Abstract
Target Audience
Radiologists and other professionals involved in imaging of oropharyngeal swallowing
Objectives
To review anatomy of the upper GI tract
To review techniques and contrast agents used in the fluoroscopic examination of the oropharynx and hypopharynx
To provide a pictorial review of some important causes of oropharyngeal dysphagia, and to link these to key findings in the clinical history to assist in establishing a clinical diagnosis
To provide self-assessment questions to reinforce key learning points
DYSPHAGIA
Swallowing disorders occur in all age groups and arise from a variety of medical conditions. Swallowing disorders are common, especially in the elderly, and may lead to dehydration, weight loss, aspiration pneumonia, airway obstruction, severely reduced quality of life, and even death {[1]; Goyal}. The prevalence of dysphagia is estimated to be greater than 20% in persons over the age of 50 [2]. In long term care facilities, one study showed that up to 60% of the elderly suffer from eating difficulties [3].
The swallowing process is typically described as having several phases: oral, pharyngeal and cervical esophageal [4]. The oral phase is sometimes further subdivided into two sub-phases (oral preparatory and oral propulsive phases). Together, these sub-phases involve the mastication and breakdown of solid food into a bolus of a swallow-ready consistency and propulsion by the tongue of both liquids and semi-solid materials into the pharynx [5]. These actions are followed by pharyngeal phase, in which driving forces generated by the tongue propel the bolus downwards through the oro-pharynx. During this phase, several valves must be closed to generate a closed pressure system and prevent adverse events including retrograde flow of material into the nose (nasal regurgitation) and aspiration (entry of material into the airway). This includes closure of the nasopharynx, closure of the laryngeal vestibule and the true vocal folds, and approximation of the tongue base to the posterior pharyngeal wall to generate a pressure seal behind the bolus [6]. Closure of the laryngeal vestibule is critical for airway protection, and occurs secondary to upward and anterior movement of the hyolaryngeal complex [7]. The final element of the pharyngeal phase involves relaxation of the upper esophageal sphincter (including the cricopharyngeus muscle) allowing the bolus to enter the cervical esophagus, and sequential contraction of the superior, middle and inferior pharyngeal constriction muscles, which facilitates pharyngeal clearance of the bolus [8]. Once the bolus passes through the upper esophageal sphincter, the esophageal phase of swallowing begins, in which material is transported towards the lower esophageal sphincter and stomach via peristaltic contractions of the smooth muscle of the thoracic esophagus [9].
The clinical signs and symptoms of dysphagia differ depending on the phase of swallowing that is affected. Therefore, the radiologist should be aware of specific signs and symptoms, which would dictate the most appropriate type of imaging technique to be performed for proper understanding and management of the problem. Oro-pharyngeal dysphagia is defined as difficulty moving food bolus from the mouth to the esophagus. It is characterized by impairments in swallowing safety (airway protection) and efficiency (bolus clearance) [10]. Typical presenting clinical signs involve coughing after the swallow, indicating possible aspiration of material into the airway [11]. Additionally, observations of multiple swallows for each bolus may indicate inefficiency and the presence of post-swallow residues in the recesses of the pharynx such as the valleculae and pyriform sinuses [12]. Clinical, non-radiographic assessment of oropharyngeal dysphagia (also known as the bedside swallowing assessment) is typically performed by speech-language pathologists, but in some cases may be conducted or assisted by other members of the allied health team with specific expertise related to feeding, eating, swallowing and nutrition. A speech-language pathologist will also typically collaborate with radiological staff in performing videofluoroscopic assessments of swallowing[13, 14]. In some cases, the patient may be referred directly for radiological assessment. The videofluoroscopic swallowing study (also known as the “modified barium swallow”) is a dynamic x-ray examination utilizing high frame rate image capture [4, 15]. An alternative approach to the instrumental examination of oropharyngeal swallowing is the fiberoptic endoscopic evaluation of swallowing (FEES) [16]. The advantages and disadvantages of the different oropharyngeal assessment options are discussed in Table 1. In contrast to the signs of oropharyngeal swallowing impairment, patients with esophageal dysphagia frequently complain of the feeling of a lump in the throat, or a sensation of food sticking at the sternal notch (the so-called “Globus” sensation [17, 18]). This symptom is frequently associated with distal esophageal lesions, such as achalasia or gastroesophageal reflux, and requires a full Upper GI study for proper investigation [19].
TABLE 1.
COMPARISIONS OF TECHNIQUES USED TO EVALUATE ORO-PHARYNGEAL DYSPHAGIA
| Procedure | Advantages | Disadvantages |
|---|---|---|
| Bedside swallow assessment | Easily performed at the bedside | Does not detect silent aspiration; less objective means of assessment. |
| Videofluoroscopy | Allows for direct assessment of oral cavity, pharynx, and esophagus; can evaluate what is occurring during the swallow without need to infer. | Radiation exposure-limits time; potential difficulty positioning patient; potential influence of the taste and texture of barium. |
| Fiberoptic endoscopic evaluation of swallowing (FEES) | Portable; does not expose patient to radiation; patient can view the assessment on screen for biofeedback when performing compensatory swallowing strategies. | Unable to view esophageal function or UES; moment of no vision occurs during swallow due to white-out, so viewing of the entire swallow is not possible. |
The videofluoroscopic swallowing study can evaluate motility of the oropharynx and hypopharynx and provides images that may identify structural, motility and mucosal abnormalities more accurately. The study can be reviewed later with referring physicians or speech-language pathologists, as well as with patients and their family members to explain management recommendations.
