Abstract
Herein, we describe the synthesis of a chemically defined CXCR4-auristatin antibody-drug conjugate (ADC) that selectively eliminates CXCR4+ over-expressing tumors. The unnatural amino acid p-acetylphenylalanine (pAcF) was site-specifically incorporated into an anti-CXCR4 IgG and conjugated to an auristatin analogue via a stable, non-cleavable oxime linkage to afford a chemically homogeneous ADC. The full-length anti-CXCR4 ADC was selectively cytotoxic to CXCR4+ cancer cells in vitro (EC50 ≈80–100 pM). Moreover, the anti-CXCR4 ADC eliminated pulmonary lesions from human osteosarcoma cells in a lung-seeding tumor model. No significant overt toxicity was observed while there was a modest decrease in the bone marrow-derived CXCR4+ cell population. Because CXCR4 is highly expressed in a majority of metastatic cancers, a CXCR4-auristatin ADC may be useful for the treatment of a variety of metastatic malignancies.
Keywords: cancer, antibody-drug conjugate, immunotherapy, antibody engineering, unnatural amino acid
Although increasingly effective drugs are being developed for the treatment of primary tumors, the treatment options for metastatic cancer are relatively limited and, consequently, the prognosis is poor. This is especially the case when multiple sites and tissues are affected, further complicating elimination of malignant versus normal cells. Recently, antibody-drug conjugates (ADCs) have emerged as a new class of immunotherapeutic agents that can deliver drugs selectively to tumors by binding to antigens highly expressed on cancers cells, followed by internalization and intracellular drug release.[1] Here, we have applied this strategy to cell surface antigens that are preferentially expressed on metastatic tumor cells. A number of studies have indicated that CXCR4 (CD184), a G-protein coupled receptor, is expressed in a variety of cancers, including breast, prostate, pancreatic, ovarian, melanoma, brain, and many forms of hematological malignancies.[2] Although CXCR4 expression levels are highly variable between these cancers, CXCR4 is known to be over-expressed in almost all metastatic tumor cells and, consequently, higher expression of CXCR4 is an adverse predictor of survival and a strong predictor of tumor relapse in patients.[3] It has also been established that SDF1α (also known as CXCL12), the cognate ligand of CXCR4 is highly expressed in local, regional, and distant metastatic sites, such as lymph nodes, bone marrow, lung, and liver, suggesting that the CXCR4/SDF1α axis plays a major role in regulating the destination of most tumor cell metastases.[4] Moreover, CXCR4 is known to be over-expressed in cancer stem cells (CSC) and induces CSCs to undergo chemotaxis and migrate below marrow stromal cells, thereby allowing these cells access to cellular niches.[2c–e]
Consequently, considerable effort has been dedicated to the development of CXCR4-targeted therapeutic agents for metastatic cancers. AMD3100 (Plerixafor, a low molecular weight CXCR4 antagonist), T140 and T22 (polypeptide CXCR4 antagonists), BKT140 (a 14-residue synthetic peptide) and CTCE-9908 (an analogue of SDF1) lead to reduced metastasis in various animal models and also show activity in non-small cell lung cancer (NSCLC), acute myeloid leukemia (AML) and multiple myeloma xenograft models.[5] Moreover, pretreatment of primary human leukemic cells with neutralizing CXCR4 antibodies blocked the entrance of homing leukemic cells into the bone marrow and spleen of transplanted NOD SCID mice.[6] A number of these CXCR4 antagonists, including AMD3100 and BKT140, as well as anti-CXCR4 antibodies (e.g., BMS-936564) are currently in clinical trials for the treatment of AML and multiple myeloma. However, it may be possible to further increase the efficacy of these agents by combining them with a selectively delivered cytotoxic agent. The ADC strategy is especially attractive since the CXCR4 receptor and its ligands have been shown to be efficiently internalized.[7] While CXCR4-targeted antibody-drug conjugates (ADC) have not yet been reported for the treatment of cancer, several types of highly cytotoxic agents, including calicheamicin, maytansinoid, and auristatin, have been conjugated to monoclonal antibodies such as anti-Her2, anti-PSMA, and anti-CD30.[8] Herein, we describe the development of an anti-CXCR4 IgG–auristatin ADC that demonstrates excellent in vitro efficacy against metastatic SJSA-1-met-luc cells (a human osteosarcoma cell line implanted in the tibia of a mouse and then derived as metastasized cells from the lung). Importantly, this ADC is capable of eliminating tumors in vivo in mice in a lung-seeding tumor model using SJSA-1-met-luc cells.
