Abstract
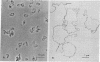
Pretreatment of yeast protoplasts with concanavalin A, according to the method used by G. A. Scarborough for Neurospora (J. Biol. Chem. 250, 1106-1111, 1975), reinforced the plasma membranes, and helped to maintain their integrity during subsequent lysis of the protoplasts. After purification by centrifuging on a Renografin density gradient, practically intact membranes were obtained. Previous labeling of the protoplasts with 125I or with [3H]concanavalin A resulted in recovery of the radioactivity in the membrane fraction. The bulk of the chitin synthetase (chitin synthase; UDP-2-acetamido-2-deoxy-D-glucose:chitin 4-beta-acetamidodeoxyglucosyltransferase; EC 2.4.1.16) recovered in the gradient was also found In this fraction; in the zymogen form. About 20% of the activity sedimented in a plasma-membrane-free fraction at lower density. Glutaraldehyde inactivated chitin synthetase when it was added to a lysate, but not when applied to intact protoplasts. It is concluded that chitin synthetase is so oriented in the membrane that it is only accessible from the inside of the cell. These results confirm our previous hypothesis that the chitin synthetase zymogen is associated with the plasma membrane, a basic assumption for the explanation of localized activation of the enzyme and initiation of septum formation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowers B., Levin G., Cabib E. Effect of polyoxin D on chitin synthesis and septum formation in Saccharomyces cerevisiae. J Bacteriol. 1974 Aug;119(2):564–575. doi: 10.1128/jb.119.2.564-575.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Farkas V. The control of morphogenesis: an enzymatic mechanism for the initiation of septum formation in yeast. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2052–2056. doi: 10.1073/pnas.68.9.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Ulane R., Bowers B. Yeast chitin synthetase. Separation of the zymogen from its activating factor and recovery of the latter in the vacuole fraction. J Biol Chem. 1973 Feb 25;248(4):1451–1458. [PubMed] [Google Scholar]
- Cabib E., Ulane R. Chitin synthetase activating factor from yeast, a protease. Biochem Biophys Res Commun. 1973 Jan 4;50(1):186–191. doi: 10.1016/0006-291x(73)91081-4. [DOI] [PubMed] [Google Scholar]
- Christensen M. S., Cirillo V. P. Yeast membrane vesicles: isolation and general characteristics. J Bacteriol. 1972 Jun;110(3):1190–1205. doi: 10.1128/jb.110.3.1190-1205.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann G. F., Wehrli E., Boehm C. Preparation and identification of yeast plasma membrane vesicles. Biochim Biophys Acta. 1974 Sep 23;363(3):295–310. doi: 10.1016/0005-2736(74)90070-4. [DOI] [PubMed] [Google Scholar]
- Keller F. A., Cabib E. Chitin and yeast budding. Properties of chitin synthetase from Saccharomyces carlsbergensis. J Biol Chem. 1971 Jan 10;246(1):160–166. [PubMed] [Google Scholar]
- Kosinová A., Farkas V., Machala S., Bauer S. Site of mannan synthesis in yeast. An autoradiographic study. Arch Microbiol. 1974;99(3):255–263. doi: 10.1007/BF00696240. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matile P., Moor H., Mühlethaler K. Isolation and properties of the plasmalemma in yeast. Arch Mikrobiol. 1967;58(3):201–211. doi: 10.1007/BF00408804. [DOI] [PubMed] [Google Scholar]
- Scarborough G. A. Isolation and characterization of Neurospora crassa plasma membranes. J Biol Chem. 1975 Feb 10;250(3):1106–1111. [PubMed] [Google Scholar]
- Schibeci A., Rattray J. B., Kidby D. K. Isolation and identification of yeast plasma membrane. Biochim Biophys Acta. 1973 Jun 7;311(1):15–25. doi: 10.1016/0005-2736(73)90250-2. [DOI] [PubMed] [Google Scholar]