Abstract
Transcranial magnetic stimulation (TMS) has shown promise as a treatment tool, with one FDA approved use. While TMS alone is able to up- (or down-) regulate a targeted neural system, we argue that TMS applied as an adjuvant is more effective for repetitive physical, behavioral and cognitive therapies, that is, therapies which are designed to alter the network properties of neural systems through Hebbian learning. We tested this hypothesis in the context of a slow motor learning paradigm. Healthy right-handed individuals were assigned to receive 5 Hz TMS (TMS group) or sham TMS (sham group) to the right primary motor cortex (M1) as they performed daily motor practice of a digit sequence task with their non-dominant hand for 4 weeks. Resting cerebral blood flow (CBF) was measured by PET at baseline and after 4 weeks of practice. Sequence performance was measured daily as the number of correct sequences performed, and modeled using a hyperbolic function. Sequence performance increased significantly at 4 weeks relative to baseline in both groups. The TMS group had a significant additional improvement in performance, specifically, in the rate of skill acquisition. In both groups, an improvement in sequence timing and transfer of skills to non-trained motor domains was also found. Compared to the sham group, the TMS group demonstrated increases in resting CBF specifically in regions known to mediate skill learning namely, the M1, cingulate cortex, putamen, hippocampus, and cerebellum. These results indicate that TMS applied concomitantly augments behavioral effects of motor practice, with corresponding neural plasticity in motor sequence learning network. These findings are the first demonstration of the behavioral and neural enhancing effects of TMS on slow motor practice and have direct application in neurorehabilitation where TMS could be applied in conjunction with physical therapy.
Keywords: TMS, Primary motor cortex, Motor learning, Digit sequence practice, Hebbian learning, Hyperbolic function, Motor system, Skill transfer, Motor learning network
Introduction
Transcranial magnetic stimulation (TMS) is a sub-type of a well-established modality, i.e. electromagnetic stimulation of excitable tissues, and provides a non-invasive alternative to direct electrical stimulation. Since Barker et al.'s (1985) first successful application of TMS in humans, investigators using TMS have published more than ten thousand peer-reviewed publications including more than 1100 in 2009 (http://www.webofknowledge.com, topic = “TMS” search). As a culmination of this extensive body of investigation, the Food and Drug Administration has approved TMS for two indications (therapeutic use in major depressive disorder (MDD) and diagnostic use in pre-surgical mapping), with additional clinical indications in advanced stages of validation (Slotema et al., 2010).
The TMS induced Hebbian alterations in synaptic efficacy (1976), including both long-term potentiation (LTP) and long-term depression (LTD) (Bütefisch et al., 2004) are thought to be the cause of its therapeutic effects. In light of this, it is somewhat surprising that the current emphasis is on the use of TMS alone as a treatment tool, while its uses as an adjuvant with other Hebbian, learning-based interventions are few. For example, TMS is being used as a sole treatment in various psychiatric disorders including MDD, schizophrenia, and anxiety disorder. TMS adjuvancy chiefly has been explored in the context of short-term motor learning (hours to few days) in normal subjects (Boyd and Linsdell, 2009; Bütefisch et al., 2004; Jung and Ziemann, 2009; Kim et al., 2004). However, to date, the effects of TMS over longer periods of motor practice (weeks) have not been examined.
To explore the adjuvant effects of TMS, we used as a model treatment the well-established, highly reliable skill acquisition paradigm introduced by Karni et al. (1995, 1998), and Ungerleider et al. (2002). The Karni paradigm is a regimen of daily repetition (typically 15 min per day) of a five-stroke digit-movement sequence. The skill acquisition in this paradigm is a classic example of Hebbian learning in both behavioral and neurophysiological domains. Thus, it shares with TMS a common mechanism of inducing neural plasticity (Bütefisch et al., 2000; Rioult-Pedotti et al., 1998). In a series of published studies, we and others have demonstrated changes in behavior as well as neuronal properties such as cerebral blood flow and connectivity following slow motor practice (Doyon and Benali, 2005; Karni et al., 1995; Ma et al., 2010, 2011; Xiong et al., 2009). Over the course of weeks, performance scores (typically measured as the number of correct sequences per unit time per session) increase monotonically to an asymptote. Such a time course of learning (~4 weeks) is comparable to the typical durations used in cognitive, behavioral, physical, and speech therapies. This 4-week approach gives this learning paradigm a much greater translational relevance than the single-session learning paradigms typically used in neuroim-aging and TMS studies.
Neural systems mediating the incremental skill acquisition following digit sequence practice (DSP) have been outlined by a series of imaging studies in humans using both PET and fMRI (Doyon and Benali, 2005; Duff et al., 2007, 2008; Grafton et al., 1992, 2002; Karni et al., 1995, 1998; Ma et al., 2010, 2011; Poldrack, 2000; Ungerleider et al., 2002; Xiong et al., 2009). Brain regions engaged during slow skill learning include the primary motor cortex (M1), premotor cortex, supplementary motor area (SMA), caudate, putamen, mesial temporal areas including hippocampus, and cerebellum (Doyon and Benali, 2005; Doyon et al., 2009; Ma et al., 2010, 2011; Orban et al., 2011; Xiong et al., 2009). These regions broadly fall under two main networks, the cortico-cerebellar and the cortico-striatal networks. M1 being common to both the networks, is therefore critically involved in skill learning. Thus, due to the role it plays throughout learning, its connections to other brain regions are important in motor learning, and its easy accessibility makes M1 an ideal target site for TMS modulation.
The most common imaging metric used in examining the neural correlates of DSP is the conditional contrast analysis (task minus control) that detects changes in activation patterns. However, no consensus has emerged on the pattern or direction (positive or negative) of the changes associated with motor learning. For example, different groups reported: continued increases in cerebral blood flow in motor areas (Karni et al., 1995, 1998; Ungerleider et al., 2002); a waxing and waning pattern of initial increases followed by a return to baseline (Hlustík et al., 2004; Xiong et al., 2009); and decreases (Poldrack, 2000). As a way to resolve these inconsistent results obtained by the traditional activation approach, our group examined changes in control state i.e., resting cerebral blood flow (CBF) during motor skill acquisition. We found that resting CBF rose significantly during a four-week course of treatment using the Karni slow-learning paradigm (Xiong et al., 2009). In this study, increases in activation (task-minus-rest contrast) were observed prior to the resting CBF changes. Once the resting CBF changes were induced, the activation pattern returned to baseline. These findings were inferred to be the result of a baseline accommodation to the neural demands of intense, daily skill rehearsal. Resting CBF measurement therefore is different from traditional conditional contrast, correlational, and connectivity analyses, all of which assume an unchanging control or resting measurements. Therefore, in the present study, we measured changes in resting blood flow to examine the effects of TMS treatment on neural networks engaged during skilled motor learning.
In proposing TMS as a treatment adjuvant, we specifically postulate that TMS adjuvancy will be effective for repetitive physical, behavioral and cognitive therapies, that is, therapies that are designed to alter the network properties of neural systems through Hebbian learning. We are therefore hypothesizing that the combination of TMS with an established primary treatment such as the Karni paradigm will enhance the efficacy of the primary treatment. In order to test this hypothesis, we applied adjuvant TMS to the M1 area representing the hand (M1hand), as participants simultaneously underwent a digit sequence training. We hypothesized that the TMS applied to the primary motor cortex would modulate the activity at the site of stimulation as well as in connected brain areas. We postulated that this modulation would manifest behaviorally as a faster learning rate and/or greater plateau when compared to DSP alone. Further, we anticipated that the imaging correlates of the TMS modulation would be evident as increases in resting state CBF in the cortico-cerebellar, and the cortico-striatal networks. We expected that the resting CBF increases would be more widespread when TMS was applied as an adjunct, when compared to DSP alone.
Secondarily, we investigated the effectiveness of TMS adjuvancy on transfer of skills to non-trained domains by testing the ability of participants to perform the training sequence with the untrained hand, and a mirrored sequence with both the trained and the untrained hands. Based on previous reports (Boutin et al., 2012a,b; Korman et al., 2003), we hypothesized that DSP would facilitate transfer during the early phase of practice (for example, at the end of first week) and that further practice would not add to this gain. However, we posited that TMS adjuvancy would result in continued gains in transfer of skills through the four week practice period.
Methods
Participants
Fifteen healthy right-handed individuals (average age ± standard error (SE) — 28.4 ± 2.1 years, 9 males) were enrolled in the study after approval by the institutional review board at the University of Texas Health Science Center at San Antonio. The right-handedness was confirmed by Edinburgh handedness inventory (average ± SE — 81 ± 5.15; Oldfield, 1971). An informed consent was obtained from all participants in accordance with the declaration of Helsinki. Participants were randomly assigned to digit sequence practice (DSP) with sham TMS (DSP + sham, n = 7) or DSP with TMS (DSP + TMS, n = 8) groups. One participant (DSP + TMS group) discontinued after 1 week, 4 participants (two in each group) ended their participation at 2 weeks, and ten participants completed the entire study. In one participant in the DSP + TMS group who completed the study, defective sensors resulted in significant loss of data over several training sessions. Therefore the data from this participant was not included in the analysis. Therefore data from 14 participants (7 in DSP + sham and 7 in DSP + TMS group) who completed the first week of the study and 9 participants (5 in DSP + sham and 4 in DSP + TMS group) who completed 4 weeks of study were further analyzed and reported here. All participants were naïve to TMS and the measurements of the study prior to enrollment. However, all participants experienced real TMS as motor threshold was estimated in all at the beginning of the study. During the treatment sessions, all participants were informed that they may not feel any muscle contraction, and at the end of the experiment, all participants stated that they were not cognizant of their group assignment.
Motor practice paradigm
The behavioral training protocol used was a five-movement, four-finger digit sequence task (Karni et al., 1995, 1998). The Karni task was performed with the subject's non-dominant hand (left hand) in order to provide a greater dynamic range for the behavioral measure of skill acquisition. The fingers were numbered as follows: index finger, 1; middle finger, 2; ring finger, 3; and little finger, 4, and the sequence: 4, 1, 3, 2, and 4 was assigned to be practiced. Practice consisted of performing the training sequence with no visual feedback as rapidly and accurately as possible. During the training program participants performed the training sequence for 16 min daily, 5 days/week, for 4 weeks.
To test the transfer of skills to the non-trained domain, we examined the ability of participants to transfer the training sequence also termed extrinsic transformation (Boutin et al., 2012a,b) by having the participants perform the training sequence with their right hand. Next, to assess the degree of generalization of the motor skills, i.e., intrinsic transformation, we tested the performance of a mirror sequence, namely 4, 2, 3, 1, and 4 with both hands. The participants performed the training sequence with the right hand, and the mirror sequence with both hands, each 2 min long, at baseline and at the end of each week of practice.