Auristatin, an antimitotic agent that prevents tubulin polymerization, has previously been conjugated to a number of antibodies, including anti-CD30, anti-PSMA, and anti-Her2 antibodies, and shown to be highly cytotoxic to tumors.[8–9] Moreover, because auristatin kills rapidly proliferating cells by interfering with microtubule function, it might be expected to be less cytotoxic to non-replicating hematopoietic stem cells that also express CXCR4. Additionally, we chose to conjugate monomethyl auristatin F (MMAF) since it is minimally cell permeable due to its C-terminal carboxylic acid.[10] To conjugate MMAF site-specifically to surface-exposed sites in full-length anti-CXCR4 IgG, we produced recombinant antibodies containing p-acetyl phenylalanine (pAcF) by unnatural amino acid (UAA) mutagenesis technology. An orthogonal tRNA/aminoacyl-tRNA synthetase (aaRS) pair specific for pAcF was co-expressed with an anti-CXCR4 gene containing a TAG codon at residue A122 in the heavy chain.[11] The tRNA/aaRS pair and mutant CXCR4 IgG genes were transiently transfected into CHO-S cells (Life Technologies) supplemented with 1.3 mM pAcF. After 10 days at 32 °C, the supernatant was harvested and anti-CXCR4 A122pAcF IgG (referred to herein as anti-CXCR4 IgGX) was purified using protein A affinity chromatography with a yield of ~1 mg/L. We used a stable, non-cleavable hydrophilic linker containing ethylene glycol units (for increased solubility) to ensure delivery of the intact ADC to the tumor followed by rapid release of the drug in the lysosome.[12]
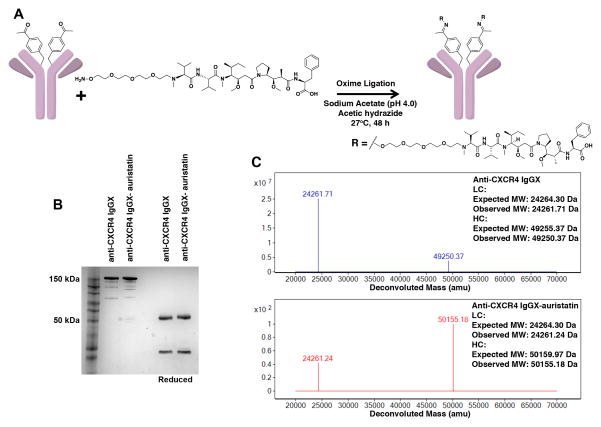
An aminooxy moiety was incorporated at the distal end of the PEG linker to selectively conjugate drug to the mutant anti-CXCR4 IgG variant via a stable oxime bond. The pAcF mutant IgG was conjugated to auristatin F-PEG2-aminooxy in sodium acetate buffer (pH 4.0) in the presence of acetic hydrazide at 28°C for 48 hours, and purified by repeated washing with PBS (pH 7.4) using an Amicon concentrator with 10 kDa MWCO (Figure 1A). Denaturing gel electrophoresis (SDS/PAGE) demonstrated that the mutant anti-CXCR4 A122pAcF IgG (anti-CXCR4 IgGX) and anti-CXCR4 A122pAcF IgG-auristatin (anti-CXCR4 IgGX-auristatin) are > 90% pure and resolved into bands of ≈150 kDa (non-reducing conditions, full-length IgG) and ~50 and ~25 kDa (reducing conditions, heavy and light chain, respectively) (Figure 1B). Electrospray-ionization mass spectrometry (ESI-MS) analysis indicated that the reduced heavy chain of anti-CXCR4 IgGX has an expected mass of 49,250 Da (Figure 1C and Figure S1). Further, ESI-MS analysis revealed a mass of 50,155 Da for the reduced heavy chain of anti-CXCR4 IgGX-auristatin (Figure 1C and Figure S1); an increase in mass by ~905 Da upon conjugation corresponds the mass of two auristatin derivatives per IgG. We did not observe any unconjugated IgG or degraded products, indicating a >95% coupling efficiency. We were thus able to produce chemically defined, homogeneous ADCs with a drug to antibody ratio (DAR) of 2.
Figure 1. Site-specific conjugation of aminooxy-auristatin to anti-CXCR4 A122pAcF IgG (anti-CXCR4 IgGX).
(A) Monomethyl auristatin F (MMAF) derivatized with a terminal alkoxy-amine is coupled by oxime ligation to anti-CXCR4 A122pAcF IgG through a pAcF residue.