RADIOLOGIC AND FUNCTIONAL ANATOMY OF THE OROPHARYNX
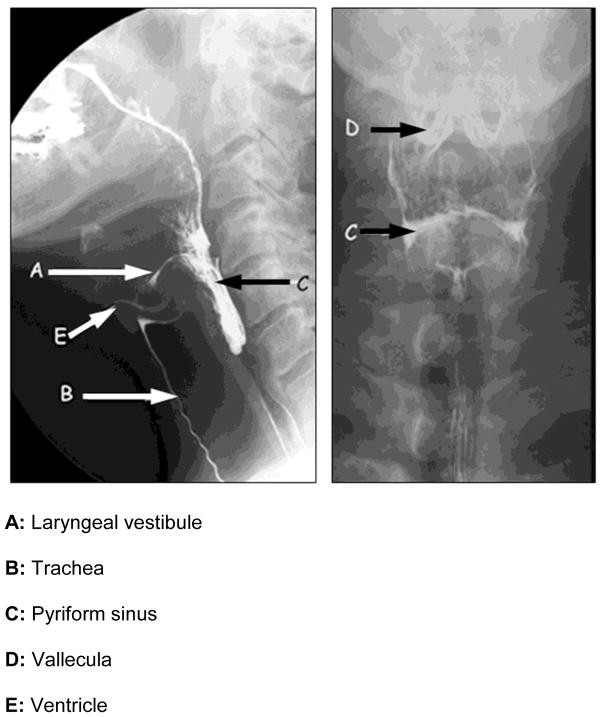
The pharynx extends from the nasal cavity to the upper esophageal sphincter at the cricopharyngeus muscle, and is formed of inner circular and outer longitudinal layers of striated muscle. The pharynx is illustrated in Figure 1 with key structures identified.
FIGURE 1.
Radiologic anatomy of the oropharynx.
As previously described, the swallowing process can be divided into 4 phases [11]. These are illustrated in the four images comprising Figure 2 as follows:
FIGURE 2.
Functional anatomy of the phases of swallowing.
Oral preparatory phase: The liquid bolus is held in a chamber between the tongue and palate [20], as shown in Figure 2a. Closure of the glossopharyngeal junction prevents spillage into the pharynx [21].
Oral propulsive phase: The bolus is squeezed towards the pharynx by tongue-palate pressure [22]. The tongue moves in an anterior-superior direction, creating a conveyer-belt like process, which moves the bolus backwards towards the oropharyngeal junction [23]. This is illustrated in Figure 2b.
Pharyngeal phase: During this phase, the velopharyngeal and laryngeal ports are closed. The hyo-laryngeal complex moves in an upward and anterior direction, positioning the entrance to the airway out of the path of the bolus and placing biomechanical traction on the upper esophageal sphincter (UES) to assist with opening [24]. At the same time, the pharynx shortens in a vertical direction via contraction of the longitudinal muscles [25]. A wave of muscle contraction from the superior to middle and then inferior pharyngeal constrictor muscles follows the bolus down through the pharynx, facilitating bolus clearance. Figure 2c illustrates the bolus in the pharyngeal phase.
Esophageal phase: After the bolus passes into the proximal cervical esophagus, as illustrated in Figure 2D, the structures of the pharynx and larynx return to their baseline position. The upper esophageal sphincter closes behind the tail of the bolus, and the laryngeal vestibule opens to allow airflow for breathing [26].
TECHNIQUE OF FLUOROSCOPIC EXAMINATION
Performance of the videofluoroscopic swallow examination requires attention to technical aspects of the protocol, as well as knowledge of the normal anatomy and physiology of the oropharynx [27]. Protocol considerations involve the preparation of appropriate oral contrast media, image acquisition utilizing standard patient positioning and cinefluorographic mode capture of images with adequate spatial and temporal resolution to enable subsequent review [15].
1) Preparing oral contrast agents for videofluoroscopic studies
Oral agents used for fluoroscopic evaluation of swallowing include either barium or non-ionic water soluble contrast [28, 29]}. Barium may be prepared in different concentrations, and/or mixed with thickening agents to simulate different liquid and food consistencies [15]. For patients who have undergone surgery involving the neck or the esophagus, water soluble contrast is used initially to assess for anastomotic leaks. Due to the reported risk of chemical pneumonitis, high osmolar iodine based agents are not recommended for use in patients who may aspirate [28].
Barium comes in both liquid and powder forms. Suspensions typically include additives to promote diffusion of the barium in a liquid medium and to limit foaming and achieve desired coating properties. Utilizing an appropriate concentration (or density) of barium is important for proper visualization during fluoroscopic monitoring of the study. Typical concentrations for oropharyngeal swallowing examination are 40% w/v or 20% w/v [30]. These concentrations are intended to provide adequate visibility or contrast, while limiting coating of the oropharyngeal mucosa, which could be mistaken for pathophysiological post-swallow residue [31]. The following information regarding the original barium product concentration is useful for preparing barium for oropharyngeal examinations:
When using the powder form: Weight/Weight (w/w = weight in grams/100 g of product) indicates the number of grams of active ingredient present in 100 g of product used for powered barium. For example, in a 85% w/w product there are 85 g of barium sulfate in 100 g of powder.
When using the liquid form: Weight/Volume (w/v = weight in grams/100 mL of product) indicates the number of grams of active ingredient present in 100 mL of product used for liquid barium. For example, in a 250% w/v (high density) product, there are 250g of barium sulfate in a volume of 100 mL of suspension.
In the United States, Varibar™ is a commercially available barium contrast product intended specifically for use in imaging the oropharynx. Varibar™ is available in several different consistencies (thin, nectar-thick, thin-honey, thick-honey and pudding-thick) at a constant 40% w/v barium concentration. In other countries, where this product is not available, clinicians may wish to consult recipes to guide them in preparing barium contrast solutions with controlled concentration. Sample recipes can be found at www.steeleswallowinglab.ca/Barium_Recipes.php [15].