Force sensitive resistor system to record performance
Each practice session was monitored by a force sensitive resistor (FSR) system developed by our group and validated against a standard video recording (Rogers et al., 2010). In this system, the voltage measured across the voltage-divider resistor is proportional to the pressure on the FSR attached to each digit tip. The voltage is sampled at 100 Hz. Recorded voltage data from all digits were processed using a MATLAB 7 (Math Works, Natick, MA) program with a graphical interface. A voltage threshold was used to determine a finger press and the time that the voltage was above threshold determined the press duration. The thresholded presses were then compared to the reference sequence to determine the number of correct sequences in the session and the duration of each sequence. All presses performed during each practice session were recorded in each participant and used to calculate the number of correct sequences, and the time taken to perform each sequence for each day of practice. The transfer sequences were also processed in the same manner to derive the number of correct sequences at each testing point.
Image guided TMS targeting
The aiming for TMS (or sham TMS) was based on treatment plans developed from high-resolution anatomical and functional MRI (3T-TIM, Siemens, Germany). Anatomical images were acquired as 1 mm3, using a 3-D Turbo-FLASH sequence with an adiabatic inversion contrast pulse (TE/TR/TI = 3.04/2100/785 ms, and flip angle = 13°). Gradient echo planar images (TE/TR = 45/2000 ms, a flip angle of 90°, and slice thickness = 6 mm, 20 slices, resolution of 3 × 3 mm2) were acquired during the abduction and adduction of the left index finger in a block design (30 s — finger abduction–adduction alternating with 30 s of rest). This task has been shown to maximally activate the first dorsal interosseous (FDI) muscle (Cramer et al., 2002; Rábago et al., 2009).
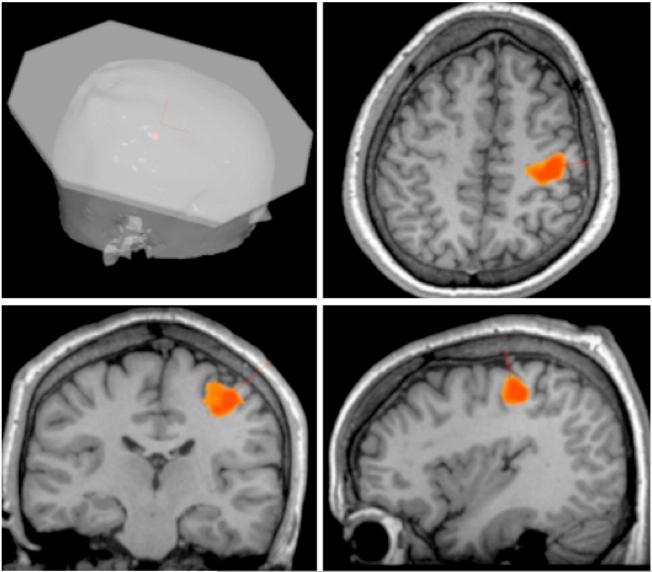
Image preprocessing was done to remove non-brain tissues, correct for image intensity fluctuations and RF inhomogeneities, and register the brain to the anatomical MRI using image processing tools from the publicly available package FSL (Smith et al., 2004) (http://www.fmrib.ox.ac.uk/fsl/). A general linear model was employed to determine voxel-wise differences in activation patterns during task and rest conditions and statistical parametric image of z scores (SPI{z}) were generated and thresholded for a z > 2.3 and a cluster significance threshold of p = 0.05 (corrected for false discovery rate). The TMS target site was selected by identifying the center-of-mass of region of maximal activation in the right M1hand area. The optimal orientation of the TMS E-field at this location was determined based on the cortical column cosine principle (Fox et al., 2004) incorporated in the TMS planning tool of the irTMS system (Lancaster et al., 2004) (see Fig. 1). On each day of TMS or sham TMS, the participants were positioned on a padded bed, their head immobilized, and the irTMS system was used to accurately place the TMS coil at the planned scalp location and orientation for the right M1hand area.
Fig. 1.
Individualized image guided TMS. TMS was applied to right M1hand area identified by a left index finger adduction and abduction task during BOLD fMRI acquisition overlaid on the individual's anatomical MRI. The TMS treatment-planning tool determined the scalp location and the orientation of the TMS coil.
TMS treatment and delivery
TMS was delivered to the right M1hand with a water-cooled figure-B coil powered by Cadwell HSMS unit (Cadwell, Inc.; Kennewick, Washington). Electromyography (EMG) was recorded from the FDI, abductor digiti minimi, and more proximal muscles such as biceps and deltoids using pre-amplified double differential MA-411 electrodes (Motion Lab Systems, Baton Rouge, LA) placed over the muscle bellies. The electrodes were connected to a Neuroscan SynAmps 32 channel head box and amplifier with a CMRR of 108 dB (Neuroscan, El Paso, Texas). Silver/silver chloride disc (1 cm dia.) electrodes were used as a skin ground placed on the left ulnar styloid process. EMG was band-pass filtered from 10 Hz to 500 Hz with a 500× gain prior to sampling (2.5 kHz). The motor evoked potential responses were elicited in the FDI muscle using single pulses of TMS delivered randomly every 5–7 s. Each participant's motor threshold (MT) was determined on the first day of practice using a standardized method (Rossini et al., 1994) defined as the minimum stimulus intensity that produced a motor evoked potential ≥ 100 μ-volts in 50% of trials during complete muscle relaxation.
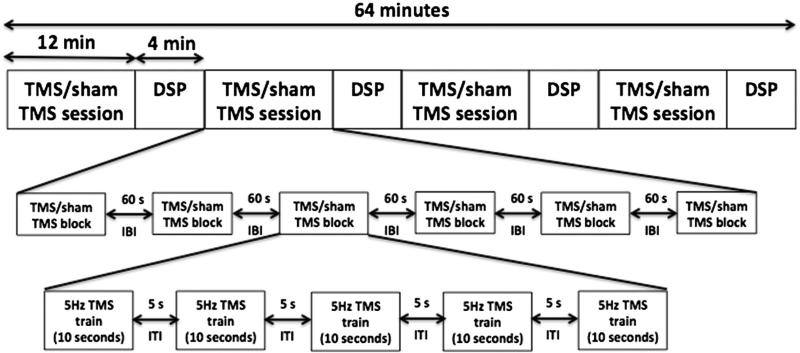
TMS treatment was applied to participants randomized to the DSP + TMS group. TMS treatment consisted of 6000 pulses delivered to the right M1hand at the rate of 5 Hz, and an intensity of 100% MT. TMS was applied at 5 Hz since 5 Hz TMS has been shown to increase neuronal excitability (Peinemann et al., 2004; Ragert et al., 2003), and improve motor performance in healthy adults (Kim et al., 2004; Siebner et al., 2000). Since low intensity TMS (subthreshold) applied to the motor cortex results in local effects only, and suprathreshold TMS results in propagation of activity to remote connected regions (Fox et al., 2006; Siebner and Rothwell, 2003), the intensity of TMS was fixed at 100% of the motor threshold to ensure propagation of the activity downstream to the cortico-spinal tracts, while keeping within the TMS safety guidelines (Rossi et al., 2009; Wassermannn, 1998). The TMS was delivered over an hour and comprised of 4 sessions, as shown in Fig. 2. Each session included 6 blocks, each of which consisted of 5 trains of TMS (50 pulses/train and inter-train interval of 5 s) with an inter-block interval of 60 s. The intersession interval was 240 s. This combination of rate, duration, and intensity is within the safety guidelines (Rossi et al., 2009; Wassermannn, 1998). TMS was given five days a week: Monday through Friday. EMG from the FDI, abductor digiti minimi, biceps, and deltoids were monitored for safety (Wassermannn, 1998). The EMG was observed for any signs of after discharges or any other abnormal EMG activity during the entire TMS session. The participants in the DSP + sham group received sham TMS. Sham TMS was applied in the same manner as real TMS, but with a spacer, 30 mm thick placed between the TMS coil and the subjects' scalp. The E-field at the front end of the spacer was 75% less than the front of real TMS coil, but the feeling of coil on the scalp and the sound from the TMS coil were similar to the real TMS condition. The DSP was interleaved with TMS sessions so that the participants practiced the digit sequence task during the inter-session intervals (see Fig. 2). The participants were instructed to execute the sequences as quickly and as accurately as possible.
Fig. 2.
Daily TMS/sham TMS treatment and digit sequence practice (DSP). The daily sitting consisted of 4 sessions of TMS/sham TMS interleaved with DSP. Each session consisted of six blocks of TMS/sham TMS with an inter-block interval (IBI) of 60 s. Each block included 5 trains of 5 Hz TMS with inter-train interval of 5 s. A train of TMS consisted of 10 s of 5 Hz TMS (50 pulses). DSP consisted of 4 min of practicing the training sequence as quickly and as accurately as possible.
Modeling of accuracy of performance
We used an accumulation model of learning based on a hyperbolic function (Mazur and Hastie, 1978) that has been shown to fit motor learning data better than an exponential function and provides additional parameters of learning. This model is defined by the equation
| (1) |
where, y is the amount of learning, t is the amount of time or training, t0 is the amount of initial training and accounts for differences in prior skill, k is the asymptote for learning, and R determines the rate of convergence to the asymptote (Mazur and Hastie, 1978; Newell et al., 2001). In this model, learning is thought to be a process by which correct response tendencies increase steadily with practice, and compete with incorrect response tendencies, which remain constant. R in this model represents this competition, and larger values of R indicate increased tendency to produce incorrect responses, i.e., slower rates of learning (Newell et al., 2001). It is important to note that while this model is well suited to analyze the accuracy (in this case the number of correct sequences per unit time), the reaction times are still best modeled by an exponential function. Using the hyperbolic function, we modeled the behavioral data scores and calculated the parameters k, t0 and R for each participant. Based on these parameters, we also estimated the time to reach 50% of asymptote (T50). A repeated measures two-factor ANOVA with days of practice and treatment (sham vs. TMS) as factors was performed on the parameters derived from the hyperbolic model for each participant.