(B) SDS/PAGE gel of anti-CXCR4-IgGX before and after coupling to auristatin. The mutant IgG includes natural heterogeneous N-linked glycosylation; ~150 kDa corresponds to the full-length IgG; ~25 kDa and ~50 kDa correspond to the reduced light chain and heavy chain, respectively. The 4–12% Tris-Glycine gel has a prestained protein ladder in the first lane and was stained with Coomassie Blue. (C) ESI-MS analysis of anti-CXCR4 IgGX before and after conjugation to auristatin. Mutant IgG spectra show the heavy and light chains after removal of glycans with PNGase F (Promega, PBS pH 7.4, 37 °C, 12 hr) and reduction with 10 mM DTT. All masses are as expected with a ~905-Da difference between the conjugated and unconjugated antibodies, corresponding to conjugation of one auristatin per heavy chain. No unreacted antibody was observed by SDS/PAGE or ESI-MS, suggesting >95% coupling efficiency.
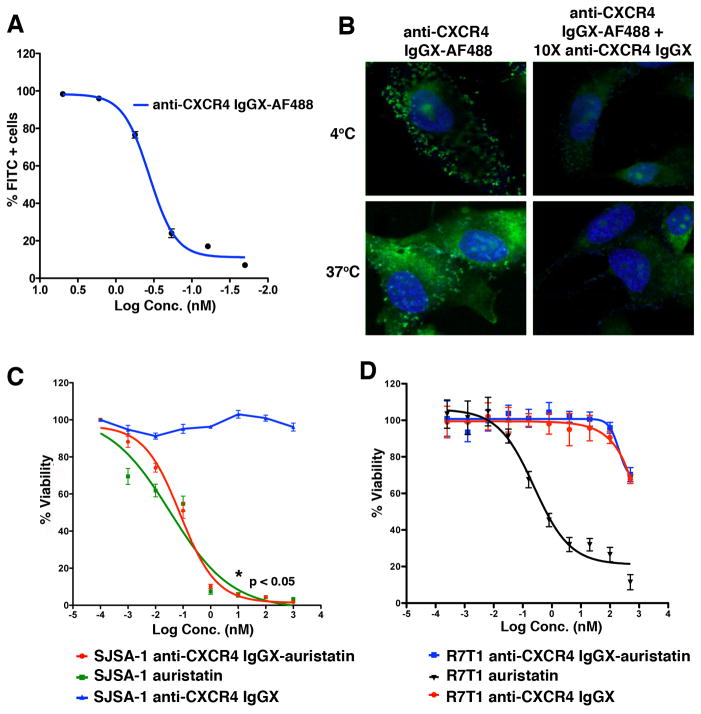
We next evaluated the specificity, affinity and internalization of the mutant antibody by conjugating it to Alexa Fluor 488 (AF488), followed by analysis of receptor binding and endocytosis using flow cytometry and confocal microscopy. Purified anti-CXCR4 IgGX was conjugated site-specifically to an aminooxy-modified AF488. SJSA-1 parental human osteosarcoma cells are known to express high levels of CXCR4.[13] From these cells, a metastatic variant was derived after intratibial injection of the parental cells in SCID mice and collection of spontaneously metastasized tumor cells from lung lesions. These cells were stably transduced with firefly luciferase (SJSA-1-met-luc), express high levels of CXCR4, and metastasize very efficiently to the lungs from the blood stream in a SCID mouse model. SJSA-1-met-luc were used here to confirm binding of the mutant anti-CXCR4 IgG. The anti-CXCR4 IgGX-AF488 conjugate bound to SJSA-1-met-luc cells with high affinity (Figure 2A and Figure S2); EC50= 0.48 nM) as measured by flow cytometry; binding was inhibited by 10-fold excess of unconjugated antibody (determined by confocal microscopy) (Figure 2B). Furthermore, anti-CXCR4 IgG-AF488 was observed in the cytoplasm within 30 minutes at 37 °C, indicating efficient internalization, which was also blocked by 10-fold excess of unconjugated antibody (Figure 2B). Since CXCR4 is expressed on hematopoietic cells, such as HSCs, lymphocytes, monocytes and NK cells, we further demonstrated binding of the anti-CXCR4 IgGX to CXCR4+ Jurkat cells. We also showed that the mutant antibody binds to CHO cells transfected with either human or mouse CXCR4 and to mouse bone marrow cells. Minimal to no binding was observed with non-transfected CHO cells and R7T1 cells (mouse CXCR4−) (Figure S2 and Figure S3).
