Abstract
Background
Continuous opioid infusion (COI) remains the mainstay of analgesic therapy in the Neonatal Intensive Care Unit (NICU). Parent/Nurse Controlled Analgesia (PNCA) has been accepted as safe and effective for pediatric patients, but few reports include use in neonates. This study sought to compare outcomes of PNCA and COI in post-surgical neonates and young infants.
Methods
Twenty infants treated with morphine PNCA were retrospectively compared to 13 infants treated with fentanyl COI in a Midwestern pediatric hospital in the United States. Outcome measures included opioid consumption, pain scores, frequency of adverse events and subsequent methadone use.
Results
The PNCA group (median 6.4 mcg/kg/hr morphine equivalents, range 0.0 – 31.4) received significantly less opioid (P < .001) than the COI group (median 40.0 mcg/kg/hr morphine equivalents; range 20.0 – 153.3), across post-operative days 0-3. Average daily pain scores (based on 0-10 scale) were low for both groups, but median scores differed nonetheless (0.8 PNCA vs 0.3 COI, P < .05). There was no significant difference in the frequency of adverse events or methadone use.
Conclusion
Results suggest PNCA may be a feasible and effective alternative to COI for pain management in post-surgical infants in the NICU. Results also suggest PNCA may provide more individualized care for this vulnerable population and in doing so, may potentially reduce opioid consumption, however more studies are needed.
Keywords: Parent/Nurse Controlled Analgesia, PNCA, PCA by Proxy, Pediatric, pain management, neonate, opioid infusion
Background
Continuous opioid infusion (COI) remains the mainstay of analgesic therapy (1-3) in the Neonatal Intensive Care Unit (NICU). Although COI is recommended by the American Academy of Pediatrics (4), COI may provide more or less analgesic than required due to the inability to quickly respond to changes in an infant’s pain level. The immediate and long-term consequences of opioids for critically ill and/or preterm infants are not well-defined (2,5,6), and it has been suggested that using smaller bolus doses and/or shorter infusion times may minimize potential adverse effects (4,7). While it may seem intuitive that less opioid is better, negative sequelae from undertreated pain may be even more harmful than the unknown consequences of opioids. Therefore, an administration technique with flexible, targeted dosing would be particularly beneficial in this population, as rapid developmental changes, gestational age, body composition, variable opioid requirements and medication pharmacodynamics can make opioid dosing for neonates difficult (2,4,8-10).
Parent/Nurse Controlled Analgesia (PNCA) allows for flexible dosing, rapid titration in response to pain or the anticipation of pain, increased parental participation, and decreases the need to repeatedly breach the intravenous (IV) line. Safety and efficacy of PNCA have been demonstrated in several pediatric populations (11-16), yet studies including neonates are sparse and are focused on Nurse Controlled Analgesia (NCA) (10,17). Furthermore, no studies were found that directly compared PNCA to COI for neonatal post-operative pain management. The purpose of this study was to evaluate the feasibility and efficacy of PNCA in post-surgical neonates and young infants and to compare PNCA to COI with respect to opioid consumption, pain scores, adverse events, and subsequent methadone use as a surrogate measure of opioid tolerance.
Methods
Participants
The hospital Human Research Review Board approved this retrospective study. Twenty-two cases of PNCA use for post-operative infants in the NICU were identified (for the purpose of this study, infant will be used to describe the neonates and young infants represented). The patients in the PNCA group had undergone surgery during the 10 month period following our initial implementation of PNCA into the NICU. Two cases involved PNCA use following a second surgery and were therefore excluded.
To compare PNCA to COI, thirteen cases involving infants who had received fentanyl COI (the standard post-operative regimen prior to implementation of PNCA in the NICU) for pain control and were similar to the infants treated with PNCA with regard to gestational age, age at time of study, and surgical procedure were identified from charts dating back 2 years before the implementation of PNCA as an option for pain management in our NICU. Equivalence in terms of surgical procedure was determined by the surgeon (MJA) using the relative size and location of the incision as the primary determining factors. The infants were also compared for gestational age, age at time of study, diagnosis, and co-morbidities. A neonatologist (MRU) and pediatric anesthesiologist (JV) concurred with the equivalence of the groups. The final sample included 33 infants, 20 in the PNCA group and 13 in the COI group.
Data Collection
Data were extracted from charts by a research nurse and double checked for accuracy by a co-investigator working independently. Data were collected for the entire time the PNCA or COI was in place. Pain scores, PNCA use and opioid consumption were analyzed for each 12 hour day shift (0600-1759) and each 12 hour night shift (1800-0559) and are reported for Post-Operative Day (POD) 0 – 3. For the majority of infants, the PNCA or COI was started on the day of surgery, labeled as POD 0. Data collection for POD 0 ended at 0559 the following morning with subsequent 24 hour time periods labeled as POD 1, 2, and 3.
An anesthesiologist prescribed all PNCAs using morphine and a recommended starting dose of 10-30mcg/kg (18) for both the button dose and basal (continuous) rate with an 8-15 minute lockout period and 60mcg/kg/hr maximum. An additional nurse bolus (equivalent to two button doses) was often available every 2-3 hours, as needed for moderate to severe pain not relieved by the PNCA. Prior to the initiation of PNCA into the NICU setting, nurses were instructed on the concepts, risks, benefits, appropriate use and functionality of PNCA. Nurses were encouraged to activate the PNCA in response to pain behaviors often associated with pain scores ≥4/10. Parents and RNs were instructed not to activate the PNCA while a patient was asleep or for reasons other than pain.
