Abstract
IMPORTANCE
The incidence of thyroid cancer in the United States has increased rapidly and Pennsylvania is the state with the highest rate of thyroid cancer in the country, although the factors driving this increase are unknown. Moreover, it remains unclear whether the increase in thyroid cancer represents a true increase in disease or is the result of overdiagnosis.
OBJECTIVE
To compare the increase in thyroid cancer incidence and tumor characteristics in Pennsylvania with the rest of the United States and gain insight into the factors influencing the increased incidence of thyroid cancer.
DESIGN, SETTING, AND PARTICIPANTS
In a population-based study, data on thyroid cancer from the Surveillance Epidemiology and End Results 9 (SEER-9) registry and the Pennsylvania Cancer Registry (PCR) from 1985 through 2009 were collected and reviewed for information regarding sex, race, histologic type of thyroid cancer, staging, and tumor size at diagnosis. International Classification of Diseases for Oncology, Third Edition code C739 (thyroid carcinoma) was used to identify 110 615 records in the SEER-9 registry and 29 030 records in the PCR.
MAIN OUTCOMES AND MEASURES
Average annual percent change (AAPC) in thyroid cancer incidence across various demographic groups in Pennsylvania.
RESULTS
The AAPC for thyroid cancer in Pennsylvania was 7.1% per year (95% CI, 6.3%–7.9%) vs 4.2% (95% CI, 3.7%–4.7%) per year in the remainder of the United States, and trends in incidence were significantly different (P < .001). Females experienced a higher AAPC (7.6% per year; 95% CI, 6.9%–8.3%) compared with males (6.1% per year; 95% CI, 4.9%–7.2%) (P < .01), and trend analysis revealed that thyroid cancer may be increasing more rapidly among black females (8.6% per year; 95% CI, 5.4%–11.9%) than among white females (7.6% per year; 95% CI, 6.8%–8.4) (P = .60; but despite the similarity in AAPC between the 2 groups, the joinpoint models fit to the data were not parallel [P < .005]). The rate of tumors with regional (7.0% per year; 95% CI, 5.8%–8.1%) or distant (1.1% per year; 95% CI, 0.3%–1.8%) spread (P < .05) and tumors that were 2 to 4 cm (7.1% per year; 95% CI, 5.2%–9.0%) (P < .05) or larger than 4 cm (6.4% per year; 95% CI, 4.5%–8.2%) (P < .05) at diagnosis also increased.
CONCLUSIONS AND RELEVANCE
The incidence of thyroid cancer is rising at a faster rate in Pennsylvania than in the rest of the nation, as is the rate of tumors that are larger and higher stage at diagnosis. These findings suggest that rising disease burden has contributed to the increased incidence of thyroid cancer. Etiologic factors promoting the rise in thyroid cancer in Pennsylvania must be investigated and may provide insight into the drivers of the national increase in thyroid cancer.
Since the mid-1970s, the incidence of thyroid cancer in the United States has more than tripled,1–3 and thyroid cancer is now the seventh leading type of cancer in the nation.4 Moreover, by 2019, thyroid cancer is projected to become the third-most common cancer in women, with an annual age-adjusted incidence of 37 per 100 000.4 The majority of this increase is due to the rising incidence of papillary thyroid cancer (PTC), which is associated with an excellent prognosis and a 10-year survival rate of 95%.1,3,5,6 However, notwithstanding its favorable long-term survival, PTC still causes significant morbidity and poses significant clinical and economic burdens.4 Accordingly, thyroid cancer represents an increasingly important disease in the United States, although the reasons underlying the rapid rise in thyroid cancer remain enigmatic.
Over the last decade several groups have argued that the apparent increase in thyroid cancer does not represent a true increase in disease but rather results from a large reservoir of undiagnosed PTC combined with overdiagnosis of small tumors that will never become clinically significant.1,7,8 In a scenario where the true incidence of thyroid cancer remains constant and diagnosis increases, the observed rise in thyroid cancer would be driven by the diagnosis of small, localized PTCs.1,7 By contrast, other groups contend that increased detection or overdiagnosis of PTC is not sufficient to explain the increase in thyroid cancer incidence. Instead, these groups argue that the increase in thyroid cancer incidence represents a true increase in disease combined with increased detection of small tumors.2,9,10 Therefore, in a scenario where the increase in thyroid cancer incidence is due to increased tumor occurrence combined with increased detection, there should be a rise in more advanced tumors that escaped early detection because of increased disease burden in the population in addition to the rise in small, localized tumors. To better distinguish between these hypotheses, we examined the rate of increase in thyroid cancer among various populations in Pennsylvania from 1985 through 2009.
Nationwide cancer data compiled in 2010 indicate that Pennsylvania is the state with the highest incidence of thyroid cancer in the nation,11 and recent evidence suggests that thyroid cancer may be increasing more rapidly in Pennsylvania than the United States as a whole.12 The incidence of thyroid cancer in Pennsylvania is well documented by the Pennsylvania Cancer Registry (PCR), which has collected statewide cancer data from hospitals, clinics, laboratories, radiation facilities, cancer centers, surgical centers, physician’s offices, and death certificates since 1985. Our aim in this study was to compare the increase in thyroid cancer incidence and tumor characteristics in the Commonwealth of Pennsylvania with those in the rest of the nation.
