Abstract
The Allostatic Load Index (ALI) has been used to establish associations between stress and health-related outcomes. This review summarizes the measurement and methodological challenges of allostatic load in occupational settings. Databases of Medline, PubPsych, and Cochrane were searched to systematically explore studies measuring ALI in working adults following the PRISMA statement. Study characteristics, biomarkers and methods were tabulated. Methodological quality was evaluated using a standardized checklist. Sixteen articles (2003–2013) met the inclusion criteria, with a total of 39 (range 6–17) different variables used to calculate ALI. Substantial heterogeneity was observed in the number and type of biomarkers used, the analytic techniques applied and study quality. Particularly, primary mediators were not regularly included in ALI calculation. Consensus on methods to measure ALI in working populations is limited. Research should include longitudinal studies using multi-systemic variables to measure employees at risk for biological wear and tear.
Keywords: Allostasis, Biomarker, Chronic stress, Employee health, Work-related stress
Introduction
Chronic stress is common in the workplace and has been associated with absenteeism1, 2), job terminations3, 4), work-related accidents5), and reduced productivity6). Work-related stress has been declared one of the biggest challenges of the 21st century by the World Health Organization7). Moreover, seventy-nine percent of 28,649 European managers participating in the European Survey of Enterprises on New and Emerging Risks (ESENER) in 2009 identified work-related stress as the second biggest risk in occupational safety8). Stress has also been recognized as an important risk factor for health-related conditions that affect workers’ quality of life including cardiovascular disease9,10,11), diabetes mellitus12, 13), asthma14), sleep disorders15), depression16), exhaustion17,18,19), and back pain20). Reducing stress in the workplace can improve employees’ work ability and well-being21) as well as productivity22) with consequent economic and social benefits for companies, workers and society as a whole23).
Stress is thought to exert negative effects on health through constant stimulation of a number of systems in the body including the hypothalamic-pituitary-adrenal (HPA) axis and autonomic nervous system (ANS). Adaptation in some form is usually required to achieve or re-establish stability (allostasis). The cost of chronic exposure to fluctuating or heightened neural or neuroendocrine response resulting from repeated or chronic stressful events is defined as allostatic load24). Approaches to measuring sources of stress and their health consequences vary substantially25). One commonly used approach for the latter, the Allostatic Load Index developed in 1997 by Seeman and colleagues, provides a possible assessment of the cumulative influence of psychosocial factors on health and wellbeing using indicators for the functioning of potentially affected systems26). Previous work demonstrates, for example, that an elevated Allostatic Load Index is associated with all-cause mortality27,28,29), cardiovascular disease26, 30), mental diseases31), migraine32), periodontitis30, 33), and other health-related conditions in the general population. Insights from this work are necessarily limited, however, as a variety of biomarkers beyond those originally described have been used.
Concepts of allostatic load and overload
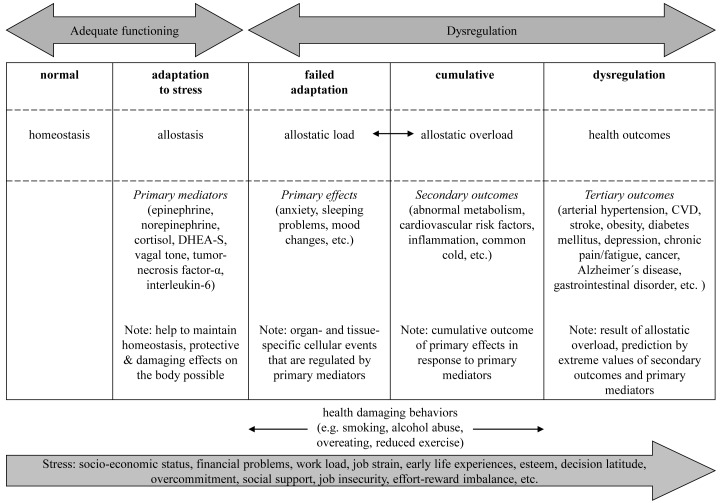
In 1860, Claude Bernard described the concept of homeostasis (Greek: homoiostasis = steady state) as the maintenance of the internal milieu34), coined by Walter Cannon for further application35). More recently, Sterling and Eyer36) introduced the term allostasis (Greek: állos = different, and stásis = congestion) in reference to processes that enabled adaptation37) to psychosocial stressors, including those needed to re-establish physiological balance38). For example, previous work documents that the release of stress-related hormones, their antagonists and anti-inflammatory cytokines (referred to as “primary mediators”) help maintain homeostasis in the face of exposure to stressful stimuli37, 39). However the interaction between these primary mediators of the stress-response cycle can also synergistically affect cellular activities to result in effects of their own (sometimes referred to as “primary effects”) such as reduced sleep quality or anxiety40, 41). Recent work indicates that the primary effects and pathophysiological processes on mitochondrial level could potentially also be measured42). The term “allostatic load” (Fig. 1), first described by McEwen and Stellar24) refers to the aggregate physiological consequences of chronic adaptation, including the wear and tear that occurs at the cellular and supracellular levels within the human body40, 43). Constant secretion of primary mediators promotes allostatic overload and secondary outcomes such as sub-clinical disturbances in markers of cardiovascular, metabolic, and immune functioning. Finally, with chronic stress dysregulation, clinical manifestations of disturbances (negative health consequences) referred to as “tertiary outcomes” may emerge such as the development of cardiovascular diseases or depression37). These tertiary outcomes are the result of allostatic load, which can be predicted from extreme values of the secondary outcomes and of the primary mediators37).