Fentanyl COIs were prescribed by the NICU team with a starting dose of 2-4mccg/kg/hr (19). A nurse bolus (equivalent to the hourly infusion) was often available for moderate to severe pain. Increases or decreases to the COI rate and bolus doses were made based on the clinical judgment of the NICU team caring for the infant.
Opioid consumption was calculated in standardized morphine equivalents (mcg/kg/hr). The amount of fentanyl (mcg/kg/hr) administered was multiplied by a factor of 20 to establish the morphine equivalents per hour or per day. Knowing the impact this conversion factor would have on the results and that the exact conversion factor for converting fentanyl to morphine remains unknown, the authors sought to be conservative in their analysis. A review of the literature revealed estimated conversion factors ranging from 4:1 to 100:1 for a variety of age groups, including neonates (2,19-25). The authors chose a conservative conversion factor that is consistent with or below the conversion factors offered in the majority of the literature reviewed (2,20-23,25).
To maintain consistency of pain score data, only infants whose pain was assessed using the FLACC (Face, Legs, Activity, Cry, Consolability) scale (26) were included. The FLACC scale has been used in previous studies including neonates and is our current pain assessment tool for infants greater than 32 weeks gestational age. Traditionally, pain scores greater than 3 (out of 10) are considered to indicate moderate to severe pain (10,26). Therefore, pain scores less than 4 were considered to indicate adequate analgesia. Somnolence was defined as a Modified Ramsay Sedation Score of less than 4 (indicating the infant aroused to consciousness with moderate tactile or loud verbal stimulus) (27).
Statistical Analysis
All statistical analyses were calculated using SPSS 20 (IBM SPSS Inc., Chicago, IL). An unadjusted P < .05 was used for statistical significance in all analyses. Descriptive statistics and plots were used to examine normality of distributions. In addition, descriptive, frequency and correlational analyses were used to provide summary information about infant characteristics. The mean (±SD) are reported, or where data are skewed, median with the Interquartile Range (IQR). Paired t-tests and Wilcoxon tests were used to compare day to night time injections and attempts for the PNCA group. Two sample t-tests and Mann Whitney tests were used to compare PCNA and COI groups. To determine differences in opioid consumption across PODs, a mixed effects model with maximum likelihood estimation and an autocorrelation matrix (AR(1)) was used. Covariates included gender, ethnicity, surgical procedure, and average daily pain scores. Unless otherwise noted, all analyses include data from POD 0 - 3. One infant in the PNCA group began PNCA on POD 4, and therefore was not included in analyses based on POD 0–3.
Results
Demographics
Demographics for the participants in each group are provided in Table 1. No between-group differences were found in terms of diagnosis, gestational age, weight or time on study. PNCA or COI was started on POD 0 for all but 3 infants. In the PNCA group, one infant each had the PNCA started on POD 1, 2, and 4. The median age at time of study differed by 2 days with the infants in the PNCA group being older (p< .05). Although statistically significant, the authors do not believe this difference was clinically significant.
Table 1.
Demographics
| PNCA (n=20) | COI (n=13) | Total Sample (N=33) | |
|---|---|---|---|
|
| |||
| n (%) | n (%) | n (%) | |
|
| |||
| Gender | |||
| Female | 10 (50) | 5 (38.5) | 15 (45.5) |
| Male | 10 (50) | 8 (61.5) | 18 (54.5) |
|
| |||
| Race | |||
| White | 7 (35) | 5 (38.5) | 12 (36.4) |
| Black | 4 (20) | 2 (15.4) | 6 (18.2) |
| Asian | 3 (15) | 0 | 3 (9.1) |
| Hispanic | 1 (5) | 3 (23.1) | 4 (12.1) |
| Other | 1 (5) | 0 | 1 (3) |
| unavailable | 4 (20) | 3 (23.1) | 7 (21.2) |
| * total 100.1% d/t rounding | |||
|
| |||
| Age (days) at time of study | |||
| Median* | 3.0 | 1.0 | 2.0 |
| IQR | 2.0-41.0 | 1.0-2.5 | 1.0-21.5 |
| *P=.006 | |||
|
| |||
| Gestational Age (weeks) | |||
| Median | 36.5 | 37.0 | 37.0 |
| IQR | 27.0 – 41.0 | 24.0 – 42.0 | 24.0 – 42.0 |
|
| |||
| Weight (kg) | |||
| Median | 3.0 | 2.8 | 2.9 |
| IQR | 2.0 – 4.1 | 2.2 – 4.4 | 2.0 – 4.4 |
|
| |||
| Surgical Area | |||
| Thoracic | 3 (15) | 2 (15.4) | 5 (15.2) |
| Upper Abdomen | 12 (80) | 5 (38.5) | 17 (51.5) |
| Lower Abdomen | 5 (25) | 6 (46.2) | 11 (33.3) |
| * total 100.1% d/t rounding | |||
|
| |||
| Total Time PNCA or COI in place (hours) | |||
| Median | 68.5 (47.8-84.5) | 64.0 (39.5-98.5) | 68.0 (45.5-89.0) |
|
| |||
| Intubated at start of PNCA or COI | 15 (75%) | 13 (100%) | 28 (85%) |
Opioid Consumption
Differences in opioid consumption were calculated, while controlling for gender, ethnicity, surgical procedure and average daily pain scores. Based on the mixed effects model, the groups differed significantly on total opioid consumption (P < .001), in that the PNCA group received significantly less opioid than the COI group during daytime, night time and combined 24 hour periods (Table 2). Daytime consumption did not differ from nighttime consumption for either group.
