Abstract
Rationale
We have recently reported that infusion of a solution containing methemoglobin (MetHb) during exposure to hydrogen sulfide results in a rapid and large decrease in the concentration of the pool of soluble/diffusible H2S in the blood. However, since the pool of dissolved H2S disappears very quickly after H2S exposure, it is unclear if the ability of MetHb to “trap” sulfide in the blood has any clinical interest and relevance in the treatment of sulfide poisoning.
Methods
In anesthetized rats, repetition of short bouts of high level of H2S infusions were applied to allow the rapid development of an oxygen deficit. A solution containing methemoglobin (600mg/kg) or its vehicle was administered one minute and a half after the end of H2S intoxication.
Results
The injection of methemoglobin solution increased methemoglobinemia to about 6%, almost instantly, but was unable to decrease the blood concentration of soluble H2S, which had already vanished at the time of infusion, or to increase combined H2S. In addition H2S-induced O2 deficit and lactate production as well as the recovery of carotid blood flow and blood pressure were similar in treated or control animals.
Conclusion
Our results do not support the view that administration of MetHb or drugs induced methemoglobinemia during the recovery phase following severe H2S intoxication in sedated rats can restore cellular oxidative metabolism, as the pool of diffusible sulfide, accessible to MetHb, disappears rapidly from the blood after H2S exposure.
Keywords: H2S toxicity, metallo-compound, Methemoglobin, rat model
Introduction
During hydrogen sulfide poisoning, H2S produces an apnea, a coma and a refractory circulatory shock leading to cardiac arrest. These effects result from a direct inhibition the mitochondrial cytochrome c oxidase, preventing in turn mitochondrial ATP production 1-3.
Sodium nitrite-induced methemoglobinemia is certainly the antidote that has been the most extensively studied 5,6. The oxidation of the molecule of ferrous iron contained in hemoglobin (Hb) dramatically increases the ability of Hb to combine H2S 7. Nitrite induced methemoglobinemia is effective to prevent H2S toxicity, when administered prior and during H2S exposure 6,8-11. Anecdotal case reports suggest beneficial effects following exposure in humans as well 12.
To overcome some of the side effects of nitrite-induced methemoglobinemia (drop in blood pressure, unpredictable methemoglobin (MetHb) levels, potentiation of the effects of H2S by Nitric Oxide, we used an infusion of a solution of methemoglobin 13 and have showed that, during H2S infusion, the presence of methemoglobin dramatically decreases the amount of free/diffusible H2S in the blood. MetHb is effective at concentrations starting at 4-5% 11 and is capable of combining with H2S as soon as it enters in the blood, as shown in both sheep 14 and rat models 11. Consequently, the pool of combined H2S, which represents the largest pool of H2S in the blood during sulfide exposure, increases in the presence of MetHb 13. The “antidotal” properties of methemoglobin against H2S poisoning must however be relevant when used in a scenario faithful to H2S intoxication in human, that is following H2S exposure. In other words, since the efficacy of a solution of methemoglobin is primarily dictated by the fate of H2S in the blood after sulfide exposure, i.e. when patients are withdrawn from the source of intoxication, what benefit is to be expected from the presence of MetHb, when all soluble H2S has already vanished from the blood 15,16.
To tackle this outstanding question, an important experimental challenge must be overcome: a balance must be found between levels of H2S that would be high enough to alter the respiratory and cardiovascular system and create an oxygen deficit, without reaching lethal levels. To produce a severe form of H2S poisoning, but without immediate lethal consequence, we used a repetition of short bouts of intravenous infusion of levels of H2S able to kill animals within 5 min, if it were infused continuously, separated by very short periods of recovery. As developed in the method section, this approach allows the progressive buildup of a large O2 deficit and lactate, akin to the creation of oxygen deficit by repetitive hemorrhage 17,18.
We developed a rat model of H2S intoxication using NaHS infusion, which allowed us to study the systemic sulfide toxicity. Rats were chosen since they behave just like larger mammals in terms of their respiratory, circulatory and metabolic responses to H2S poisoning, in marked contrast to mice 19,20. We are presenting 1) the characteristics of this model during and following severe H2S intoxication and 2) the effects of a direct infusion of a methemoglobin solution versus saline, administered after the cessation of H2S exposure, on the kinetics and magnitude of the recovery of O2 deficit/debt, lactate production and circulatory responses.
Material and Methods
Animal preparation
Adult Sprague-Dawley rats were studied (see weight and number of animals used in the protocol section). All experiments were conducted in accordance with the Guideline for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication No. 85-23, revised 1996). The study was approved by the Pennsylvania State University College of Medicine Institutional Care and Use Committee. Rats were anaesthetized with 3.5% isoflurane in O2, followed by urethane (1.2 g/kg, IP). Animals were then tracheostomized (14G surflo catheter, TERUMO, NJ) as previously described 15. A catheter (PE-50 tubing) was inserted into the right femoral artery for continuous monitoring of systemic arterial blood pressure (ABP), while another catheter was inserted into the left carotid artery for arterial blood sampling. An additional catheter was inserted into the right femoral vein for NaHS infusion, while a double lumen catheter was inserted into right jugular vein for injection of methemoglobin solution and continuous infusion of muscular relaxant (10 mg/kg/h, Rocuronium Bromide, Hospira Inc. Lake Forest, IL). The left femoral artery and right carotid artery were exposed and isolated from the vein, nerve, and surrounding tissues and a transonic flow probe (MA1PRB, Transonic Systems Inc., Ithaca, NY) was placed around both vessels. Body temperature was monitored with a rectal probe (Thermalert TH-5, Physitemp, Clifton NJ) and maintained around 37°C using a heating pad and a lamp. At the end of the experiment, rats were euthanized by a lethal injection of barbiturate IV (200mg/kg) into the right heart through the jugular catheter, followed by an aortic dissection.