PET imaging
PET data were acquired with a CTI EXACT HR scanner (Knoxville, TN). Sixty-three contiguous slices (2.5-mm thick) in a transaxial field of view of 15.5 cm were acquired. Water labeled with oxygen-15 (, half-life 122 s) was administered intravenously (155 MBq ) and cerebral blood flow (CBF) was measured using a bolus technique (Fox et al., 2000, 2006). Data was collected after bolus arrival in the brain (15–20 s after injection) for 90 s. Images were corrected by measured attenuation using 68Ge/68Ga transmission scans and reconstructed at an in-plane resolution of 7-mm full width at half maximum (FWHM) and an axial resolution of 6.5-mm FWHM. Participants' heads were immobilized in the PET scanner using individually fitted, thermally molded, plastic face masks (Fox and Raichle, 1984). Each participant was studied in two sessions: before beginning DSP and immediately after completion of 4 week DSP. During both sessions, the participants underwent two measurements of CBF during eyes closed rest condition.
PET data preprocessing
Image preprocessing was performed using previously validated methods and in-house software. PET images were corrected for head motion using the MCFLIRT tool in FSL 4.0 (http://www.fmrib.ox.ac.uk/fsl/) and PET and MRI images were spatially transformed relative to the stereotaxic atlas of Talairach and Tournoux (1988) (Lancaster et al., 1995, 1997). Regional tissue uptake of was globally normalized to whole rCBF brain mean value with images scaled to a mean of 1000 counts. These value and spatially normalized images were tri-linearly interpolated, re-sampled (60 slices, 8 mm3 voxels), and Gaussian filtered to a final resolution of 9.9 mm (FWHM). Further data analyses were performed using MIPS software (Multiple Image Processing Station, Research Imaging Institute, UT Health Science Center at San Antonio, TX) and MANGO (Multi Analysis GUI, ric.uthscsa.edu/mango).
PET data analysis
Conditional contrast analysis
For each subject, voxel-by-voxel pairwise contrasts were generated for baseline and after 4 weeks of DSP to identify regional CBF changes present during rest. Within-subject regional changes were then averaged across the subjects in each of the two groups. A maxima and minima search was then used to identify local extrema within a search volume measuring 1000 mm3 (Fox and Mintun, 1989; Fox et al., 1988; Mintun et al., 1989). A gamma 1 statistic measuring skewness and gamma 2 statistic measuring kurtosis of the distribution of the extrema established before post hoc analysis were used as an omnibus test to assess overall significance. We confirmed that for the 4 week DSP vs. baseline contrasts, the gamma 2 statistic for all the masked voxels and for the extrema set were significant. The group-mean subtraction images from both sessions were then converted to statistical parametric images of z scores (SPI{z}). Brain regions with increases in CBF post DSP with z score > 3, p > 0.005 (false discovery rate corrected q = 0.05), and cluster size > 200 mm3 are reported.
Volume of interest analysis
To further confirm the hemodynamic differences between preand post-DSP conditions in the two groups, the brain regions that were observed to have increased CBF at 4 weeks were probed in the value normalized PET data. Eleven volumes of interest (VOIs) were selected subject by subject for investigating motor learning effects. These brain areas have been shown to be involved in motor skill learning (Doyon and Benali, 2005; Doyon et al., 2009; Karni et al., 1998; Xiong et al., 2009), and were observed to have increased CBF in the conditional contrast analysis. These included the right and left primary motor areas (M1), right sided supplementary motor area (SMA), lateral premotor cortex (PMd), cingulate cortex, putamen, hippocampus, amygdala left sided cerebellum, and bilateral inferior parietal lobule (BA 40). The locations of the VOIs were centered on the center of mass of clusters of increased CBF observed the post vs. pre DSP resting state either in the sham group or the TMS group. The size of each VOI was standardized and was 5 × 5 × 5 voxels (1000 mm3). The averaged value normalized PET counts from each VOI from the two groups were assessed for significant differences by a paired T-test.
Results
Performance: accuracy
There were no statistically significant differences in age, degree of handedness, baseline performance, location of M1hand, and the motor threshold between the two groups (see Table 1). However, ANOVA of the number of correct sequences revealed significant difference between baseline and subsequent week performance in both groups (DSP + sham: F (4, 24) = 24, p < 0.00003, DSP + TMS: F (4, 19) = 4.2, p = 0.018). Similar to our previous reports (Xiong et al., 2009), there were no significant differences between week 2 and week 4 of the learning in either group, indicating that most of the learning happened in the first two weeks.
Table 1.
Characteristics of DSP + sham and DSP + TMS groups expressed as average ± SEM. p indicates the significance level determined by ANOVA analysis.
| DSP + sham | DSP + TMS | p | |
|---|---|---|---|
| M:F | 3:4 | 5:2 | |
| Age (years) | 28.57 ± 3.16 | 27.14 ± 3.25 | 0.76 |
| Handedness | 76.57 ± 7.88 | 89.0 ± 6.66 | 0.25 |
| M1hand (Talairach coordinates) | |||
| x | 32.09 ± 2.65 | 32.79 ± 1.41 | 0.82 |
| y | –18.86 ± 1.5 | –23.92 ± 2.15 | 0.07 |
| z | 54.45 ± 1.29 | 51.73 ± 2.87 | 0.40 |
| Motor threshold (% machine output) | 60.0 ± ±4.3 | 52.29 ± 3.12 | 0.17 |
| Baseline performance (# correct sequences) | 26.0 ± 2.76 | 33.71 ± 2.44 | 0.06 |
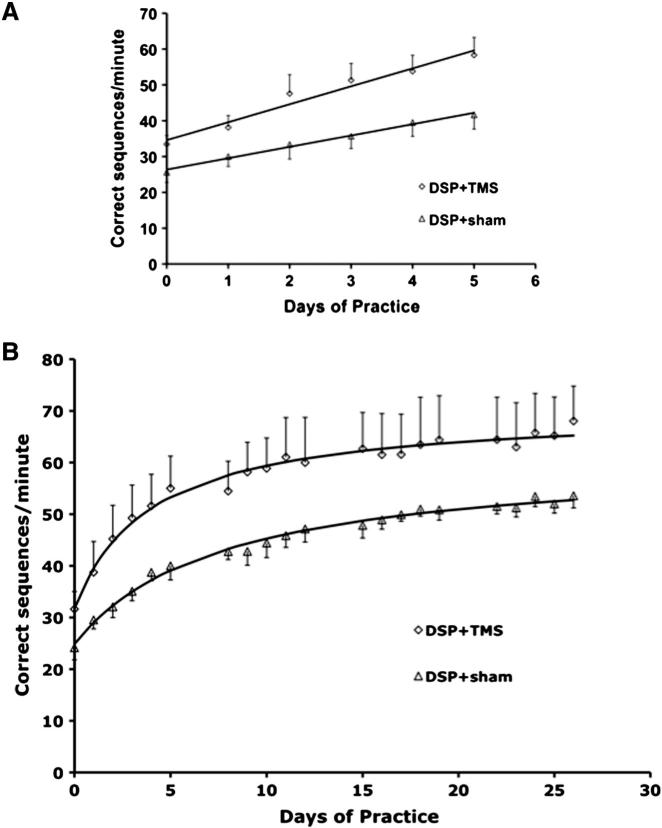
Fig. 3 shows the average performance of the finger sequence task in DSP + sham and DSP + TMS groups during the first week (panel A) and over the entire 4 weeks of practice (panel B). A two-factor ANOVA with repeated measures comparing the behavioral data between the two groups (DSP + sham and DSP + TMS) and 5 time points (baseline, week 1, week 2, week 3, and week 4) revealed a significant difference between the two groups (F (1, 44) = 11.82, p = 0.0017). Specifically, the two-factor ANOVA across the two groups and the time points of baseline and week 1 was significantly different for groups (F (1,13) = 6.27, p = 0.027), indicating that the two groups diverge during the first week. The performance during the first week was modeled as a linear function (Fig. 3 panel A). The rate of increase in performance in the DSP + TMS group was 5.01 ± 0.77 sequences/day (average ± SE) and 3.17 ± 0.33 sequences/day in the DSP + sham group (p = 0.048).
Fig. 3.
Performance over the course of training of digit sequence task in DSP + sham (open triangles) and DSP + TMS (open diamonds) groups. The solid lines indicate the modeled function for the two groups. Top panel A: First week of training. Both groups demonstrated significant improvement in performance from baseline. The DSP + TMS group had a significantly faster rate of learning, evidenced by a greater slope. Bottom panel B: Four weeks of training. Both groups demonstrated significant improvement in performance from baseline. The DSP + TMS group had a significantly faster rate of learning, evidenced by a significantly shorter model parameter R.
The performance data over the entire 4 weeks fit the hyperbolic function well in all the participants (n = 9). The parameters k, R, t0, and T50 were calculated for each participant, and averaged, and are listed in Table 2. The parameter R, the rate of learning expressed as days (a larger R indicates a longer time taken to reach plateau) was significantly shorter for the DSP + TMS group when compared to DSP + sham group (2.79 ± 0.69 days versus 5.02 ± 0.68 days; F (1, 7) = 5.86, p = 0.046), i.e., the DSP + TMS group reached the plateau significantly faster than the DSP + sham group. Similarly, the T50 also reflected this difference with the DSP + TMS group reaching 50% of asymptote in 4.78 ± 0.91 days while the DSP + sham group reached this point in 8.17 ± 1.01 days (F (1, 7) = 6.77, p = 0.035). The parameters k (the asymptote for learning), and t0 (a measure of baseline skill) were not significantly different between the two groups.
Table 2.
Modeled parameters for DSP + sham and DSP + TMS groups expressed as average ± SEM. T0 is the baseline amount of learning, k is the asymptote, R is the rate to reach the asymptote, and T50 is the number of days to reach 50% of asymptote. p is the significance level determined by ANOVA. R and T50 in the TMS + MP group were significantly shorter.
| Model parameters | DSP + sham | DSP + TMS | p |
|---|---|---|---|
| Slope (1 week) | 3.17 ± 0.33 | 5.01 ± 0.77 | 0.048 |
| t0 (days) | 3.53 ± 0.71 | 2.27 ± 0.60 | 0.202 |
| k (# correct sequences) | 61.67 ± 1.61 | 72.02 ± 9.09 | 0.245 |
| R (days) | 5.02 ± 0.68 | 2.79 ± 0.69 | 0.046 |
| T50 (days) | 8.17 ± 1.01 | 4.78 ± 0.91 | 0.035 |
Performance: timing
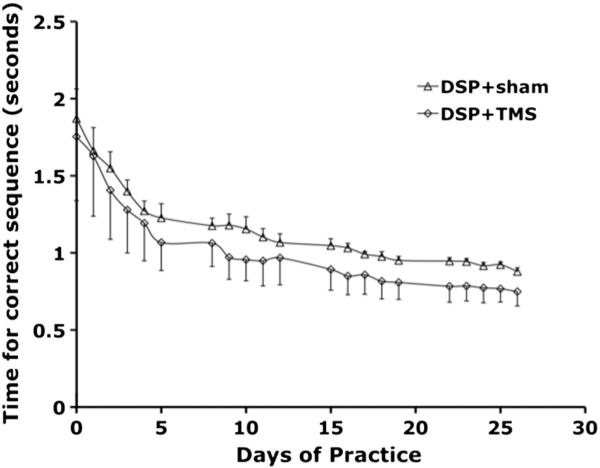
At baseline, the average time to complete a sequence was 1.87 s for the DSP + sham group and 1.75 s for the DSP + TMS group (p = 0.80). There was a significant improvement in the time to complete the sequences over the training period in both groups (Fig. 4). A two way ANOVA with repeated measures demonstrated a significant decrease in sequence completion times in both groups (F (20, 249) = 6.73, p ≤ 0.00003) as well as between the two groups (F (1, 249) = 9.18, p = 0.0028).