Figure 2. In vitro activity of anti-CXCR4 IgGX-auristatin.
(A) Binding of anti-CXCR4 IgGX-AF488 to SJSA-1-met-luc cells. Cells were incubated with increasing concentrations of the conjugate at 4 °C for 30 mins and binding was analyzed by flow cytometry. (B) SJSA-1-met-luc cells were plated at 80% confluence and treated with 50 nM anti-CXCR4 IgGX-AF488 in the presence or absence of 500 nM unconjugated anti-CXCR4 IgGX. Cells were incubated at either 4 °C or 37 °C for 30 mins, fixed and imaged using a Zeiss confocal microscope. Dose dependent in vitro cytotoxicity of anti-CXCR4 IgGX-auristatin with (C) SJSA-1-met-luc (CXCR4+) and (D) R7T1 (CXCR4−) cells. Cells were treated with increasing concentrations of anti-CXCR4 IgGX, anti-CXCR4 IgGX-auristatin or unconjugated auristatin for 72 hrs at 37 °C, 5% CO2 and viability measured using CellTiter Glo (Promega). Percent viability is normalized to untreated controls. (n=3, mean and S.D.)
To determine the in vitro efficacy of the ADCs, SJSA-1-met-luc cells were grown to 80% confluence and treated with either anti-CXCR4 IgGX-auristatin, unconjugated mutant IgG or monomethyl auristatin E (MMAE) (positive control for cytotoxicity) for 72 hours and cell viability was measured by bioluminescent quantitation of released ATP using CellTiter Glo. Cell viability was significantly decreased (*p < 0.05) with increasing concentrations of anti-CXCR4 IgGX-auristatin (EC50 of 0.08 ± 0.03 nM (mean ± SD)) and unconjugated MMAE (EC50 of 0.03 ± 0.06 nM (mean ± SD)), while no significant activity was observed with unconjugated mutant antibody at up to 1 μM concentrations (Figure 2C). Further, no cytotoxicity was observed following treatment of R7T1 cells (CXCR4−) with either anti-CXCR4 IgGX-auristatin or unconjugated anti-CXCR4 IgGX (Figure 2D). Taken together, these data indicate that the observed cell killing by the ADC is due to CXCR4 dependent internalization of auristatin conjugated to the mutant anti-CXCR4 antibody.
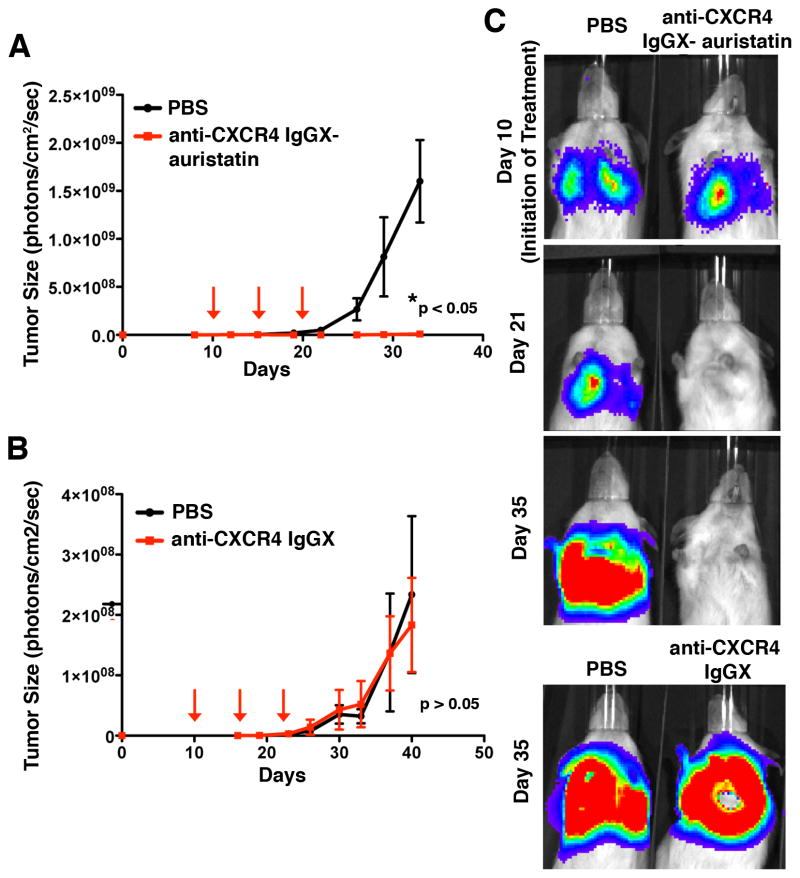
We then evaluated the efficacy of the anti-CXCR4 ADC in a mouse tumor xenograft model (lung-seeding model). SJSA-1-met-luc tumor growth rates were determined in vivo by longitudinal noninvasive bioluminescence imaging. The SJSA-1-met-Luc cells (10,000/mouse) were injected via tail vein into NOD/SCID (C. B17) mice (day zero) followed by noninvasive bioluminescence imaging, which indicated tumor cell signal accumulating in the lungs of the mice. Most cells were cleared away from the lungs within 2 days as indicated by a decrease in the observed bioluminescence. By day 10, an increase in bioluminescence was observed in the lungs, indicating that the cells that remained in the lungs had seeded and formed tumors. Treatment was initiated when pulmonary tumor lesions reached ~1×106 photons per cm2/sec (~day 10; Figure 3, Figure S4, and Figure S5). Mice were randomized into 3 groups and injected with vehicle (PBS), anti-CXCR4 IgGX-auristatin (3 doses, 2.5mg/kg, every 5 days, i.v., on the basis of previous pharmacokinetic and efficacy experiments with a similarly generated anti-Her2 IgG-auristatin conjugate),[8a] or unconjugated anti-CXCR4 IgGX (3 doses, 2.5mg/kg, every 5 days, i.v.). In mice treated with the anti-CXCR4 