Measurements and data analysis
The tracheal catheter was connected to a small animal ventilator (SAR-1000, CWE) and rats were mechanically ventilated (frequency of breathing: 80-90 breath.min−1, minute ventilation~350-400 ml/min). The expiratory flow was measured using a pneumotachograph (1100 Series, Hans Rudolph, Shawnee, KS). The expiratory circuit was connected to a series of two 5 ml mixing chambers. Mixed expired O2, CO2 and H2S fractions were measured continuously from the second mixing chamber using O2 (Oxystar-100, CWE), CO2 (model 17630, VacuMed, Ventura, CA) and H2S (Interscan RM series, Simi Valley, CA; range: 0-200.0 ppm) analyzers15.
Breathing frequency (f) and tidal volume (VT) were determined from the expiratory flow signal and minute ventilation (VE) was computed as f.VT. Oxygen consumption (VO2) was computed in Standard Temperature and Pressure, Dry (STPD) conditions as previously described 18.
The arterial catheter was connected to a pressure transducer (TA-100, CWE Inc., Ardmore, PA), while the transonic flow probes were connected to a perivascular flowmeter (TS420, Transonic Systems Inc., Ithaca, NY). All signals were digitized at 200 Hz using an analog-to-digital data acquisition system (Power Lab 16/35, AD Instruments, Colorado Springs, CO) and stored for later analysis. Mean ABP, VO2, minute ventilation, carotid blood flow (CBF) and femoral blood flow (FBF) were displayed online for monitoring.
Arterial partial pressures in O2 (PaO2) and CO2 (PaCO2), as well as lactate concentration were measured using i-STAT1 blood gas analyzer (ABAXIS, Union city, CA).
H2S determination in the blood
We followed the procedure proposed and validated by Wintner et al. 21 which we have previously used and described 15. In brief, arterial blood (200 μl) was added with a syringe to a solution of monobromobiname (MBB; 20 mM in 200 μl of acetonitrile) and 200 μl HEPES (50 mM, pH 8.0) in a sealed vial. After 10 minutes, a volume of 100 μl 0.1 N HCl was added to prevent any further reaction between MBB and H2S. The mixture was then extracted and the residue was dissolved and purified by Supercritical Fluid Extraction. Sulfide-dibimane was measured by High Performance Liquid Chromatography (HPLC) analysis using a Shimadzu HPLC system consisting of two 10ADVP pumps, a SCL-10AVP controller, and a Rheodyne injector, interfaced with a Hitachi L 7485 fluorescence detector. Data were recorded using a Hitachi D2500 integrator. The fluorescence excitation wavelength was 390 nm and the emission wavelength was 470 nm. Under these chromatographic conditions sulfide-bimane eluted at 19.4 min. The levels of sulfide-bimane in rat blood were determined based on standard. Several examples of chromatograms, obtained with this technic, can be found in 2 previous publications from our laboratory15,16.
Concentration of gaseous H2S in the blood
Expired H2S was determined as previously described 13,15. The fraction of H2S was continuously measured from the second mixing chamber, allowing the calculation of the partial pressure of expired H2S (PEH2S) and of the alveolar pressure (PAH2S) assuming to be equivalent to H2S arterial partial pressures (PaH2S) (see 15 for further details). The concentration of gaseous H2S in the blood (CgH2S) was calculated as: CgH2S =0.00012*PaH2S, with 0.00012 being the coefficient of solubility of H2S (0.09 mol.l.760−1 mmHg at 37°C in saline) as previously described 15. Assuming that H2S is under the form of H2S gas and its sulfhydryl anion HS− at a ratio of 1:3 in the arterial blood 22,23, the concentration of total dissolved H2S was estimated as three times CgH2S.
Methemoglobin concentration in the blood
Arterial gas partial pressure in O2 and CO2 and MetHb concentrations were determined using a GEM Premier 4000 gas analyzer (Instrumentation Laboratory, Bedford, MA, USA). The accuracy of MetHb measurement was validated using a spectrophotometric method in duplicate 24. This technique was also used to determine the concentration of MetHb in the Hb solution, which was found to be about 98% 11.
H2S intravenous infusion
H2S was administered intravenously as solution of sodium hydrosulfide hydrate (0.8 mg/ml NaHS, Sigma Aldrich, St Louis, MO) in sterile saline, prepared immediately prior each experiment and kept in airtight syringes. H2S was infused using a syringe pump (Fusion 100, Chemyx Inc., Stafford, TX) in the femoral vein.
Methemoglobin solution
Methemoglobin solution was prepared from human hemoglobin powder (Sigma Aldrich, St Louis, MO), mostly constituted (92-100%) of methemoglobin. Hemoglobin powder was diluted in sterile saline at the concentration of 100 mg/ml.
Experimental protocols
1. Effects of methemoglobin infusion on O2 deficit, lactic acidosis and circulation following H2S exposure (protocol 1)
This model was designed to produce a significant oxygen deficit and lactic acidosis along with a low blood pressure without killing the animal within one hour. To achieve this goal, we established a protocol consisting in repetitive bouts of potentially lethal IV dose. The lethal dose of NaHS was established in a series of pilot experiments, showing that 25% of animals died within minutes at a rate of infusion of 5-6 μmole/min while no animals survived more than 5 min at a rate of 10 μmole/min. Below this rate of infusion, no clear oxygen deficit would develop within at least 10 minutes of exposure. As presented below a intermittent infusion of NaHS at a rate of 20 micromole/min produces a progressive reduction in blood pressure and VO2, allowing enough time to administer the antidote after sulfide exposure.