Methods
The institutional review board at Penn State College of Medicine approved the use of SEER-9 and PCR data. National thyroid cancer rates were derived from the Surveillance Epidemiology and End Results 9 (SEER-9) registries, which includes cancer data from Atlanta, Georgia; Connecticut; Detroit, Michigan; Hawaii; Iowa; New Mexico; San Francisco-Oakland, California; Seattle-Puget Sound, Washington; and Utah. The data within SEER-9 span from 1973 to 2010, cover approximately 9.4% of the US population, and contain records of over 4.3 million tumors.13 As a whole, these data are thought to be representative of the cancer prevalence within the general US population. Thyroid cancer data in the Commonwealth of Pennsylvania from 1985 through 2009 were extracted from the PCR, which collects data on all new cancers diagnosed or treated in Pennsylvania. International Classification of Diseases for Oncology, Third Edition (ICD-O-3) code C739 (thyroid carcinomas) was used to identify cases, and populations were separated by race based on SEER criteria. For both the SEER-9 and PCR data sets, age-adjusted thyroid cancer rates and standard errors of the rate were calculated in Microsoft Excel (Microsoft Corporation) using the method of Tiwari et al.14
To examine trends in age-adjusted thyroid cancer rates among different groups, joinpoint models were fit to the data using the Joinpoint Regression Program (Version 4.0.4; National Cancer Institute; 2013). This program was chosen because it uses a parametric method to fit a flexible, precise, piece-wise model to the data using a statistically optimal number of “join points” to separate periods of time where the change in age-adjusted cancer rate is significantly different.15 Furthermore, joinpoint regressions are specifically designed to fit Poisson distributed data, which is appropriate for low-incidence cancers such as thyroid cancer.15 The slope of each piece of the regression can be summarized by the estimated annual percentage change (APC), and an average annual percentage change (AAPC) can be calculated as a weighted average of the slope of the joinpoint regression curve over a fixed period. The AAPC can therefore be used to compare changes in cancer rate between groups overtime. For convenience, we bracketed the data into 3 periods: 1985 through 1994, 1995 through 2004, and 2005 through 2009, with the exception of tumor size data for which the AAPC was calculated over 2004 through 2009 owing to limited data availability. Additionally, in some instances it was not possible to calculate AAPCs for every time period due to small sample size. All data sets were analyzed using identical parameters within the Joinpoint Regression Program. To determine whether the age-adjusted rate of thyroid cancer is increasing at a similar rate between groups, we conducted pairwise statistical comparison of joinpoint models between groups using the “parallel” pairwise comparison function in the Joinpoint Regression Program, which tests whether 2 mean join-point functions are parallel, given different Y-intercepts.16
Results
Using the inclusion criteria specified, we identified 110 615 records of thyroid cancer in the SEER-9 database and 29 030 cases in the PCR. The Table displays the number of thyroid cancers and AAPC in age-adjusted thyroid cancer rate in Pennsylvania compared with the rest of the country. To more easily compare trends between groups, the AAPCs presented in the Table were calculated over the 3 periods (1985–1994, 1995–2004, and 2005–2009). Over the study period (1985–2009), the AAPC of thyroid cancer in Pennsylvania (7.1% per year; 95% CI, 6.3%–7.9%) was significantly higher than the AAPC in the remainder of the country (4.2% per year; 95% CI, 3.7%–4.7%) (P < .01). Examination of US vs Pennsylvania trends in age-adjusted thyroid cancer rate (Figure 1A) revealed that the trends were significantly different (ie, nonparallel) (P < .001). We also evaluated the incidence of thyroid cancer among different sex and racial groups in Pennsylvania. Males and females experienced significant increases in age-adjusted thyroid cancer rate (P < .05). The AAPC for females (7.6% per year; 95% CI, 6.9%–8.3%) was significantly higher than the AAPC for males (6.1% per year; 95% CI, 4.9%–7.2%) (P < .01), and the trends in APC between males and females were nonparallel (P < .001).
Table.
