan et al31 for additional references.) Although other studies32,33 measured inner retinal thickness and field sensitivity, the macula was only coarsely sampled using the 6° × 6°-grid spacing of the 24-2 visual field, and inner retinal thickness was obtained using the combined RNFL and RGC + IPL. The local RNFL, of course, is not a measure of local RGC loss because it contains fibers passing from other locations.
To compare local RGC + IPL thickness with local SAP sensitivity in the macula, we segmented RGC + IPL thickness and compared these measures with visual field loss on the 2° × 2°-grid spacing of the 10-2 visual field. In making these comparisons, RGC displacement near the fovea was taken into consideration.
METHODS
PARTICIPANTS
Nineteen eyes of 19 controls (mean [SD] age, 51.3 [9.7] years) had normal vision, no history of elevated intraocular pressure, and a normal optic disc appearance. Fourteen eyes of 14 patients (mean [SD] age, 57.7 [14.7] years) with glaucoma (10 normal-tension, 2 exfoliative, and 2 primary open-angle) were tested using SAP (Humphrey Field Analyzer II, SITA standard with appropriate trial lenses; Carl Zeiss Meditech, Inc, Dublin, California) and frequency-domain OCT (3D-OCT 1000; Topcon Inc, Paramus, New Jersey). All the patients exhibited glaucomatous optic neuropathy based on a clinical fundus examination and had reliable (fixation losses ≤30%, false-positive and false-negative rates ≤15%) abnormal 24-2 visual fields, defined as an abnormal glaucoma hemifield test (GHT) and an abnormal mean deviation (MD) (P ≤ .05) or pattern standard deviation (PSD) (P ≤ .05). To be included, patients were required to have a visual acuity of 20/50 or better and refractive error less than 6.00 diopters. Patients with a history of ocular surgery or of other ocular or neurologic diseases that could affect structural or functional measurements were excluded from the study. Written informed consent was obtained from all the participants. Procedures followed the tenets of the Declaration of Helsinki, and the protocol was approved by the institutional review boards of Columbia University and New York Eye and Ear Infirmary, New York.
MEASURES
Functional measures were obtained from 10-2 SAP tests. If both eyes were considered glaucomatous, the worse eye was chosen based on the 10-2 MD (mean [SD] MD, −7.7 [4.6] dB). All 10-2 SAP results were significantly abnormal (MD or PSD, P ≤ .01), met the reliability criteria as defined previously herein, and were performed within 1 year (mean [SD], 4.9 [3.6] months, with all but 1 within 8 months) of the frequency-domain OCT test used for structural measures. The scanning protocol consisted of 6 × 6-mm cube scans with macula (central) fixation. (See Figure 1B, grayscale overlay on fundus, for the region scanned.) The mean (SD) quality score of the scans was 58.7 (8.4). The scans consisted of 128 B-scans of 512 A-scans each. Figure 1A shows a sample scan.
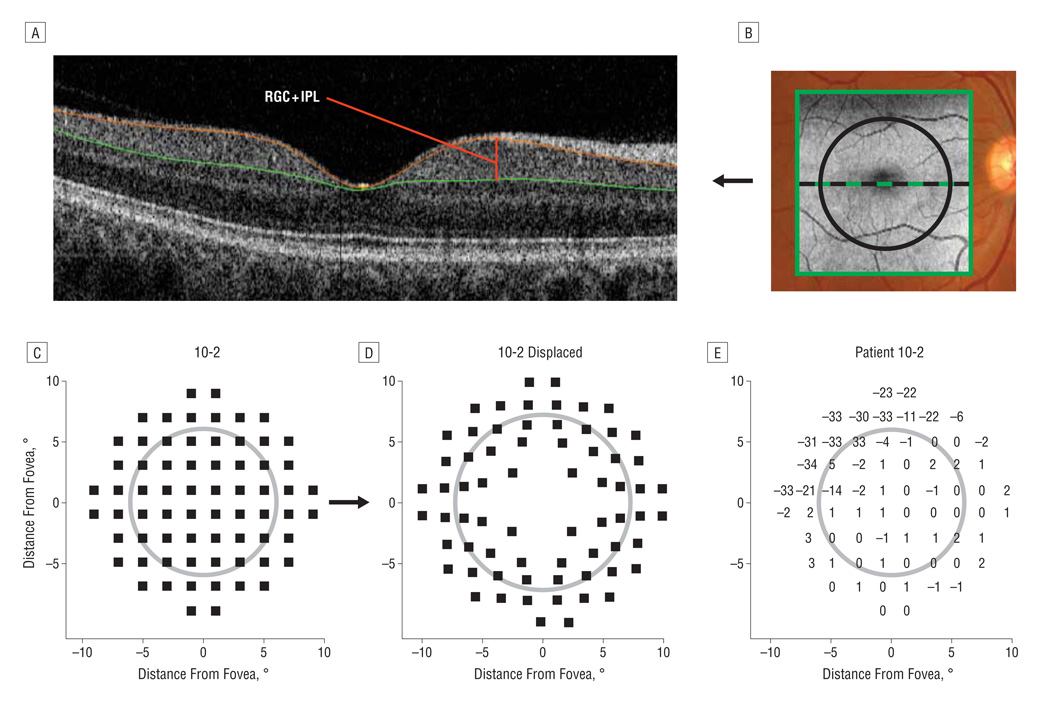
Figure 1.