Twelve rats weighing 433 ± 50 g were studied in this protocol: after an adequate recovery period from surgical procedure for about one hour and following at least 10 minutes of hemodynamic and respiratory stability, a first arterial blood was sampled. Two minutes later, NaHS infusion was started at a rate of 2 ml.min−1 (20-22 μmol.min−1) and was sustained until mean arterial blood pressure reached 50 mmHg, 40 s was allowed before resuming H2S infusion as illustrated in figure 1. The same sequence was repeated until an O2 deficit of about 15 ml/kg was reached, then H2S infusion was stopped. Arterial blood was sampled again at 1, 5, 10 and 15 min following H2S exposure. One minute and 30 seconds into recovery, a methemoglobin (100 mg/ml, 3 ml in total) or a saline (3 ml, Control group) solution was infused intravenously.
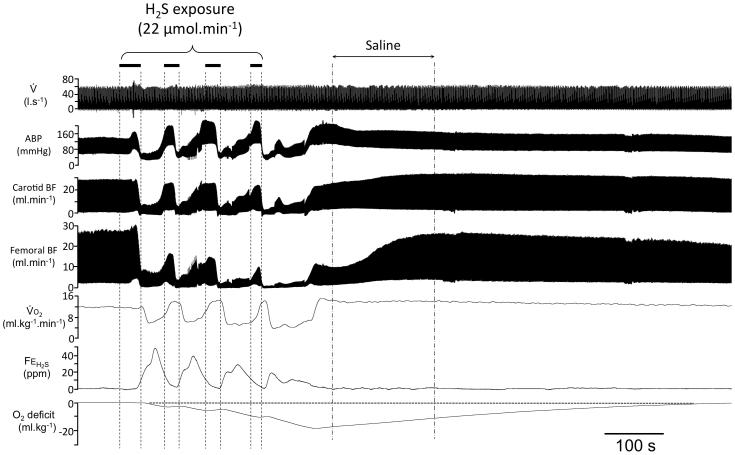
Figure 1.
Recording obtained in one rat during and following intoxication with H2S produced by four repetitions of NaHS infusion at a rate of 22 μmol min−1. This intoxication was in this example followed by a saline injection during the recovery. From top to bottom: respiratory flow (V̇), Arterial Blood Pressure (ABP), Carotid Blood Flow (CBF), Femoral Blood Flow (FBF), Oxygen uptake (VO2), expired fraction of H2S (FEH2S), and O2 deficit are shown. The solid horizontal bars indicate the periods of NaHS infusions. Arterial blood pressure and carotid blood flow dramatically decreased during NaHS infusion. The oxygen deficit accumulated during the NaHS infusion reached −18 ml.kg−1 along with an important lactic acidosis − in this example 7.4 mM, one minute following the cessation of NaHS infusion−. Following the cessation of sulfide infusion, ABP and blood flows increased progressively with an exponential like pattern towards baseline values. Note that VO2 remained elevated during the whole recovery “repaying” the O2 deficit/debt within about 10 minutes.
2. Effects of methemoglobin infusion on H2S kinetics following H2S exposure (protocol 2)
This protocol was designed to characterize the changes in H2S concentrations after moderate single steady infusion of H2S, following a saline or MetHb injection. Twelve rats 569 ± 77 g were studied in this protocol. Each animal received an infusion of NaHS at a rate of 5.5-6.5 micromol/min, which allowed mean arterial blood pressure to drop by about 10%, which typically required 4-5 minutes. Then NaHS infusion was stopped; one minute and a half into recovery a 2ml saline solution was infused IV for 90 s. Thirty minute later, a second IV NaHS infusion was performed followed this time by the infusion of a MetHb solution (2 ml during 90 s) following the same protocol as with saline. H2S concentrations were compared between the two conditions (Saline/control versus MetHb). Blood was sampled before NaHS infusion (baseline), 3 minutes into infusion and then 5, 10 and 15 min into recovery.
Statistical Analysis
All results are presented as mean± SD. All variables of interest were compared between the control animals (saline) and those receiving methemoglobin. They were also analyzed over time, i.e., prior to and at the end of each of the periods of H2S infusion as well as before and after injection of saline or methemoglobin, using ANOVA for repeated measurements. All statistical analyses were conducted using GraphPad Prism 6 (Graphpad Software, La Jolla, CA, USA). Post-hoc comparisons were performed using a Bonferroni correction; p < 0.05 was regarded as significant.