Table 2.
Opioid consumption (morphine equivalents) for 24h day, 12h day, and 12h nights
| PNCA (n = 20) | COI (n = 13) | |
|---|---|---|
|
| ||
| Daily Opioid Consumed | ||
| Median | 6.4 | 40.0* |
| IQR | 1.8 – 13.6 | 34.6 – 57.1 |
|
| ||
| Daytime Consumption | ||
| Median | 5.9 | 40.0* |
| IQR | 2.1 – 12.8 | 31.7 – 58.3 |
|
| ||
| Nighttime Consumption | ||
| Median | 9.5 | 40.0* |
| IQR | 2.3 – 14.6 | 38.33 – 60.0 |
IQR: Inter Quartile range
No daytime vs. nighttime differences in consumption were found for either group
P < .001 for differences between PNCA and COI groups
For the PNCA group, the average amount of opioid consumed was 6.4 (1.8 – 13.6) mcg/kg/hr. Daytime consumption was 5.9 (2.1 – 12.8) mcg/kg/hr and nighttime consumption was 9.5 (2.3 – 14.6). The starting button dose was 10.0 (9.2 – 11.8) mcg/kg and the starting basal rate was 10.7 (10.0 – 12.0) mcg/kg/hr. Basal infusions were initiated for 16 infants at the time the PNCA was initiated. Fourteen of the 16 basal rates were discontinued prior to the PNCA being completely discontinued, which contributed to the overall average opioid consumption (6.4 (1.8 – 13.6) mcg/kg/hr) being less than the starting basal rate (10.7 (10.0 – 12.0) mcg/kg/hr). Given the small number of infants without a basal infusion, no comparisons were made between infants with or without a basal infusion. The average number of injections and attempts was consistently below 1 per hour. There was no difference in the average number of injections per hour during day shifts (0.2; 0.0 – 1.1) compared to night shifts (0.3; 0.0 – 2.3), or in the average number of attempts per hour during the day shifts (0.2; 0.0 – 1.3) compared to the night shifts (0.3; 0.0 – 2.6). Three infants received one nurse administered bolus each throughout the study period. Two infants in the PNCA group were transitioned to methadone after the PNCA was discontinued, one of whom was started on methadone eight days after the PNCA had been discontinued. No infants in the COI group received methadone.
For the COI group, the average amount of opioid consumed (converted to morphine equivalents) was 40.0 (34.6 – 57.1) mcg/kg/hr. Daytime consumption was 40.0 (31.7 – 58.3) mcg/kg/hr and nighttime consumption was 40.0 (38.3 – 60.0) mcg/kg/hr. The starting dose was 40.0 mcg/kg/hr (40.0 – 60.0). Eight infants required additional bolus therapy and the frequency of administration ranged from 0-2 boluses per infant, per 12 hour shift, totaling 10 boluses administered on day shifts, and 15 boluses administered on night shifts. The average daily pain score was never high in either group, but opioid consumption was much higher in the COI group (Figure 1).
Figure 1.
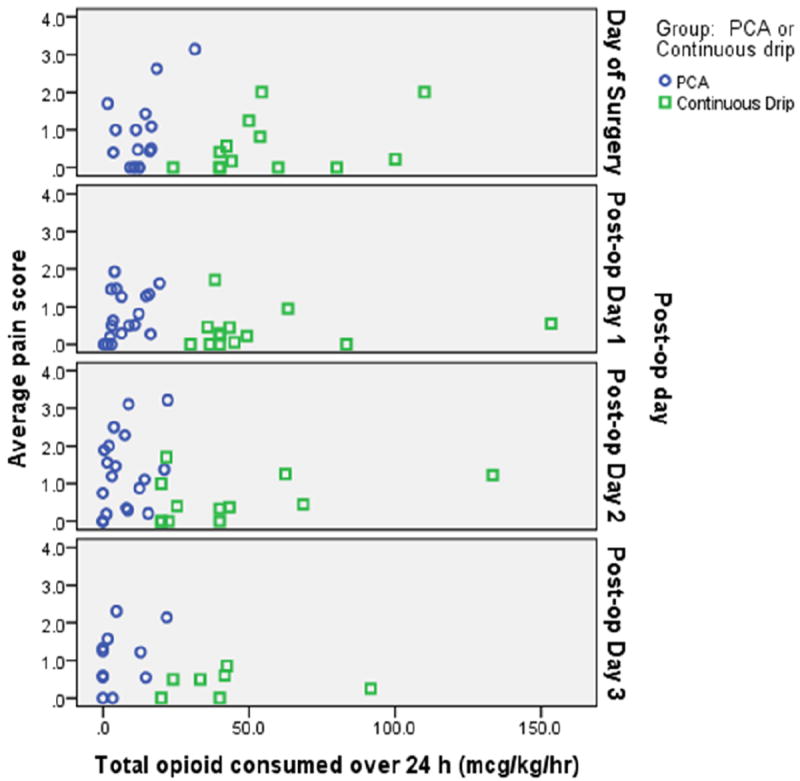
shows amount of opioid consumed by average daily pain score for each infant for each Post-operative day
Differences between premature infants’ (gestational age <37 weeks) and full term infants’ total opioid consumption over a 24 hour period, were examined using a 2 (Group: PNCA vs. COI) × 2 (Gestational Age: < 37 weeks/Preemie vs. ≥ 37 weeks) ANOVA. Results showed a significant Group × Gestational Age interaction (P = .004). Gestational age had no effect on total opioid consumption for the PNCA group. However for the COI group, the full term infants (45.0; 37.1 – 71.7) consumed a greater amount of opioid than the premature infants (40.0; 30.8 – 43.3) (Figure 2).