An example of how the thickness measurements are obtained and an example of the adjustment for retinal ganglion cell (RGC) displacement. A, A horizontal cross section (B-scan) of the macular region as obtained from frequency-domain optical coherence tomography (fdOCT), with the segmented boundaries shown in color. The RGC + inner plexiform layer (RGC + IPL) thickness is shown as the red line. B, An overlay of the fdOCT cube scan with the sections manually segmented marked with black lines. The original 10-2 visual field points (C) and displaced points (D) corresponding to the RGC location based on a model derived from histological analysis.32 E, A patient’s 10-2 visual field with total deviation values from age-matched controls. The 7.2° black circle in B–E is for reference.
ANALYSIS
For analysis of functional measures, the total deviation (TD) values (in decibels), provided by the 10-2 SAP test, was obtained for each patient. The TD values represent the local loss in sensitivity compared with age-matched controls (Figure 1E).
The local structural thickness was determined for the individual B-scans of the cube scan. In particular, for each B-scan, the boundaries of anatomical layers were determined using a previously validated automated segmentation algorithm,34 which was then hand-corrected using manual segmentation via a custom program as previously described,29,30 a technique that has been shown to provide reliable and repeatable results.35 The hand correction was performed by an experienced operator (A.S.R.) masked to the patient’s other data. The results were similar to those obtained by another operator (J.C.) using a manual segmentation procedure29 on a subset of the scans. Figure 1A shows the borders segmented and indicates the RGC + IPL for which we calculated thickness values. Because the boundary between the RGC layer and the IPL can sometimes be hard to determine, we measured the combined RGC + IPL. For each cube scan, we segmented 128 B-scans. The RGC + IPL thickness measures were then smoothed in 2 dimensions using an averaging filter.
Comparing RGC + IPL thickness with local loss in SAP sensitivity, it is important to consider displacement of the RGCs in the macula.27 The average location of the RGCs associated with each SAP test point was approximated using equations derived from the histological analysis–based work of Drasdo et al.36 For ease of presentation, all left eyes were flipped across the vertical midline to appear as right eyes, and all structural data were flipped across the horizontal midline for display in “field view.” Figure 1C shows the location of the 10-2 visual field SAP test points, and Figure 1D shows the location after adjusting for RGC displacement. All the quantitative analyses were performed using technical computing software (Matlab; MathWorks, Natick, Massachusetts).
MODEL
To assess the relationship of the data, a simple linear model was used based on previous work17–19 relating SAP sensitivity to peripapillary RNFL thickness. The model has 3 assumptions: (1) The RGC + IPL thickness R is composed of 2 components, the thickness s (for signal) due to the RGC bodies and the residual b (for base level). Thus, R = s + b, where b includes the portions of the IPL and RGC layer not affected by glaucoma, which may include glial cells, blood vessels, bipolar cell axons, and amacrine cell projections. (2) As SAP field sensitivity changes, the value of s decreases, and b remains constant. (3) As SAP sensitivity decreases, the signal portion s of the total RGC + IPL thickness R decreases linearly when SAP is expressed in linear units (not decibels). Thus, R = (s0 − b) T + b for T ≤ 1.0 and R = s0 for t > 1.0, where s0 is the median of control RGC + IPL thickness at a particular eccentricity and T is the relative sensitivity, defined as 100.1D, where D is the TD value of SAP sensitivity from age-matched controls in decibels. The variable b was estimated as the median of the RGC + IPL thickness values at locations where the SAP sensitivity was less than −15 dB as previously described.18
RESULTS
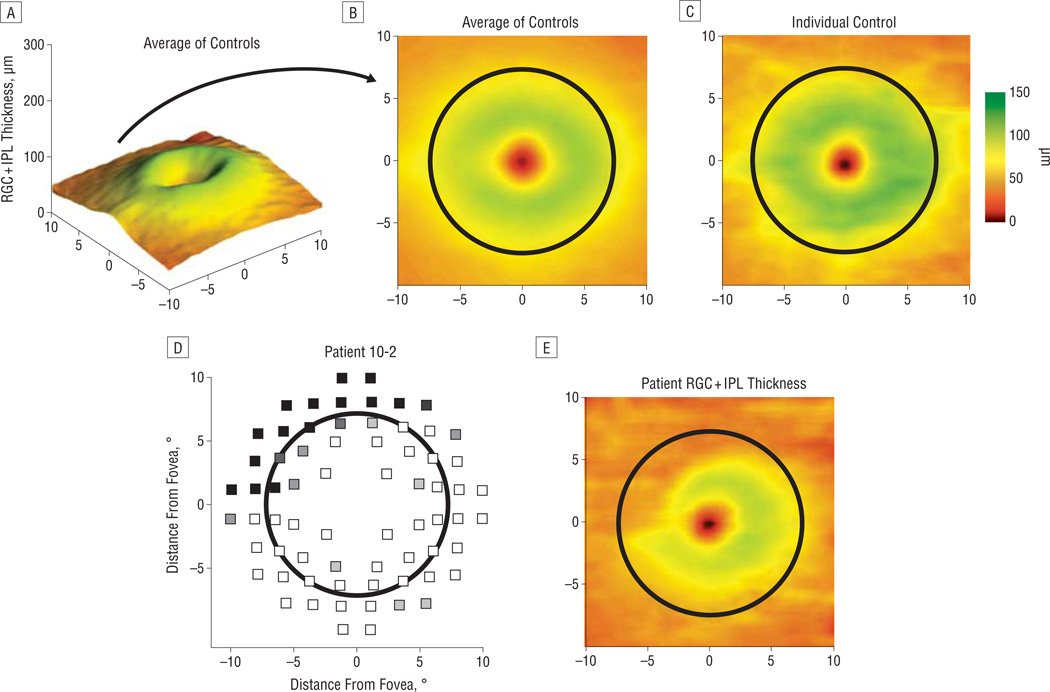
The mean RGC + IPL thickness for the 19 controls is presented in Figure 2A as a 3-dimensional thickness plot and in Figure 2B as a 2-dimensional pseudocolor plot over the 6 × 6-mm (approximately 20° × 20°) central region. Green represents the region of greatest thickness, and black represents the region of lowest thickness. Most of the thicker region lies inside approximately 7.2° on the retina (black circle), which corresponds to 6° for the visual field test stimuli before accounting for ganglion cell displacement. The RGC + IPL thickness of an individual control is presented in Figure 2C.