2) Volume of oral contrast and swallowing protocol
A standardized protocol for type and volumes of oral contrast is highly recommended as the best approach to answer clinical questions while limiting radiation exposure. One approach to protocol standardization is the Modified Barium Swallow Impairment Profile, or MBSImp [32]. In our institution, a similar standard protocol involves up to 16 boluses, divided into 7 core tasks and up to 9 other swallowing tasks allowing for the exploration of pathophysiology with other bolus types (e.g., different consistencies or larger volumes) or the probing of therapeutic effectiveness with different maneuvers (such as positional changes, voluntary breath-hold maneuvers or effortful swallows). The 7 core tasks begin with an initial bolus hold challenge using a 10 cc thin liquid bolus, which the patient is instructed to hold in their mouth for 5 seconds before swallowing; this is intended to challenge oral bolus control and containment. This is followed by 3 teaspoon-sized swallows of a thin liquid barium, for which we use a 20% w/v contrast suspension. These thin liquid swallows provide the standardized context for evaluating aspiration risk. The remaining core tasks provide the standardized context for evaluating swallowing efficiency and residue risk using 3 teaspoon-sized swallows of a 40% w/v spoon-thick liquid barium.
3) Image Acquisition
Patient Position
The patient is initially placed in a lateral position to review all the swallowing phases and to assess for abnormalities in the timing of swallowing, i.e,. delayed oral phase, delayed initiation of the pharyngeal phase, etc. In cases where asymmetry or anatomical/structural differences are suspected, such as pharyngeal diverticula or post-surgical anastomotic leaks, Antero-posterior and/or oblique views are included [33–35]. Valsalva maneuvers may be performed with patient in an Antero-posterior position to distend pharyngeal structures to assess for asymmetry (which may arise from unilateral nerve paresis).
Image acquisition
It is preferred to acquire images using “Last Image hold cine” to reduce radiation dosage and allow for later review of the study is preferable. A high image acquisition rate (using either continuous or high frequency pulsed fluoroscopy) is recommended to ensure that brief or subtle findings such as penetration or tongue pumping are not overlooked. It is reported that evidence of aspiration is missed more often when only 15 images per second are viewed (vs. 30 images per second) [36]. Furthermore, to counter the commonly-voiced opinion that radiation exposure may be reduced by using lower fluoroscopy pulse rates, it has been shown that overall procedure length is shorter, meaning that fewer swallows are required to obtain answers to clinical questions, when image acquisition rate is set at 30 images per second vs. 15 images per second [36]. Thus, adherence to the ALARA principle (i.e., as low as reasonably achievable) is best achieved by collecting 30 images per second and following a standardized protocol.
VIDEOFLUOROSCOPIC APPEARANCE OF SPECIFIC ETIOLOGIES OF ORO-PHARYNGEAL AND CERVICAL ESOPHAGEAL DYSPHAGIA
There are many causes of dysphagia in the oropharynx and cervical esophagus, ranging from motility disorders to structural abnormalities, and all of these require videofluoroscopic evaluation to reveal pathophysiology, using the techniques described above. Several specific examples will be discussed below, divided into structural and functional etiologies. Some fluoroscopic findings, such as ‘tongue pumping’ may point to specific conditions such as Parkinson’s disease, which may require further imaging for diagnostic confirmation. Such imaging is beyond the scope of this Educational Review.
a) Structural causes
Structural causes of dysphagia include both intrinsic and extrinsic causes. Extrinsic pathologies, such as tumour, enlarged thyroid or cervical osteophytes, can result in secondary symptoms from mass effect. Further imaging such as ultrasound for thyroid gland abnormalities and CT or MRI scans for head and neck tumor would be required for further evaluation of these issues.
1. Intrinsic cause: Cricopharyngeal bar or prominent cricopharyngeus muscle
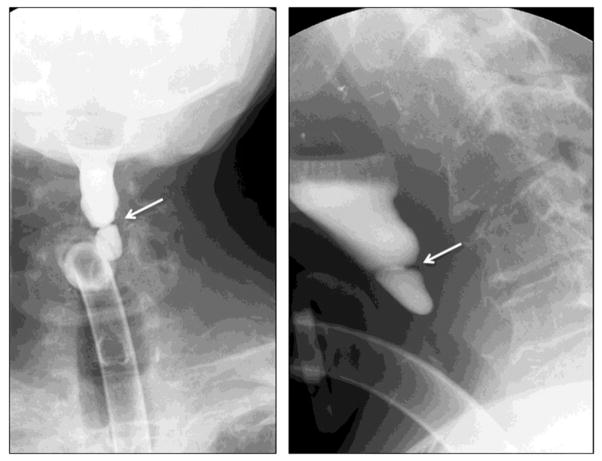
The cricopharyngeal bar is a smooth, posterior bar- or band-like protrusion into the lumen and the barium column, seen on the lateral view, at the junction of the hypopharynx and cervical esophagus, at about the level of C5–C6. An example is shown in Figure 3 from a single patient, in whom a small cricopharyngeal bar developed into an obstructive condition over several years. It is considered to reflect either a spasm of the cricopharyngeus muscle, and/or a failure of inhibition of tonic cricopharyngeus muscle contraction. The exact cause of cricopharyngeal prominence is unknown, but a high level of co-occurrence with gastroesophageal reflux (GERD) is considered to suggest one possible causative mechanism. Cricopharyngeal bar may be seen in 5–10% of asymptomatic patients. In symptomatic patients, a prominent cricopharyngeus may be due to neurologic diseases that cause pharyngeal paresis (CVA), neuro-muscular diseases (e.g., myasthenia gravis, dermatomyositis) or be a compensatory response to GERD. The increased resting tone of the cricopharyngeus muscle has been implicated in the formation of a Zenker’s diverticulum, which will be discussed below. The criocopharyngeal bar may progress over time to cause significant dysphagia.