Fig. 4.
Sequence time over the course of training of digit sequence task in DSP + sham (open triangles) and DSP + TMS (open diamonds) groups. Both groups demonstrated significant decreases in time taken to perform the sequences from baseline. The DSP + TMS groups had a significantly greater decrease (p = 0.0028).
Performance: transfer
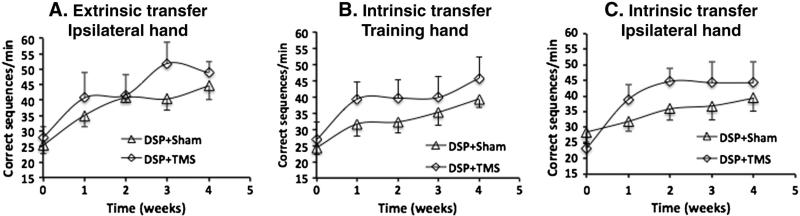
At baseline there were no differences between the DSP + sham and DSP + TMS groups in the performance of the training sequence with the right hand (p = 0.26) as well as the mirrored sequence with left (p = 0.68) and right (p = 0.59) hands. A two way ANOVA with repeated measures revealed significant effect of time on the number of correct sequences between baseline and each week of training in both the DSP + sham and for the DSP + TMS groups, across all three transfer conditions (Fig. 5). Thus, there was significant transfer of skills for performing the trained sequence with the right hand (F (4, 20) = 19.07, p < 0.0005), as well as for performing the mirrored sequence with both hands (left hand — F (4, 20) = 19.21, p < 0.0005; right hand — F (4, 20) = 14.619, p < 0.0005) over the duration of training. Specifically pairwise comparison of the trained sequence task with the right hand indicated that in both groups, the baseline was significantly different from all other time points (p < 0.01), week one was also different from weeks 3 and 4 (p < 0.05), and no further differences between weeks 2, 3 and 4, indicating perhaps a plateau in skill transfer by the end of the second week. In case of the mirror sequence task with the left hand in both groups, the baseline was significantly different than all other time points (p < 0.006), and so was week 4 (p < 0.003), indicating that the intrinsic transfer of skills was ongoing in both groups. For the mirror sequence task with the right hand in both groups, the baseline was significantly different than all other time points (p < 0.01), week 1 was different than weeks 2, 3, and 4 (p < 0.02), and no further differences between weeks 2, 3 and 4. However, there was a time × group interaction ((F 4, 19) = 2.724, p = 0.053) indicating that the rate of performance change was different for the two groups over the training period.
Fig. 5.
Transfer of motor skills in DSP + sham and DSP + TMS groups: A. Extrinsic transfer: The performance of the training sequence with the hand ipsilateral to the TMS stimulation (right hand). B. Intrinsic transfer-training hand: The performance of the mirrored sequence with the training hand (left hand). C. Intrinsic transfer-ipsilateral hand: The performance of the mirrored sequence with the hand ipsilateral to the TMS stimulation (right hand). All the three transfer sequences improved significantly over the training period in both the groups.
Resting CBF correlates of rTMS treatment
Conditional contrast analysis
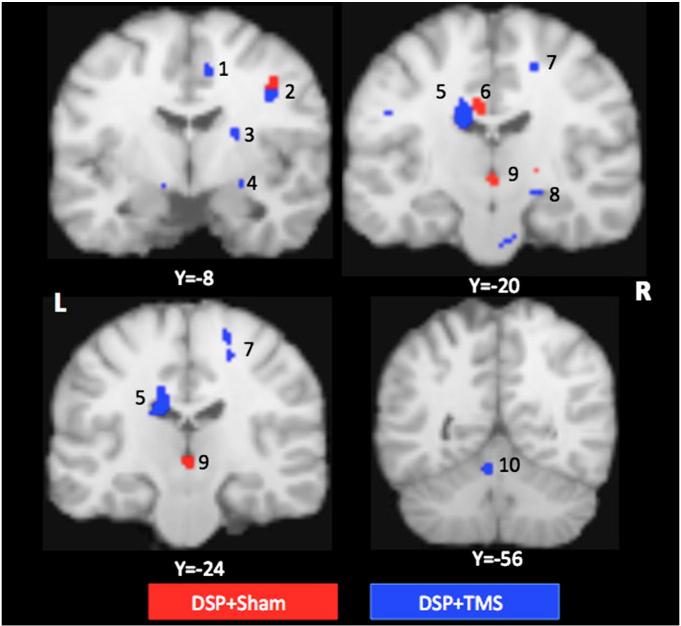
Significant increases in resting CBF was observed in several brain regions at 4 weeks compared to baseline in both the DSP + sham and DSP + TMS groups and are listed in Table 3, and illustrated in Fig. 6. Consistent with previous published literature (Xiong et al., 2009), resting CBF was increased in the right M1hand, and PMd following 4 weeks of DSP in both the DSP + sham and DSP + TMS groups. In addition, we found increased resting CBF in brain areas specifically engaged during the fast learning phase of motor learning in both groups. The DSP + sham group had increases in resting CBF in the right precuneus, thalamus, bilateral posterior cingulate cortex, and left sided cerebellum. In the DSP + TMS group, increased CBF was observed in the right putamen, left caudate, left cerebellum, right me-dial temporal lobe including the amygdala, and parahippocampus (see Table 3, and Fig. 6).
Table 3.
Locations of peak extrema (x, y and z in Talairach co-ordinates), Brodmann area, volumes, z score, and p values of brain regions that demonstrated significant change in resting rCBF at 4 weeks in DSP + sham and DSP + TMS groups.
| x | y | z | Brain area | Brodmann area (BA) | Cluster size (mm3) | z score | p value |
|---|---|---|---|---|---|---|---|
| DSP + sham group | |||||||
| 42 | –16 | 54 | Precentral gyrus | 4 | 456 | 3.16 | 0.00078 |
| 40 | –8 | 38 | Precentral gyrus | 6 | 584 | 3.35 | 0.00041 |
| 4 | –30 | 36 | Cingulate gyrus | 31 | 480 | 3.20 | 0.00069 |
| –20 | –38 | 36 | Cingulate gyrus | 31 | 384 | 3.30 | 0.00049 |
| –4 | –20 | 30 | Cingulate gyrus | 23 | 464 | 3.43 | 0.00030 |
| 4 | –68 | 32 | Cuneus | 7 | 928 | 3.93 | 0.00004 |
| 16 | –64 | 40 | Precuneus | 7 | 320 | 3.08 | 0.00105 |
| 55 | –60 | 36 | Angular gyrus | 39 | 376 | 3.43 | 0.00030 |
| 2 | –30 | 2 | Thalamus/pulvinar | 744 | 3.67 | 0.00012 | |
| 2 | –22 | –4 | Red nucleus | 600 | 3.40 | 0.00034 | |
| –22 | –76 | –37 | Cerebellum/inferior semi-lunar lobule | 448 | 3.67 | 0.00012 | |
| –19 | –88 | –30 | Cerebellum/tuber | 296 | 3.34 | 0.00043 | |
| DSP + TMS group | |||||||
| 22 | –24 | 56 | Precentral gyrus | 4 | 272 | 2.96 | 0.0016 |
| 22 | –22 | 48 | Precentral gyrus | 4 | 416 | 3.24 | 0.0006 |
| 40 | –8 | 32 | Precentral gyrus | 6 | 592 | 3.10 | 0.0010 |
| 12 | –6 | 42 | Cingulate gyrus | 24 | 488 | 3.60 | 0.0002 |
| 0 | –41 | 52 | Paracentral lobule | 5 | 280 | 3.57 | 0.0002 |
| –8 | –36 | 46 | Precuneus | 7 | 536 | 3.31 | 0.0005 |
| 22 | –24 | –8 | Parahippocampal gyrus | 28 | 864 | 4.99 | <0.00003 |
| –10 | –22 | 24 | Caudate/tail | 824 | 4.41 | <0.00003 | |
| 28 | 0 | 12 | Lentiform nucleus/putamen | 728 | 3.41 | 0.0003 | |
| 24 | –10 | 14 | Lentiform nucleus/putamen | 744 | 3.03 | 0.0012 | |
| –8 | –12 | –10 | Substantia nigra | 496 | 3.41 | 0.0003 | |
| 26 | –10 | –8 | Amygdala | 320 | 3.02 | 0.0013 | |
| –4 | –58 | –18 | Cerebellum/culmen | 488 | 3.66 | 0.0001 | |
| –13 | –58 | –6 | Cerebellum/culmen | 472 | 3.34 | 0.0004 | |
| –2 | –72 | –16 | Cerebellum/vermis | 392 | 3.23 | 0.0006 | |
Fig. 6.
Brain regions demonstrating increases in resting CBF after 4 weeks in DSP + sham group (regions in red) and DSP + TMS group (regions in blue). 1. Cingulate cortex (BA 24), 2. premotor cortex, 3. putamen, 4. amygdala, 5. caudate nucleus, 6. cingulate gyrus (BA23), 7. primary motor cortex, 8. parahippocampus, 9. thalamus, and 10. cerebellum. The PET data are overlaid on Colin Brain, and the coordinates are in Talairach system. The left hemisphere is on the left side of the image.