Joinpoint Regression Analysis: Race and Sex (1985–2009)
| Variable | No. of Patients | AAPC in Age-Adjusted Thyroid Cancer Rates in Pennsylvania by Category, % (95% CI) | |||
|---|---|---|---|---|---|
| 1985–1994 | 1995–2004 | 2005–2009 | 1985–2009 | ||
| Pennsylvania vs national | |||||
| Pennsylvania | 29 030 | 4.1 (3.0 to 5.1) | 9.6 (8.3 to 11.0) | 7.3 (6.1 to 8.6) | 7.1 (6.3 to 7.9) |
| National | 110 615 | 1.7 (0.7 to 2.8) | 5.5 (5.2 to 5.9) | 6.7 (6.3 to 7.2) | 4.2 (3.7 to 4.7) |
| Race and sex | |||||
| Male | 6918 | 3.7 (1.5 to 5.9) | 7.6 (6.6 to 8.6) | 8.1 (7.0 to 9.2) | 6.1 (4.9 to 7.2) |
| Female | 22 112 | 4.5 (3.2 to 5.7) | 10.5 (9.4 to 11.6) | 7.2 (6.4 to 7.9) | 7.6 (6.9 to 8.3) |
| White | 26 300 | 4.4 (3.1 to 5.8) | 10.0 (8.2 to 11.9) | 7.4 (6.4 to 8.5) | 7.1 (6.2 to 8.1) |
| Black | 1765 | 3.6 (−1.1 to 8.5) | 10.3 (5.2 to 15.5) | 12.3 (3.5 to 21.8) | 8.0 (4.7 to 11.3) |
| White male | 6426 | 5.6 (4.8 to 6.4) | 5.6 (4.8 to 6.4) | 13.3 (7.2 to 19.7) | 6.9 (5.8 to 8.0) |
| Black male | 310 | ND | ND | ND | 6.0 (4.3 to 7.8) |
| White female | 19 874 | 4.9 (3.7 to 6.0) | 11.0 (9.4 to 12.5) | 7.1 (6.3 to 8.0) | 7.6 (6.8 to 8.4) |
| Black female | 1455 | 4.4 (−0.2 to 9.3) | 11.0 (6.0 to 16.2) | 12.1 (3.8 to 21.2) | 8.6 (5.4 to 11.9) |
| Tumor type | |||||
| Papillary | 23 488 | 6.3 (4.7 to 8.0) | 10.9 (7.9 to 13.9) | 11.4 (8.2 to 14.6) | 8.8 (7.1 to 10.5) |
| Anaplastic | 263 | ND | ND | ND | 4.2 (1.9 to 6.6) |
| Follicular | 2184 | ND | ND | ND | 1.4 (0.6 to 2.2) |
| Oxyphilic | 1036 | ND | ND | ND | −0.2 (−1.1 to 0.7) |
| Lymphoma | 599 | ND | ND | ND | −0.4 (−1.9 to 1.1) |
| Other | 1183 | ND | ND | ND | −1.3 (−1.9 to −0.7) |
| Tumor stage | |||||
| In situ | 65 | ND | ND | ND | −13.1 (−19.9 to −5.6) |
| Local | 20 755 | 6.5 (5.1 to 7.9) | 10.6 (7.7 to 13.7) | 10.5 (7.4 to 13.7) | 8.5 (6.9 to 10.2) |
| Regional | 5938 | 4.7 (3.1 to 6.4) | 7.7 (6.5 to 8.9) | 10.2 (8.3 to 12.1) | 7.0 (5.8 to 8.1) |
| Distant | 1399 | ND | ND | ND | 1.1 (0.3 to 1.8) |
| Tumor size, cm | (2004–2009) | ||||
| <2 | 7708 | ND | ND | ND | 11.0 (9.2 to 12.8) |
| 2–4 | 3391 | ND | ND | ND | 7.1 (5.2 to 9.0) |
| >4 | 1249 | ND | ND | ND | 6.4 (4.5 to 8.2) |
Abbreviations: AAPC, average annual percent change; ND, no data.
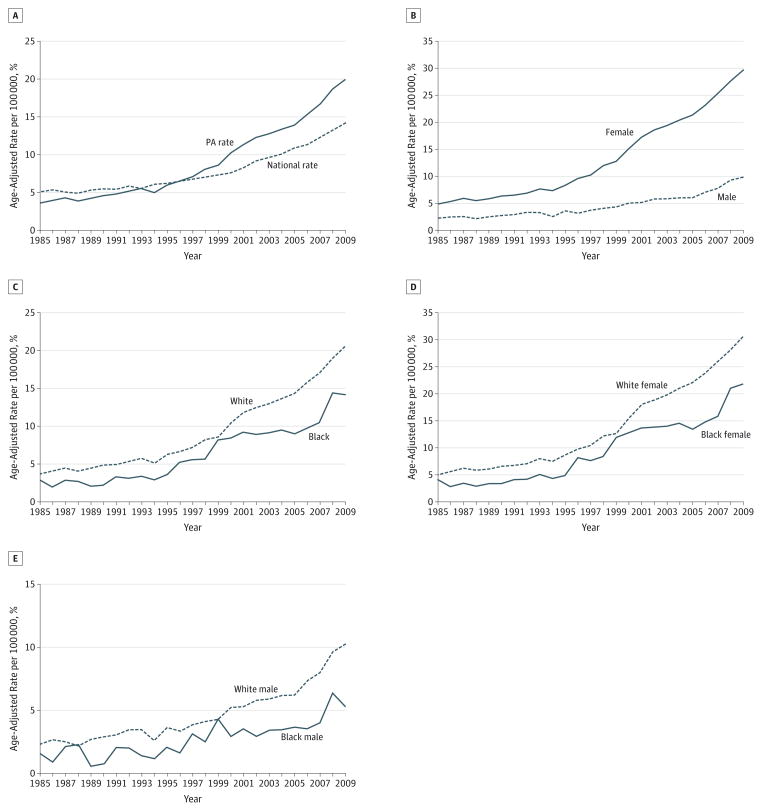
Figure 1.