IgGX-auristatin, initial pulmonary tumor lesions were barely detectable within 8 days, as compared the tumor burden in PBS treated mice, which displayed continuously growing lesions at a steady rate (Figure 3A, C and Figure S4, n= 6 mice/group; mean and SEM, *p < 0.05). Mice treated with anti-CXCR4 IgGX-auristatin showed a complete absence of tumor burden even 15 days after the final treatment dose (Figure 3A, C and Figure S4), demonstrating excellent efficacy for this site-specific ADC with two drug molecules per antibody. As anticipated, no significant differences were observed between mice treated with either PBS or unconjugated or anti-CXCR4 IgGX (Figure 3B, C and Figure S5, n = 6 mice/group, mean and SEM, p > 0.05). Since the unconjugated mutant antibodies did not exhibit in vitro cytotoxicity on SJSA-1-met-luc cells and SCID mice do not have adaptive immune systems, a significant treatment effect of unconjugated antibodies either by cell toxicity or antibody-dependent cell-mediated cytotoxicity (ADCC) was not expected. Importantly, total body weights of mice treated with the ADC remained unchanged throughout the duration of the study (Figure S6), suggesting that the therapy is not grossly toxic to the animals.
Figure 3. In vivo efficacy of anti-CXCR4 IgGX-auristatin.
Lung tumors were allowed to develop in NOD/SCID (C.B17) mice for 10 days after i.v. injection of 104 SJSA-1-met-luc cells. Lesion development and response to antibody treatment was monitored using longitudinal noninvasive bioluminescence imaging (IVIS 200). (A) Mice injected i.v. with either anti-CXCR4 IgGX-auristatin (3 doses of 2.5mg/kg once every 5 days) or PBS (n=6 mice/group; mean and SEM, *p < 0.05). (B) Mice injected with either anti-CXCR4 IgGX (3 doses of 2.5mg/kg once every 5 days) or PBS (n=6 mice/group, mean and SEM, p>0.05). (C) Representative images from IVIS imaging showing bioluminescence corresponding to tumor size on various days during the study.
Hematopoietic stem cells (HSCs) and various cells of the hematopoietic lineage express CXCR4 and may be potential targets for anti-CXCR4 ADCs.[14] Depletion of these cell types could pose a significant clinical concern for the translation of an anti-CXCR4 ADC to the clinic. However, HSCs in the bone marrow are known to be mostly quiescent and, as such, may not be affected by a microtubule binder like auristatin that primarily targets rapidly proliferating cells. [14a, 15] In order to test the potential effects of the anti-CXCR4 ADC on HSCs in vivo, we injected BALB/c mice with either PBS or anti-CXCR4 IgGX-auristatin (2 doses, 2.5mg/kg, every 5 days, i.v.). We then isolated bone marrow from these mice and analyzed the HSCs in the bone marrow by flow cytometry (Figure S7A).[16] HSCs were identified as Lin−/c-kit+/sca1+ (LSK) populations and were further classified as multipotent progenitor cells (MPP; Lin−/c-kit+/sca1+/CD48+/CD150+), short-term HSCs (ST-HSCs; Lin−/c-kit+/sca1+/CD34+/CD135−), and long-term HSCs (LT-HSCs; Lin−/c-kit+/sca1+/CD34−/CD135−), (Figure S7B and Table S1). Consistent with the non-proliferating nature of endogenous HSCs, a modest decrease (24%) in HSC populations was observed in mice treated with the ADC as compared to PBS treated mice (Figure S7C).