Figure 2.
The average thickness of controls along with individual examples of a control and a patient with glaucoma. The retinal ganglion cell + inner plexiform layer (RGC + IPL) thickness from the average of all controls as a 3-dimensional projection (A) and as a 2-dimensional pseudocolor map (B). C, Two-dimensional map for an individual control. D, A patient’s 10-2 visual field after morphing to account for RGC displacement, with grayscale representing the depth of field defect (black is worse). E, The same patient’s RGC + IPL thickness.
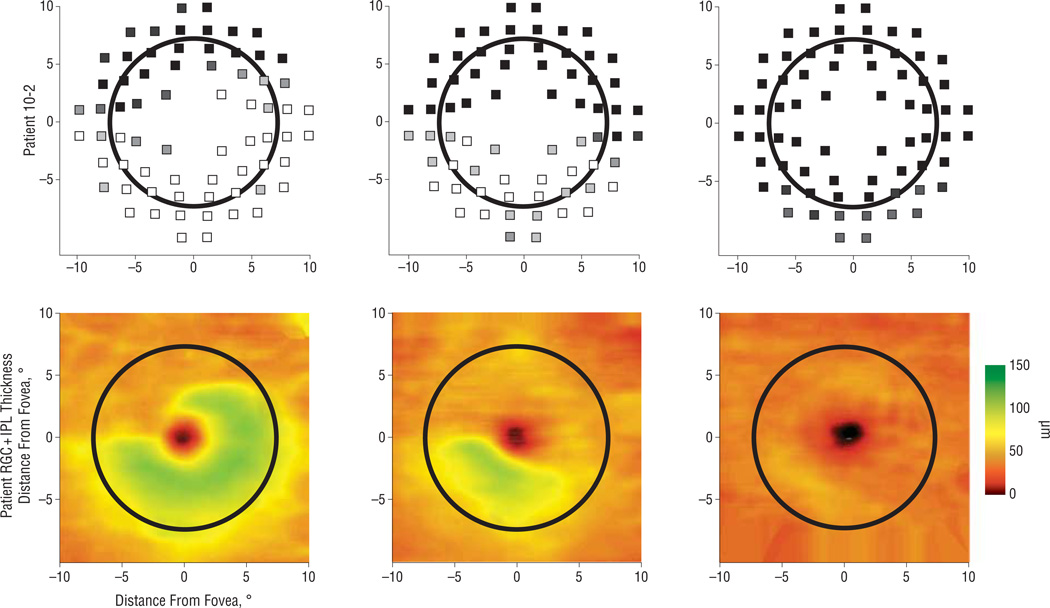
For patients with defects, there was a conspicuous qualitative agreement between the visual field defect and the corresponding local RGC + IPL thickness. For example, a patient with a deep superonasal field defect on the 10-2 visual field (Figure 2D) exhibited a corresponding decrease in RGC + IPL thickness in the inferotemporal retina (Figure 2E, field view). Other examples can be seen in Figure 3.
Figure 3.
Three examples of qualitative agreement between displaced 10-2 visual fields (top row, as in Figure 2D) and retinal ganglion cell + inner plexiform layer (RGC + IPL) thickness (bottom row). The different patients show varying states of disease. The thickness map has been flipped along the horizontal meridian to correspond to the visual field.