Incidence of Thyroid Cancer Among Different Groups in Pennsylvania (PA)
Graphs depict the age-adjusted rate of thyroid cancer for PA vs United States (A), males vs females in PA (B), whites vs blacks in PA (C), white females vs black females in PA (D), and white males vs black males in PA (E).
When the study population was stratified by race, we found that whites experienced a 16.8% increase in age-adjusted thyroid cancer rate, while blacks experienced an 11.3% increase (Figure 1C). The AAPC for whites over the study period was 6.1% per year (95% CI, 4.9%–7.3%), while the AAPC for blacks was 8.0% per year (95% CI, 4.7%–11.3%) (Table); however, the differences in AAPC over the entire study period between whites and blacks were not statistically significant (P = .60). Nonetheless, statistical comparison of the joinpoint models fit to the data for whites and blacks revealed that the models were nonparallel (P = .002).
Stratification of the study population by race and sex revealed that white females experienced the largest increase in age-adjusted thyroid cancer incidence over the study period (25.5%), followed by black females (17.7%), white males (8.0%), and black males (3.8%) (Figure 1D and E). The AAPC for black females was 8.6% per year (95% CI, 5.4%–11.9%); however, this was not significantly different from the AAPC for white females (7.6% per year; 95% CI, 6.8%–8.4) (P = .60) (Table). Despite the similarity in AAPC between the 2 groups, the join-point models fit to the data were not parallel (P < .005), indicating that the incidence of thyroid cancer may be increasing at a faster rate among black females than white females. White and black males also experienced similar increases in AAPC over the study period (P = .40), with an AAPC for white males of 6.9% per year (95% CI, 5.8%–8.0%), while for black males the AAPC was 6.0% per year (95% CI, 4.3%–7.8%). Furthermore, when the joinpoint models fit to the data for black and white males were compared, parallelism could not be rejected (P = .85).
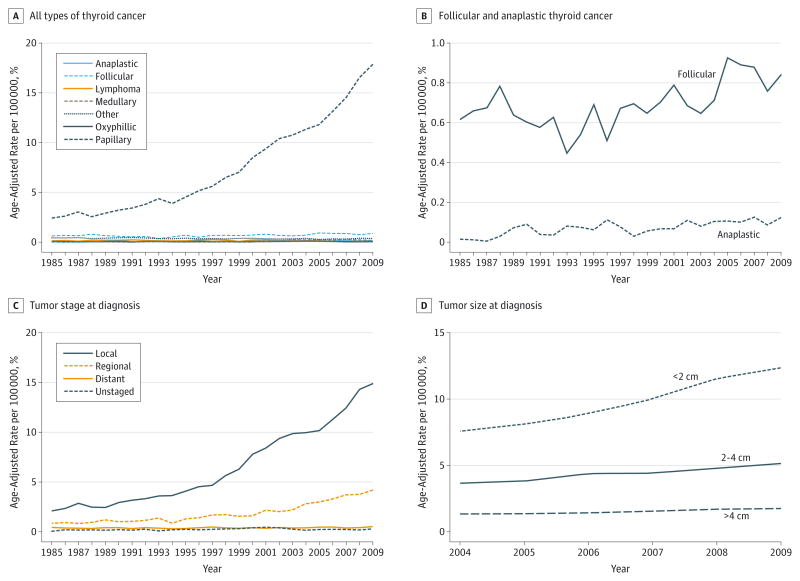
To understand which histologic type of thyroid cancer was driving the observed increase in incidence, we examined the AAPC for different types of thyroid cancer (Table). In Pennsylvania, the incidence of PTC, anaplastic thyroid cancer, and follicular thyroid cancer increased significantly over the study period (Figure 2A and B, and Table). Papillary thyroid cancer had the highest AAPC of 8.8% per year (95% CI, 7.1%–10.5%), followed by anaplastic thyroid cancer with an AAPC of 4.2% per year (95% CI, 1.9%–6.6%), and follicular thyroid cancer with an AAPC of 1.4% per year (95% CI, 0.6%–2.2%). No significant change was observed in the incidence of oxyphilic (Hurthle cell) thyroid cancer or thyroid lymphoma (Table). As expected, the joinpoint model fit to the data for PTC was not parallel to models fit to data for any other type of thyroid cancer (P < .001).
Figure 2.
Thyroid Cancer Trends in Pennsylvania
Graphs depict the age-adjusted rate of thyroid cancer in Pennsylvania segregated by all histologic types of thyroid cancer (A), follicular and anaplastic thyroid cancer (B), tumor stage at diagnosis (C), or tumor size at diagnosis (D).