In conclusion, we generated a site-specific anti-CXCR4 IgGX-auristatin conjugate with a DAR of 2 that was able to selectively target and eliminate CXCR4+ metastatic cancer cells, both in vitro and in vivo. This approach also significantly spared CXCR4+ hematopoietic cells in vivo, suggesting that it may represent a promising new approach to the treatment of metastatic cancer.
Supplementary Material
Footnotes
This is manuscript No. 28020 from the Scripps Research Institute. This work was supported by NIH grant R01 GM097206 (PGS), R01 CA170737 (BFH), and R01 CA170140 (BFH)
Supporting information for this article (including experimental details) is available on the WWW under http://
Contributor Information
Dr. Sumith A. Kularatne, Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute 10550 N. Torrey Pines Rd, La Jolla, CA 92037 (USA).
Dr. Vishal Deshmukh, Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute 10550 N. Torrey Pines Rd, La Jolla, CA 92037 (USA).
Dr. Jennifer Ma, California Institute for Biomedical Research (Calibr) 11119 N. Torrey Pines Road, La Jolla, CA 92037 (USA)
Dr. Virginie Tardif, Department of Immunology and Microbial Science, The Scripps Research Institute, La Jolla, CA 92037 (USA)
Dr. Reyna K. V. Lim, California Institute for Biomedical Research (Calibr) 11119 N. Torrey Pines Road, La Jolla, CA 92037 (USA)
Holly M. Pugh, California Institute for Biomedical Research (Calibr) 11119 N. Torrey Pines Road, La Jolla, CA 92037 (USA)
Dr. Ying Sun, Ambrx, Inc., 10975 N. Torrey Pines Rd, La Jolla, CA 92037
Anthony Manibusan, Ambrx, Inc., 10975 N. Torrey Pines Rd, La Jolla, CA 92037.
Aaron J. Sellers, Ambrx, Inc., 10975 N. Torrey Pines Rd, La Jolla, CA 92037
Dr. Richard S. Barnett, Ambrx, Inc., 10975 N. Torrey Pines Rd, La Jolla, CA 92037
Shailaja Srinagesh, Ambrx, Inc., 10975 N. Torrey Pines Rd, La Jolla, CA 92037.
Jane S. Forsyth, Department of Molecular and Experimental Medicine, The Scripps Research Institute, La Jolla, CA 92037 (USA)
Dr. Wolf Hassenpflug, Department of Molecular and Experimental Medicine, The Scripps Research Institute, La Jolla, CA 92037 (USA)
Dr. Feng Tian, Ambrx, Inc., 10975 N. Torrey Pines Rd, La Jolla, CA 92037
Dr. Tsotne Javahishvili, Ambrx, Inc., 10975 N. Torrey Pines Rd, La Jolla, CA 92037
Dr. Brunhilde Felding-Habermann, Department of Molecular and Experimental Medicine, The Scripps Research Institute, La Jolla, CA 92037 (USA)
Dr. Brian R. Lawson, California Institute for Biomedical Research (Calibr) 11119 N. Torrey Pines Road, La Jolla, CA 92037 (USA). Department of Immunology and Microbial Science, The Scripps Research Institute, La Jolla, CA 92037 (USA)
Dr. Stephanie A. Kazane, Email: skazane@calibr.org, California Institute for Biomedical Research (Calibr) 11119 N. Torrey Pines Road, La Jolla, CA 92037 (USA)
Dr. Peter G. Schultz, Email: schultz@scripps.edu, Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute 10550 N. Torrey Pines Rd, La Jolla, CA 92037 (USA). California Institute for Biomedical Research (Calibr) 11119 N. Torrey Pines Road, La Jolla, CA 92037 (USA)
References