Fig. 1.
Stress-regulating process from homeostasis to allostatic overload. DHEA-S: Dihydroepiandrosterone sulfate, CVD: cardiovascular disease.
Measuring allostasis and allostatic load
Health-related effects of stress can be measured and quantified using tools such as the Allostatic Load Index. This index was originally based on data from 10 physiological or physical measurements including six secondary outcomes: systolic and diastolic blood pressure, total cholesterol (TC), high-density-lipoprotein (HDL), glycosylated hemoglobin (HbA1c), waist-to-hip ratio (WHR), and four primary mediators: dehydroepiandrosterone sulfate (DHEA-S), as well as urinary epinephrine, norepinephrine, and cortisol26). According to the original description, these data are transformed into a summary score by assessing the distribution of each value within the screened sample and assigning those within the highest risk quartile (lowest quartile of DHEA-S and HDL-values) the value “1”. These binary indicators, all of which are equally weighted, are then added. The original index, ranging from 0 to 10 with higher values indicating higher physiological strain and lower values indicating better adaptation to stress, and was validated in the McArthur Study of Successful Aging28).
To enable the development of future interventions that might mitigate the health effects of work-related stress, methods used in assessing key associations must be thoughtfully considered and carefully evaluated. For example, numerous methods exist for quantifying physiological responses to stress, each with varying levels of sensitivity and specificity. The extent to which methodological variability exists in quantifying these responses is unclear, yet this may impact the quality of evidence upon which interventions may be based. Additionally, the extent to which measures of allostatic load represent this complex concept fully may influence accuracy. There is common agreement among researchers that allostatic load measurement should include neuroendocrine and immunological biomarkers44,45,46), because multiple mediators are involved in the process of stress adaptation and interact in a non-linear way to affect many organ systems within the body47). Nevertheless, there is a lack of agreement on the combination of different biomarkers that best reflect a gold standard, leading people to approach the “diagnosis” of allostatic overload in a variety of ways.
Therefore, the objective of this study was to examine the ways in which the Allostatic Load Index has been measured, the frequency with which the concept of allostatic load was fully represented by operational measures, and the range of biomarkers used in working populations. Studies with poor methodological quality are more likely to be affected by bias than those of very good quality48). Additionally, studies that incompletely represent elements of the allostatic load model may be subject to inaccurate conclusions from their results unless some effort is made to validate the new approach. To ensure suitability for daily use of the Allostatic Load Index by occupational health practitioners a gold standard with standardized definitions is required. We hypothesized that there is limited evidence and consensus for A) specific biomarkers, B) defined thresholds of used variables, and C) techniques calculating an Allostatic Load Index.
Methods
Search strategy
We searched multiple databases, including Medline (1966–2013) accessed using both PubMed (US National Library of Medicine) and DIMDI (Deutsches Institut für Medizinische Dokumentation und Information), the databases of PSYNDEX (1981–2013), PASCAL (1973–2013), ISOC-Psicologica (1975–2013), ERIC (Education Resources Information Center) (1966–2013), NARCIS (National Academic Research and Collaborations Information System) (1907–2013) accessed via PubPsych (Leipniz-Zentrum für Psychologische Information und Dokumentation), and the Cochrane Central Register of Controlled Trials (1993–2013). Articles eligible for review were those published up to December 31, 2013. Search terms included “allosta*” and “allosta?” or the English key word “allostatic load”, respectively.