FIGURE 3.

Development of a progressively worsening cricopharyngeal bar over time.
2. Intrinsic cause: Cervical esophageal webs
Cervical webs represent thin mucosal folds, which are frequently located along the anterior wall of the lower hypopharynx and proximal (upper) cervical esophagus. An example is shown in Figure 4. These may occasionally be circumferential, and appear as 1 to 2 mm in width shelf-like lumen-filling defects on videofluoroscopic studies. Cervical webs have been linked to underlying conditions such as GERD, epidermolysis bullosa dystrophica, and benign mucus membrane pemphigoid. Controversy exists regarding a possible association between cervical esophageal webs and iron deficiency anemia (Plummer–Vinson syndrome).
FIGURE 4.

Lateral view of cervical esophagus, demonstrating a focal ring-like web in the cervical esophagus.
3. Intrinsic cause: Pharyngeal Diverticula
Several different types of diverticulum may be readily visible, either on oropharyngeal or upper GI barium studies. These include pharyngeal diverticula and pouches, Zenker’s Diverticulum and Killian-Jamieson Diverticulum. Of these, the Zenker’s Diverticulum is the most common and is most frequently implicated in pharyngeal dysphagia. As mentioned above, Zenker’s diverticula typically occur in the context of cricopharyngeal dysfunction.
The Zenker’s Diverticulum is a pouch that develops in an area of anatomic weakness in the posterior part of the hypopharynx, between the oblique fibers of thyropharyngeus and the transverse fibers of cricopharyngeus portions of the inferior pharyngeal constrictor muscle. This area is known as the Triangle of Killian or Killian’s Dehiscence. The mouth of a Zenker’s diverticulum is consequently located just above the cricopharyngeal muscle and the narrowest portion of the upper esophageal sphincter. Imaging to confirm and determine the severity of a Zenker’s diverticulum requires at least three different images (AP, lateral, oblique), as illustrated in Figure 5. The effects of the diverticulum on the cervical esophagus should also be explored, to determine the extent to which compression is contributing to significant dysphagia.
FIGURE 5.
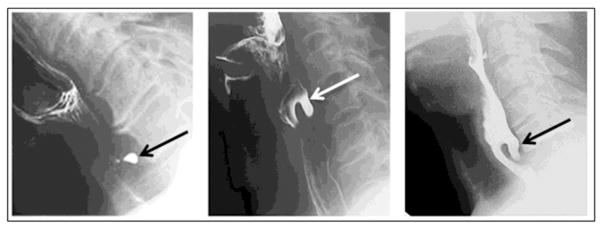
Different views of a Zenker’s diverticulum, extending inferiorly and compressing the cervical esophagus.
By contrast, Killian–Jamieson Diverticula originate below the cricopharyngeus, as illustrated in Figure 6. Notably, diverticula may not be visible in the lateral view, shown in the right hand panel of the figure.
FIGURE 6.
Antero-posterior and lateral views in a patient with a left lateral Killian-Jamieson Diverticulum.
4. Extrinsic cause: Enlarged Thyroid Gland
An enlarged thyroid gland, as seen in multinodular goiter or thyroid carcinoma can cause dysphagia, especially when it wraps around the trachea anteriorly and/or the esophagus posteriorly. An Antero-posterior videofluoroscopic view is best to assess the site and degree of compression, as illustrated in Figure 7. An ultrasound should be performed to assess the thyroid gland for any potential malignancy, especially if the patient presents with hoarse voice or another change in voice quality. Compression of the recurrent laryngeal nerve by a goiter or invasion by a thyroid malignancy may result in vocal cord dysfunction, contributing to dysphonia.
FIGURE 7.

Antero-posterior view showing an enlarged right lobe of the thyroid causing compression and lateral displacement of the cervical esophagus.
5. Cervical Spine Osteophytes
Large syndesmophyte/osteophyte complexes in diffuse idiopathic skeletal hyperostosis (DISH), or degenerative disc diseases can cause dysphagia. Additionally, iatrogenic injury can arise from anterior surgical approaches for treatment of these conditions, such as cervical spine fusion [37]. Figure 8 illustrates a patient with cervical osteophytes in the C4, 5 and 6 region.
FIGURE 8.
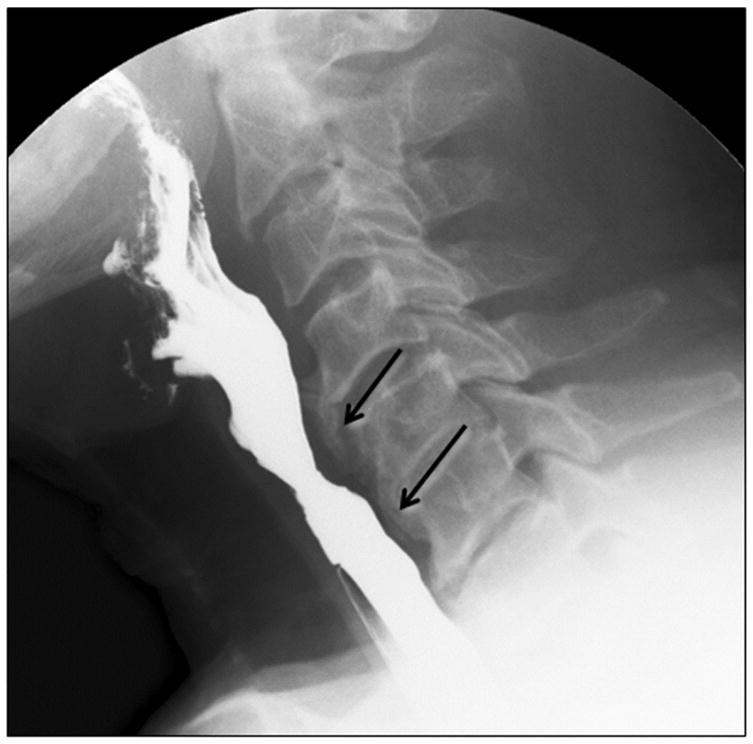
Lateral view showing anterior osteophytes in the region of C 4, 5 and 6 causing narrowing of the cervical esophagus
Large osteophytes cause direct mechanical blockage of the esophagus or hypopharynx, or may misdirect the bolus towards the airway. Dysphagia may even be caused by small osteophytes, if they are located at the fixed points of the pharynx or esophagus, for example, close to the cricoid cartilage or the upper esophageal sphincter. Osteophytes have the potential to cause an inflammatory reaction in nearby soft tissue, which may extend around either the pharynx or esophagus. Additionally, neuropathy can result from osseous impingement of either sensory or motor cranial nerves.
