Novel targeted therapies for the treatment of acute myeloid leukemia (AML) and other advanced myeloid malignancies are urgently needed. Recently we reported results from in vitro studies comparing the BCL-2, BCL-XL and BCL-w inhibitor ABT-737 (preclinical compound with similar inhibitory profile to ABT-263/navitoclax) with the selective BCL-2 inhibitor ABT-199, as single-agent and in combination with 5-azacytidine (5-Aza) in AML-derived cell lines [1]. This study demonstrated greater in vitro potency of ABT-737 in most AML-derived cell lines, as compared to ABT-199, including greater sensitization to 5-Aza. Additionally, we demonstrated that the combination of ABT-737 and 5-Aza exhibits strong synergy in short-term ex vivo cultures of myeloid malignancies, including de novo and secondary AML, myelodysplastic syndrome (MDS) and myeloproliferative neoplasms (MPNs). Tsao et al. reported synergistic activity between 5-Aza and ABT-737 in AML as well [2]. Recently, Pan et al. published a comparison between single-agent ABT-737 or ABT-263 and ABT-199 in vitro and in animal models, demonstrating potent ABT-199 activity in AML, although ABT-263 was more potent in some primary samples [3]. However, none of these studies directly compared the potential of ABT-737 versus ABT-199 to sensitize the anti-leukemic activity of 5-Aza. Therefore, we directly compared the ability of ABT-737 versus ABT-199 to synergize with 5-Aza in myeloid malignancies using short-term ex vivo cultures of primary AML and MDS/chronic myelomonocytic leukemia (CMML) samples.
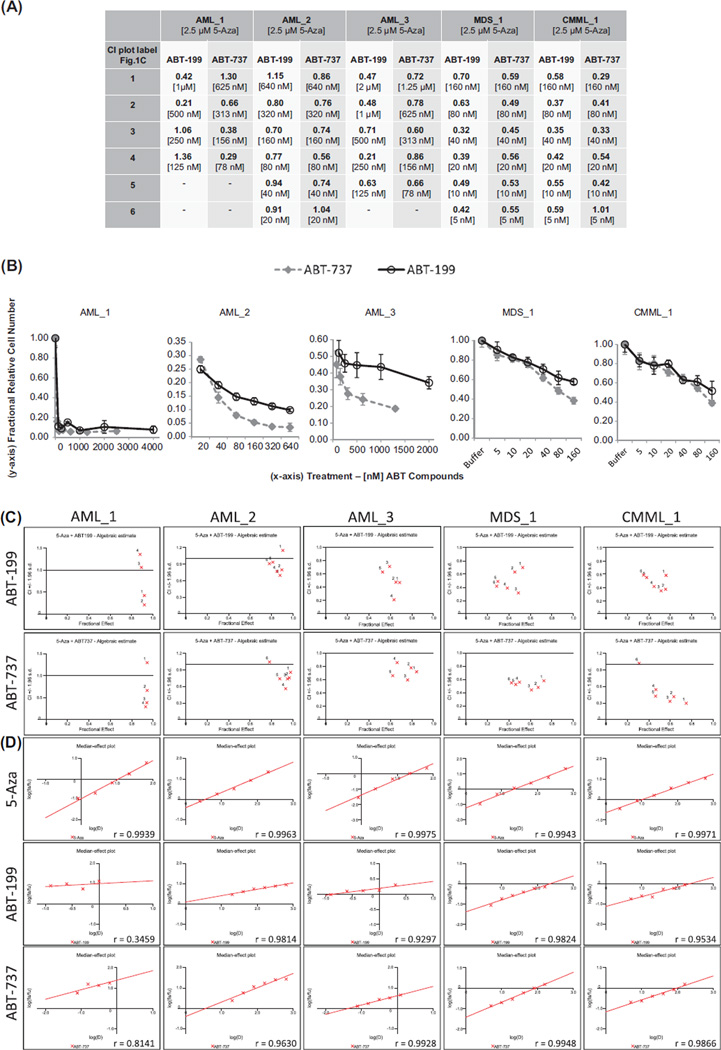
In primary AML and MDS/CMML samples tested in ex vivo drug dose combination response assays (n = 5), all samples showed synergy, and the synergistic effect with 5-Aza was similar for ABT-199 and ABT-737 [Figures 1(A), 1(C) and 1(D)], making this the first published evidence, to our knowledge, that ABT-199 also synergizes with 5-Aza in a limited number of AML and MDS/CMML samples ex vivo. The level of ex vivo synergy for ABT-737 and 5-Aza was comparable to that previously published [1]. Synergy occurred at low nM doses of ABT-737 and ABT-199, mostly in the range of 40–160 nM for both compounds, doses which most frequently corresponded to maximal synergy. The respective 5-Aza concentrations shown in Figure 1 are 10–15% maximal effective concentration (EC10–15) doses for AML samples and EC10–20 doses for MDS/CMML samples, corresponding to 2.5 µM 5-Aza. Antagonism with 5-Aza was observed at high nanomolar doses (> 640 nM) of both ABT compounds, or with exceedingly high (> 200 µM), clinically irrelevant doses of 5-Aza (data not shown).