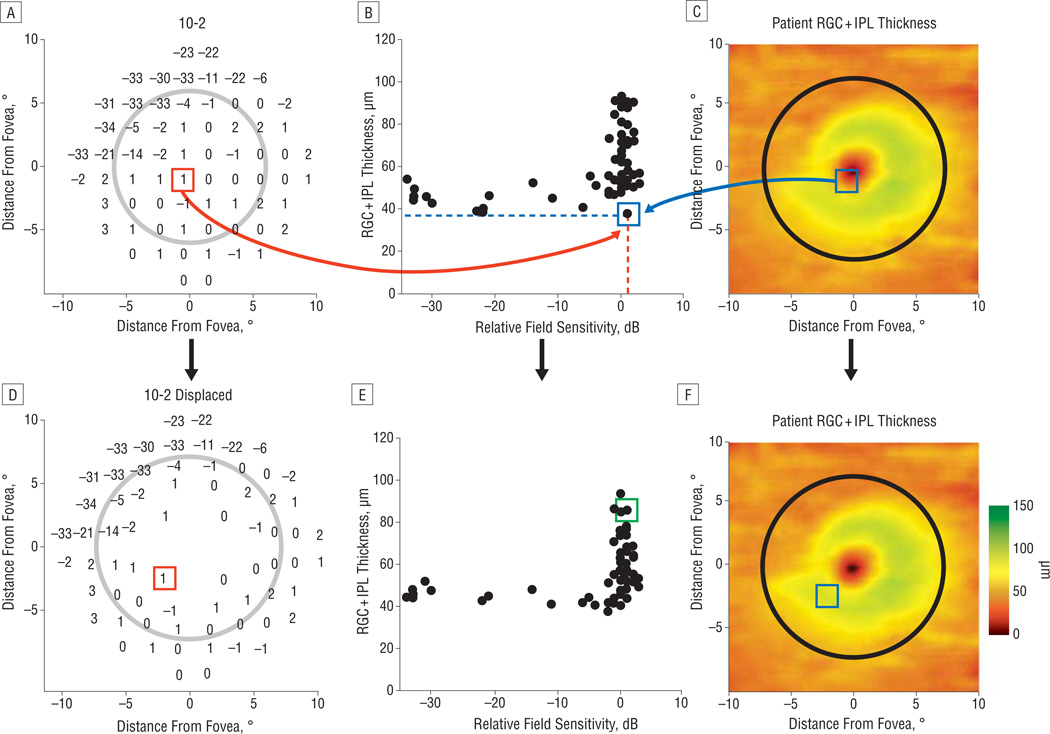
For a quantitative comparison, local RGC + IPL thickness (blue box in Figure 4C and blue dotted line in Figure 4B) was plotted vs relative SAP sensitivity (TD) (red box in Figure 4A and red dotted line in Figure 4B) as shown in Figure 4B (green square). The plot in Figure 4B shows an orderly relationship between RGC + IPL thickness and visual field loss, with the RGC + IPL thickness decreasing rapidly to an asymptotic level with loss of sensitivity. This relationship is clearer after accounting for displacement of the RGCs (Figure 4D–F). For example, the data point outlined in green in Figure 4B was initially an outlier because a normal sensitivity (0 dB of TD) corresponded to a thin region of the RGC + IPL, but it corresponded to a thicker region after displacement.
Figure 4.
An example of the structure-function relationship of a particular patient along with an illustration of the reduced variability resulting from adjusting for retinal ganglion cell (RGC) displacement. A patient’s 10-2 visual field (A) and RGC + inner plexiform layer (RGC + IPL) thickness (C) and the quantitative relationship between all the 10-2 visual field total deviation (relative sensitivity) values and the RGC + IPL thickness at the corresponding location (B). One location in the visual field at the x, y coordinates of 1°,−1° (red box) is shown with the corresponding data point on the structure-function plot (green box; part E) and on the corresponding location in the thickness plot (blue box; part B). Without accounting for the spatial displacement of ganglion cells, the value for thickness and threshold is an outlier on the structure-function plot (B). D–F, The relationship improves and the point in the green box now resides in the distribution of the structure-function values after accounting for displacement of the RGCs.
QUANTITATIVE ASSESSMENT OF RELATIONSHIP
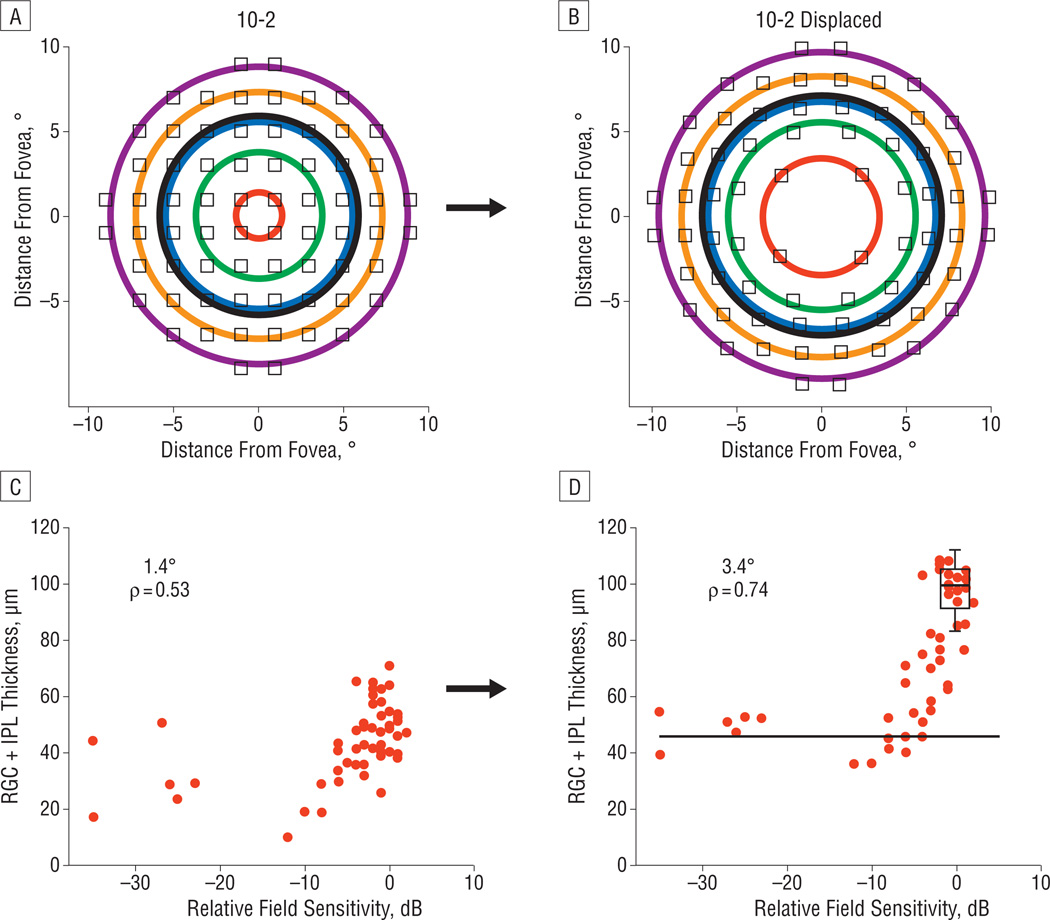
By combining the data from all the eccentricities in Figure 4, we are implicitly assuming that the functional relationship between RGC + IPL thickness and SAP loss is the same at all retinal locations. To avoid this assumption, the data were divided by groups of SAP test points into concentric rings (Figure 5A) ranging from 1.4° to 8.8° in radius for the visual field test points and, after accounting for ganglion cell displacement, corresponding to eccentricities ranging from 3.4° to 9.7° on the retina (Figure 5B). Figure 5C shows the data from the eccentricity ring closest to the fovea, which corresponds to 1.4° for the visual field test points, for all 14 patients. Figure 5D illustrates the decreased scatter after adjusting for displacement. Displacement improves the Spearman rank correlation coefficient from 0.53 to 0.74. The horizontal black line marks the asymptotic (residual) value, calculated as the median RGC + IPL thickness of all data points with SAP TD worse than or equal to −15 dB. The box plot indicates the median, quartiles, and 95% limits of the control data for this eccentricity.