6. Post-operative causes of dysphagia
Surgery to the head, neck or thorax, such as procedures required to resect tumors, may result in complications such as anastomotic leak, vascular injury, cranial nerve damage, which may result in dysphagia [38]. Reconstructive flaps to primarily fill spatial defects, without innervation, may interrupt both neural and muscle contraction sequences that are important for swallowing function. Patients with esophageal cancers require resection and either anastomosis with gastric pull up (Ivor Lewis procedure) or neo-esophageal reconstruction with jejunal pull up or colonic interposition; these procedures involve the risk of anastomotic leak and abscess formation. Patients who undergo laryngectomy or esophagectomy for cancer may develop benign or malignant strictures in the long-term leading to dysphagia. Sinuses or fistula tracts may also develop in the soft tissue of the pharynx and esophagus, either in the immediate post-operative period or after many years. Figure 9 illustrates a fistula, in which contrast is noted to pass from the left side of the cervical esophagus into an irregular collection. The contrast is then seen to course anteriorly in a linear tract to the left side of the neck and on to the skin. These observations are consistent with a leak and fistula from the cervical esophagus, leading to the left side of the neck.
FIGURE 9.
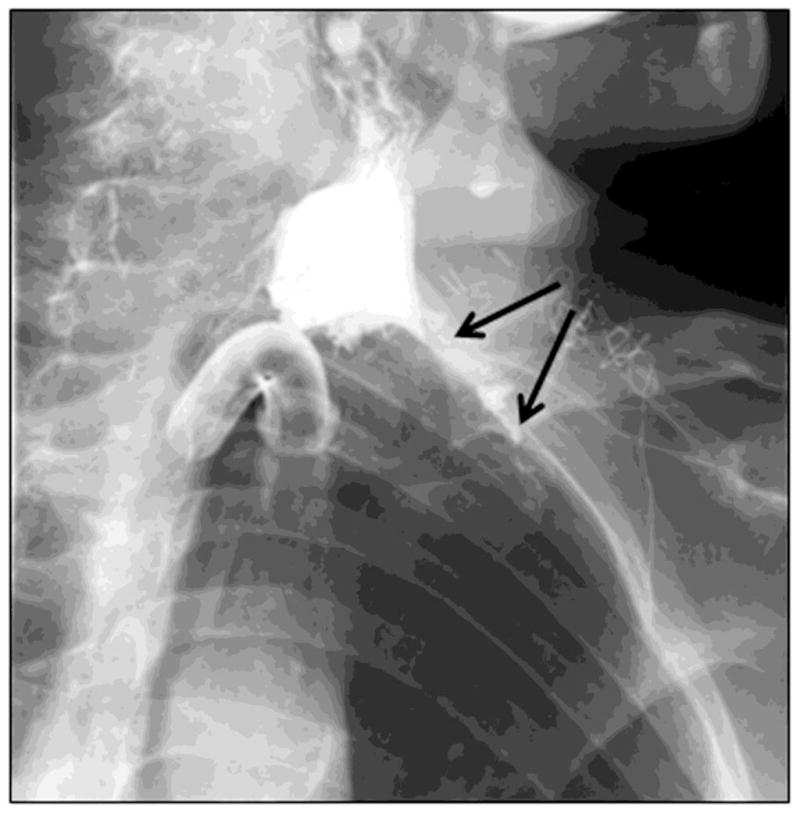
A left posterior oblique view showing a leak and fistula from left lateral aspect of the cervical esophagus in a patient with laryngectomy.
7. Post-radiation stricture
Patients with head and neck tumors frequently undergo radiation treatment either preoperatively, or post tumor surgery, or as primary treatment. Those receiving chemoradiation have a slightly increased incidence of developing benign strictures [39]. It has been suggested that radiation doses less than 60Gy have a lower risk of esophageal stricture. In one study, about 3.4% of patients developed proximal esophageal strictures [39]. The pathophysiology of the stricture is thought to involve progressive endarteritis and ischemia. Dysphagia from stricture in the pharynx or esophagus after radiation therapy for laryngeal malignancy can be demonstrated with videofluoroscopy or upper GI series. Findings may vary from short smooth strictures to complete obliteration, such as the case illustrated in Figure 10. Recurrence of malignant disease cannot be excluded on barium examinations and therefore CT scan should be done in these patients.
FIGURE 10.
Antero-posterior and lateral views showing complete obstruction in the cervical esophagus at the C4/C5 level 2 years after laryngectomy and radiation therapy for laryngeal carcinoma. Note the post radiation mucosal web in the hypopharynx.
b) Functional neuromuscular disorders
Functional neuromuscular disorders may contribute to a wide variety of pathophysiology in dysphagia, as summarized in Table 2.