Results
1) Effects of Methemoglobin infusion following repetition of short bouts of high levels of H2S (protocol 1)
Figure 1 shows an example of the response to the repetition of several bouts of high level H2S infusion (20 micromol/min). Among the twelve rats that were studied, 3 rats died during or just after the cessation of H2S infusion and were excluded from the analysis. In order to minimize the number of rats used in the study, a second test was also performed each time H2S exposure was followed by a saline injection. Following the second series of H2S exposure, the injection of either saline or MetHb solution was randomly performed. In contrast, when MetHb was performed in first, no second H2S exposure was completed. Accordingly, six rats received a saline injection following the first exposure to H2S bouts. Among these six rats, a second bout of H2S exposure was performed followed by a saline injection in two rats and a MetHb injection in the remaining four rats. The three other rats only received a MetHb injection following the first exposure to H2S bouts. Overall, fifteen tests were analyzed in 9 rats, 8 using saline injection, and 7 with Methemoglobin injection, following H2S exposure. As shown in table 1 (see also below), there was no difference in any of the variables of interest before injection in the rats that received MetHb or saline. In addition, no statistical difference was found between the first and second trial in the magnitude of oxygen deficit or peak/nadir values of hemodynamics (blood pressure, blood flow). However, a significant lower cumulative dose of H2S was needed during the second bout of NaHS (42.1 ± 10.9 vs 27.1 ± 7.7 μmol (p=0.01)) to obtain the same level of O2 deficit (−15.6 ± 4 vs −17.3 ± 4.2 ml.kg−1 ; NS) and blood lactic acidosis (6.0 ± 0.9 vs 5.3 ± 1.7 mM; NS) as compared to the first trial.
Table 1.
Oxygen uptake, carotid and femoral blood flow, arterial blood pressure, arterial blood gases and lactates before (baseline) and at the end of repetition of intravenous H2S infusions. Data are expressed as Mean±SD.
|
Control
(n=8) |
Methemoglobin
(n=7) |
|
|||
|---|---|---|---|---|---|
| Baseline | mean |
SD |
mean |
SD |
p* |
| Weight (kg) | 0.41 | 0.026 | 0.43 | 0.051 | NS |
| VO2 (ml/kg/min) | 11.7 | 1.9 | 12.7 | 2.3 | NS |
| Carotid BF (ml/min) | 8.6 | 2.8 | 8.3 | 2.5 | NS |
| Femoral BF (ml/min) | 5.5 | 1.8 | 4.1 | 1.7 | NS |
| Arterial BP (mmHg) | 82 | 17 | 80 | 12 | NS |
| PaO2 (mmHg) | 69 | 9 | 67 | 14 | NS |
| PaCO2 (mmHg) | 37 | 6 | 36 | 14 | NS |
| Blood lactates (mM) | 2.38 | 0.6 | 1.9 | 0.7 | NS |
| Repetition of H2S infusions | |||||
| N° repetitions | 4.8 | 1.4 | 4.3 | 1.5 | NS |
| CgH2S (μM) | 15.6 | 2.9 | 15.8 | 2.6 | NS |
| Cumulative dose (μM) | 36.6 | 12.3 | 33.4 | 12.6 | NS |
| Total duration of infusion (s) | 101.6 | 34.1 | 92.8 | 34.9 | NS |
| End H2S infusion | |||||
| VO2 nadir (ml/kg/min) | 4.4 | 1.0 | 4.8 | 1.4 | NS |
| O2 deficit nadir (ml) | -16 | 4.9 | -16.8 | 3.1 | NS |
| Carotid BF nadir (ml/min) | 2.4 | 1.7 | 2.1 | 1.6 | NS |
| Femoral BF nadir (ml/min) | 0.6 | 0.8 | 0.4 | 0.2 | NS |
| Arterial BP nadir (mmHg) | 44 | 11 | 37 | 9 | NS |
| PaO2 (mmHg) | 63 | 9 | 76 | 21 | NS |
| PaCO2 (mmHg) | 38 | 14 | 35 | 7 | NS |
| Blood lactates (mM) | 5.7 | 1.2 | 5.8 | 1.5 | NS |
VO2 = oxygen uptake; Carotid BF= Carotid Blood Flow; Femoral BF= Femoral Blood Flow; Arterial BP= Arterial Blood Pressure N° repetitions= number of repetitions of H2S infusion, CgH2S=Concentration of gaseous H2S in the blood; SD = Standard deviation;
ANOVA
Typically, the series of repetitions of H2S infusions resulted in a dramatic decrease in ABP, carotid and femoral blood flows and VO2 (figure 1 and 2) along with an increase in lactic acid (table 2). After each bout of NaHS infusion, mean arterial blood pressure, carotid and femoral blood flows have the tendency to return, although not fully, towards baseline values during the 40 seconds in between two repetitions of NaHS infusion (Figure 1). Table 1 summarizes the baseline data during and at the end of NaHS infusion.
Figure 2.
Temporal evolution of mean ABP (panel A), carotid blood flow (CBF, panel B), femoral blood flow (panel C) and VO2 (panel D) during and following H2S intoxication with saline (open circles) or MetHb (closed circles). Mean ABP, CBF and VO2 decreased during sulfide infusion but recovered during the recovery with a similar kinetics and magnitude following the injection of MetHb or saline. Femoral blood flow (FBF) increased less with MetHb than saline, so FBF was significantly lower during the MetHb (p=0.01) and at 5 min (p=0.007) compared to saline injection.
Table 2.
Mean ±SD of blood lactates and kinetics of recovery.
|
Control
(n=8) |
Methemoglobin
(n=7) |
|
|||
|---|---|---|---|---|---|
| Blood lactates (mM) in recovery | mean |
SD |
mean |
SD |
p* |
| 1 min | 5.7 | 1.2 | 5.8 | 1.5 | NS |
| 5 min | 3.5 | 0.6 | 3.2 | 0.6 | NS |
| 10 min | 2.1 | 0.6 | 2.3 | 0.6 | NS |
| 15 min | 1.5 | 0.4 | 1.6 | 0.5 | NS |
| Kinetics of recovery (s) | |||||
| Time from stop H2S to nadir O2 deficit | 68 | 18 | 89 | 40 | NS |
| Time from nadir to payment O2 deficit | 395 | 130 | 533 | 242 | NS |
1.1) Recovery with Saline/control injection (n=8)
In the tests wherein saline was used, the repetitions of intravenous H2S infusions provided an oxygen deficit of −16 ± 4.9 ml.kg−1 at the end of H2S infusion along with an increase in blood lactates (5.7 ± 1.2 mM). On average ABP decreased by 45 ± 17% while mean CBF and mean FBF were reduced by 73.6 ±15.5% and 90.6 ±9.3%, respectively.