Figure 2.

shows differences in opioid consumption between groups based on gestational age (premature vs. full term).
Pain Scores
Average daily pain scores remained consistently below 2/10 for both groups (Table 3), but differed significantly between groups (PNCA 0.8 (0.0 – 3.2) vs. COI 0.3 (0.0 – 2.0); P < .005). Groups differed (P < .005) in median 12 hour daytime (PNCA 0.5 (.0 – 1.4), COI .0 (0-0.5), and in 12 hour night time (PNCA 1.0 (.0 -1.7) vs. COI 0.3 (.0 – 1.0) (P <.05) pain scores. Maximum pain scores were 8/10 during the day shifts and 9/10 during the night shifts and corresponded to two infants in the PNCA group who had abdominal surgery (Figure 3). Average pain scores for premature infants did not differ from those of full-term infants in 24 hour daily (premature 0.4 vs full term 0.6), 12 hour daytime (premature 0.3 vs full term 0.2), or 12 hour nighttime (premature 0.4 vs full term 0.7), pain scores. Ten percent of the infants in the PNCA group and 3% of the infants in the COI group had pain scores >4/10 during day time hours. No differences were found during night time hours. However, side by side comparisons of all pain scores for infants in both groups by POD (Figure 3) illustrate that the two treatment modalities provided equivalent pain control.
Table 3.
Pain Scores
| Median (IQR) Pain 24 hrs | Median (IQR) Pain Day | Median (IQR) Pain Night | ||
|---|---|---|---|---|
|
| ||||
| PNCA | Post-op Day 0 | 0.5 (0.0 – 1.3) | 0.0 (0.0 – 0.5) | 0.6 (0.0 – 1.4) |
|
| ||||
| Post-op Day 1 | 0.6 (0.3 – 1.4) | 0.6 (0.1 – 1.4) | 0.5 (0.0 – 1.3) | |
|
| ||||
| Post-op Day 2 | 1.2 (0.2 – 2.0) | 1 (0.0 – 1.9) | 1.2 (0.6 – 2.3) | |
|
| ||||
| Post-op Day 3 | 0.9 (0.2 – 1.5) | 0.5 (0.0 – 1.5) | 2.0 (0.9 – 2.1) | |
|
| ||||
| COI | Post-op Day 0 | 0.2 (0.0 – 1.0) | 0.0 (0.0 – 1.3) | 0.3 (0.0 – 1.0) |
|
| ||||
| Post-op Day 1 | 0.2 (0.0 – 0.5) | 0.0 (0.0 – 0.6) | 0.2 (0.0 – 0.6) | |
|
| ||||
| Post-op Day 2 | 0.4 (0.0 – 1.2) | 0.0 (0.0 – 0.6) | 0.7 (0.0 – 2.2) | |
|
| ||||
| Post-op Day 3 | 0.5 (0.0 – 0.6) | 0.2 (0.0 – 0.5) | 0.0 (0.0 – 0.9) | |
Median daily pain score ratings by post-operative day
Figure 3. Pain Assessments for each Patient across Time.
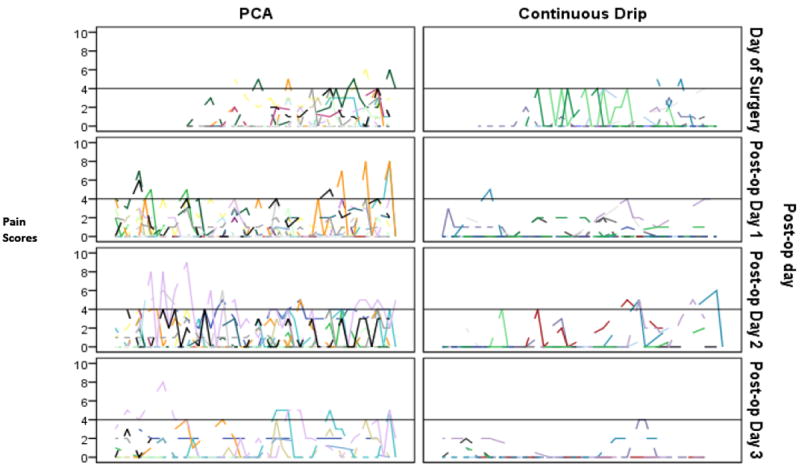
The reference line corresponds to a pain score of 4 which was determined by the authors to be the threshold of acceptable pain intensity.
Adverse Events
Eight (40%) infants in the PNCA group and 8 (62%) infants in the COI group were noted to have somnolence (Modified Ramsay Score less than 4) during POD 0-1. One infant in each group was noted to have somnolence beyond POD 1. Because all but 1 (PNCA group) of the infants who experienced somnolence were intubated, it is possible somnolence was a goal of therapy.
Two infants in the COI group (15%) required naloxone while receiving fentanyl 1mcg/kg/hr. The first infant had apnea requiring CPAP and bag mask breaths shortly after extubation. The COI was discontinued and naloxone administered. The second infant had a planned extubation and subsequent apnea. The COI was not discontinued following naloxone administration. No infants in the PNCA group received naloxone.