TABLE 2.
ETIOLOGIES OF FUNCTIONAL NEUROMUSCULAR CAUSES OF DYSPHAGIA
| CENTRAL NERVOUS SYSTEM | PERIPHERAL SYSTEM | MYONEURAL JUNCTION | SKELETAL MUSCLE | OTHER DISORDERS |
|---|---|---|---|---|
|
|
|
|
|
1. Tongue pumping
Swallowing impairment in Parkinson’s disease may be seen in any or all of the phases of swallowing – oral, pharyngeal and esophageal. Tongue pumping, which involves a repetitive backward and forward rocking motion of the tongue, is considered to be pathognomonic of Parkinson’s disease. This is best evaluated on lateral fluoroscopy of the swallowing study, which must include the oral cavity [4].
2. Laryngeal Vestibular Penetration
Laryngeal vestibular penetration occurs when barium enters the laryngeal vestibule but does not pass below the level of glottis through the true vocal folds [40]. Transient penetration (also called high penetration) has been described to occur in healthy people [41], and involves spontaneous clearance from the laryngeal vestibule. This is usually a result of a delay between bolus arrival near the entrance to the airway and closure of the laryngeal vestibule with retroversion of the epiglottis over the entrance. This condition may arise from poor oral control of a bolus (sometimes called premature spill) or from delayed initiation of the pharyngeal swallow itself. When material enters the supraglottic space of the laryngeal vestibule, the expected reflex response is rapid initiation of a swallow via excitation of receptors of the internal branch of the superior laryngeal nerve [42].
3. Aspiration
Aspiration is the term used to describe the passage of foreign material (including food and liquid), through the true vocal cords into the trachea. When this happens, the expected reflex response is a cough due to excitation of recurrent laryngeal nerve receptors. Patients with dysphagia may present with aspiration leading to either an immediate, delayed or absent cough response. Additionally, the cough may or may not be effective at ejecting material back into the hypopharynx. The severity of aspiration is determined using a subjective impression of the amount of material aspirated (e.g., trace, moderate or severe amounts) and quantification of the depth of aspiration and response in terms of coughing. The 8-point Penetration-Aspiration Scale [40] has become the standard metric for aspiration severity (see Table 3), and captures both the depth of airway invasion (e.g., above versus below the true vocal folds), and whether or not material is ejected to a higher anatomical level of safety or remains at its lowest position. A score of 8 represents “silent aspiration” in which material is aspirated below the true vocal folds without any overt clinical signs. During a videofluoroscopic examination, separate evaluations of aspiration should be made for each bolus consistency (i.e., thin, nectar-thick, honey-thick or spoon-thick barium). An important purpose beyond completion of standardized bolus challenges in the videofluoroscopy is the exploration of the effectiveness of bolus texture modification or behavioral maneuvers in limiting aspiration.
TABLE 3.
8-POINT PENETRATION-ASPIRATION SCALE
| Category | Score | Description | Classification |
|---|---|---|---|
| Normal | 1 | Contrast does not enter the airway | Normal (no aspiration) |
| Penetration | 2 | Contrast enters the supraglottic space, but is then ejected from the airway | |
| 3 | Contrast enters the supraglottic space, but is not ejected from the airway | Abnormal (aspiration risk) | |
| 4 | Contrast contacts the vocal folds, but is then ejected | ||
| 5 | Contrast contacts the vocal folds, but is not ejected | ||
| Aspiration | 6 | Contrast passes the glottis but no subglottic residue is visible | |
| 7 | Contrast passes the glottis; visible subglottic residue despite patient’s response | ||
| 8 | Contrast passes the glottis; visible subglottic residue, absent patient’s response |
4. Post-swallow residue in the pharynx
Bolus material may collect and remain in the spaces of the pharynx (valleculae, pyriform sinuses) after the swallow. This is generally considered a sign of weak bolus propulsion or inadequate upper esophageal sphincter opening. Residue is a risk for secondary post-swallow aspiration. Residue severity is frequently captured using subjective ordinal scales describing the extent to which the space housing the residue is judged to be full (e.g., up to 25% full, > 25% full). Recently, a more detailed approach to measuring residue severity has been described, in which pixel area measures both of the lateral view appearance of residue and of the spatial housing are made, and normalized to an anatomical scalar derived using measures of the length of the cervical spine. This method is known as the Normalized Residue Ratio Scale [43].
CONCLUSION
Dysphagia in the oropharyngeal or cervical esophageal stages of swallowing is common in the elderly and will become an increasing problem with the expected demographic increase in the geriatric population. This review article has demonstrated many important causes and presentations of oropharyngeal dysphagia, which are sometimes overlooked during the conventional Upper GI study. Videofluoroscopic evaluation for assessment of both structural abnormalities and motility disorders of the oropharynx using various compositions of barium contrast is currently the standard of practice. Utilizing best practice radiographic techniques and having knowledge of swallowing mechanisms and various diseases are important for assessment of dysphagia. Dynamic fluoroscopic imaging remains an essential and important tool for assessing functional disorders of swallowing. Early recognition of dysphagia risk will lead to better patient management. Detailed videofluoroscopic assessment can guide treatment decisions with the goal of decreasing the secondary complications of dysphagia, such as aspiration pneumonia, dehydration, malnutrition, and depression, and thereby contributing to improved outcomes both in health and quality of life.
Acknowledgments
Grants: None
Footnotes
Disclosures: NONE
Contributor Information
Nasir M Jaffer, Email: njaffer@mtsinai.on.ca, Associate Professor, Faculty of Medicine Department of Medical Imaging, University of Toronto, Mount Sinai Hospital, Room 565, 600 University Avenue, Toronto, ONT. M5G 1X5, Phone 416-586-4800 Ext 278, Fax: 416 586 8695.