- 1.Senter PD. Curr Opin Chem Biol. 2009;13:235–244. doi: 10.1016/j.cbpa.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 2.a) Domanska UM, Kruizinga RC, Nagengast WB, Timmer-Bosscha H, Huls G, de Vries EG, Walenkamp AM. Eur J Cancer. 2013;49:219–230. doi: 10.1016/j.ejca.2012.05.005. [DOI] [PubMed] [Google Scholar]; b) Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, Taichman RS, Pienta KJ, Wang J. Cancer Metastasis Rev. 2010;29:709–722. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Gassmann P, Haier J, Schluter K, Domikowsky B, Wendel C, Wiesner U, Kubitza R, Engers R, Schneider SW, Homey B, Muller A. Neoplasia. 2009;11:651–661. doi: 10.1593/neo.09272. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Kim M, Koh YJ, Kim KE, Koh BI, Nam DH, Alitalo K, Kim I, Koh GY. Cancer Res. 2010;70:10411–10421. doi: 10.1158/0008-5472.CAN-10-2591. [DOI] [PubMed] [Google Scholar]; e) Sobolik T, Su YJ, Wells S, Ayers GD, Cook RS, Richmond A. Mol Biol Cell. 2014;25:566–582. doi: 10.1091/mbc.E13-07-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darash-Yahana M, Pikarsky E, Abramovitch R, Zeira E, Pal B, Karplus R, Beider K, Avniel S, Kasem S, Galun E, Peled A. FASEB J. 2004;18:1240–1242. doi: 10.1096/fj.03-0935fje. [DOI] [PubMed] [Google Scholar]
- 4.a) Gelmini S, Mangoni M, Serio M, Romagnani P, Lazzeri E. J Endocrinol Invest. 2008;31:809–819. doi: 10.1007/BF03349262. [DOI] [PubMed] [Google Scholar]; b) Libura J, Drukala J, Majka M, Tomescu O, Navenot JM, Kucia M, Marquez L, Peiper SC, Barr FG, Janowska-Wieczorek A, Ratajczak MZ. Blood. 2002;100:2597–2606. doi: 10.1182/blood-2002-01-0031. [DOI] [PubMed] [Google Scholar]
- 5.a) McDermott DH, Lopez J, Deng F, Liu Q, Ojode T, Chen H, Ulrick J, Kwatemaa N, Kelly C, Anaya-O’Brien S, Garofalo M, Marquesen M, Hilligoss D, DeCastro R, Malech HL, Murphy PM. J Cell Mol Med. 2011;15:2071–2081. doi: 10.1111/j.1582-4934.2010.01210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Peled A, Abraham M, Avivi I, Rowe JM, Beider K, Wald H, Tiomkin L, Ribakovsky L, Ribak Y, Ramati Y, Aviel S, Galun E, Shaw HL, Eizenberg O, Hardan I, Shimoni A, Nagler A. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-13-1302. [DOI] [PubMed] [Google Scholar]; c) Richert MM, Vaidya KS, Mills CN, Wong D, Korz W, Hurst DR, Welch DR. Oncol Rep. 2009;21:761–767. [PubMed] [Google Scholar]; d) Tamamura H, Hori A, Kanzaki N, Hiramatsu K, Mizumoto M, Nakashima H, Yamamoto N, Otaka A, Fujii N. FEBS Lett. 2003;550:79–83. doi: 10.1016/s0014-5793(03)00824-x. [DOI] [PubMed] [Google Scholar]; e) Tamamura H, Xu Y, Hattori T, Zhang X, Arakaki R, Kanbara K, Omagari A, Otaka A, Ibuka T, Yamamoto N, Nakashima H, Fujii N. Biochem Biophys Res Commun. 1998;253:877–882. doi: 10.1006/bbrc.1998.9871. [DOI] [PubMed] [Google Scholar]; f) Yu M, Gang EJ, Parameswaran R, Stoddart S, Fei F, Schmidhuber S, Park E, Hsieh YT, Yang AS, Groffen J, Heisterkamp N, Kim YM. Blood Cancer J. 2011;1:e14. doi: 10.1038/bcj.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tavor S, Petit I, Porozov S, Avigdor A, Dar A, Leider-Trejo L, Shemtov N, Deutsch V, Naparstek E, Nagler A, Lapidot T. Cancer Res. 2004;64:2817–2824. doi: 10.1158/0008-5472.can-03-3693. [DOI] [PubMed] [Google Scholar]