Next, we examined the AAPC for thyroid cancer in Pennsylvania segregated by tumor stage or tumor size (Table and Figure 2C and D). Notably, the AAPC for thyroid cancer that was localized to the thyroid at the time of diagnosis was 8.5% per year (95% CI, 6.9%–10.2%) (P < .05); however, the AAPC for tumors that had spread regionally at the time of diagnosis was significant at 7.0% per year (95% CI, 5.8%–8.1%) (P < .05). Furthermore, the AAPC of cancers with distant metastasis at diagnosis also significantly increased over the study period, with an AAPC of 1.1% per year (95% CI, 0.3%–1.8%) (P < .05). The trend for the incidence of localized tumors was not parallel to the trends for tumors with regional (P < .001) or distant spread (P < .001), indicating that the greatest increase in thyroid cancer has been among tumors localized to the thyroid at diagnosis (Figure 2C). Since 2004, the PCR has also collected data on the size of thyroid tumors at the time of diagnosis (Figure 2D). From 2004 through 2009, the AAPC for tumors smaller than 2 cm at the time of diagnosis was 11.0% per year (95% CI, 9.2%–12.8%) (Table); however, we also found that over this period the incidence of tumors that were larger at the time of diagnosis also increased. The AAPC for tumors between 2 and 4 cm at diagnosis was 7.1% per year (95% CI, 5.2%–9.0%), and the AAPC for tumors larger than 4 cm at diagnosis was 6.4% per year (95% CI, 4.8%–8.2%). When joinpoint models fit to the data for the smaller than 2 cm, 2 to 4 cm, and larger than 4 cm groups were subjected to pairwise comparison, parallelism could not be rejected for any of the pairs (P > .99 for all pairwise tests).
Discussion
Over the 24 years covered by this study, the incidence of thyroid cancer in the United States increased by an average of 4.2% per year. Strikingly, we found that the incidence of thyroid cancer in Pennsylvania is increasing at a faster rate than the rest of the country. Geographical variations in thyroid cancer incidence have been identified in other studies. For example, New Caledonia in the South Pacific has one of the highest incidences of thyroid cancer yet reported,17,18 and elevated rates of thyroid cancer have been observed in regions of Italy surrounding Mt Etna and Mt Vesuvius19,20 and in a contiguous area encompassing eastern Pennsylvania, New Jersey, and southern NewYork within the United States.21 Accordingly, these data imply that local environmental factors may play role in the incidence of thyroid cancer.
Although the factors responsible for the increased incidence of thyroid cancer remain unclear, we found the sex and racial distribution of thyroid cancer in Pennsylvania to be similar to national trends.3,22 This suggests that the etiologic factors driving the increased incidence of thyroid cancer in Pennsylvania may be applicable on a national scale. Specifically, we observed the highest incidence among white females, followed by black females, white males, and black males (Figure 1). The highest rate of increase in thyroid cancer incidence was identified among the black population, with black females exhibiting the fastest rate of increase, followed by white females, black males, and white males. However, the differences in AAPC calculated over the entire study period failed to reach statistical significance, likely due to a high degree of variability in thyroid cancer incidence among the black population (Table and Figure 1C–E). The trends in thyroid cancer incidence for blacks vs whites and black females vs white females were nonparallel (Figure 1C and D), suggesting that thyroid cancer may be increasing at a faster rate among blacks, specifically black females, compared with whites. The mechanism driving the more rapid increase in thyroid cancer among blacks requires further study. One possibility is that recent increases in access to health insurance and health care may contribute to increased detection of thyroid cancer in the black population.
The majority of the increase in thyroid cancer in Pennsylvania is due to PTC (Figure 2A), although we also observed significant increases in the incidence of anaplastic and follicular thyroid cancer (Figure 2B). This finding is important because some research indicates that a subset of anaplastic thyroid cancer may be derived from de-differentiated PTC.23 Therefore, increases in anaplastic thyroid cancer may directly result from increased PTC disease burden. The observation that thyroid cancer is increasing at a faster rate in Pennsylvania compared with the rest of the country but exhibits racial and sex distributions similar to national trends suggests that the etiologic factors underlying the national increase in thyroid cancer may be concentrated in Pennsylvania, although the specific factors underlying any increase in thyroid cancer incidence remain unclear.
Risk factors for the development of thyroid cancer have traditionally included exposure to radiation (therapeutic or accidental),24 female sex,22 and iodine deficiency or excess.25,26 Less understood potential risk factors include Hashimoto disease,27 cruciferous vegetable consumption,28 low phytoestrogen consumption,29 female hormones,30 and obesity.31 Although the Three Mile Island nuclear accident in central Pennsylvania released significant amounts of radioactivity, several studies have shown that the Three Mile Island accident is not likely to be responsible for large increases in the incidence of thyroid cancer in Pennsylvania.12,32,33 By contrast, obesity and dietary habits may independently contribute to the development of thyroid cancer.31,34–37 According to Centers for Disease Control and Prevention data from 2010, Pennsylvania is the state with the 13th highest prevalence of obesity, with 29.1% of the population having a body mass index of 30 or greater (calculated as weight in kilograms divided by height in meters squared).38 Only 2 states included in the SEER-9 database, Michigan and Iowa, had higher rates of obesity than Pennsylvania in 2011. Therefore, if obesity is a major risk factor for thyroid cancer, the high rate of obesity in Pennsylvania may be correlated with the increased incidence of thyroid cancer in Pennsylvania compared with national data from the SEER-9 registry. However, it is also possible that obesity is a surrogate marker for other risk factors associated with thyroid cancer, so further research is needed to establish whether a definitive link exists between obesity and thyroid cancer.