Figure 1.
5-Azacytidine synergy in combination with ABT-199 or ABT-737. 5-Azacytidine was applied to ex vivo cultures as a single dose upon experiment initiation and the readout for synergy analyses with CalcuSyn was relative cell number as measured with Cell Titer-Glo (Promega) at 96 h. (A) Combination index (CI) values for 2.5 µM 5-azacytidine combined with the indicated dose range of ABT-199 or ABT-737. Row headings in (A) correspond to the 4–6 dose labels for ABT-199 or ABT-737 plotted in CI versus fractional effect plots shown in (C), and as listed in brackets under each CI value in (A). Label “1” corresponds to the highest dose of ABT compound, while labels “4” to “6” correspond to the lowest dose. Nine doses of 5-azacytidine and six doses of each ABT compound were tested in all experiments; however, higher ABT compound doses were necessarily dropped for CalcuSyn analysis if they did not fit into the linear range of response. (B) Single-agent activity of ABT-199 and ABT-737. Nanomolar ABT concentrations are plotted on the x-axis, while relative cell number expressed as a fraction of the buffer treated control cells is plotted on the y-axis. For AML_2, MDS_1 and CMML_1 equipotent doses of each ABT compound were used, whereas AML_1 and AML_3 received different dose ranges for each ABT compound. (C) CI versus fractional effect plots for ABT-199 and ABT-737. CI values < 0.9 are synergistic, 0.9–1.1 are nearly additive and > 1.1 are antagonistic. A fractional effect of 0 is the minimal effect, while 1 is the maximal effect. (D) Median-effect plots for 5-azacytidine, ABT-199 and ABT-737 treatments. r-Values for each drug dose–response fit are listed in the lower right corner of each plot.
We also compared the single-agent activity of ABT-199 versus ABT-737 ex vivo. As highlighted above, we previously showed that, as single agent, ABT-737 was more potent than ABT-199 in vitro. However, consistent with recently published data [3], we find that ex vivo ABT-199 and ABT-737 were more similarly potent in AML samples, with EC50 values < 20 nM for both agents [Figure 1(B) and Tables I and II]. Thus, the AML-derived cell line panel we previously evaluated had a greater dependency on BCL-XL and/or BCL-w in vitro than the primary samples we analyzed ex vivo. This is also consistent with our recent observations from BH3 profiling assays. BH3 profiling is a functional assay that uses BH3 peptides to indirectly assess induction of mitochondrial outer membrane permeabilization as a surrogate measurement of apoptosis. The BH3 peptides used are referred to as BH3 metrics when associated with BH3 profiling outputs. Previously, we showed that HRK (specific BH3 binding partner for BCL-XL) was important as a BH3 metric in vitro [4,5]; however, NOXA (MCL-1 specific binding partner) as a BH3 metric more strongly correlated with clinical 5-Aza response [1]. While single-agent EC50 values of ABT-737 and ABT-199 were more similar ex vivo than in vitro ABT-737 was still significantly more potent in some ex vivo samples [Figure 1(B) and Table 1]. Our observations are consistent with a recent report [3] showing that although ABT-199 and ABT-737/263 are often equipotent in ex vivo AML samples, some samples are more sensitive to ABT-737/263, while fewer samples are more sensitive to ABT-199. Th us, BCL-XL and BCL-w may play a more important role in modulating apoptosis in a number of cases of AML. Conversely, BCL-2 and hence ABT-199 may have a greater role in other cases of AML. Collectively, these data also support our previous suggestion, that dual BCL-2 family targeting drugs (i.e. BCL-XL and BCL-2, or BCL-XL and MCL-1) could hold more potential for the treatment of some myeloid neoplasms. However, whether more selective or broader BCL-2 family targeting compounds will have greater activity in myeloid malignancies remains an open question, since oncogenic dependency and compound selectivity influence therapeutic index. For example, the on-target side-effect of thrombocytopenia arising from BCL-XL inhibition by navitoclax has prevented further development in leukemias for now, while ABT-199 is currently being investigated as single-agent in an ongoing trial in patients with AML (ClinicalTrials.gov; NCT01994837). Which agent may be more advantageous, and for which patients, can ultimately only be determined clinically.