Study selection
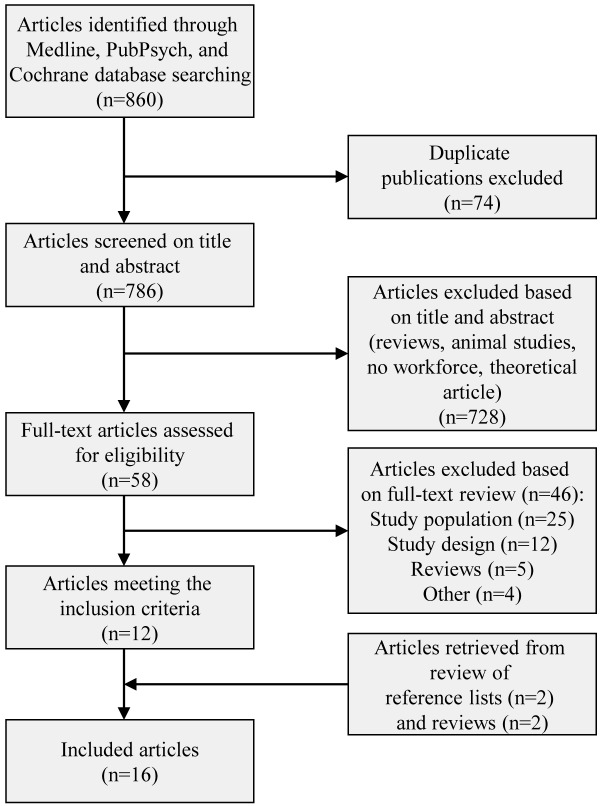
Methods of the analysis and inclusion criteria were specified in advance (Appendix), following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement49). Titles and abstracts of each article were read by two reviewers (DM, MNJ) to identify whether the manuscript met all of the following eligibility criteria: (1) calculation of an Allostatic Load Index from measures of physiological functioning or physical characteristics; (2) human study; (3) employed participants; (4) full text article; (5) article written in English, German, French, Spanish, or Chinese. Duplicate publications, editorials, book chapters, lectures, commentaries, and animal studies were excluded as was research on children and retirees (Fig. 2), given our focus on studies conducted in working adults. In the event an abstract was not available, the full text was screened to determine whether the article should be retained in the sample. When disagreements about the eligibility of a study arose, both reviewers screened the full text of the article. Reference lists for all articles (both those included and excluded from the sample) were screened to identify additional articles for review. Initial agreement between the two reviewers for inclusion was 100% (Cohen’s κ of 1.0).
Fig. 2.
Study selection.
The search strategy identified 860 articles: 757 from Medline (757 by PubMed and 719 by DIMDI), 95 from PubPsych (51 by PASCAL, 31 by PSYNDEX, 5 by ERIC, 6 by NARCIS, 2 by ISOC), and 8 from the Cochrane Register. After excluding 74 duplicates, 786 abstracts were screened. Fifty-eight full-text articles were reviewed following the initial application of exclusion criteria; an additional 46 were excluded due to samples restricted to non-working individuals (older adults or children) or due to other reasons reported in Fig. 2. Reference list screening identified four additional eligible publications, resulting in a total sample of 16 articles published between 2003 and 2013. Three studies50,51,52) measuring allostatic load in different subgroups within the same sample were retained as were two studies53, 54) that explored different hypotheses within the same sample.
Data abstraction
After reviewing the full text of each article, the following elements were abstracted: year of publication, authors, business or industrial setting, number and type of biomarkers that were incorporated into calculating the Allostatic Load Index, reported Allostatic Load Index (mean value), study design, number of participants, age of the study sample (mean, standard deviation, and range), gender (% male), and study findings. To remain consistent with the original description of Seeman26) and to follow previously used classifications55,56,57), biomarkers were categorized either as primary mediators or as secondary outcomes according to Fig. 1. Further primary mediators were classified into three categories: neuroendocrine factors (hypothalamic-pituitary-adrenal [HPA] axis and sympathetic-adrenal-medullary [SAM] axis), neurophysiological factors (vagal activity), and anti-inflammatory markers, while secondary outcomes were classified into four categories: metabolic factors, inflammatory markers, cardiovascular factors, and organ functions. Additionally, any information related to the method used to calculate the Allostatic Load Index was abstracted along with confounders assessed in each study.