Figure 5.
An illustration of the grouping of standard automated perimetry test points by eccentricity and the structure-function relationship of the most central test points, both before and after adjusting for retinal ganglion cell (RGC) displacement. The original 10-2 visual field points (A) and displaced points (B) to account for RGC location, grouped in rings of various eccentricities for analysis (3.4°, 5.6°, 6.8°, 8.3°, and 9.7° on the retina after accounting for displacement). C, The relationship between RGC + inner plexiform layer (IPL) thickness and corresponding 10-2 visual field total deviation values for the innermost eccentricity ring (1.4°). D, The improved correlation after accounting for displacement (now 3.4° on the retina). The black horizontal line is the asymptotic residual thickness, and the box plot represents the median, quartiles, and central 95% of the distribution of control RGC + IPL thickness values.
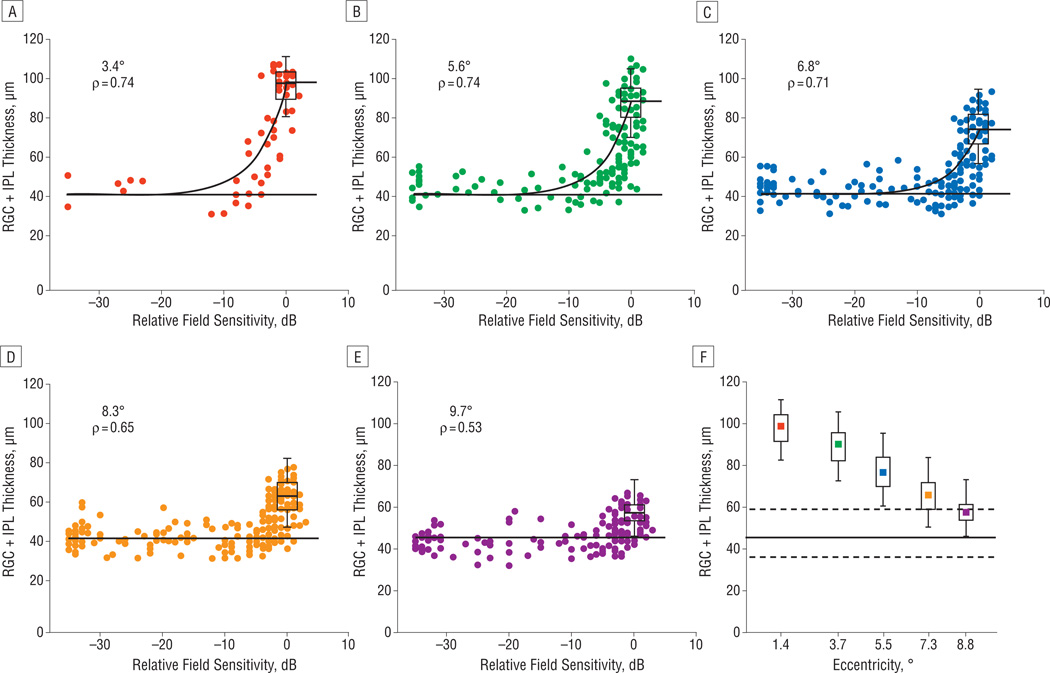
Figure 6 presents the data for all 6 rings. For rings within approximately 7.2° on the retina (red, green, and blue), the data show a stronger relationship than do the data outside this region (orange and purple). Although the inner 3 eccentricity rings (red, green, and blue) show a notable decrease in RGC + IPL thickness to an asymptotic level as the SAP sensitivity decreases, the change with respect to SAP sensitivity (ie, disease state) is less appreciable for the outer 2 rings (orange and purple). The correlations reflect this qualitative impression, with higher values (0.71–0.74) observed within 7.2° and lower values (0.53–0.65) outside this region. The control data also reflect this trend (Figure 6F), where the median and central 95% decrease with eccentricity and, for the outer 2 rings (orange and purple), overlap considerably with the 95% limits of the residual (dotted horizontal lines).