Table I.
p-Values associated with single-agent ABT-737 and ABT-199 comparisons in AML samples with diff erential activity*.
| AML_2 (nM) ABT-737 and ABT-199 | 20 | 40 | 80 | 160 | 320 | 640 |
| p-Value | 0.0071 | 0.012 | 1.4e–4 | 1.0e–4 | 2.4e–5 | 7.4e–4 |
| AML_3 (nM) ABT-199/ABT-737 | NT | NT | 125/78 | 250/156 | 500/313 | 1000/625 |
| p-Value | NT | NT | 0.22 | 0.059 | 1.89e–5 | 0.0029 |
AML, acute myeloid leukemia; NT, not tested.
For AML_2, equipotent doses of ABT-199 and ABT-737 were tested and are thus compared. For AML_3, diff erent dose ranges of ABT-199 and ABT-737 were tested, and therefore a higher dose of ABT-199 is compared to a lower dose of ABT-737 to further illustrate greater sensitivity to ABT-737.
Table II.
Characteristics of primary samples testedex vivo.
| Sample characteristics: diagnosis, cytogenetics | |
| AML_1 | Relapsed AML, t(11;17)(q23;q25) (68% by FISH), trisomy 21 (10% by FISH) |
| AML_2 | De novo AML, trisomy 8 (25% by FISH), FLT3 neg., NPM1 neg. |
| AML_3 | Relapsed AML, antecedent MDS, diploid karyotype |
| MDS_1 | MDS (RA with unilineage dysplasia), diploid karyotype, normal MDS FISH panel |
| CMML_1 | Low-risk CMML untreated, diploid karyotype, normal MDS, CMML FISH panel |
AML, acute myeloid leukemia; FISH, fluorescence in situ hybridization; neg., negative; MDS, myelodysplastic syndrome; RA, refractory anemia; CMML, chronic myelomonocytic leukemia.
The pre-clinical data presented by our and Dr. Konopleva’s groups is promising with regard to the activity of ABT-737 in combination with 5-Aza. Herein we present the first evidence that ABT-199 also potently synergizes with 5-Aza in primary AML and MDS/CMML samples ex vivo. Based on our preclinical data, it can be suggested that such a combination may also be an effective strategy for patients refractory/resistant to monotherapy with 5-Aza or BCL-2 family inhibitors. Navitoclax may still have a role in some cases of AML where additional anti-apoptotic BCL-2 family members such as BCL-XL or BCL-w are operating, such as in erythroid-differentiated disease with high BCL-XL expression [6,7]. Additionally, MCL-1 is thought to be a critical anti-apoptotic gene in AML [8,9]. Evidence suggesting that 5-Aza can reduce MCL-1 protein levels in primary samples [2] provides further impetus to support 5-Aza in combination with ABT-199 or navitoclax, because neither of these compounds inhibits MCL-1.