Quality assessment/Assessment of potential bias
Although there is no recognized gold standard for assessing methodological quality of observational studies, we used a checklist approach modified from previous work58, 59) that identified study features associated with potential sources of bias and the presence of key conceptual components (Table 1). Two reviewers (DM, MNJ) independently assessed these criteria for each study, with any disagreement resolved by consensus. Initial agreement between the two reviewers in rating study quality was 92% (147 of the 160 items, Cohen’s κ of 0.77). Two authors were contacted by a member of the research team to clarify details on methods used in conducting their study. Additional information obtained in this way did not result in different ratings. When present, the criterion was scored as “1”; the value “0” was used if data were missing or if specific information was not provided. We used a multiple hurdle approach. If a study failed item 4 (use of at least one primary mediator and three secondary outcomes for ALI calculation) of the quality assessment, the study quality was rated as poor. This parameter was considered the most important quality component irrespective of other valuations. Additionally a quality score (0–10) was calculated by summing the ratings and dividing these by the total number of criteria. Each study was then categorized as being of poor, fair, good, or very good methodological quality if it met <60%, 60–79%, 80–99%, or 100% of items, respectively. The quality assessment led to a judgment about the risk of bias (poor quality = high risk; fair quality = unclear risk; good and very good quality = low risk) and the internal validity of this review in accordance with the Cochrane Handbook60).
Table 1. Checklist for assessing methodological quality.
| Study objective | |
| 1 | A specific, clearly stated hypothesis is described. |
| Study population | |
| 2 | The main features of the population (e.g. age, gender, industrial setting, place of recruitment) are described. |
| 3 | Sampling is random and not selective (data presented) (e.g. exclusion of participants with diabetes mellitus, cardiovascular disease, or arterial hypertension). |
| Assessment of Allostatic Load Index | |
| 4 | At least one primary mediator and three secondary outcomes are included in calculating the Allostatic Load Index (Fig. 1). |
| 5 | The Allostatic Load Index is calculated using the standardized method of risk quartiles 26). |
| 6 | Allostatic Load Index components are directly measured using standard techniques. |
| Analysis and data presentation | |
| 7 | Analyses are adjusted for potential sources of confounding. |
| 8 | Measures of associations are presented (OR including 95% confidence intervals for logistic regression, β for linear regression). |
| 9 | Cut-off values are presented for each variable used in calculating the Allostatic Load Index. |
| 10 | The number of cases in the multivariate analysis is at least 10 times the number of independent variables in the analysis. |
Results
Study and sample characteristics
Table 2 summarizes study characteristics including the year, first author, country in which the study was conducted, work setting, biomarkers used in calculating an Allostatic Load Index, study design, and sample characteristics including mean age and gender composition. Nearly all studies used a cross-sectional study design. A single longitudinal study was identified61), but the Allostatic Load Index was determined at a single point in time and associations with health-related outcomes were measured cross-sectionally.
Table 2. Characteristics and study findings from studies on allostatic load (AL) in the workforce.