The data presented herein address the question of whether the increased incidence in thyroid cancer is due to an increase in disease and/or increased detection in several ways. First, we observed significant, positive AAPCs for tumors that had undergone regional or distant metastasis at the time of diagnosis, even though tumors that were localized to the thyroid underwent the largest AAPC over the study period (Table and Figure 2C). These data suggest that an overdiagnosis model, which would predict an increase in incidence for localized tumors while the incidence of more advanced tumors would remain the same or decrease, is not sufficient to explain the rise in thyroid cancer incidence in Pennsylvania. It is notable, however, that the incidence of localized tumors was not parallel to the incidence of tumors with regional or distant spread at the time of diagnosis, suggesting that increased detection may also play an important role in the increased incidence of thyroid cancer (Figure 2C). Furthermore, it is also possible that higher rates of elective central compartment neck dissection may contribute to the increased incidence of tumors with regional metastasis at diagnosis. However, we also found that the incidences of tumors that were larger at the time of diagnosis (ie, >2 cm) were increasing at similar rates compared with tumors that were diagnosed before reaching 2 cm in size (Table and Figure 2D). Because larger tumors may be palpable on physical examination, these findings indicate an increase in the incidence of clinically significant tumors, suggesting that an actual increase in disease has contributed to the rising incidence of thyroid cancer.
It is important to note that the increased use of imaging may contribute significantly to the incidental detection of larger tumors. Recent evidence indicates that almost 40% of tumors discovered incidentally on imaging studies are larger than 4 cm and nearly 50% of stage III or IV tumors are first identified on imaging studies. Accordingly, the frequent use of imaging may explain the increased incidence of more advanced thyroid cancers.39 However, other groups with similar findings have concluded that that improved detection may not represent the only factor contributing to the increased incidence of thyroid cancer.40
In interpreting the data presented herein, the limitations of our study must be considered. Primarily, the data used in this study are from a historical source, and therefore this study is retrospective. In addition, differences in access to health care may have influenced the rate of thyroid cancer among different populations. In particular, we note that blacks comprise only 6.7% of the cases of thyroid cancer despite the fact that 11.4% of the population in Pennsylvania is black according to 2010 census data.41 This observation raises the possibility that blacks have a lower incidence of thyroid cancer in Pennsylvania, or that thyroid cancer is underdiagnosed in this population. The incidence of thyroid cancer among the black population was highly variable, which may result from fluctuations in access to care42 and a relatively small sample size; although we note that despite these year-to-year variations, we still observed a significant increase in APC for this group. Another limitation is that the PCR has only collected data on the size of thyroid tumors at the time of diagnosis since 2004, thereby limiting the conclusions that can be drawn from trends in tumor size. However, data on the extent of thyroid cancers at the time of diagnosis have been collected throughout the entire study period, lending support to our assertion that the increase in thyroid cancer APC we observed in Pennsylvania is due to an actual increase in disease incidence.
Conclusions
We report that the rate of thyroid cancer in Pennsylvania is increasing more rapidly than the national average, as defined by the SEER-9 data set. Consistent with national trends, we observed the highest rate of thyroid cancer among white females in Pennsylvania; however, we found the fastest rate of change among black females. Moreover, PTC was the histologic type of thyroid carcinoma that underwent the most rapid change in incidence. The majority of tumors were localized to the thyroid at diagnosis; however, we also observed significant increases in tumors that had spread regionally or distantly at diagnosis. Furthermore, while we observed the largest increase in rate among tumors smaller than 2 cm at diagnosis, we also found that the incidence of tumors larger than 2 cm at diagnosis also increased over the study period. As a whole, these data show that the rate of thyroid cancer is increasing in Pennsylvania at a faster rate than in other parts of the United States. Although the reasons underlying this increase in disease are not yet known, further research may help define potential environmental or genetic factors that can explain the rise of thyroid cancer.
Acknowledgments
Funding/Support: Dr Bann is supported by National Institutes of Health–National Cancer Institute grant F30 CA165774.
Role of Funder/Sponsor: The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
Previous Presentation: This study was presented at the Fifth World Congress of the International Federation of Head and Neck Oncologic Societies and the Annual Meeting of the American Head & Neck Society; July 27, 2014; New York, New York.
Additional Contributions: The Department of Public Health Sciences at the Penn State Hershey Cancer Institute assisted in conducting this research.
Author Contributions: Drs Bann and Goldenberg had full access to all of the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Goyal, Goldenberg.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Bann, Goldenberg.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Bann, Camacho, Goldenberg.
Obtained funding: Bann.
Administrative, technical, or material support: Goyal, Goldenberg.