Figure 6.
The structure-function relationship of the remaining data points, sorted by eccentricity, as well as a summary of control data compared with patient residual thickness across all eccentricities. A–F, The relationship between retinal ganglion cell + inner plexiform layer (RGC + IPL) thickness and corresponding 10-2 visual field total deviation values for various eccentricity rings (3.4°–9.7°). The Spearman rank correlations (ρ) decrease with eccentricity. The black horizontal line is the assumed asymptotic residual thickness, and the box plot represents the median, quartiles, and central 95% of the distribution of control RGC + IPL thickness values. The solid black curve in A–C is the prediction of a linear model describing the relationship for the eccentricity rings within approximately 7.2° on the retina. F, The box plots of control values (as before) plotted with increasing eccentricity. The solid black line is the asymptotic residual thickness, and the dotted lines above and below it encompass the central 95% of the data used to calculate the residual.
As a tool to assess the structure vs function relationship, we fitted a simple linear model to the data within 7.2° on the retina. The simple linear model uses the predicted residual (horizontal black line) as the y-intercept and the median of control data as the value for normal (0 dB of TD) sensitivity. The fit was reasonable, although there is a suggestion of a systematic bias where RGC + IPL thicknesses associated with relatively small visual field losses (eg, −3 to −10 dB) are smaller than predicted by the model.
COMMENT
At a qualitative level, there is a clear relationship (eg, Figures 2D and E and 3) between regions of visual field scotomas and corresponding regions of RGC + IPL thinning, which was noted in all the patients studied. A quantitative analysis indicated that the overall relationship was orderly, particularly when the data were segregated by eccentricity (Figure 6A–E), with correlations ranging from 0.53 to 0.74. The relationship was improved considerably by accounting for anatomical displacement of the RGCs based on a model by Drasdo et al.36 The effect of displacement was greatest at the central eccentricity ring, as might be expected given that the largest degree of displacement occurs closest to the fovea. For the most central ring, the correlation improved from 0.53 to 0.74 with displacement (Figure 5C and D).
The quantitative relationship observed was considerably weaker outside approximately 7.2° on the retina (approximately 6° on the visual field). This is not surprising given that the RGC + IPL thickness decreased markedly beyond this region, even in normal eyes. In particular, for eccentricities beyond approximately 7.2° on the retina, a strong relationship is not expected because the RGC + IPL thickness values at these eccentricities associated with near-normal sensitivities (approximately 0 dB) do not differ markedly from the RGC + IPL thickness values associated with severe visual loss (≤−15 dB). This is due to the known thinning of the RGC layer to a single layer of cells as the more peripheral regions of the macula are approached.
Of interest was the observed asymptotic loss across all eccentricities (Figure 6A–E), with a median RGC + IPL thickness residual value associated with severe sensitivity loss of approximately 45 µm. This observed residual is approximately one-half of the initial control median RGC + IPL thickness for the ring closest to the fovea (3.4° on the retina), and for the outermost eccentricity considered (8.8° on the retina), the residual comprises greater than 80% of the initial control median RGC + IPL thickness. Thus, the residual amount is too large (and too uniform) to be explained by a factor such as the inclusion of blood vessels. Although we cannot rule out a thickening due to gliosis or neuronal remodeling, the residual is more likely due to a relative preservation of the IPL because we have recently seen this in patients months after ischemic optic neuropathy.37
The simple linear model, an extension of a similar model applied to the RNFL,19 performs reasonably well for the eccentricities within approximately 7.2°. There is a suggestion of a systematic deviation at low visual field sensitivities, suggesting that initial glaucomatous damage might affect RGC + IPL thickness more than expected from the loss of SAP sensitivity. This deviation, if reliable, may be explained, at least in part, by models that consider spatial summation (eg, cortical pooling)3,4 or neuronal remodeling.38
Regarding the general quantitative agreement, the intraindividual and interindividual variability on both axes20 limits the ability of one measure to precisely predict the other. However, relatively large RGC + IPL thicknesses are clearly associated with near-normal sensitivities, and severely abnormal visual field points are strongly associated with small RGC + IPL thicknesses. As is shown in the qualitative examples (Figures 2D and E and 3), in a clinical setting, the RGC + IPL thickness should be capable of showing normal or abnormal regions that agree spatially with the visual field. The degree to which the RGC + IPL thickness can stage glaucomatous damage will depend on the ability to reduce interindividual variability. For questions such as progression, observing changes in repeat visits by the same patient, which is not affected by the variability between subjects, may hold more promise. In any case, once visual field damage becomes more severe than approximately −10 dB, the ability of the RGC + IPL thickness to predict the gradations in visual field loss in the same location is limited.
Finally, note that the study population consisted mostly of patients with normal-tension glaucoma. Although we did not notice any obvious difference in the structure-function agreement for patients with exfoliative and primary open-angle glaucoma, further work should consider different types of glaucoma more closely. In addition, although we did not observe an age effect in this relatively small sample, a larger study should address this issue as well. A future study should also look more closely at RGC + IPL thickness in a subpopulation of glaucoma suspects (ie, those with evidence of an optic neuropathy but normal visual field sensitivity).
In conclusion, the patients with glaucoma in this study showed a reasonably strong relationship between loss in SAP sensitivity and decrease in local RGC + IPL thickness. This relationship is improved after accounting for anatomical displacement of the RGCs. Combining local RGC + IPL thickness with local SAP field loss may help stage the degree of early glaucomatous damage to the macula.