As illustrated in Figures 2A, following the cessation of NaHS infusion, mean ABP increased from 40.3 ± 10.5 to 125.8 ± 33.6 mmHg even before the injection of 3 ml saline, and remained higher than baseline until the 15th minutes of recovery, with an average of 103±22 mmHg (p=0.001). Femoral blood flow also rose above the baseline value gradually following the IV injection of saline (Figure 2B). Carotid blood flow increased progressively eventually reaching a higher level than baseline (p=0.0006; Figure 2C).
Within the first 15 min of recovery, oxygen consumption increased and remained higher than baseline by 25.1±9.3% (p<0.0001), 19.3±13.1% (p=0.002), 13±13.2% (p=0.02) at 5, 10 and 15 min of recovery respectively, as illustrated in Figure 2D. On average, VO2 rose from 12.2 ±2.1 at baseline to 13.7 ± 2.4 ml.kg−1.min−1 at 15 min of recovery (p=0.002). It takes about 10 minutes to recover from the oxygen deficit in the saline group (Figure 3).
Figure 3.
O2 deficit with saline injection (open circles) or MetHb injection (closed circles). The large O2 deficit produced by H2S infusion recovered in both conditions, and was “repaid” with 15 min. MetHb had no effect on the magnitude or kinetics of the oxygen deficit (before injection) or its repayment (after injection).
1.2) Recovery with Methemoglobin injection (n=7)
As mentioned above, no statistical difference was found between the groups that received saline vs MetHb prior to the injection of the antidote or its vehicle: we found the same level of oxygen deficit and lactic acidosis as in saline tests, 16.8 ±3.1 ml.kg−1 just before the injection of the antidote (NS from saline) and 5.8 ± 1.5 mM (NS from saline) respectively. Mean ABP decreased by 52 ± 16%, while mean CBF and mean FBF were reduced by 76.3 ± 11.6% and 90 ±3.5%, respectively (NS from saline group). Oxygen consumption was dramatically reduced and reached its nadir within the 10 sec following the end of the last bout of NaHS infusion, at 4.8 ± 1.4 ml.kg−1.min−1 (p<0.0001; from baseline; NS from saline condition).
Following MetHb infusion, no differences were observed in the change in MABP (Figure 2A), carotid blood flow (CBF, Figure 2B) VO2 (Figure 2D), or oxygen deficit (figure 3) when compared to saline/control group. However, femoral blood flow (FBF) returned to baseline more slowly following MetHb than following saline infusion (see Figure 2C). As in saline tests, oxygen consumption remained elevated following MetHb injection by 21.3 ± 20.2% (p=0.02), 15.2 ±10.5% (p=0.004), 12 ±7.9% (p=0.005) at 5, 10 and 15 min of recovery respectively.
As shown in Table 2, the use of MetHb did not affect the amplitude or the kinetics of recovery nor did it speed up the reduction in lactic acidosis. Of note is that the time to recover the oxygen deficit following H2S intoxication appeared to be if anything longer following MetHb than after saline (533±242 s vs 395±130 s), but without reaching statistical significance (figure 3).
2) Effects of intravenous injection of methemoglobin on sulfide concentrations following constant infusion of H2S (protocol 2)
Among the twelve rats studied according to this protocol, 4 rats died during or within one minute following H2S infusion in control conditions, while one rat died just at the onset of MetHb injection. As a result, 8 control recovery periods and 7 recovery periods with MetHb were analyzed.
2.1) Recovery and saline infusion (n=8)
Intravenous NaHS infusion at a rate 5.9 ±2.4 μmol.min−1 caused a rapid rise in both CgH2S and CMBBH2S. CgH2S increased up to 2.3 ± 2.5 μM and CMBBH2S to 19.4 ±14.9 μM in keeping with the rate of H2S infusion. This was associated with a moderate decrease in mean arterial blood pressure and mean femoral blood flow without reaching significance as illustrated on Figure 4. CgH2S decreased by 90% in the very first minute following the cessation of NaHS infusion, while CMBBH2S dropped to 1.6 ±0.3 μM (p<0.0001) at 5 min (Figure 4A). Saline was infused one minute and half after the cessation of NaHS and CMBBH2S remained unchanged up to 15 min of recovery (Figure 4B).
Figure 4.
Concentration of gaseous H2S (CgH2S, Panel A) and total H2S (CMBBH2S, Panel B), before and after saline or MetHb infusion. Both gaseous and total H2S concentrations in the blood decreased within the first one minute following the cessation of H2S infusion, i.e. before injection of MetHb or saline. No effect of MetHb on CgH2S or CMBBH2S was found. *Significantly different from the data during H2S infusion, p<0.0001.
2.2) Recovery and methemoglobin infusion (n=7)
In the rats that received methemoglobin solution during recovery, the rate of NaHS infusion was identical to the saline group, averaging 6.2 ±2.5 μmol/min. This infusion resulted in an increase in CgH2S to 3.0 ±2.9 μM and CMBBH2S to 19.6 ±16.9 μM. CgH2S decreased as soon as NaHS infusion was stopped. The injection of methemoglobin, which was performed 1.5 min into recovery, did not affect the normal decline in CgH2S and CMBBH2S at 5, 10 and 15 min (Figures 4A and 4B). Blood MetHb concentration reached 5.9 ± 0.6 % 5 min after MetHb IV injection (p<0.0001). The only difference between saline and MetHb groups was a persistent increase in blood pressure following MetHb infusion. The difference remained significant up to 15 minutes.