Two infants in the PNCA group required reintubation, but neither event was associated with opioid-induced sedation or respiratory depression. One infant had self-extubated (cause unknown) and was immediately reintubated. The second infant had a planned extubation, but developed respiratory distress (not depression) requiring reintubation.
Discussion
The immediate and long-term consequences of opioid exposure for critically ill and/or preterm infants are not well-defined (2,5,6). Although recent studies have reported conflicting results regarding long-term effects, adverse outcomes have been reported (28,29). Given the negative sequelae associated with undertreated pain (30) coupled with evidence of adverse long-term consequences of opioid use in neonates (28,29,31), it would be ideal to quickly respond to increased pain and safely provide targeted pain control using the least amount of opioid necessary to manage pain, without causing excess sedation. Results of this study suggest PNCA may offer such advantages.
The main finding of this study is that PNCA appears to be feasible and effective in the neonatal population. An additional finding suggests that although the precise fentanyl to morphine conversion factor is unknown, the PNCA group received significantly less opioid than the COI group, with no compromise in comfort and no apparent increase in morbidity. The starting dose for the COI group was significantly higher than the starting dose of the PNCA, yet 61% of infants in the COI group required nurse administered boluses. Because the boluses were equivalent to the hourly rate (40mcg/kg in morphine equivalents), when those boluses were required, the infants received significantly more opioid than the average of less than one (10mcg/kg in morphine equivalents) PNCA dose administered per hour in the PNCA group. Not only could this difference in the method of opioid administration contribute to the diffidence seen in opioid consumption, it may have contributed to the difference seen in the number of infants who experienced increased somnolence in the COI group.
It has been suggested that the risk of adverse effects associated with opioid use in neonates can be minimized by using smaller bolus doses and/or shortened infusion times (7). Examples of such effects include tolerance and central sensitization; with the former potentially developing more quickly with continuous infusions than with intermittent doses (7). Additionally, low dose morphine exposure in neonates has been linked with poorer performance on an IQ visual subtest at 5 years of age (28), and preemptive morphine use has been linked with adverse behavioral, physical, and short-term memory outcomes (29). In light of these concerns and suggested solutions, there are 2 important perspectives on the results of this study: the findings may indicate that infants treated with PNCA benefited from this method of opioid administration. The results may also indicate that neonates treated with COI may have received more opioid than necessary for good pain management, and this in turn, could be putting them at risk for serious short-term and long-term effects.
While not all were statistically significant, a number of the between-group differences found in this study suggest that infants in the COI group may have received more opioid than necessary to maintain comfort. First, the difference in opioid consumption seen between groups could possibly be attributed to the basal rates in the PNCA group being discontinued early on. This could mean that once the basal rate was stopped, nurses and parents titrated opioids using the low PNCA button doses alone. Therefore, as pain decreased over time, lingering and/or incidental pain was treated as it occurred without exposing the infants to continuously infusing opioids during times when opioids were not needed. Second, despite differences in opioid consumption, pain scores were low and relatively similar between groups, yet fewer infants in the COI group had pain scores ≥4/10 and more infants in the COI group were somnolent between POD 0-1 than in the PNCA group. While these findings could suggest better pain control in the COI group, alternatively, they may suggest that for this small group of patients, PNCA was better able to target pain without causing as much sedation.
While neonates have been reported to be at greater risk for opioid adverse events (10,32), it is difficult to determine what role, if any, opioids played in the need for naloxone for two infants in the COI group; theoretically, a basal rate on a PNCA could have produced the same result. In contrast, it was clear that opioids did not contribute to the two infants in the PNCA group that required re-intubation. However, for the one infant who had self extubated (PNCA group), pain and/or agitation cannot be ruled out as contributing factors, in which case perhaps more opioid would have been beneficial. Equally unclear is the rationale for methadone use for two patients in the PNCA group, one of whom was started on methadone eight days after the PNCA was stopped. In our NICU, methadone is typically used to wean opioids, or to treat withdrawal syndrome. It is unclear whether either of these was the case for these two infants, or whether it was a result of practitioner preference. Larger, prospective studies are needed in order to measure the effect of PNCA compared to COI on the development of opioid tolerance and possible withdrawal.
A few factors may have contributed to the large differences found in opioid consumption for the 2 groups in this study. First, fentanyl is known to create rapid tolerance which may have resulted in increased COI rates and thus consumption (6). Furthermore, the results may have been influenced by fentanyl’s relatively rapid clearance rate. However, Berde et al. (33) have described a “context-sensitive half-time,” which suggests that fentanyl clearance is reduced with repeated doses or prolonged infusions, and this may be particularly true for post-abdominal surgery in neonates. Lastly, although the conversion factor chosen for this study may be considered an under-estimation of the differences in opioid consumption based on the range reported across published reports (2,20-23,25), some may consider the differences an over-estimation (19,24). While these findings must be taken with caution, they do suggest that PNCA may allow for appropriate pain management with minimal use of opioid
The amount of morphine consumed in the PNCA group is similar to values reported in the literature. For example, morphine consumption was similar to that reported in a study including neonatal NCA (10) but was much less than that reported in studies of P/NCA in older infants and children (10-12,15). These findings may reflect differences in pharmacokinetics (8,34) and are consistent with prior studies demonstrating that neonates require less opioid (8,35). Only the premature infants in the COI group consumed less opioid than the full term infants. While these results are consistent with previous studies (8,34,35), if prematurity were the only contributing factor, one would expect to see this difference in both groups, not exclusively in the COI group.