VOI analysis
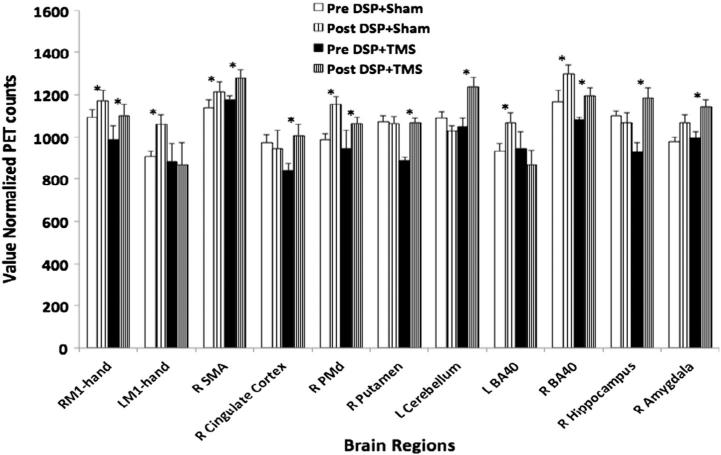
VOI analysis confirmed the findings from the conditional contrast analysis (see Fig. 7). The resting CBF in the right M1hand, SMA, PMd, and inferior parietal lobule (BA 40) was significantly increased in both groups after 4 weeks of DSP (p < 0.05). In addition, the resting CBF in the left sided M1hand and BA 40 was significantly increased (p < 0.05) at the end of training in the DSP + sham group, while CBF in the right cingulate motor cortex, putamen, hippocampus, amygdala, and the left cerebellum was significantly increased (p < 0.05) after 4 weeks only in the DSP + TMS group (Fig. 7).
Fig. 7.
Right: resting state CBF amplitude changes following 4 weeks of DSP + sham and DSP + TMS. TMS was applied to right M1hand area. Significant increases in rsCBF were noted in right M1hand, supplementary motor area (SMA), premotor cortex (PMd), and inferior parietal lobule (BA 40) in both groups after 4 weeks of DSP. The DSP + sham group demonstrated significant increases in the left M1hand, and left BA 40 following training. Additional increases in rsCBF were noted in the right sided cingulate cortex, putamen, hippocampus, amygdala, as well as cerebellum in the DSP + TMS group. * = p < 0.05.
Safety of rTMS
TMS was tolerated by all participants, and no serious adverse effects were noted during the study duration in either group. Each participant in the DSP + TMS group received a total of 120,000 pulses of TMS (6000 pulses a day for 20 days). Minor adverse effects observed were transient hearing loss in one participant in the DSP + sham group (hearing returned to baseline 3 months later), and discomfort at the site of stimulation and tingling of teeth in two participants in the DSP + TMS group. This, is the first demonstration of the safety of 5 Hz TMS delivered to the primary motor cortex at 100% of motor threshold over a period of 4 weeks.
Discussion
In this report, we investigated the adjuvant application of TMS to augment motor performance in healthy volunteers. We applied 5 Hz TMS concomitantly as participants practiced a digit sequence task daily for 4 weeks. We replicated the improvements in motor performance seen following training on a digit sequence task (Karni et al., 1995; Xiong et al., 2009). We also demonstrate that adjuvant application of TMS with the digit sequence task significantly augments motor performance. The effect of TMS was demonstrated to be an increase in the rate of learning early in the training period with concomitant decrease in time taken to perform each digit sequence. Next, we demonstrate that the slow motor practice also results in improved generalization of motor skills indicated by sustained transfer in both extrinsic and intrinsic domains. This transfer effect was present in the TMS adjuvant group as well, and in fact, intrinsic transfer of motor skills was significantly improved by TMS adjuvancy. We also demonstrate for the first time, the neural correlates of the TMS induced augmentation in motor performance in the context of slow motor learning. The positive effects of TMS adjuvancy are mediated via an increase in the resting state activity in the network that is engaged during motor skill acquisition. The TMS induced increases in resting cerebral blood flow was seen in the cortico-striatal and cortico-cerebellar networks, and extended to cortical areas mediating attention and memory. Our findings are consistent with previous reports of beneficial effects of non-invasive stimulation in healthy adults (Boyd and Linsdell, 2009; Bütefisch et al., 2004; Kim et al., 2004; Pascual-Leone et al., 1998). To the best of our knowledge, this is the first demonstration of the enhancing effects of 5 Hz TMS adjuvancy on slow motor practice over a long time period (4 weeks).
Motor learning paradigm as a model treatment
As a treatment model, the digit sequence task used in this study offers numerous advantages. First, the skill acquisition is a classic example of Hebbian learning both behaviorally and neurophysiologically. In a series of published studies, we and others have used functional neuroimaging and neural-system modeling to demonstrate changes in the network properties of the multi-regional network recruited by the task (Doyon et al., 2009; Ma et al., 2010, 2011; Xiong et al., 2009). Second, the time course of learning (~4 weeks) is as comparable to the typical durations used in cognitive and behavioral therapies, physical therapy and speech therapy. This property gives this learning paradigm much greater translational relevance than the single-session learning paradigms typically used in neuroimaging and TMS studies. Third, the performance of interest is readily recorded by virtue of being overt, i.e., involving a motor behavior. In our current study, we used a finger-mounted force sensing system (Rogers et al., 2010), which automatically records each finger stroke. Fourth, performance gains are highly reliable and readily modeled. The accumulation model of Mazur and Hastie (1978) fit these data extremely well, even at the single-subject level. Fifth, the treatment is completely “portable”, in that it can be self administered in virtually any setting. Sixth, substantial performance gains accrue in normal subjects, allowing treatment efficacy and logistics (identifying the optimal rate, duration, and intensity of TMS, length of TMS administration etc.) to be thoroughly explored and refined prior to initiating clinical trials in patient populations. Seventh, the task can be readily performed in PET or MRI scanners (unlike most physical therapies), allowing imaging assessment of the mechanism action of treatment during actual task performance. Collectively these properties make the digit sequence task used in this study an outstanding model treatment for testing the hypothesis of TMS adjuvancy for Hebbian learning.
Primary motor cortex as a target for TMS treatment
In the current study, we demonstrate that TMS applied to M1hand area augments both the motor behavior and the resting brain activity that accompanies DSP. Behaviorally, both rate of skill learning, and the time to perform each correct sequence were significantly improved in the DSP + TMS group when compared with the DSP + sham group. Neural correlates of this augmentation were seen as increases in resting CBF in the cortico-striatal and cortico-cerebellar networks. Similar to our findings, studies with short duration of TMS and transcranial direct current stimulation (tDCS) applied to M1 have demonstrated augmentation in motor skill learning (Bütefisch et al., 2004; Jung and Ziemann, 2009; Kim et al., 2004; Reis et al., 2009).
Several studies point to the critical role M1 plays across various stages of skill learning. For example, destroying dopaminergic projections to the motor cortex abolishes skill acquisition (Hosp et al., 2011) indicating that M1 is needed for skill acquisition. Local changes in synaptic efficacy such as LTP (Ziemann et al., 2004) as well as increases in the size of motor maps and corticospinal excitability (Pascual-Leone et al., 1995) have been demonstrated in M1 following sequence motor learning. Inhibitory theta burst TMS has been shown to impair motor learning involving rapid finger movements, but only when applied during the early stages of motor practice (Platz et al., 2012a,b). Daily application of tDCS to M1 daily had a beneficial effect, while weekly application did not alter motor behavior supporting the notion that M1 is engaged during the early stages of learning (Boggio et al., 2007). Such findings demonstrate the critical role of M1 in early stages of skill acquisition. Synaptogenesis and motor map reorganization in M1 have been shown to occur during the late phases of skill learning (Kleim et al., 2004) indicating to the role of M1 in consolidation of motor skill. M1 is also a major cortical output to descending motor commands. Thus, important aspects of skilled motor performance are maintained in the primary motor cortex and its output system (Gentner et al., 2010) and may also relate to storage of muscle combinations arising through a practice related neuronal activity. Our current findings, and existing literature collectively indicate that M1hand is indeed an important target for TMS treatment. Its ready accessibility, and ease of TMS application make M1 a principal target for TMS treatment especially in motor rehabilitation studies.
Effects of TMS on motor behavior
Here, we have demonstrated that adjuvant TMS applied to M1hand influenced motor behavior in the first week, as shown by faster rate of skill acquisition (Table 2 and Fig. 3). The rate of learning or time constant R in DSP + sham group of 5.02 is similar to previously published data (Xiong et al., 2009), and was significantly reduced to 2.79 days in the DSP + TMS group. There were differences in baseline performance between the two groups (p = 0.06) even though the two groups did not differ in the time taken to perform the sequence (p = 0.80), perhaps indicating to a wide range in individual motor skills. However, each participant showed significant improvement following DSP and the modeling of the slope of the learning curve independently showed that TMS accelerated skill learning. Such TMS induced behavioral enhancement has been reported previously in the context of short term sequence learning (Kim et al., 2004; Pascual-Leone et al., 1999) and even in the absence of any motor practice (Yoo et al., 2008). We observed that the behavioral gain in this study was mediated by increased speed (Fig. 4). Increases in speed following adjuvant TMS can be a result of improved error correction and sequence ordering that happens early in learning (Penhune and Steele, 2012). Contrary to changes in speed that happens early in learning, strategy changes such as chunking emerge much later in the learning process (Orban et al., 2011; Penhune and Steele, 2012). In our study, even though we did not specifically examine late stage strategy change, we observed that the initial gain in timing was maintained throughout 4 weeks. Thus, modulation of M1 with TMS during motor practice increased the rate of learning and speed of sequence performance across early and late stages of skill acquisition.
We also examined another important aspect of motor learning, namely transfer of skills. We found that practicing the training sequence also enhanced the performance of that sequence with the untrained hand (right hand in this study) and performance of a mirrored sequence with both hands. The transfer of skills to non-trained domains is an integral part of skill acquisition and is acquired through two separate cortical systems. One system mediated by attention and memory networks, encodes spatial co-ordinates early in training and is flexible and therefore more readily generalized (Korman et al., 2003). Such a transfer mechanism is also termed intrinsic transformation. However, prolonged practice appears to enhance skill acquisition via a system based on motor co-ordinates that is not conducive to transfer of skills to other motor domains. This movement specific representation is also called extrinsic transformation. Thus, prolonged practice appears to hinder successful transfer (Boutin et al., 2012a; Korman et al., 2003). Contrary to our initial hypothesis, we found that prolonged practice (4 weeks) resulted in significant transfer of skill learning to non-trained domains, and it was not adversely affected by TMS adjuvancy (see Fig. 6). Recently, Boutin et al. (2012b) have demonstrated that testing with non-trained sequences between extended practice sessions, especially during early periods promoted greater generalization of both extrinsic and intrinsic components. They propose that the retrieval caused by testing during the process of memory encoding (during the practice period) enhances transfer. Our findings support this observation since the participants performed the transfer tasks during skill acquisition, at the end of each week of training. Brain regions such as the motor cortex, cingu-late gyrus, superior parietal lobule, inferior parietal lobule, and cerebellum have been shown to correlate with transfer of learning (Seidler and Noll, 2008). These very regions also demonstrated an increased resting CBF following slow motor practice in the present study, more so with TMS adjuvancy. The significant improvement in intrinsic transfer seen with TMS treatment (Fig. 6) could be explained by the increases in resting CBF in the motor cortex, cingulate cortex, and cerebellum in the TMS group. Recently, Lefebvre et al. (2012) have shown that dual tDCS, and not sham conditions resulted in greater generalization to other motor tasks in stroke patients undergoing motor training. Thus, slow practice that includes repeated testing and adjuvant TMS appears to improve transfer of skills, a factor that is especially important in the context of neuro-rehabilitation.