| First author (year) | Country, Industry | Original biomarkers comprising Allostatic Load Index |
Original biomarkers used (%) |
Other biomarkers assessed |
AL, mean | Study design | Participants, n | Gender male, % | Age in years | Associations (AL↑) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SBP | DBP | WHR | HDL | TC | HbA1c | DHEA-S | Cortisol | Epi | Norepi | mean | SD** | range** | |||||||||
| Bellingrath (2009) | Germany, Teachers |
✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 100 | body fat, CRP, D-Dimer, Fibrinogen, Glucose, TG, TNFα |
5.0 | cross-sectional | 104 | 0 | 45.0 | ±9.75 | 25-61 | effort-reward imbalance↑, exhaustion↑ |
| De Castro (2010) | USA, Latino day workers |
✔ | ✔ | ✔ | 30 | BMI, CRP, salivary cortisol | 1.57 | cross-sectional | 30 | 100 | 45.8 | ±13.2 | - | SES↓, work safety↓, smoking↑, alcohol consumption↑, physical health↓ |
|||||||
| Fischer (2009) | Germany, Aircraft industry workers |
✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 60 | CRP, D-Dimer, LDL | not described | cross-sectional | 468 | 89 | 41.2 | - | 18-61 | progenitor cells↓ (smoker >46 years) |
||||
| Hasson (2009) | Sweden, Health Care (A) and IT (B) |
✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 70 | LDL, LDL/HDL, Prolactin, Pulse, TG |
3.20 A: 3.09 B: 3.46 | cross-sectional | 339 A: 241 B: 98 | 0 | A: 46.5 B: 41.2 | A: ±9.9 B: ±10.7 | - | A+B: age↑, educational attainment↓, self-rated health↓ |
|||
| Johansson (2007) | Sweden, various | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 60 | Peak expiratory airflow | 1.97 | longi-tudinal | 369 | 0 | 43 | - | - | no characteristics under study |
||||
| Juster (2011) | Canada, various | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 80 | α-Amylase, Albumin, Creatinine, CRP, Fibrinogen, Insulin, TG |
2.69 | cross-sectional | 30 | 36.7 | 45.4 | ±2.12 | 27-65 | cortisol↓, chronic stress↑, burnout↑, not depression |
||
| Juster (2012) | Canada, various | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 80 | α-Amylase, Albumin, Creatinine, CRP, Fibrinogen, Insulin, TG |
2.69 | cross-sectional | 30 | 36.7 | 45.4 | ±2.12 | 27-65 | physical complaints↑, masculinity↑ |
||
| Juster (2013) | Canada, various male (A), female (B) |
✔ | ✔ | ✔ | ✔ | ✔ | 50 | BMI, CRP, glucose, heart rate variability, HOMA, insulin, interleukin-6, LDL, TG, TNFα, |
not described | cross-sectional | 199 | 40.7 | A: 39.4 B: 42.8 | A: ±11.31 B: ±11.38 | 20-64 | occupational status↑(A) ↓(B), age↑, decision latitude↓(B) |
|||||
| Langelaan (2007) | Netherlands, Telecom managers |
✔ | ✔ | ✔ | ✔ | ✔ | 50 | BMI, CRP, Glucose | 1.72-2.03* | cross-sectional | 290 | 100 | 43.0 | ±8.0 | - | age↑, not burnout, not exhaustion |
|||||
| Li (2007) | China, Industrial workers |
✔ | ✔ | ✔ | ✔ | 40 | Adiponectin, BMI, HOMA ß-cell function, HOMA-IR, LDL, TG, Visfatin, |
2.50-3.15* | cross-sectional | 504 | 50 | 37.94 | ±9.47 | - | age↑, men, job control↓ | ||||||
| Li (2007) 1 | China, Industrial workers, 4 different branches |
✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 70 | BMI, Cholesterol/HDL, CRP, HOMA, Prolactin, TG |
3.85-4.78* | cross-sectional | 963 | 49.4 | 36.91 | ±10.4 | - | work-related stress↑, job demands↑, job control↓ |
|||
| Lipowicz (2013) | Poland, various | ✔ | ✔ | ✔ | 30 | alkaline phosphatase, bilirubin, body fat, creatinine clearance, erythrocyte sedimentation rate, glucose, Peak expiratory airflow, total plasma protein |
2.54 | cross-sectional | 3887 | 100 | - | - | 25-60 | education↓, urbanite↑, married, SES↓, physical activity↓, ex-smoker |
|||||||
| Näswall (2011) | Sweden, various | ✔ | ✔ | ✔ | ✔ | ✔ | 50 | TC/HDL, Peak expiratory airflow, |
1.89 | cross-sectional | 159 | 0 | - | - | - | self-rated health↓, not job insecurity |
|||||
| Schnorpfeil (2003) | Germany, Aircraft industry workers |
✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 100 | Albumin, BMI, CRP, TNFα | 3.15 | cross-sectional | 324 | 83.9 | 40.6 | ±9.3 | 21.3-60.5 | men, age↑, job demands↑ |
| Sun (2007) | China, 5 different industrial branches |
✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 70 | BMI, TC/HDL, CRP, Fibrinogen, IGR, TG |
3.69-4.54* | cross-sectional | 1219 | 52 | 38.08 | ±9.17 | 23-58 | age↑, decision latitude↓, job demands↑, Type A personality↑, educational attainment↓ |
|||
| Von Thiele (2006) | Sweden, Health care workers |
✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 70 | Glucose, LDL, LDL/HDL, Prolactin, Pulse, TG |
3.43 | cross-sectional | 241 | 0 | 45.8 | ±9.75 | - | exhaustion↑, age↑, insufficient recovery from work-stress↑ |
|||
| Use of original biomarkers (%) | 94 | 94 | 88 | 88 | 63 | 75 | 44 | 44 | 25 | 19 | |||||||||||
SBP: systolic blood pressure; DBP: diastolic blood pressure; WHR: waist-to-hip ratio; HbA1c: glycosylated hemoglobin; HDL: serum high-density-lipoprotein; TC: total cholesterol; DHEA-S: dehydroepiandrosterone sulfate; Cortisol: urine levels of cortisol; Epi: epinephrine; Norepi: norepinephrine; BMI: body mass index, CRP: C-reactive protein, HOMA: homeostasis model assessment, IGR: insulin-glucose ratio, LDL: low-density-lipoprotein, TG: triglycerides, TNFα: tumor-necrosis factor alpha, SES: socioeconomic status
*indicates range of means when multiple subgroups were under study
**presented if information provided in the study
Data were presented on a total of 9,156 employees (69.5% male) aged 18–65 yr from seven countries and various industrial settings. The number of participants in each study ranged from 30 to 3,887 (mean=572, median=307). Five studies recruited female participants only61,62,63,64,65) and three studies were restricted to male participants66,67,68). Seven studies explored employees in specific work settings62,63,64, 66, 67, 69, 70).