Although both the PNCA and COI groups in the current study had opioids infusing, the basal rate in the PNCA group was significantly lower than the infusion rate in the COI group. Basal rates are used frequently with pediatric PNCA (11,12,15,16), yet remain controversial (36-40). In this study, basal rates were used for 80% of the infants in the PNCA group, all of whom weighed less than 5 kg, but basal rates are not always used in this patient population (10). Despite this difference, opioid consumption in our study was somewhat similar to levels reported in a large study of NCA that included neonates (10), and may suggest that basal rates do not substantially impact the overall opioid consumption, as reported with older children (41). As the majority of basal rates were able to be stopped prior to stopping the PNCA completely, one could also infer that nurses or parents activated the PNCA as needed to deliver the amount of opioid required to achieve comfort, whether a basal rate was in place or not. The low PNCA use in this study is similar to previous studies of PNCA (11,12,15) and continues to support PNCA as a manageable option for nurses and parents. Additionally, PNCA did not appear to impact the length of time opioids were required, and the amount of time PNCA was in place is comparable to other studies (10,14).
Limitations
Although the findings of this study are promising, there are limitations. First, the sample size is small, with fewer infants in the COI group. Attempts to balance the numbers would have resulted in other dissimilarities which the authors felt would have confounded the results more than having unequal groups. Second, because our standard COI consisted of fentanyl, medications and management differed between groups. However, several studies have compared fentanyl to morphine and each offers important contributions to our understanding of the differences between these medications (19,21,24,42,43) . Lastly, because of the retrospective nature of this study, several dynamics remain unknown (e.g. Did RNs consistently activate the PNCA in response to pain scores ≥4? Was the PNCA activated for reasons other than surgical pain and was its use appropriate?). It remains unknown how many parents actually participated in PNCA activation. As one reason for implementing PNCA in the NICU was to increase parental involvement, to learn if they participated in PNCA activation and how that participation impacted care could provide additional insight. Future studies designed in a prospective, randomized, controlled fashion with a larger sample would be better suited to address such matters.
Conclusion
Results of this study suggest that PNCA may be a feasible and effective alternative to COI for the management of post-operative pain in neonates. Despite concerns about the long-term consequences of opioid exposure during the neonatal period, there are minimal data offering alternatives to COI in this patient population. PNCA may offer an effective alternative and may provide more individualized care with less sedation. Although results may suggest less opioid consumption in the PNCA group, due to the limitations identified above, caution must be taken when interpreting or generalizing those results. Future research in the form of a randomized controlled trial with a larger sample size, using the same opioid, and investigating even more significant outcomes (e.g. ventilator days, neurocognitive outcomes) is warranted and may add support to the use of PNCA for neonates following surgery, and perhaps for neonates requiring opioids for reasons other than post-operative pain management.
Acknowledgments
The authors wish to thank the Jane B. Pettit Pain Management Center for continued support of pediatric pain management and Sharon Eisch, RN, for her contributions to this study.
“This study was supported by UL1 TR000055 – Clinical and Translational Science Award.”
Footnotes
Disclosures: This study was approved by The Human Research Review Board. This research was carried out without funding.
The authors have no conflicts of interest.
References
- 1.De Lima J, Carmo KB. Practical pain management in the neonate. Best Pract Res Clin Anaesthesiol. 2010 Sep;24(3):291–307. doi: 10.1016/j.bpa.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Simons SH, Anand KJ. Pain control: opioid dosing, population kinetics and side-effects. Semin Fetal Neonatal Med. 2006 Aug;11(4):260–267. doi: 10.1016/j.siny.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 3.van Dijk M, Bouwmeester NJ, Duivenvoorden HJ, Koot HM, Tibboel D, Passchier J, et al. Efficacy of continuous versus intermittent morphine administration after major surgery in 0-3-year-old infants; a double-blind randomized controlled trial. Pain. 2002 Aug;98(3):305–313. doi: 10.1016/S0304-3959(02)00031-3. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics. Prevention and management of pain and stress in the neonate. American Academy of Pediatrics Committee on Fetus and Newborn Committee on Drugs Section on Anesthesiology Section on Surgery Canadian Paediatric Society Fetus and Newborn Committee. Pediatrics. 2000 Feb;105(2):454–461. [PubMed] [Google Scholar]