Effects of TMS on motor network
In the current study, we have identified the neural correlates of improved motor performance following TMS adjuvancy. We demonstrate that TMS adjuvancy during motor practice targets the brain regions engaged in motor learning and that the effect of TMS is more extensive than motor practice alone. TMS resulted in increased resting CBF in brain areas that are normally recruited during motor learning, such as the primary motor cortex, SMA, PMd, and inferior parietal lobule, and additional increases in resting CBF were noted in the cingulate cortex, putamen, cerebellum, hippocampus and amygdala. Thus, there is a greater engagement of cortical regions engaged in attention, and memory, subcortical regions such as the putamen, and the cerebellum. Conversely we also found that motor practice alone resulted in increases in the contralateral motor cortex and inferior parietal lobule that was absent in the DSP + TMS group. Thus motor skill learning may engage disparate neural systems such as recruiting the contralateral hemisphere (as in the case of motor practice), and using cortical and subcortical regions in the same hemisphere (as in the case of TMS adjuvancy).
Brain areas implicated in motor learning are differentially engaged during various stages of skill learning (Doyon and Benali, 2005; Doyon et al., 2009; Ma et al., 2010, 2011; Orban et al., 2011; Xiong et al., 2009). For example, the frontal associative region areas, associative sections of striatum (dorsal and anterior parts of putamen), and motor cortical areas (including M1, SMA, PMd), and cerebellum, and part of the cortico-cerebellar network are activated during task performance in the fast learning state. Recent studies have also demonstrated the role of medial temporal regions including the hippocampus, and amygdala during this early phase (Albouy et al., 2008; DeCoteau et al., 2007; Schendan et al., 2003). As the skill acquisition evolves, the activation pattern moves from the associative striatum to the sensorimotor striatum (posterior ventral part of putamen), namely the cortico-striatal network. Similar shift has been seen in the cerebellum where the activation pattern during task performance moves from the cerebellar cortices to the cerebellar nuclei (Doyon et al., 2009). These findings have been incorporated into a neurobiological model of motor skill learning (Doyon and Benali, 2005; Doyon et al., 2009) that proposes an integrated view of the brain plasticity mediating motor memory formation at different stages of the acquisition process. Such a theoretical model permits us to make testable predictions with regard to the level at which an intervention (TMS, tDCS, physical therapy etc.) would mediate its effects. Based on this neurobiological model we hypothesized that TMS intervention should occur in the early learning phase when several brain regions including M1 are undergoing rapid changes. Indeed the behavioral and imaging findings presented here support with this argument.
TMS stimulation of M1 appears to augment the cortico-striatal loops as well as the cortico-cerebellar loops engaged during early stages of motor learning as indicated by resting CBF changes reported here. While DSP alone resulted in increases in resting CBF in the M1, premotor cortex, cingulate cortex, thalamus, and cerebellum, TMS adjuvancy resulted in increases in resting CBF extending to the striatum, substantia nigra, amygdala, and parahippocampus. TMS appears to improve motor performance by engaging the same network involved in skill learning, and by causing more pervasive changes. Thus, resting CBF increases in the amygdala, parahippocampus, striatum, substantia nigra, and cerebellum seen with TMS adjuvancy indicates stronger engagement of memory and motor networks. Prior to this study, the effects of TMS treatment motor networks have been investigated in the setting of task specific activation paradigms, that too in the context of TMS applied without DSP. Pascual-Leone et al. (1998) have demonstrated that 10 Hz subthreshold TMS resulted in increased activation in the stimulated M1 during task, while 1 Hz reduced activation at M1, but increased activation in the SMA and contralateral M1 — indicating that modulatory effects of TMS are not limited to the site of stimulation. Similarly, increased activation in motor network was observed following 10 Hz rTMS single session of 1000 pulses (Yoo et al., 2008). Thus, the behavioral and resting CBF changes reported here fit well in the theoretical framework, and indicate to the stage of skill learning when TMS can be applied to augment motor performance. We have demonstrated that TMS applied early in motor learning improves motor performance. These findings however are considered preliminary because of the small number of participants as well as subtle changes in the resting CBF, and further studies with larger sample size are needed to confirm our observations.
Mechanism of action of TMS
The behavioral and baseline blood flow augmentation resulting from TMS adjuvancy observed in this study can result from several mechanisms. Factors such as enhanced cortical excitability, increased effective synaptic transmission — LTP (Kim et al., 2006), expanded movement representation (Kleim et al., 2003), and greater offline consolidation (Reis et al., 2009) can all improve behavior and resting CBF. These changes result in a more synchronized firing and an enhanced synaptic transmission, a consequence of which is improved speed. Indeed, the resting CBF increases in M1 and connected brain regions reported here support this argument. A recent resting state fMRI study has indicated that tDCS can alter the intrinsic functional architecture of M1 such as increasing local connectedness, and enhancing long distance functional communication within M1 (Polanía et al., 2012). It is possible that TMS can result in similar functional reorganization at the site of stimulation. The mechanism of action of TMS induced facilitation of motor transfer is not well understood, and merits further investigation.
TMS parameters: rate, intensity, and duration
Two important parameters that influence the outcome of TMS adjuvancy are its rate and intensity. Higher frequencies of TMS generally have shown facilitation while rates less than 1 Hz have demonstrated inhibition at least in the motor system (Chen et al., 1997; Ridding and Rothwell, 2007). Consistent with this principle, 5 Hz and 10 Hz TMS applied to the primary motor cortex has demonstrated increased neuronal excitability (Peinemann et al., 2004; Ragert et al., 2003), and improved motor performance (Kim et al., 2004; Pascual-Leone et al., 1999; Siebner et al., 2000; Yoo et al., 2008). In order to identify the optimal rate to drive the motor cortex our group used a TMS/PET baboon model (Salinas et al., 2011) to investigate the rate of TMS needed to optimally stimulate the motor cortex. Cerebral blood flow was measured during 3 Hz, 5 Hz, 10 Hz, and 15 Hz TMS applied to M1. The data demonstrated that the CBF response was optimal at 5 Hz rTMS not only at the site of stimulation, but also in the connected motor areas (Salinas et al., 2013). In addition, we found no significant CBF changes at any rTMS frequency in the homologous contralateral M1hand region. These results are consistent with those demonstrated in previous human rTMS studies (Siebner et al., 2001). We therefore propose that TMS applied at 5 Hz can optimally modulate the activity at primary motor cortex and in connected areas.
Low intensity TMS (subthreshold) applied to the motor cortex results in local effects. This is consistent with the finding that at 5 Hz, subthreshold intensity of TMS did not improve motor performance, even though excitability of M1 was increased (Agostino et al., 2007). Suprathreshold TMS results in propagation of activity to remote connected regions (Fox et al., 2006; Siebner and Rothwell, 2003). Therefore in this study, the intensity of TMS was fixed at 100% of the motor threshold to ensure propagation of the activity downstream to the cortico-spinal tracts. While it is acknowledged that the rate and intensity probably interact, there are no studies that have examined this systematically. For example, contrary to that observed at 5 Hz, and 10 Hz TMS applied at subthreshold intensities results in LTP like effects (Kim et al., 2004; Pascual-Leone et al., 1999; Yoo et al., 2008). Future studies need to investigate the rate and intensity interaction to determine the optimal rate and intensity of TMS that can be applied in a safe manner.
Another important TMS parameter is the duration of TMS treatment. There is emerging evidence from the study of the motor system that the duration of effects of TMS is proportional to total number of TMS pulses (Peinemann et al., 2004; Quartarone et al., 2005) with a demonstration that a large number of pulses of TMS in one session (900 pulses or more) is needed to alter the excitability of the corticospinal output system, and even greater number of TMS pulses (≥1500) to alter spinal motor neuron excitability (Quartarone et al., 2005). In the present study, we delivered 6000 pulses of TMS at 5 Hz daily, in order to alter the excitability of the corticospinal output as well as the result in long lasting effects.
Advantage of resting state CBF studies
Recent studies of motor learning have demonstrated that it is important to investigate the baseline changes in CBF (Xiong et al., 2009). Significant training induced increase in power spectrum in the 0.08 Hz frequency band in resting state fMRI data reflecting an increase in spontaneous firing in the right M1 area, supports the resting CBF findings (Xiong et al., 2009). Understanding the direction of change in resting CBF helps interpret changes in task related activation patterns and connectivity within the motor learning network following DSP and TMS intervention. We replicated the previous reports of increased resting CBF in the motor cortex, SMA and premotor cortex following motor practice. In addition, TMS appears to further increase the resting state CBF in other regions engaged during motor skill acquisition. The TMS induced CBF effects demonstrate that one possible explanation for the decreased activation observed in the associative striatum, the medial temporal lobe, and the cerebellum during the late stages of skill learning could be due to an increase in the baseline or resting CBF in these regions. Since the resting state CBF changes were observed at a later stage of learning (at 4 weeks, when a behavioral plateau was achieved) these regions may in fact be important during later stages of learning as well, especially following TMS treatment. Further, the resting CBF changes shed light on the possible mechanism of action of TMS. Resting CBF changes during motor learning are thought to reflect closely with increased neuronal activity and synaptogenesis (Xiong et al., 2009). Similarly, TMS effects like enhancing synaptic strength (Kleim et al., 2003), increased dendritic hypertrophy (Adkins-Muir and Jones, 2003), and synaptogenesis in M1 can also result in increases in resting CBF. Exercise induced angiogenesis and up-regulation of cytochrome oxidase enzyme have also been suggested as possible mechanisms for increased resting CBF (Xiong et al., 2009). Effects of chronic administration of rTMS on angiogenesis, and cytochrome oxidase enzyme pathway may be akin to slow motor practice, but have not been investigated.