Range of biomarkers measuring allostatic load
A total of 39 different biomarkers (Table 3) were used representing six different primary mediators and 33 different secondary outcome variables, with studies in our sample including between six and 17 measures (median=12.5; IQR=5.5) to calculate an index of allostatic load as reported in Table 2. A wide range of thresholds for each biomarker was reported depending on the screened study sample.
Table 3. Biomarkers used in calculating the Allostatic Load Index in working populations.
| Group | Type | Biomarker | Description | Threshold ranges reported |
|---|---|---|---|---|
| Primary mediators | Neuroendocrine | Cortisol (urine) | Adrenal glucocorticoid and indicator of HPA-axis activity | 24.83–25.6 µg/g creatinine |
| 60.0 µg/l | ||||
| 418.5 nmol/l | ||||
| Cortisol (saliva) | 10.7 ng/ml | |||
| 410.4–839.8 nmol/l | ||||
| DHEA-S (µg/dl) | Adrenal hormone and functional HPA-axis antagonist | 13.3–51.5 | ||
| Epinephrine (urine) | Adrenal and brain catecholamine as neurotransmitter and indicator of sympathetic nervous system activity | 4.75–9.0 nmol/l 5.55 µg/g creatinine |
||
| Norepinephrine (urine) | Brain catecholamine as neurotransmitter and indicator of sympathetic nervous system activity | 64.0 µg/g creatinine 173.0 nmol/l |
||
| Neurophysiological | Heart rate variability (SDNN, standard deviation of beat-to-beat intervals) (ms) | Physiological phenomenon of variation in the time interval between heartbeats measured by the variation in beat-to-beat intervals | 118 | |
| Anti-inflammatory | TNF-α (pg/ml) | Cytokine affecting inflammation, tissue repair, immune defence, and lipid metabolism; increased in obesity | 1.44–2.2 | |
| Secondary outcomes | Metabolic | Insulin (µU/ml) | Pancreatic hormone for regulating glucose levels | 46.85 |
| Glucose (mg/dl) | Blood glucose; primary source of energy | 97.3–122.0 | ||
| Total cholesterol (mg/dl) | Basic element of steroid hormones, former indicator of atherosclerotic risk | 177.9–249.0 | ||
| HDL High-density-lipoprotein (mg/dl) | Cardioprotective form of cholesterol, transport of cholesterol from peripheral tissues to liver, indicator of atherosclerotic risk | 37.0–76.0 | ||
| LDL Low-density-lipoprotein (mg/dl) | Cardio-damaging form of cholesterol, transport of cholesterol to peripheral tissues, indicator of atherosclerotic risk | 116.0–137.3 | ||
| Triglyceride (mg/dl) | Cardio-damaging form of fat, important source of energy | 101.5–141.75 | ||
| Total cholesterol-HDL ratio | Indicator of atherosclerotic risk | 3.71 | ||
| HbA1c (%) | Average glucose level over the previous 12 wk, indicating degree of blood glucose regulation | 4.6–5.8 | ||
| Waist-to-hip ratio | Indicator of location of adipose tissue deposits based on ratio of waist circumference to hip circumference | 0.83–0.97 | ||
| Body Mass Index (kg/m²) | Indicator of obesity based on weight and height | 25.2–28.5 | ||
| Body fat (%) | Percentage of a person’s body that is not composed of water, muscle, bone, and vital organs, equivalent to essential fat plus storage fat | 22.0–37.3 | ||
| IGR | Parameter for differential diagnosis of hypoglycemia | 1.76 | ||
| HOMA-IR | Measure of insulin resistance | 2.05 | ||
| HOMA-β | Measure of pancreatic ß-cell function | 3.94 | ||
| Adiponectin (ng/ml) | Hormone synthesized in fat cells for regulation of perceived hunger and increased effect of insulin; decreased with high insulin resistance | 5.79 | ||
| Inflammatory | CRP (mg/l) | acute phase inflammatory protein | 1.4–6.0 | |