Several mechanistic and clinical questions remain unanswered regarding monotherapy with BCL-2 family inhibitors, as well as their potential to be combined with 5-Aza. A large body of evidence underlies the theory that a therapeutic index for cytotoxic chemotherapy is, at least in part, a function of apoptotic differences between normal and malignant cells/tissues, termed “apoptotic primedness,” not merely differences in proliferation rate as conventionally thought [10,11]. Thus, it is conceivable that BCL-2 family targeting agents may be effective as single-agent therapy, especially in diseases dependent on BCL-2, such as chronic lymphocytic leukemia, where clinical responses have already been observed [12]. In AML, the contribution of anti-apoptotic proteins BCL-2, BCL-XL and MCL-1 in maintaining leukemogenesis alone or combined is not completely resolved. Furthermore, it is not known whether 5-Aza induces additional pro-apoptotic signals that increase the “apoptotic primedness,” resulting in increased therapeutic benefit in combination with BCL-2 family targeting compounds. It is possible that dual or multiple targeting of BCL-2 family proteins may be therapeutically impractical due to side-effects; however, it is likely that a therapeutic index may even be greater for broader BCL-2 family inhibition given that neoplastic myeloid cells persist at a higher apoptotic threshold, likely mediated by redundant expression of BCL-2 and other anti-apoptotic family members. Evidence that BCL-2 and MCL-1 may be selectively up-regulated in leukemic stem cells compared to normal hematopoietic stem cells also supports this concept [8,9,13].
In summary, we show that ABT-199 compared to ABT-737 can result in similarly potent 5-Aza sensitization in AML and MDS ex vivo. Therefore, clinical trials combining 5-Aza with navitoclax, as we previously proposed, or with ABT-199 for the treatment of advanced myeloid malignancies are warranted.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Bogenberger JM, Kornblau SM, Pierceall WE, et al. BCL-2 family proteins as 5-Azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia. 2014 Jan 23; doi: 10.1038/leu.2014.44. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao T, Shi Y, Kornblau S, et al. Concomitant inhibition of DNA methyltransferase and BCL-2 protein function synergistically induce mitochondrial apoptosis in acute myelogenous leukemia cells. Ann Hematol. 2012;91:1861–1870. doi: 10.1007/s00277-012-1537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan R, Hogdal LJ, Benito JM, et al. Selective BCL-2 inhibition by ABT-199 causes on target cell death in acute myeloid leukemia. Cancer Discov. 2014;4:362–375. doi: 10.1158/2159-8290.CD-13-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogenberger JM, Pierceall WE, Lena R, et al. BH3 profiling predicts 5-azacytidine response in malignant myeloid cells. Blood. 2012;120(Suppl. 1) Abstract 1432. [Google Scholar]
- 5.Tibes R, Kornblau SM, Pierceall WE, et al. BH3 profiling as predictor of 5-azacytidine and decitabine clinical responses. Blood. 2013;122:603. [Google Scholar]
- 6.Silva M, Richard C, Benito A, et al. Expression of Bcl-x in erythroid precursors from patients with polycythemia vera. N Engl J Med. 1998;338:564–571. doi: 10.1056/NEJM199802263380902. [DOI] [PubMed] [Google Scholar]
- 7.Zeuner A, Pedini F, Francescangeli F, et al. Activity of the BH3 mimetic ABT-737 on polycythemia vera erythroid precursor cells. Blood. 2009;113:1522–1525. doi: 10.1182/blood-2008-03-143321. [DOI] [PubMed] [Google Scholar]
- 8.Glaser SP, Lee EF, Trounson E, et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev. 2012;26:120–125. doi: 10.1101/gad.182980.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Opferman JT, Iwasaki H, Ong CC, et al. Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science. 2005;307:1101–1104. doi: 10.1126/science.1106114. [DOI] [PubMed] [Google Scholar]
- 10.Certo M, Del Gaizo Moore V, Nishino M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 11.Sarosiek KA, Ni Chonghaile T, Letai A. Mitochondria: gatekeepers of response to chemotherapy. Trends Cell Biol. 2013;23:612–619. doi: 10.1016/j.tcb.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seymour JF, Davids MS, Pagel JM, et al. Updated results of a phase I first-in-human study of the BCL-2 inhibitor ABT-199 (GDC-0199) in patients with relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) J Clin Oncol. 2013;31(Suppl.) Abstract 7018. [Google Scholar]
- 13.Lagadinou ED, Sach A, Callahan K, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12:329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]