Discussion
We found that in a rat model of hydrogen sulfide poisoning, the injection of a methemoglobin solution in the very first minutes of recovery, was unable to affect the concentration of H2S in the blood, H2S-induced O2 deficit, and lactate accumulation.
Methodological considerations
The effects of a constant IV NaHS infusion in protocol 2, showed that almost 25% of rats died within minutes when using a rate of 5.5-6.5 μmol/min, but with little or no effects on circulation and the rate of oxygen uptake. Exploratory tests in a sample of rats have shown that no animals would survive more than 5 min at a constant IV NaHS infusion rate of 10 μM/min (100% mortality). H2S administered intermittently at a must higher rate allowed a severe reduction in VO2 and in arterial blood pressure along with lactate accumulation, but with the same mortality (25 %) as a during moderate rate of infusion, due to the very rapid off-kinetics of H2S in the blood and the tissues.
Among the 12 rats that were studied in order to complete protocol 1, 3 rats died during or just after the cessation of H2S infusion. Among the surviving rats, those “treated” by saline injections received a second injection of NaHS followed by a second treatment, in attempt to reduce the number of animals. Clearly lower cumulative doses of H2S were needed during the second series of injections to produce a decrease in arterial blood pressure and VO2 of same magnitude as in the first series. In that sense, this current protocol differs from the “traditional” way of looking at dose-effect response as we were primarily interested in the recovery of the circulatory and “metabolic” depression induced by H2S and thus needed to produce similar effects on these variables in all exposed animals. The dose-effects relationship for H2S is so steep that responses can be quite variable. Even during the first series of injection, the cumulative quantity of H2S was rather variable, averaging 42.1 ± 10.9 micromol, with a coefficient of variability of 25 % (SD/Mean). The short bouts of infusions were thus repeated until our target reduction in arterial blood pressure was obtained. With this approach there was no difference in any of the variables of interest before injection in the rats that received MetHb versus saline, as presented in table 1. In addition, no statistical difference was found between the first and second trial in the magnitude of oxygen deficit or peak/nadir values of blood pressure or blood flow.
Methemoglobin as an antidote following H2S intoxication
Methemoglobinemia has long been proposed as a specific treatment of hydrogen sulfide poisoning not only in animals models 6,25, but also in humans 10,12,25. Its counteracting effects are based on the combination of H2S with the ferric iron (Fe3+) present on MetHb 26 and also on the apparent ability of MetHb to catalyze the oxidation of H2S 8. This effect was found with either sodium nitrite-induced methemoglobinemia 27 or a methemoglobin solution 11. We have shown that levels as low as 3% methemoglobinemia are sufficient to abolish the stimulation of breathing triggered by IV bolus of H2S 11. To our knowledge, all previous studies on the efficacy of MetHb have demonstrated beneficial effects against H2S-induced lethality, particularly in rodents, when nitrite-induced methemoglobinemia was injected prior to 6,25 or during H2S exposure15. We have recently found 13 that infusion of a similar levels of methemoglobin in rat during H2S exposure produces an immediate reduction in CgH2S by more than half, while CMBBH2S increased by several folds. The latter reflects the creation of a new sink (ferric iron) capable of combining free H2S.
The absence of effects of methemoglobin infusion on H2S concentrations following H2S intoxication can be understood in light of the kinetics of H2S in the blood13. Indeed, our group has already shown that at least two compartments of H2S can be described in the body: a soluble form related to the H2S partial pressure which disappears with virtually no delay to undetectable levels a soon as the exposure ceases, and complex combined forms, which remain present at low concentrations in the blood and tissue for quite sometimes after the cessation of exposure 15. Since we injected MetHb solution 1-2 minutes following H2S infusion, we can assume that little dissolved H2S if any was present to be trapped in the blood.
Lack of improvement in circulation and O2 deficit with MetHb infusion following H2S infusion
We hypothetized that MetHb could improve the recovery of O2 utilization by the mitochondria, following the cessation of hydrogen sulfide intoxication via its specific ability to combine soluble H2S, but also possibly though its anti-NO effects 28-30. All the animals, during severe forms of H2S intoxication, presented signs of impediment of mitochondrial oxidative metabolism, as reflected by a decrease in VO2 leading to a large O2 deficit of about 16 ml/kg and a hyperlactacidemia of more than 7 mM in some animals. We were unable to observe any changes in the recovery of the markers of oxidative metabolism following MetHb infusion. This supports the view that, since the soluble forms of H2S have already disappeared at the moment of methemoglobin infusion 13,15, MetHb is unable to alter the recovery from sulfide exposure. As MetHb does not diffuse inside the cells and can not interact with combined H2S, no faster restoration of a normal activity of the mitochondrial electron chain is to be expected.