- 5.Anand KJ, Hall RW. Pharmacological therapy for analgesia and sedation in the newborn. Arch Dis Child Fetal Neonatal Ed. 2006 Nov;91(6):F448–53. doi: 10.1136/adc.2005.082263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franck LS. Some pain, some gain: reflections on the past two decades of neonatal pain research and treatment. Neonatal Netw. 2002 Aug;21(5):37–41. doi: 10.1891/0730-0832.21.5.37. [DOI] [PubMed] [Google Scholar]
- 7.Taddio A, Katz J. Pain, opioid tolerance and sensitisation to nociception in the neonate. Best Pract Res Clin Anaesthesiol. 2004 Jun;18(2):291–302. doi: 10.1016/j.bpa.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Bouwmeester NJ, van den Anker JN, Hop WC, Anand KJ, Tibboel D. Age- and therapy-related effects on morphine requirements and plasma concentrations of morphine and its metabolites in postoperative infants. Br J Anaesth. 2003 May;90(5):642–652. doi: 10.1093/bja/aeg121. [DOI] [PubMed] [Google Scholar]
- 9.Lynn AM, Nespeca MK, Bratton SL, Shen DD. Intravenous morphine in postoperative infants: intermittent bolus dosing versus targeted continuous infusions. Pain. 2000 Oct;88(1):89–95. doi: 10.1016/S0304-3959(00)00313-4. [DOI] [PubMed] [Google Scholar]
- 10.Howard RF, Lloyd-Thomas A, Thomas M, Williams DG, Saul R, Bruce E, et al. Nurse-controlled analgesia (NCA) following major surgery in 10,000 patients in a children’s hospital. Paediatr Anaesth. 2010 Feb;20(2):126–134. doi: 10.1111/j.1460-9592.2009.03242.x. [DOI] [PubMed] [Google Scholar]
- 11.Czarnecki ML, Ferrise AS, Jastrowski Mano KE, Garwood MM, Sharp M, Davies H, et al. Parent/nurse-controlled analgesia for children with developmental delay. Clin J Pain. 2008 Nov-Dec;24(9):817–824. doi: 10.1097/AJP.0b013e3181773b69. [DOI] [PubMed] [Google Scholar]
- 12.Czarnecki ML, Salamon KS, Jastrowski Mano KE, Ferrise AS, Sharp M, Weisman SJ. A preliminary report of parent/nurse-controlled analgesia (PNCA) in infants and preschoolers. Clin J Pain. 2011 Feb;27(2):102–107. doi: 10.1097/AJP.0b013e3181f0972c. [DOI] [PubMed] [Google Scholar]
- 13.Anghelescu DL, Burgoyne LL, Oakes LL, Wallace DA. The safety of patient-controlled analgesia by proxy in pediatric oncology patients. Anesth Analg. 2005 Dec;101(6):1623–1627. doi: 10.1213/01.ANE.0000184198.13285.33. [DOI] [PubMed] [Google Scholar]
- 14.Malviya S, Voepel-Lewis T, Tait AR, Merkel S, Lauer A, Munro H, et al. Pain management in children with and without cognitive impairment following spine fusion surgery. Paediatr Anaesth. 2001 Jul;11(4):453–458. doi: 10.1046/j.1460-9592.2001.00686.x. [DOI] [PubMed] [Google Scholar]
- 15.Monitto CL, Greenberg RS, Kost-Byerly S, Wetzel R, Billett C, Lebet RM, et al. The safety and efficacy of parent-/nurse-controlled analgesia in patients less than six years of age. Anesth Analg. 2000 Sep;91(3):573–579. doi: 10.1097/00000539-200009000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Voepel-Lewis T, Marinkovic A, Kostrzewa A, Tait AR, Malviya S. The prevalence of and risk factors for adverse events in children receiving patient-controlled analgesia by proxy or patient-controlled analgesia after surgery. Anesth Analg. 2008 Jul;107(1):70–75. doi: 10.1213/ane.0b013e318172fa9e. [DOI] [PubMed] [Google Scholar]
- 17.Morton NS, Errera A. APA national audit of pediatric opioid infusions. Paediatr Anaesth. 2010 Feb;20(2):119–125. doi: 10.1111/j.1460-9592.2009.03187.x. [DOI] [PubMed] [Google Scholar]
- 18.Anand KJ. International Evidence-Based Group for Neonatal Pain. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001 Feb;155(2):173–180. doi: 10.1001/archpedi.155.2.173. [DOI] [PubMed] [Google Scholar]
- 19.Suarez A, Knoppert DC, Lee DS, Pletsch D, Seabrook JA. Opioid infusions in the neonatal intensive care unit. J Pediatr Pharmacol Ther. 2010 Apr;15(2):142–146. [PMC free article] [PubMed] [Google Scholar]
- 20.Berde CB, Sethna NF. Analgesics for the treatment of pain in children. N Engl J Med. 2002 Oct 3;347(14):1094–1103. doi: 10.1056/NEJMra012626. [DOI] [PubMed] [Google Scholar]
- 21.Patanwala AE, Duby J, Waters D, Erstad BL. Opioid conversions in acute care. Ann Pharmacother. 2007 Feb;41(2):255–266. doi: 10.1345/aph.1H421. [DOI] [PubMed] [Google Scholar]
- 22.Tibboel D, Anand KJ, van den Anker JN. The pharmacological treatment of neonatal pain. Semin Fetal Neonatal Med. 2005 Apr;10(2):195–205. doi: 10.1016/j.siny.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Anand KJ, Willson DF, Berger J, Harrison R, Meert KL, Zimmerman J, et al. Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics. 2010 May;125(5):e1208–25. doi: 10.1542/peds.2009-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saarenmaa E, Huttunen P, Leppaluoto J, Meretoja O, Fellman V. Advantages of fentanyl over morphine in analgesia for ventilated newborn infants after birth: A randomized trial. J Pediatr. 1999 Feb;134(2):144–150. doi: 10.1016/s0022-3476(99)70407-5. [DOI] [PubMed] [Google Scholar]