Acknowledgments
Funding/Support: This study was supported by grants from the National Institute of Health (EY02115); Research to Prevent Blindness; the Department of Veterans Affairs Rehabilitation and Research Division (Dr Kardon); Topcon Inc; the Joseph and Geraldine LaMotta Research Fund of the New York Glaucoma Research Institute; and the Glaucoma Research and Education Fund of Lenox Hill Hospital (Dr de Moraes). Topcon Inc also supplied the ocular coherence tomography machine used in this study.
Footnotes
Author Contributions: Mr Raza had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Disclosure: Drs Hood, Liebmann, and Zhang report being consultants for Topcon Inc.
REFERENCES
- 1.Spaeth GL, Lopes JF, Junk AK, Grigorian AP, Henderer J. Systems for staging the amount of optic nerve damage in glaucoma: a critical review and new material. Surv Ophthalmol. 2006;51(4):293–315. doi: 10.1016/j.survophthal.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Quigley HA, Dunkelberger GR, Green WR. Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol. 1989;107(5):453–464. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 3.Garway-Heath DF, Caprioli J, Fitzke FW, Hitchings RA. Scaling the hill of vision: the physiological relationship between light sensitivity and ganglion cell numbers. Invest Ophthalmol Vis Sci. 2000;41(7):1774–1782. [PubMed] [Google Scholar]
- 4.Swanson WH, Felius J, Pan F. Perimetric defects and ganglion cell damage: interpreting linear relations using a two-stage neural model. Invest Ophthalmol Vis Sci. 2004;45(2):466–472. doi: 10.1167/iovs.03-0374. [DOI] [PubMed] [Google Scholar]
- 5.Harwerth RS, Quigley HA. Visual field defects and retinal ganglion cell losses in patients with glaucoma. Arch Ophthalmol. 2006;124(6):853–859. doi: 10.1001/archopht.124.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuman JS, Hee MR, Puliafito CA, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol. 1995;113(5):586–596. doi: 10.1001/archopht.1995.01100050054031. [DOI] [PubMed] [Google Scholar]
- 8.Garway-Heath DF, Holder GE, Fitzke FW, Hitchings RA. Relationship between electrophysiological, psychophysical, and anatomical measurements in glaucoma. Invest Ophthalmol Vis Sci. 2002;43(7):2213–2220. [PubMed] [Google Scholar]
- 9.Garway-Heath DF. Comparison of structural and functional methods. In: Weinreb RN, Greve EL, editors. Glaucoma Diagnosis: Structure and Function. The Hague, the Netherlands: Kugler Publications; 2004. pp. 135–143. [Google Scholar]
- 10.Leung CK, Chong KK, Chan WM, et al. Comparative study of retinal nerve fiber layer measurement by StratusOCT and GDx VCC, II: structure/function regression analysis in glaucoma. Invest Ophthalmol Vis Sci. 2005;46(10):3702–3711. doi: 10.1167/iovs.05-0490. [DOI] [PubMed] [Google Scholar]
- 11.Bowd C, Zangwill LM, Medeiros FA, et al. Structure-function relationships using confocal scanning laser ophthalmoscopy, optical coherence tomography, and scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2006;47(7):2889–2895. doi: 10.1167/iovs.05-1489. [DOI] [PubMed] [Google Scholar]
- 12.Mai TA, Reus NJ, Lemij HG. Structure-function relationship is stronger with enhanced corneal compensation than with variable corneal compensation in scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2007;48(4):1651–1658. doi: 10.1167/iovs.06-1003. [DOI] [PubMed] [Google Scholar]
- 13.Kim KH, Choi J, Lee CH, Cho BJ, Kook MS. Relationship between scanning laser polarimetry with enhanced corneal compensation and with variable corneal compensation. Korean J Ophthalmol. 2008;22(1):18–25. doi: 10.3341/kjo.2008.22.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drasdo N, Mortlock KE, North RV. Ganglion cell loss and dysfunction: relationship to perimetric sensitivity. Optom Vis Sci. 2008;85(11):1036–1042. doi: 10.1097/OPX.0b013e31818b94af. [DOI] [PubMed] [Google Scholar]
- 15.Harwerth RS, Vilupuru AS, Rangaswamy NV, Smith EL., III The relationship between nerve fiber layer and perimetry measurements. Invest Ophthalmol Vis Sci. 2007;48(2):763–773. doi: 10.1167/iovs.06-0688. [DOI] [PubMed] [Google Scholar]
- 16.Harwerth RS, Wheat JL, Rangaswamy NV. Age-related losses of retinal ganglion cells and axons. Invest Ophthalmol Vis Sci. 2008;49(10):4437–4443. doi: 10.1167/iovs.08-1753. [DOI] [PubMed] [Google Scholar]
- 17.Harwerth RS, Wheat JL, Fredette MJ, Anderson DR. Linking structure and function in glaucoma. Prog Retin Eye Res. 2010;29(4):249–271. doi: 10.1016/j.preteyeres.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hood DC, Anderson SC, Wall M, Kardon RH. Structure versus function in glaucoma: an application of a linear model. Invest Ophthalmol Vis Sci. 2007;48(8):3662–3668. doi: 10.1167/iovs.06-1401. [DOI] [PubMed] [Google Scholar]