Clinical relevance of the present study in the treatment of H2S poisoning
The fact that no improvement in hemodynamics, O2 deficit or lactate production could be demonstrated following a severe H2S intoxication after MetHb infusion can not exclude, however that a clinical benefit would be produced by this antidote. Only long-term follow-up, i.e. in a chronic animal model, would help us to clarify such an effect. Indeed, human studies have shown that subjects surviving from acute H2S poisoning present with severe post-anoxic injury responsible for long-term after-effects of sulfide intoxication, including motor and cognitive impairment 31, which may be explained as well by the associated shock and reduction in oxygen supply. The potential beneficial effects of an antidote injected following H2S exposure on the long-term memory loss and cognitive impairment remains to be evaluated. The lack of intracellular effects of methemoglobin appears to be a fundamental limit for its use; other antidotes such as high dose hydroxocobalamin (Vit B12) for example 32, which can diffuse into the cells 33 via non-transcobalamin mechanisms 34 may prove to be better candidate than MetHb to treat sulfide poisoning after sulfide exposure 13.
In conclusion, our results have shown no acute benefits of MetHb infusion during the recovery phase following severe H2S intoxication in sedated rats. No differences in hemodynamics or O2 deficit recovery were observed when compared to saline injection. We postulate that this lack of effect is in large part accounted for by the natural fate of soluble H2S in the body and the action of methemoglobin, which is limited to the intra-vascular compartment. However, the potential effects on the long-term neurological consequences in the animal surviving remain to be evaluated.
Acknowledgements
This work was supported by the CounterACT Program, National Institutes of Health Office of the Director (NIH OD), and the National Institute of Neurological Disorders and Stroke (NINDS), Grant Number 1R21NS080788-01.
Dr. B. Chenuel is supported by a scholarship from “Association des Chefs de Service du CHU de Nancy” and “Association Nancéienne pour l’Encouragement à la Recherche Physiologique”.
The authors would to thank Nicole Tubbs from her skillful technical assistance and Dr. Bogdan Prokopczyk and Neil Trushin from the Department of Pharmacology at Penn State University College of Medicine for their expertise in the determination of H2S using HPLC.
Footnotes
Declaration of Interest:
The authors report no declarations of interest
References
- 1.Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. Journal of bioenergetics and biomembranes. 2008;40(5):533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- 2.Dorman DC, Moulin FJ, McManus BE, Mahle KC, James RA, Struve MF. Cytochrome oxidase inhibition induced by acute hydrogen sulfide inhalation: correlation with tissue sulfide concentrations in the rat brain, liver, lung, and nasal epithelium. Toxicological sciences : an official journal of the Society of Toxicology. 2002;65(1):18–25. doi: 10.1093/toxsci/65.1.18. [DOI] [PubMed] [Google Scholar]
- 3.Leschelle X, Goubern M, Andriamihaja M, Blottiere HM, Couplan E, Gonzalez-Barroso MD, Petit C, Pagniez A, Chaumontet C, Mignotte B, Bouillaud F, Blachier F. Adaptative metabolic response of human colonic epithelial cells to the adverse effects of the luminal compound sulfide. Biochimica et biophysica acta. 2005;1725(2):201–212. doi: 10.1016/j.bbagen.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. H2S signals through protein S-sulfhydration. Science signaling. 2009;2(96):ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith RP. Cobalt salts: effects in cyanide and sulfide poisoning and on methemoglobinemia. Toxicology and applied pharmacology. 1969;15(3):505–516. doi: 10.1016/0041-008x(69)90052-0. [DOI] [PubMed] [Google Scholar]
- 6.Smith RP, Kruszyna R, Kruszyna H. Management of acute sulfide poisoning. Effects of oxygen, thiosulfate, and nitrite. Arch Environ Health. 1976;31(3):166–169. doi: 10.1080/00039896.1976.10667212. [DOI] [PubMed] [Google Scholar]
- 7.Smith RP. The oxygen and sulfide binding characteristics of hemoglobins generated from methemoglobin by two erythrocytic systems. Mol Pharmacol. 1967;3(4):378–385. [PubMed] [Google Scholar]
- 8.Beck JF, Bradbury CM, Connors AJ, Donini JC. Nitrite as antidote for acute hydrogen sulfide intoxication? Am Ind Hyg Assoc J. 1981;42(11):805–809. doi: 10.1080/15298668191420738. [DOI] [PubMed] [Google Scholar]
- 9.Guidotti TL. Hydrogen sulphide. Occup Med (Lond) 1996;46(5):367–371. doi: 10.1093/occmed/46.5.367. [DOI] [PubMed] [Google Scholar]
- 10.Smith RP. Nitrite treatment for hydrogen sulfide poisoning. Ann Intern Med. 1981;95(6):782. doi: 10.7326/0003-4819-95-6-782_1. [DOI] [PubMed] [Google Scholar]
- 11.Van de Louw A, Haouzi P. Ferric Iron and Cobalt (III) compounds to safely decrease hydrogen sulfide in the body? Antioxidants & redox signaling. 2013;19(5):510–516. doi: 10.1089/ars.2012.4513. [DOI] [PubMed] [Google Scholar]
- 12.Hall AH, Rumack BH. Hydrogen sulfide poisoning: an antidotal role for sodium nitrite? Vet Hum Toxicol. 1997;39(3):152–154. [PubMed] [Google Scholar]