Limitations
Our study does have a few limitations. We did not investigate the effect of TMS only (without any DSP) on motor behavior. While TMS alone is able to up- (or down-) regulate a targeted neural system as applied in the treatment of depression, we argue that it is unlikely that TMS alone will train the system in a specific, skilled pattern of firing. Additional studies will have to be carried out to systematically examine this issue. Another important aspect of this study is that TMS was applied throughout 4 weeks of skill acquisition, spanning over various stages of skill acquisition. While the present study clearly demonstrates the effect of TMS on the early stage of skill learning, it cannot specify the effects of TMS at other stages of skill learning. Future studies will concentrate on examining the effect of TMS at each phase of motor skill learning.
Another drawback of this study is that we did not examine if the beneficial effects of TMS extended past the training duration. Indeed, for a TMS to be a truly effective treatment, its effects should persist beyond the duration of treatment. Other studies have demonstrated that the effects of motor practice are retained for several months both in normal persons (Kleim and Jones, 2008; Pendt et al., 2011) and in patients with Parkinson's disease (Pendt et al., 2011), and cerebral palsy (Geerdink et al., 2013). While concomitant 5 Hz TMS to the premotor cortex during motor learning resulted in greater retention (Boyd and Linsdell, 2009), tDCS did not enhance long-term retention in healthy persons (Reis et al., 2009). Therefore, future studies should investigate the long-lasting effects of TMS adjuvancy in a systematic manner in normal individuals as well as in various neurological disorders.
Future directions
Based on these findings in healthy individuals, we propose to use the same parameters to study the effect of TMS on motor rehabilitation in patients with stroke. Few studies have examined the augmentative effects of TMS and tDCS in patients with stroke. Improved motor function in stroke patients has been demonstrated following one session of subthreshold 10 Hz TMS (Kim et al., 2006) and 10 days of adjuvant suprathreshold 3 Hz TMS (Khedr et al., 2005). In a recent study, 10 days of adjuvant 10 Hz subthreshold TMS (or sham TMS) was applied to the M1 of the affected hemisphere along with motor practice in patients with subacute stroke (Chang et al., 2010). TMS group had additional behavioral improvement, an importantly, the effects persisted long term, at 3 months after intervention. Further examination of the neural correlates of this intervention demonstrated a greater modulation of activities in the cortico-basal ganglia–thalamocortical circuits (Chang et al., 2012). We anticipate that the rTMS protocol outlined in this study will result in similar behavioral and imaging findings. However, there are also reports of TMS not augmenting motor behavior. For example, two weeks of subthreshold 20 Hz TMS to M1 applied as adjuvant treatment with constraint induced therapy was not found to be beneficial (Malcolm et al., 2007). The inconsistencies in the rate, the intensity, and the duration of TMS, as well as the variability in the location of stroke, degree of deficits, and different mechanisms of action of physical therapies contribute to these disparate findings. We propose that the TMS parameters applied in this study would be appropriate for treatment trials in patients with stroke.
Conclusion
This study indicates that TMS applied concomitantly augments behavioral effects of motor practice by modifying neuronal activity in brain areas engaged during early phase of skill learning. Specifically, TMS to the M1hand improved motor performance by reducing the time taken for each sequence that resulted in a faster rate of learning. Both motor practice and TMS adjuvancy resulted in greater transfer of skills. The neural correlates of improved motor performance were increased in resting CBF in brain regions such as the cingulate motor cortex, putamen, medial temporal lobe, and cerebellum that are specifically engaged during early learning phase. The safety and feasibility of daily rTMS treatments over 4 weeks were demonstrated. These findings are the first to show the enhancing effects of TMS on slow motor practice over a 4-week period and have direct application in neurorehabilitation where TMS could be applied in conjunction with physical therapy.
Acknowledgments
This work was funded by grants from the Veterans Administration (Merit Award to Peter. T. Fox), and the National Institute of Deafness and Communication Disorders (R21-DC009467-01A1 to S. Narayana).
Footnotes
Conflict of interest
The authors have no conflicts of interest.
References
- Adkins-Muir DL, Jones TA. Cortical electrical stimulation combined with rehabilitative training: enhanced functional recovery and dendritic plasticity following focal cortical ischemia in rats. Neurol. Res. 2003;25(8):780–788. doi: 10.1179/016164103771953853. [DOI] [PubMed] [Google Scholar]
- Agostino R, Iezzi E, Dinapoli L, Gilio F, Conte A, Mari F, Berardelli A. Effects of 5 Hz subthreshold magnetic stimulation of primary motor cortex on fast finger movements in normal subjects. Exp. Brain Res. 2007;180(1):105–111. doi: 10.1007/s00221-006-0838-3. [DOI] [PubMed] [Google Scholar]
- Albouy G, Sterpenich V, Balteau E, Vandewalle G, Desseilles M, ng-Vu T, et al. Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron. 2008;58:261–272. doi: 10.1016/j.neuron.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of the human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor. Neurol. Neurosci. 2007;25(2):123–129. [PubMed] [Google Scholar]
- Boutin A, Badets A, Salesse RN, Fries U, Panzer S, Blandin Y. Practice makes transfer of motor skills imperfect. Psychol. Res. 2012a;76(5):611–625. doi: 10.1007/s00426-011-0355-2. [DOI] [PubMed] [Google Scholar]
- Boutin A, Panzer S, Salesse RN, Blandin Y. Testing promotes effector transfer. Acta Psychol. (Amst.) 2012b;141(3):400–407. doi: 10.1016/j.actpsy.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Boyd LA, Linsdell MA. Excitatory repetitive transcranial magnetic stimulation to left dorsal premotor cortex enhances motor consolidation of new skills. BMC Neurosci. 2009;10:72. doi: 10.1186/1471-2202-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bütefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG. Mechanisms of use-dependent plasticity in the human motor cortex. Proc. Natl. Acad. Sci. U. S. A. 2000;97(7):3661–3665. doi: 10.1073/pnas.050350297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bütefisch CM, Khurana V, Kopylev L, Cohen LG. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J. Neurophysiol. 2004;91(5):2110–2116. doi: 10.1152/jn.01038.2003. [DOI] [PubMed] [Google Scholar]
- Chang WH, Kim YH, Bang OY, Kim ST, Park YH, Lee PK. Long-term effects of rTMS on motor recovery in patients after subacute stroke. J. Rehabil. Med. 2010;42(8):758–764. doi: 10.2340/16501977-0590. [DOI] [PubMed] [Google Scholar]
- Chang WH, Kim YH, Yoo WK, Goo KH, Park CH, Kim ST, Pascual-Leone A. rTMS with motor training modulates cortico-basal ganglia–thalamocortical circuits in stroke patients. Restor. Neurol. Neurosci. 2012;30(3):179–189. doi: 10.3233/RNN-2012-110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48(5):1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Weisskoff RM, Schaechter JD, Nelles G, Foley M, Finklestein SP, et al. Motor cortex activation is related to force of squeezing. Hum. Brain Mapp. 2002;16(4):197–205. doi: 10.1002/hbm.10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoteau WE, Thorn C, Gibson DJ, Courtemanche R, Mitra P, Kubota Y, et al. Learning-related coordination of striatal and hippocampal theta rhythms during acquisition of a procedural maze task. Proc. Natl. Acad. Sci. U. S. A. 2007;104:5644–5649. doi: 10.1073/pnas.0700818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr. Opin. Neurobiol. 2005;15(2):161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, et al. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav. Brain Res. 2009;199(1):61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Duff E, Xiong J, Wang B, Cunnington R, Fox P, Egan G. Complex spatio-temporal dynamics of fMRI BOLD: a study of motor learning. NeuroImage. 2007;34(1):156–168. doi: 10.1016/j.neuroimage.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff EP, Johnston LA, Xiong J, Fox PT, Mareels I, Egan GF. The power of spectral density analysis for mapping endogenous BOLD signal fluctuations. Hum. Brain Mapp. 2008;29(7):778–790. doi: 10.1002/hbm.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Mintun MA. Noninvasive functional brain mapping by change distribution analysis of averaged PET images of H152O tissue activity. J. Nucl. Med. 1989;30:141–149. [PubMed] [Google Scholar]
- Fox PT, Raichle ME. Stimulus rate dependence of regional cerebral blood flow in human striatal cortex, demonstrated by positron emission tomography. J. Neurophysiol. 1984;51(5):1109–1120. doi: 10.1152/jn.1984.51.5.1109. [DOI] [PubMed] [Google Scholar]
- Fox PT, Mintun MA, Reiman EM, Raichle ME. Enhanced detection of focal brain responses using intersubject averaging and change-distribution analysis of subtracted PET images. J. Cereb. Blood Flow Metab. 1988;8:642–653. doi: 10.1038/jcbfm.1988.111. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Zamarripa F, Xiong JH, Lancaster JL. Brain correlates of stuttering and syllable production. a PET performance-correlation analysis. Brain. 2000;23(Pt 10):1985–2004. doi: 10.1093/brain/123.10.1985. [DOI] [PubMed] [Google Scholar]
- Fox PT, Narayana S, Tandon N, Sandoval H, Fox SP, Kochunov P, et al. Column-based model of electric field excitation of cerebral cortex. Hum. Brain Mapp. 2004;22(1):1–14. doi: 10.1002/hbm.20006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Narayana S, Tandon N, Fox SP, Sandoval H, Kochunov P, et al. Intensity modulation of TMS induced cortical excitation: primary motor cortex. Hum. Brain Mapp. 2006;27(6):478–487. doi: 10.1002/hbm.20192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerdink Y, Aarts P, Geurts AC. Motor learning curve and long-term effectiveness of modified constraint-induced movement therapy in children with unilateral cerebral palsy: a randomized controlled trial. Res. Dev. Disabil. 2013;34(3):923–931. doi: 10.1016/j.ridd.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Gentner R, Gorges S, Weise D, aufm Kampe K, Buttmann M, Classen J. Encoding of motor skill in the corticomuscular system of musicians. Curr. Biol. 2010;20(20):1869–1874. doi: 10.1016/j.cub.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Woods RP, Mazziotta JC, Phelps ME. Somatotopic mapping of the primary motor cortex in humans: activation studies with cerebral blood flow and positron emission tomography. J. Neurophysiol. 1992;66(3):735–743. doi: 10.1152/jn.1991.66.3.735. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry RB. Motor sequence learning with the nondominant left hand. A PET functional imaging study. Exp. Brain Res. 2002;146(3):369–378. doi: 10.1007/s00221-002-1181-y. [DOI] [PubMed] [Google Scholar]
- Hebb DO. Physiological learning theory. J. Abnorm. Child Psychol. 1976;4(4):309–314. doi: 10.1007/BF00922529. [DOI] [PubMed] [Google Scholar]