- 19.Hood DC, Kardon RH. A framework for comparing structural and functional measures of glaucomatous damage. Prog Retin Eye Res. 2007;26(6):688–710. doi: 10.1016/j.preteyeres.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hood DC, Anderson SC, Wall M, Raza AS, Kardon RH. A test of a linear model of glaucomatous structure-function loss reveals sources of variability in retinal nerve fiber and visual field measurements. Invest Ophthalmol Vis Sci. 2009;50(9):4254–4266. doi: 10.1167/iovs.08-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson CA, Cioffi GA, Liebmann JR, Sample PA, Zangwill LM, Weinreb RN. The relationship between structural and functional alterations in glaucoma: a review. Semin Ophthalmol. 2000;15(4):221–233. doi: 10.3109/08820530009037873. [DOI] [PubMed] [Google Scholar]
- 22.Kotecha A, O’Leary N, Melmoth D, Grant S, Crabb DP. The functional consequences of glaucoma for eye-hand coordination. Invest Ophthalmol Vis Sci. 2009;50(1):203–213. doi: 10.1167/iovs.08-2496. [DOI] [PubMed] [Google Scholar]
- 23.Schiefer U, Papageorgiou E, Sample PA, et al. Spatial pattern of glaucomatous visual field loss obtained with regionally condensed stimulus arrangements. Invest Ophthalmol Vis Sci. 2010;51(11):5685–5689. doi: 10.1167/iovs.09-5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenfield DS, Bagga H, Knighton RW. Macular thickness changes in glaucomatous optic neuropathy detected using optical coherence tomography. Arch Ophthalmol. 2003;121(1):41–46. doi: 10.1001/archopht.121.1.41. [DOI] [PubMed] [Google Scholar]
- 25.Lederer DE, Schuman JS, Hertzmark E, et al. Analysis of macular volume in normal and glaucomatous eyes using optical coherence tomography. Am J Ophthalmol. 2003;135(6):838–843. doi: 10.1016/s0002-9394(02)02277-8. [DOI] [PubMed] [Google Scholar]
- 26.Kanadani FN, Hood DC, Grippo TM, et al. Structural and functional assessment of the macular region in patients with glaucoma. Br J Ophthalmol. 2006;90(11):1393–1397. doi: 10.1136/bjo.2006.099069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hood DC, Raza AS, de Moraes CGV, et al. Initial arcuate defects within the central 10 degrees in glaucoma. Invest Ophthalmol Vis Sci. 2011;52(2):940–946. doi: 10.1167/iovs.10-5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakamoto A, Hangai M, Nukada M, et al. Three-dimensional imaging of the macular retinal nerve fiber layer in glaucoma with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51(10):5062–5070. doi: 10.1167/iovs.09-4954. [DOI] [PubMed] [Google Scholar]
- 29.Wang M, Hood DC, Cho JS, et al. Measurement of local retinal ganglion cell layer thickness in patients with glaucoma using frequency-domain optical coherence tomography [published correction appears in Arch Ophthalmol. 2010;128(9):1150] Arch Ophthalmol. 2009;127(7):875–881. doi: 10.1001/archophthalmol.2009.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hood DC, Lin CE, Lazow MA, Locke KG, Zhang X, Birch DG. Thickness of receptor and post-receptor retinal layers in patients with retinitis pigmentosa measured with frequency-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50(5):2328–2336. doi: 10.1167/iovs.08-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan O, Chopra V, Lu AT, et al. Detection of macular ganglion cell loss in glaucoma by Fourier-domain optical coherence tomography. Ophthalmology. 2009;116(12):2305–2314. e1–e2. doi: 10.1016/j.ophtha.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim NR, Lee ES, Seong GJ, Kim JH, An HG, Kim CY. Structure-function relationship and diagnostic value of macular ganglion cell complex measurement using Fourier-domain OCT in glaucoma. Invest Ophthalmol Vis Sci. 2010;51(9):4646–4651. doi: 10.1167/iovs.09-5053. [DOI] [PubMed] [Google Scholar]
- 33.Cho JW, Sung KR, Lee S, et al. Relationship between visual field sensitivity and macular ganglion cell complex thickness as measured by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51(12):6401–6407. doi: 10.1167/iovs.09-5035. [DOI] [PubMed] [Google Scholar]
- 34.Yang Q, Reisman CA, Wang Z, et al. Automated layer segmentation of macular OCT images using dual-scale gradient information. Opt Express. 2010;18(20):21293–21307. doi: 10.1364/OE.18.021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hood DC, Cho J, Raza AS, Dale EA, Wang M. Reliability of a computer-aided manual procedure for segmenting optical coherence tomography scans. Optom Vis Sci. 2011;88(1):113–123. doi: 10.1097/OPX.0b013e3181fc3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drasdo N, Millican CL, Katholi CR, Curcio CA. The length of Henle fibers in the human retina and a model of ganglion receptive field density in the visual field. Vision Res. 2007;47(22):2901–2911. doi: 10.1016/j.visres.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moura AL, Raza AS, Cho J, et al. Retinal ganglion cell and inner plexiform layer thickness measured with fdOCT in regions of severe sensitivity loss in patients with ischemic optic neuropathy [ARVO abstract] Invest Ophthalmol Vis Sci. 2010;51 E-Abstract 659. [Google Scholar]
- 38.Morgan JE. Retinal ganglion cell shrinkage in glaucoma. J Glaucoma. 2002;11(4):365–370. doi: 10.1097/00061198-200208000-00015. [DOI] [PubMed] [Google Scholar]