- 7.a) Roland J, Murphy BJ, Ahr B, Robert-Hebmann V, Delauzun V, Nye KE, Devaux C, Biard-Piechaczyk M. Blood. 2003;101:399–406. doi: 10.1182/blood-2002-03-0978. [DOI] [PubMed] [Google Scholar]; b) Signoret N, Oldridge J, Pelchen-Matthews A, Klasse PJ, Tran T, Brass LF, Rosenkilde MM, Schwartz TW, Holmes W, Dallas W, Luther MA, Wells TN, Hoxie JA, Marsh M. J Cell Biol. 1997;139:651–664. doi: 10.1083/jcb.139.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Signoret N, Rosenkilde MM, Klasse PJ, Schwartz TW, Malim MH, Hoxie JA, Marsh M. J Cell Sci. 1998;111(Pt 18):2819–2830. doi: 10.1242/jcs.111.18.2819. [DOI] [PubMed] [Google Scholar]
- 8.a) Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, Kazane SA, Halder R, Forsyth JS, Santidrian AF, Stafin K, Lu Y, Tran H, Seller AJ, Biroc SL, Szydlik A, Pinkstaff JK, Tian F, Sinha SC, Felding-Habermann B, Smider VV, Schultz PG. Proc Natl Acad Sci U S A. 2012;109:16101–16106. doi: 10.1073/pnas.1211023109. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, Rejniak SX, Gordon KA, DeBlanc R, Toki BE, Law CL, Doronina SO, Siegall CB, Senter PD, Wahl AF. Blood. 2003;102:1458–1465. doi: 10.1182/blood-2003-01-0039. [DOI] [PubMed] [Google Scholar]; c) Ma D, Hopf CE, Malewicz AD, Donovan GP, Senter PD, Goeckeler WF, Maddon PJ, Olson WC. Clin Cancer Res. 2006;12:2591–2596. doi: 10.1158/1078-0432.CCR-05-2107. [DOI] [PubMed] [Google Scholar]
- 9.Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, DeBlanc RL, Gearing RP, Bovee TD, Siegall CB, Francisco JA, Wahl AF, Meyer DL, Senter PD. Nat Biotechnol. 2003;21:778–784. doi: 10.1038/nbt832. [DOI] [PubMed] [Google Scholar]
- 10.a) Sutherland MS, Sanderson RJ, Gordon KA, Andreyka J, Cerveny CG, Yu C, Lewis TS, Meyer DL, Zabinski RF, Doronina SO, Senter PD, Law CL, Wahl AF. J Biol Chem. 2006;281:10540–10547. doi: 10.1074/jbc.M510026200. [DOI] [PubMed] [Google Scholar]; b) Jackson D, Gooya J, Mao S, Kinneer K, Xu L, Camara M, Fazenbaker C, Fleming R, Swamynathan S, Meyer D, Senter PD, Gao C, Wu H, Kinch M, Coats S, Kiener PA, Tice DA. Cancer Res. 2008;68:9367–9374. doi: 10.1158/0008-5472.CAN-08-1933. [DOI] [PubMed] [Google Scholar]
- 11.Dickerson CT, Marquis DM, Obungu V, Peng SB, Vaillancourt PE. Google Patents. 2009. [Google Scholar]
- 12.a) Okeley NM, Miyamoto JB, Zhang X, Sanderson RJ, Benjamin DR, Sievers EL, Senter PD, Alley SC. Clin Cancer Res. 2010;16:888–897. doi: 10.1158/1078-0432.CCR-09-2069. [DOI] [PubMed] [Google Scholar]; b) Kovtun YV, Goldmacher VS. Cancer Lett. 2007;255:232–240. doi: 10.1016/j.canlet.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Perissinotto E, Cavalloni G, Leone F, Fonsato V, Mitola S, Grignani G, Surrenti N, Sangiolo D, Bussolino F, Piacibello W, Aglietta M. Clin Cancer Res. 2005;11:490–497. [PubMed] [Google Scholar]
- 14.a) Rettig MP, Ansstas G, DiPersio JF. Leukemia. 2012;26:34–53. doi: 10.1038/leu.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Rosu-Myles M, Gallacher L, Murdoch B, Hess DA, Keeney M, Kelvin D, Dale L, Ferguson SS, Wu D, Fellows F, Bhatia M. Proc Natl Acad Sci U S A. 2000;97:14626–14631. doi: 10.1073/pnas.97.26.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson A, Trumpp A. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 16.a) Spangrude GJ, Heimfeld S, Weissman IL. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]; b) Challen GA, Boles N, Lin KK, Goodell MA. Cytometry A. 2009;75:14–24. doi: 10.1002/cyto.a.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.