- 25.Walter-Nicolet E, Annequin D, Biran V, Mitanchez D, Tourniaire B. Pain management in newborns: from prevention to treatment. Paediatr Drugs. 2010 Dec 1;12(6):353–365. doi: 10.2165/11318900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.Manworren RC, Hynan LS. Clinical validation of FLACC: preverbal patient pain scale. Pediatr Nurs. 2003 Mar-Apr;29(2):140–146. [PubMed] [Google Scholar]
- 27.Hoffman GM, Nowakowski R, Troshynski TJ, Berens RJ, Weisman SJ. Risk reduction in pediatric procedural sedation by application of an American Academy of Pediatrics/American Society of Anesthesiologists process model. Pediatrics. 2002 Feb;109(2):236–243. doi: 10.1542/peds.109.2.236. [DOI] [PubMed] [Google Scholar]
- 28.de Graaf J, van Lingen RA, Simons SH, Anand KJ, Duivenvoorden HJ, Weisglas-Kuperus N, et al. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children’s functioning: five-year follow-up of a randomized controlled trial. Pain. 2011 Jun;152(6):1391–1397. doi: 10.1016/j.pain.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson SA, Ward WL, Paule MG, Hall RW, Anand KJ. A pilot study of preemptive morphine analgesia in preterm neonates: effects on head circumference, social behavior, and response latencies in early childhood. Neurotoxicol Teratol. 2012 Jan-Feb;34(1):47–55. doi: 10.1016/j.ntt.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Anand KJ, Aranda JV, Berde CB, Buckman S, Capparelli EV, Carlo W, et al. Summary proceedings from the neonatal pain-control group. Pediatrics. 2006 Mar;117(3 Pt 2):S9–S22. doi: 10.1542/peds.2005-0620C. [DOI] [PubMed] [Google Scholar]
- 31.Fitzgerald M, Walker SM. Infant pain management: a developmental neurobiological approach. Nat Clin Pract Neurol. 2009 Jan;5(1):35–50. doi: 10.1038/ncpneuro0984. [DOI] [PubMed] [Google Scholar]
- 32.Purcell-Jones G, Dormon F, Sumner E. The use of opioids in neonates. A retrospective study of 933 cases. Anaesthesia. 1987 Dec;42(12):1316–1320. doi: 10.1111/j.1365-2044.1987.tb05283.x. [DOI] [PubMed] [Google Scholar]
- 33.Berde CB, Jaksic T, Lynn AM, Maxwell LG, Soriano SG, Tibboel D. Anesthesia and analgesia during and after surgery in neonates. Clin Ther. 2005 Jun;27(6):900–921. doi: 10.1016/j.clinthera.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 34.Kart T, Christrup LL, Rasmussen M. Recommended use of morphine in neonates, infants and children based on a literature review: Part 2--Clinical use. Paediatr Anaesth. 1997;7(2):93–101. doi: 10.1111/j.1460-9592.1997.tb00488.x. [DOI] [PubMed] [Google Scholar]
- 35.Bouwmeester NJ, Hop WC, van Dijk M, Anand KJ, van den Anker JN, Tibboel D. Postoperative pain in the neonate: age-related differences in morphine requirements and metabolism. Intensive Care Med. 2003 Nov;29(11):2009–2015. doi: 10.1007/s00134-003-1899-4. [DOI] [PubMed] [Google Scholar]
- 36.Doyle E, Robinson D, Morton NS. Comparison of patient-controlled analgesia with and without a background infusion after lower abdominal surgery in children. Br J Anaesth. 1993 Nov;71(5):670–673. doi: 10.1093/bja/71.5.670. [DOI] [PubMed] [Google Scholar]
- 37.Doyle E, Harper I, Morton NS. Patient-controlled analgesia with low dose background infusions after lower abdominal surgery in children. Br J Anaesth. 1993 Dec;71(6):818–822. doi: 10.1093/bja/71.6.818. [DOI] [PubMed] [Google Scholar]
- 38.Kelly JJ, Donath S, Jamsen K, Chalkiadis GA. Postoperative sleep disturbance in pediatric patients using patient-controlled devices (PCA) Paediatr Anaesth. 2006 Oct;16(10):1051–1056. doi: 10.1111/j.1460-9592.2006.01932.x. [DOI] [PubMed] [Google Scholar]
- 39.Yildiz K, Tercan E, Dogru K, Ozkan U, Boyaci A. Comparison of patient-controlled analgesia with and without a background infusion after appendicectomy in children. Paediatr Anaesth. 2003 Jun;13(5):427–431. doi: 10.1046/j.1460-9592.2003.01061.x. [DOI] [PubMed] [Google Scholar]
- 40.McNeely JK, Trentadue NC. Comparison of patient-controlled analgesia with and without nighttime morphine infusion following lower extremity surgery in children. J Pain Symptom Manage. 1997 May;13(5):268–273. doi: 10.1016/s0885-3924(96)00324-7. [DOI] [PubMed] [Google Scholar]
- 41.Berde CB, Lehn BM, Yee JD, Sethna NF, Russo D. Patient-controlled analgesia in children and adolescents: a randomized, prospective comparison with intramuscular administration of morphine for postoperative analgesia. J Pediatr. 1991 Mar;118(3):460–466. doi: 10.1016/s0022-3476(05)82169-9. [DOI] [PubMed] [Google Scholar]
- 42.Orge FH, Lee TJ, Walsh M, Gordon K. Comparison of fentanyl and morphine in laser surgery for retinopathy of prematurity. J AAPOS. 2013 Apr;17(2):135–139. doi: 10.1016/j.jaapos.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 43.Franck LS, Vilardi J, Durand D, Powers R. Opioid withdrawal in neonates after continuous infusions of morphine or fentanyl during extracorporeal membrane oxygenation. Am J Crit Care. 1998 Sep;7(5):364–369. [PubMed] [Google Scholar]