Study supervision: Goyal, Goldenberg.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Cramer JD, Fu P, Harth KC, Margevicius S, Wilhelm SM. Analysis of the rising incidence of thyroid cancer using the Surveillance, Epidemiology and End Results national cancer data registry. Surgery. 2010;148(6):1147–1153. doi: 10.1016/j.surg.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18(3):784–791. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aschebrook-Kilfoy B, Schechter RB, Shih YC, et al. The clinical and economic burden of a sustained increase in thyroid cancer incidence. Cancer Epidemiol Biomarkers Prev. 2013;22(7):1252–1259. doi: 10.1158/1055-9965.EPI-13-0242. [DOI] [PubMed] [Google Scholar]
- 5.Aschebrook-Kilfoy B, Ward MH, Sabra MM, Devesa SS. Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid. 2011;21(2):125–134. doi: 10.1089/thy.2010.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albores-Saavedra J, Henson DE, Glazer E, Schwartz AM. Changing patterns in the incidence and survival of thyroid cancer with follicular phenotype—papillary, follicular, and anaplastic: a morphological and epidemiological study. Endocr Pathol. 2007;18(1):1–7. doi: 10.1007/s12022-007-0002-z. [DOI] [PubMed] [Google Scholar]
- 7.Kent WD, Hall SF, Isotalo PA, Houlden RL, George RL, Groome PA. Increased incidence of differentiated thyroid carcinoma and detection of subclinical disease. CMAJ. 2007;177(11):1357–1361. doi: 10.1503/cmaj.061730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies L, Ouellette M, Hunter M, Welch HG. The increasing incidence of small thyroid cancers: where are the cases coming from? Laryngoscope. 2010;120(12):2446–2451. doi: 10.1002/lary.21076. [DOI] [PubMed] [Google Scholar]
- 9.Gursoy A. Rising thyroid cancer incidence in the world might be related to insulin resistance. Med Hypotheses. 2010;74(1):35–36. doi: 10.1016/j.mehy.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Li N, Du XL, Reitzel LR, Xu L, Sturgis EM. Impact of enhanced detection on the increase in thyroid cancer incidence in the United States: review of incidence trends by socioeconomic status within the surveillance, epidemiology, and end results registry, 1980–2008. Thyroid. 2013;23(1):103–110. doi: 10.1089/thy.2012.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cancer Institute. [Accessed October 7, 2013];State cancer profiles: incidence. 2013 http://statecancerprofiles.cancer.gov/cgi-bin/quickprofiles/profile.pl?00&080.
- 12.Goyal N, Camacho F, Mangano J, Goldenberg D. Thyroid cancer characteristics in the population surrounding Three Mile Island. Laryngoscope. 2012;122(6):1415–1421. doi: 10.1002/lary.23314. [DOI] [PubMed] [Google Scholar]
- 13.Surveillance Research Program NCI. SEER*Stat Databases: November 2012 Submission. 2012 http://seer.cancer.gov/data/seerstat/nov2012/
- 14.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15(6):547–569. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Fay MP, Yu B, Barrett MJ, Feuer EJ. Comparability of segmented line regression models. Biometrics. 2004;60(4):1005–1014. doi: 10.1111/j.0006-341X.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- 17.Ballivet S, Salmi LR, Dubourdieu D, Bach F. Incidence of thyroid cancer in New Caledonia, South Pacific, during 1985–1992. Am J Epidemiol. 1995;141(8):741–746. doi: 10.1093/oxfordjournals.aje.a117496. [DOI] [PubMed] [Google Scholar]
- 18.Truong T, Rougier Y, Dubourdieu D, et al. Time trends and geographic variations for thyroid cancer in New Caledonia, a very high incidence area (1985–1999) Eur J Cancer Prev. 2007;16(1):62–70. doi: 10.1097/01.cej.0000236244.32995.e1. [DOI] [PubMed] [Google Scholar]
- 19.Pellegriti G, De Vathaire F, Scollo C, et al. Papillary thyroid cancer incidence in the volcanic area of Sicily. J Natl Cancer Inst. 2009;101(22):1575–1583. doi: 10.1093/jnci/djp354. [DOI] [PubMed] [Google Scholar]
- 20.Biondi B, Arpaia D, Montuori P, et al. Under the shadow of Vesuvius: a risk for thyroid cancer? Thyroid. 2012;22(12):1296–1297. doi: 10.1089/thy.2012.0002. [DOI] [PubMed] [Google Scholar]
- 21.Mangano JJ. Geographic variation in US thyroid cancer incidence and a cluster near nuclear reactors in New Jersey, New York, and Pennsylvania. Int J Health Serv. 2009;39(4):643–661. doi: 10.2190/HS.39.4.c. [DOI] [PubMed] [Google Scholar]