- 13.Haouzi P, Sonobe T, Torsell-Tubbs N, Prokopczyk B, Chenuel B, Klingerman CM. In Vivo Interactions Between Cobalt or Ferric Compounds and the Pools of Sulphide in the Blood During and After H2S Poisoning. Toxicological sciences : an official journal of the Society of Toxicology. 2014 doi: 10.1093/toxsci/kfu140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haouzi P, Bell H, Philmon M. Hydrogen sulfide oxidation and the arterial chemoreflex: effect of methemoglobin. Respiratory physiology & neurobiology. 2011;177(3):273–283. doi: 10.1016/j.resp.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Klingerman CM, Trushin N, Prokopczyk B, Haouzi P. H2S concentrations in the arterial blood during H2S administration in relation to its toxicity and effects on breathing. American journal of physiology Regulatory, integrative and comparative physiology. 2013;305(6):R630–638. doi: 10.1152/ajpregu.00218.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haouzi P, Chenuel B, Sonobe T, Klingerman CM. Are H2S-trapping compounds pertinent to the treatment of sulfide poisoning? Clinical toxicology. 2014;52(5):566. doi: 10.3109/15563650.2014.923906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haouzi P, Van de Louw A. Uncoupling mitochondrial activity maintains body [Formula: see text] during hemorrhage-induced O2 deficit in the anesthetized rat. Respiratory physiology & neurobiology. 2013;186(1):87–94. doi: 10.1016/j.resp.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Van de Louw A, Haouzi P. Oxygen deficit and H2S in hemorrhagic shock in rats. Critical care. 2012;16(5):R178. doi: 10.1186/cc11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haouzi P, Bell HJ, Notet V, Bihain B. Comparison of the metabolic and ventilatory response to hypoxia and H2S in unsedated mice and rats. Respiratory physiology & neurobiology. 2009;167(3):316–322. doi: 10.1016/j.resp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Haouzi P, Notet V, Chenuel B, Chalon B, Sponne I, Ogier V, Bihain B. H2S induced hypometabolism in mice is missing in sedated sheep. Respiratory physiology & neurobiology. 2008;160(1):109–115. doi: 10.1016/j.resp.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Wintner EA, Deckwerth TL, Langston W, Bengtsson A, Leviten D, Hill P, Insko MA, Dumpit R, VandenEkart E, Toombs CF, Szabo C. A monobromobimane-based assay to measure the pharmacokinetic profile of reactive sulphide species in blood. Br J Pharmacol. 2010;160(4):941–957. doi: 10.1111/j.1476-5381.2010.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almgren T, Dyrssen D, Elgquist B, Johannsson O. Dissociation of hydrogen sulfide in seawater and comparison of pH scales . Marine Chemistry. 1976;4:289–297. [Google Scholar]
- 23.Olson KR. A Practical Look at the Chemistry and Biology of Hydrogen Sulfide. Antioxidants & redox signaling. 2011 doi: 10.1089/ars.2011.4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahman MM, Kim SJ, Kim GB, Hong CU, Lee YU, Kim SZ, Kim JS, Kang HS. Nitrite-induced methemoglobinaemia affects blood ionized and total magnesium level by hydrolysis of plasma adenosine triphosphate in rat. Basic Clin Pharmacol Toxicol. 2009;105(5):294–300. doi: 10.1111/j.1742-7843.2009.00450.x. [DOI] [PubMed] [Google Scholar]
- 25.Smith RP, Gosselin RE. Hydrogen sulfide poisoning. J Occup Med. 1979;21(2):93–97. doi: 10.1097/00043764-197902000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Smith RP, Gosselin RE. On the mechanism of sulfide inactivation by methemoglobin. Toxicology and applied pharmacology. 1966;8(1):159–172. doi: 10.1016/0041-008x(66)90112-8. [DOI] [PubMed] [Google Scholar]
- 27.Kohn MC, Melnick RL, Ye F, Portier CJ. Pharmacokinetics of sodium nitrite-induced methemoglobinemia in the rat. Drug Metab Dispos. 2002;30(6):676–683. doi: 10.1124/dmd.30.6.676. [DOI] [PubMed] [Google Scholar]
- 28.Buehler PW, Alayash AI. All hemoglobin-based oxygen carriers are not created equally. Biochimica et biophysica acta. 2008;1784(10):1378–1381. doi: 10.1016/j.bbapap.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Rohlfs RJ, Bruner E, Chiu A, Gonzales A, Gonzales ML, Magde D, Magde MD, Jr., Vandegriff KD, Winslow RM. Arterial blood pressure responses to cell-free hemoglobin solutions and the reaction with nitric oxide. The Journal of biological chemistry. 1998;273(20):12128–12134. doi: 10.1074/jbc.273.20.12128. [DOI] [PubMed] [Google Scholar]
- 30.Smani Y, Fifre A, Labrude P, Vigneron C, Faivre B. Pharmacological and physicochemical factors in the pressor effects of conjugated haemoglobin-based oxygen carriers in vivo. Journal of hypertension. 2007;25(3):599–608. doi: 10.1097/HJH.0b013e3280119000. [DOI] [PubMed] [Google Scholar]
- 31.Tvedt B, Skyberg K, Aaserud O, Hobbesland A, Mathiesen T. Brain damage caused by hydrogen sulfide: a follow-up study of six patients. American journal of industrial medicine. 1991;20(1):91–101. doi: 10.1002/ajim.4700200109. [DOI] [PubMed] [Google Scholar]
- 32.Truong DH, Mihajlovic A, Gunness P, Hindmarsh W, O'Brien PJ. Prevention of hydrogen sulfide (H2S)-induced mouse lethality and cytotoxicity by hydroxocobalamin (vitamin B(12a)) Toxicology. 2007;242(1-3):16–22. doi: 10.1016/j.tox.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Astier A, Baud FJ. Complexation of intracellular cyanide by hydroxocobalamin using a human cellular model. Human & experimental toxicology. 1996;15(1):19–25. doi: 10.1177/096032719601500104. [DOI] [PubMed] [Google Scholar]
- 34.Hall CA, Begley JA, Green-Colligan PD. The availability of therapeutic hydroxocobalamin to cells. Blood. 1984;63(2):335–341. [PubMed] [Google Scholar]