- Hlustík P, Solodkin A, Noll DC, Small SL. Cortical plasticity during three-week motor skill learning. J. Clin. Neurophysiol. 2004;21(3):180–191. doi: 10.1097/00004691-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J. Neurosci. 2011;31(7):2481–2487. doi: 10.1523/JNEUROSCI.5411-10.2011. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung P, Ziemann U. Homeostatic and nonhomeostatic modulation of learning in human motor cortex. J. Neurosci. 2009;29(17):5597–5604. doi: 10.1523/JNEUROSCI.0222-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377(6545):155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. PNAS. 1998;95:250–252. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65(3):466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- Kim YH, Park JW, Ko MH, Jang SH, Lee PK. Facilitative effect of high frequency subthreshold repetitive transcranial magnetic stimulation on complex sequential motor learning in humans. Neurosci. Lett. 2004;367(2):181–185. doi: 10.1016/j.neulet.2004.05.113. [DOI] [PubMed] [Google Scholar]
- Kim YH, You SH, Ko MH, Park JW, Lee KH, Jang SH, Yoo WK, Hallett M. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37(6):1471–1476. doi: 10.1161/01.STR.0000221233.55497.51. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 2008;51(1):S225–S239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- Kleim JA, Bruneau R, VandenBerg P, MacDonald E, Mulrooney R, Pocock D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol. Res. 2003;25(8):789–793. doi: 10.1179/016164103771953862. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J. Neurosci. 2004;24(3):628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman M, Raz N, Flash T, Karni A. Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. Proc. Natl. Acad. Sci. U. S. A. 2003;100(21):12492–12497. doi: 10.1073/pnas.2035019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Glass TG, Lankipalli BR, Downs H, Mayberg HS, Fox PT. A modality-independent approach to spatial normalization of tomographic images of the human brain. Hum. Brain Mapp. 1995;3:209–223. [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, et al. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum. Brain Mapp. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Narayana S, Wenzel D, Luckemeyer J, Roby J, Fox PT. Evaluation of an image-guided robotically-positioned transcranial magnetic stimulation system. Hum. Brain Mapp. 2004;22(4):329–340. doi: 10.1002/hbm.20041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, Laloux P, Peeters A, Desfontaines P, Jamart J, Vandermeeren Y. Dual-tDCS enhances online motor skill learning and long-term retention in chronic stroke patients. Front. Hum. Neurosci. 2012;6:343. doi: 10.3389/fnhum.2012.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Wang B, Narayana S, Hazeltine E, Chen X, Robin DA, et al. Changes in regional activity are accompanied with changes in inter-regional connectivity during 4 weeks motor learning. Brain Res. 2010;1318C:64–76. doi: 10.1016/j.brainres.2009.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Narayana S, Robin DA, Fox PT, Xiong J. Changes occur in resting state network of motor system during 4 weeks of motor skill learning. NeuroImage. 2011;58(1):226–233. doi: 10.1016/j.neuroimage.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm MP, Triggs WJ, Light KE, Gonzalez Rothi LJ, Wu S, Reid K, et al. Repetitive transcranial magnetic stimulation as an adjunct to constraint induced therapy: an exploratory randomized controlled trial. Am. J. Phys. Med. Rehabil. 2007;86(9):707–715. doi: 10.1097/PHM.0b013e31813e0de0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE, Hastie R. Learning as accumulation: a reexamination of the learning curve. Psychol. Bull. 1978;85(6):1256–1274. [PubMed] [Google Scholar]
- Mintun MA, Fox PT, Raichle ME. A highly accurate method of localizing regions of neuronal activation in the human brain with positron emission tomography. J. Cereb. Blood Flow Metab. 1989;9(1):96–103. doi: 10.1038/jcbfm.1989.13. [DOI] [PubMed] [Google Scholar]
- Newell KM, Liu YT, Mayer-Kress G. Time scales in motor learning and development. Psychol. Rev. 2001;108(1):57–82. doi: 10.1037/0033-295x.108.1.57. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orban P, Peigneux P, Lungu O, Debas K, Barakat M, Bellec P, et al. Functional neuroanatomy associated with the expression of distinct movement kinematics in motor sequence learning. Neuroscience. 2011;179:94–103. doi: 10.1016/j.neuroscience.2011.01.040. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J. Neurophysiol. 1995;74(3):1037–1045. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Cañete C, Catalá MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J. Clin. Neurophysiol. 1998;15(4):333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tarazona F, Catalá MD. Applications of transcranial magnetic stimulation in studies on motor learning. Electroencephalogr. Clin. Neurophysiol. Suppl. 1999;51:157–161. [PubMed] [Google Scholar]
- Peinemann A, Reimer B, Löer C, Quartarone A, Münchau A, Conrad B, Siebner HR. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin. Neurophysiol. 2004;115(7):1519–1526. doi: 10.1016/j.clinph.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Pendt LK, Reuter I, Müller H. Motor skill learning, retention, and control deficits in Parkinson's disease. PLoS One. 2011;6(7):e21669. doi: 10.1371/journal.pone.0021669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhune VB, Steele CJ. Parallel contributions of cerebellar, striatal and M1 mechanisms to motor sequence learning. Behav. Brain Res. 2012;226(2):579–591. doi: 10.1016/j.bbr.2011.09.044. [DOI] [PubMed] [Google Scholar]
- Platz T, Roschka S, Christel MI, Duecker F, Rothwell JC, Sack A. Early stages of motor skill learning and the specific relevance of the cortical motor system — a combined behavioural training and theta burst TMS study. Restor. Neurol. Neurosci. 2012a;30(3):199–211. doi: 10.3233/RNN-2012-110204. [DOI] [PubMed] [Google Scholar]
- Platz T, Roschka S, Doppl K, Roth C, Lotze M, Sack AT, et al. Prolonged motor skill learning — a combined behavioural training and theta burst TMS study. Restor. Neurol. Neurosci. 2012b;30(3):213–224. doi: 10.3233/RNN-2012-110205. [DOI] [PubMed] [Google Scholar]
- Polanía R, Paulus W, Nitsche MA. Reorganizing the intrinsic functional architecture of the human primary motor cortex during rest with non-invasive cortical stimulation. PLoS One. 2012;7(1):e30971. doi: 10.1371/journal.pone.0030971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Imaging brain plasticity: conceptual and methodological issues — a theoretical review. NeuroImage. 2000;12(1):1–13. doi: 10.1006/nimg.2000.0596. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Morgante F, Sant'angelo A, Battaglia F, et al. Distinct changes in cortical and spinal excitability following high-frequency repetitive TMS to the human motor cortex. Exp. Brain Res. 2005;161(1):114–124. doi: 10.1007/s00221-004-2052-5. [DOI] [PubMed] [Google Scholar]
- Rábago CA, Lancaster JL, Narayana S, Zhang W, Fox PT. Automated-parameterization of the motor evoked potential and cortical silent period induced by transcranial magnetic stimulation. Clin. Neurophysiol. 2009;120(8):1577–1587. doi: 10.1016/j.clinph.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragert P, Dinse HR, Pleger B, Wilimzig C, Frombach E, Schwenkreis P, et al. Combination of 5 Hz repetitive transcranial magnetic stimulation (rTMS) and tactile coactivation boosts tactile discrimination in humans. Neurosci. Lett. 2003;348(2):105–108. doi: 10.1016/s0304-3940(03)00745-6. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc. Natl. Acad. Sci. U. S. A. 2009;106(5):1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat. Rev. Neurosci. 2007;8(7):559–567. doi: 10.1038/nrn2169. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat. Neurosci. 1998;1(3):230–234. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Rogers W, Zhang W, Narayana S, Lancaster JL, Robin DA, Fox PT. Force sensing system for automated assessment of motor performance during fMRI. J. Neurosci. Methods. 2010;190(1):92–94. doi: 10.1016/j.jneumeth.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A, Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr. Clin. Neurophysiol. 1994;91(2):79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Salinas FS, Szabó CÁ, Zhang W, Jones L, Leland MM, Wey HY, Duong TQ, Fox PT, Narayana S. Functional neuroimaging of the baboon during concurrent image-guided transcranial magnetic stimulation. NeuroImage. 2011;57(4):1393–1401. doi: 10.1016/j.neuroimage.2011.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas FS, Narayana S, Zhang W, Fox PT, Szabó CÁ. Repetitive transcranial magnetic stimulation elicits rate dependent brain network responses in non human primates. Brain Stimul. 2013 doi: 10.1016/j.brs.2013.03.002. http://dx.doi.org/10.1016/j.brs.2013.03.002 (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- Schendan HE, Searl MM, Melrose RJ, Stern CE. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Noll DC. Neuroanatomical correlates of motor acquisition and motor transfer. J. Neurophysiol. 2008;99(4):1836–1845. doi: 10.1152/jn.01187.2007. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp. Brain Res. 2003;148(1):1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Peller M, Willoch F, Minoshima S, Boecker H, Auer C, et al. Lasting cortical activation after repetitive TMS of the motor cortex: a glucose metabolic study. Neurology. 2000;54(4):956–963. doi: 10.1212/wnl.54.4.956. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Takano B, Peinemann A, Schwaiger M, Conrad B, Drzezga A. Continuous transcranial magnetic stimulation during positron emission tomography: a suitable tool for imaging regional excitability of the human cortex. NeuroImage. 2001;14(4):883–890. doi: 10.1006/nimg.2001.0889. [DOI] [PubMed] [Google Scholar]
- Slotema CW, Blom JD, Hoek HW, Sommer IE. Should we expand the tool-box of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J. Clin. Psychiatry. 2010;71(7):873–884. doi: 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme Medical Publishers; NY.: 1988. [Google Scholar]
- Ungerleider LG, Doyon J, Karni A. Imaging brain plasticity during motor skill learning. Neurobiol. Learn. Mem. 2002 Nov;78(3):553–564. doi: 10.1006/nlme.2002.4091. [DOI] [PubMed] [Google Scholar]
- Wassermannn EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr. Clin. Neurophysiol. 1998;108(1):1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Xiong J, Ma L, Wang B, Narayana S, Duff EP, Egan GF, et al. Long-term motor training induced changes in regional cerebral blood flow in both task and resting states. NeuroImage. 2009;45(1):75–82. doi: 10.1016/j.neuroimage.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo WK, You SH, Ko MH, Tae Kim S, Park CH, Park JW, Hoon Ohn S, Hallett M, Kim YH. High frequency rTMS modulation of the sensorimotor networks: behavioral changes and fMRI correlates. NeuroImage. 2008;39(4):1886–1889. doi: 10.1016/j.neuroimage.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Ilić TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J. Neurosci. 2004;24(7):1666–1672. doi: 10.1523/JNEUROSCI.5016-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]