- 22.Aschebrook-Kilfoy B, Kaplan EL, Chiu BC, Angelos P, Grogan RH. The acceleration in papillary thyroid cancer incidence rates is similar among racial and ethnic groups in the United States. Ann Surg Oncol. 2013;20(8):2746–2753. doi: 10.1245/s10434-013-2892-y. [DOI] [PubMed] [Google Scholar]
- 23.Quiros RM, Ding HG, Gattuso P, Prinz RA, Xu X. Evidence that one subset of anaplastic thyroid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations. Cancer. 2005;103 (11):2261–2268. doi: 10.1002/cncr.21073. [DOI] [PubMed] [Google Scholar]
- 24.Nikiforov YE. Radiation-induced thyroid cancer: what we have learned from Chernobyl. Endocr Pathol. 2006;17(4):307–317. doi: 10.1007/s12022-006-0001-5. [DOI] [PubMed] [Google Scholar]
- 25.Feldt-Rasmussen U. Iodine and cancer. Thyroid. 2001;11(5):483–486. doi: 10.1089/105072501300176435. [DOI] [PubMed] [Google Scholar]
- 26.Dijkstra B, Prichard RS, Lee A, et al. Changing patterns of thyroid carcinoma. Ir J Med Sci. 2007;176(2):87–90. doi: 10.1007/s11845-007-0041-y. [DOI] [PubMed] [Google Scholar]
- 27.Lun Y, Wu X, Xia Q, et al. Hashimoto’s thyroiditis as a risk factor of papillary thyroid cancer may improve cancer prognosis. Otolaryngol Head Neck Surg. 2013;148(3):396–402. doi: 10.1177/0194599812472426. [DOI] [PubMed] [Google Scholar]
- 28.Truong T, Baron-Dubourdieu D, Rougier Y, Guénel P. Role of dietary iodine and cruciferous vegetables in thyroid cancer: a countrywide case-control study in New Caledonia. Cancer Causes Control. 2010;21(8):1183–1192. doi: 10.1007/s10552-010-9545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horn-Ross PL, Hoggatt KJ, Lee MM. Phytoestrogens and thyroid cancer risk: the San Francisco Bay Area thyroid cancer study. Cancer Epidemiol Biomarkers Prev. 2002;11(1):43–49. [PubMed] [Google Scholar]
- 30.Horn-Ross PL, Canchola AJ, Ma H, Reynolds P, Bernstein L. Hormonal factors and the risk of papillary thyroid cancer in the California Teachers Study cohort. Cancer Epidemiol Biomarkers Prev. 2011;20(8):1751–1759. doi: 10.1158/1055-9965.EPI-11-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson E, De P, Nuttall R. BMI, diet and female reproductive factors as risks for thyroid cancer: a systematic review. PLoS One. 2012;7(1):e29177. doi: 10.1371/journal.pone.0029177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levin RJ. Incidence of thyroid cancer in residents surrounding the Three Mile Island nuclear facility. Laryngoscope. 2008;118(4):618–628. doi: 10.1097/MLG.0b013e3181613ad2. [DOI] [PubMed] [Google Scholar]
- 33.Levin RJ, De Simone NF, Slotkin JF, Henson BL. Incidence of thyroid cancer surrounding Three Mile Island nuclear facility: the 30-year follow-up. Laryngoscope. 2013;123(8):2064–2071. doi: 10.1002/lary.23953. [DOI] [PubMed] [Google Scholar]
- 34.Engeland A, Tretli S, Akslen LA, Bjørge T. Body size and thyroid cancer in two million Norwegian men and women. Br J Cancer. 2006;95(3):366–370. doi: 10.1038/sj.bjc.6603249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 36.Kitahara CM, Platz EA, Freeman LE, et al. Obesity and thyroid cancer risk among US men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev. 2011;20(3):464–472. doi: 10.1158/1055-9965.EPI-10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcello MA, Sampaio AC, Geloneze B, Vasques AC, Assumpção LV, Ward LS. Obesity and excess protein and carbohydrate consumption are risk factors for thyroid cancer. Nutr Cancer. 2012;64(8):1190–1195. doi: 10.1080/01635581.2012.721154. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. [Accessed November 17, 2013];Overweight and obesity. 2013 http://www.cdc.gov/obesity/data/adult.html.
- 39.Malone MK, Zagzag J, Ogilvie JB, Patel KN, Heller KS. Thyroid cancers detected by imaging are not necessarily small or early stage. Thyroid. 2014;24(2):314–318. doi: 10.1089/thy.2012.0651. [DOI] [PubMed] [Google Scholar]
- 40.Yoo F, Chaikhoutdinov I, Mitzner R, Liao J, Goldenberg D. Characteristics of incidentally discovered thyroid cancer. JAMA Otolaryngol Head Neck Surg. 2013;139(11):1181–1186. doi: 10.1001/jamaoto.2013.5050. [DOI] [PubMed] [Google Scholar]
- 41.United States Census Bureau. [Accessed November 17, 2013];State & County QuickFacts: Pennsylvania. 2013 http://quickfacts.census.gov/qfd/states/42000.html.
- 42.Hollenbeak CS, Wang L, Schneider P, Goldenberg D. Outcomes of thyroid cancer in African Americans. Ethn Dis. 2011;21(2):210–215. [PubMed] [Google Scholar]