Dr Edmund, Radiology Resident, Faculty of Medicine Department of Medical Imaging, University of Toronto, Mount Sinai Hospital, 600 University Avenue, Toronto, ONT. M5G 1X5, Phone 416 5896 4800 Ext 5278, Fax: 416 586 8695.
Frederick Wing-Fai Au, Email: frederick.au@uhn.ca, Address: Toronto General Hospital, Department of Medical imaging, 585 University Avenue East NCSB 1c-571, Toronto, Ontario M5G 2N2, Phone: 416-340 3372, Fax: 416-593 0502.
Catriona M. Steele, Email: catriona.steele@uhn.ca, Senior Scientist and Director, Swallowing Rehabilitation Research Laboratory, Toronto Rehabilitation Institute - University Health Network, Associate Professor, Speech-Language Pathology, University of Toronto, 550 University Avenue, #12-101, Toronto, ON, M5G 2A2, Tel: (416) 597 3422 X 7603, Fax: (416) 597 7131.
References
- 1.Palmer JB, Drennan JC, Baba M. Evaluation and treatment of swallowing impairments. American Family Physician. 2000;61:2453–2462. [PubMed] [Google Scholar]
- 2.Prasse JE, Kikano GE. An overview of dysphagia in the elderly. Advanced Studies in Medicine. 2004;4(10):527–533. [Google Scholar]
- 3.Steele CM, Greenwood C, Ens I, Robertson C, Seidman-Carlson R. Mealtime difficulties in a home for the aged: not just dysphagia. Dysphagia. 1997;12:43–50. doi: 10.1007/pl00009517. [DOI] [PubMed] [Google Scholar]
- 4.Logemann JA. Manual for the videofluorographic study of swallowing: Second edition. Austin, TX: Pro-Ed, Inc; 1993. [Google Scholar]
- 5.Hiiemae KM, Palmer JB. Tongue movements in feeding and speech. Crit Rev Oral Biol Med. 2003;14:413–429. doi: 10.1177/154411130301400604. [DOI] [PubMed] [Google Scholar]
- 6.Clavé P, Rofes L, Arreola V, et al. Diagnosis and management of oropharyngeal dysphagia and its nutritional and respiratory complications in the elderly. Gastroenterology Research and Practice. 2011:Article ID 818979. doi: 10.1155/2011/818979. http://dx.doi.org/10.1155/2011/818979. [DOI] [PMC free article] [PubMed]
- 7.Pearson WG, Jr, Langmore SE, Zumwalt AC. Evaluating the structural properties of suprahyoid muscles and their potential for moving the hyoid. Dysphagia. 2011;26:345–351. doi: 10.1007/s00455-010-9315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahrilas PJ, Logemann JA, Lin S, Ergun GA. Pharyngeal clearance during swallowing: a combined manometric and videofluoroscopic study. Gastroenterology. 1992;103:128–136. doi: 10.1016/0016-5085(92)91105-d. [DOI] [PubMed] [Google Scholar]
- 9.Cook IJ, Dodds WJ, Dantas RO, et al. Timing of videofluoroscopic, manometric events, and bolus transit during the oral and pharyngeal phases of swallowing. Dysphagia. 1989;4:8–15. doi: 10.1007/BF02407397. [DOI] [PubMed] [Google Scholar]
- 10.Clave P, Arreola V, Romea M, et al. Accuracy of the volume-viscosity swallow test for clinical screening of oropharyngeal dysphagia and aspiration. Clin Nutr. 2008;27(6):806–815. doi: 10.1016/j.clnu.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Logemann JA. Dysphagia: evaluation and treatment. Folia PhoniatrLogop. 1995;47:140–164. doi: 10.1159/000266348. [DOI] [PubMed] [Google Scholar]
- 12.McCullough GH, Rosenbek JC, Wertz RT, et al. Utility of clinical swalloiwng examination measures for detecting aspiration post-stroke. J Speech Lang Hear Res. 1997;48(6):1280–1293. doi: 10.1044/1092-4388(2005/089). [DOI] [PubMed] [Google Scholar]
- 13.ASHA. Guidelines for Speech-Language Pathologists Performing Videofluoroscopic Swallowing Studies. American Speech Language Hearing Association. 2003 [Google Scholar]
- 14.ASHA. Preferred Practice Patterns for the Profession of Speech-Language Pathology. American Speech Language Hearing Association. 2004 [Google Scholar]
- 15.Peladeau-Pigeon M, Steele CM. Technical aspects of a videofluoroscopic swallowing study. Canadian Journal of Speech-Language Pathology and Audiology. 2013;37:216–226. [Google Scholar]
- 16.Langmore SE, Schatz K, Olsen N. Fiberoptic endoscopic examination of swallowing safety: a new procedure. Dysphagia. 1988;2:216–219. doi: 10.1007/BF02414429. [DOI] [PubMed] [Google Scholar]
- 17.Lundquist A, Olsson R, Ekberg O. Clinical and radiologic evaluation reveals high prevalence of abnormalities in young adults with dysphagia. Dysphagia. 1998;13:202–207. doi: 10.1007/PL00009572. [DOI] [PubMed] [Google Scholar]
- 18.Chen CL, Tsai CC, Chou AS, Chiou JH. Utility of ambulatory pH monitoring and videofluoroscopy for the evaluation of patients with globus pharyngeus. Dysphagia. 2007;22:16–19. doi: 10.1007/s00455-006-9033-8. [DOI] [PubMed] [Google Scholar]
- 19.American Gastroenterological Association medical position statement on management of oropharyngeal dysphagia. Gastroenterology. 1999;116:452–454. doi: 10.1016/s0016-5085(99)70143-5. [DOI] [PubMed] [Google Scholar]
- 20.Kahrilas PJ, Lin S, Logemann JA, Ergun GA, Facchini F. Deglutitive tongue action: volume accommodation and bolus propulsion. Gastroenterology. 1993;104:152–162. doi: 10.1016/0016-5085(93)90847-6. [DOI] [PubMed] [Google Scholar]
- 21.Dantas RO, Dodds WJ, Massey BT, Shaker R, Cook IJ. Manometric characteristics of the glossopalatal sphincter. Digestive Diseases and Sciences. 1990;35:161–166. doi: 10.1007/BF01536757. [DOI] [PubMed] [Google Scholar]
- 22.Hiiemae KM, Palmer JB. Tongue movements in feeding and speech. Crit Rev Oral Biol Med. 2003;14:413–429. doi: 10.1177/154411130301400604. [DOI] [PubMed] [Google Scholar]
- 23.Steele CM, Van Lieshout P. Tongue movements during water swallowing in healthy young and older adults. J Speech Lang Hear Res. 2009;52:1255–1267. doi: 10.1044/1092-4388(2009/08-0131). [DOI] [PubMed] [Google Scholar]
- 24.Mendelsohn MS, McConnel FM. Function in the pharyngoesophageal segment. Laryngoscope. 1987;97:483–489. doi: 10.1288/00005537-198704000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Palmer JB, Tanaka E, Ensrud E. Motions of the posterior pharyngeal wall in human swallowing: a quantitative videofluorographic study. Arch Phys Med Rehabil. 2000;81:1520–1526. doi: 10.1053/apmr.2000.17829. [DOI] [PubMed] [Google Scholar]
- 26.Shaker R. Functional relationship of the larynx and upper GI tract. Dysphagia. 1993;8:326–330. doi: 10.1007/BF01321771. [DOI] [PubMed] [Google Scholar]
- 27.Jones B. Radiologic Evaluation of the Dysphagic Patient. Nutrition in Clinical Practice. 1999;14:S10–12. [Google Scholar]
- 28.Harris JA, Bartelt D, Campion M, et al. The use of low-osmolar water-soluble contrast in videofluoroscopic swallowing exams. Dysphagia. 2013;28:520–527. doi: 10.1007/s00455-013-9462-0. [DOI] [PubMed] [Google Scholar]
- 29.Popa Nita S, Murith M, Chisholm H, Engmann J. Matching the rheological properties of videofluoroscopic contrast agents and thickened liquid prescriptions. Dysphagia. 2013;28:245–252. doi: 10.1007/s00455-012-9441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fink TA, Ross JB. Are we testing a true thin liquid? Dysphagia. 2009;24:285–289. doi: 10.1007/s00455-008-9203-y. [DOI] [PubMed] [Google Scholar]
- 31.Steele CM, Molfenter SM, Peladeau-Pigeon M, Stokely SL. Challenges in preparing contrast media for videofluoroscopy. Dysphagia. 2013;28:464–467. doi: 10.1007/s00455-013-9476-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin-Harris B, Brodsky MB, Michel Y, et al. MBS measurement tool for swallow impairment--MBSImp: establishing a standard. Dysphagia. 2008;23:392–405. doi: 10.1007/s00455-008-9185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dodds WJ, Stewart ET, Logemann JA. Physiology and radiology of the normal oral and pharyngeal phases of swallowing. AJR AmJ Roentgenol. 1990;154:953–963. doi: 10.2214/ajr.154.5.2108569. [DOI] [PubMed] [Google Scholar]
- 34.Palmer JB, Kuhlemeier KV, Tippett DC, Lynch C. A protocol for the videofluorographic swallowing study. Dysphagia. 1993;8:209–214. doi: 10.1007/BF01354540. [DOI] [PubMed] [Google Scholar]
- 35.Bonilha HS, Humphries K, Blair J, et al. Radiation exposure time during MBSS: influence of swallowing impairment severity, medical diagnosis, clinician experience, and standardized protocol use. Dysphagia. 2013;28:77–85. doi: 10.1007/s00455-012-9415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonilha HS, Blair J, Carnes B, et al. Preliminary Investigation of the Effect of Pulse Rate on Judgments of Swallowing Impairment and Treatment Recommendations. Dysphagia. 2013;28(4):528–538. doi: 10.1007/s00455-013-9463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin RE, Neary MA, Diamant NE. Dysphagia following anterior cervical spine surgery. Dysphagia. 1997;12:2–8. doi: 10.1007/pl00009513. [DOI] [PubMed] [Google Scholar]
- 38.Pai PS. Review: Complications in head and neck surgery. Otorhinolaryngology Clinics: An international journal. 2010;2:61–67. [Google Scholar]
- 39.Laurell G, Kraepelien T, Mavroidis P, et al. Stricture of the proximal esophagus in head and neck carcinoma patients after radiotherapy. Cancer. 2003;97:1693–1700. doi: 10.1002/cncr.11236. [DOI] [PubMed] [Google Scholar]
- 40.Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 41.Allen JE, White CJ, Leonard RJ, Belafsky PC. Prevalence of penetration and aspiration on videofluoroscopy in normal individuals without dysphagia. Otolaryngol Head Neck Surg. 2010;142:208–213. doi: 10.1016/j.otohns.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 42.Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia. 2010;25:323–333. doi: 10.1007/s00455-010-9301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearson WG, Jr, Molfenter SM, Smith ZM, Steele CM. Image-based Measurement of Post-Swallow Residue: The Normalized Residue Ratio Scale. Dysphagia. 2012 doi: 10.1007/s00455-012-9426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]