| D-Dimer (mg/l) | fibrin cleavage product resulting from activated blood coagulation and fibrinolysis; elevated by stress | 0.38 | ||
| Erythrocyte sedimentation rate (mm/h) | rate at which red blood cells sediment in a period of one hour as a non-specific measure of inflammation | 5.0–13.0 | ||
| Fibrinogen (g/l) | protein and factor of blood coagulation, influences thrombosis; elevated by stress | 3.3–4.69 | ||
| Interleukin-6 (pg/ml) | pro-inflammatory cytokine and anti-inflammatory myokine stimulating immune response | 1.17–1.27 | ||
| Visfatin (ng/ml) | inflammatory adipokine | 14.97 | ||
| Cardio-vascular | Systolic blood pressure (mmHg) | indicator of intravascular pressure at end of left ventricular contraction | 115.2–160.0 | |
| Function | Diastolic blood pressure (mmHg) | indicator of intravascular pressure at end of left ventricular relaxation | 71.2–95.0 | |
| Pulse (bpm) | heart rate | 69.3–77.0 | ||
| Organ function | Albumin (urine) (g/l) | early indicator of subclinical renal damage | 4.3–42.5 | |
| α-Amylase (U/l) | enzyme synthesized in pancreatic gland and salivary glands for enzymatic cleavage of glucose | 32.25 | ||
| Alkaline phosphatase (mU/ml) | enzyme present in all tissues throughout the entire body, particularly concentrated in liver, bile duct, and kidney | 65.0–69.0 | ||
| Bilirubin (mg/dl) | yellow breakdown product of normal haemoglobin catabolism | 0.8–0.9 | ||
| Creatinine (mg/dl) | breakdown product of muscle creatinine phosphate, filtered and excreted by the liver | 0.16 | ||
| Creatinine clearance rate (ml/min) | volume of blood plasma that is cleared of creatinine per unit time, measure of renal filtration function | 75.0–97.5 | ||
| Peak expiratory flow (l/min) | maximum pulmonary airflow and expiratory speed | 260–370 | ||
| Prolactin (ng/ml) | pituitary hormone stimulating milk production in mammary glands, elevated by stress and sleep deprivation | 10.0–11.0 | ||
| Total plasma protein (g/100 ml) | total amount of protein in blood plasma made up of albumin and globulin | 7.8–7.9 | ||
Representation of the allostatic load concept
As mentioned, representation of the allostatic load concept is reflected by the presence of specific types of biomarker measures (primary mediators and secondary outcomes), enabling the calculation of a summary index and thereby indicating the degree of overload. Only two studies63, 69) assessed all 10 measures identified in the original description (median=7.0; IQR=2.3). The majority of studies (88%) included four measures of the original secondary outcomes with systolic and diastolic blood pressure (94%), HDL (88%), and WHR (88%) being the most prominent. Primary mediators including DHEA-S (44%), and urinary cortisol (44%), epinephrine (25%) and norepinephrine (19%) were less frequently assessed in our sample with six studies (38%) measuring no primary mediators at all51, 61, 65,66,67,68).
All studies created risk quartiles for the distribution of components and used these in calculating some form of an Allostatic Load Index26). In one case, z-score values were also determined for each individual and summed66). This standardized formulation allows the weight of each biomarker to differ depending on its deviation from the sample mean56). Different analytic techniques to identify a threshold for “high” or “low” Allostatic Load Index were used. Most authors treated the Allostatic Load Index as a continuous variable, although dichotomous approaches to indicate allostatic overload were also noted. The latter categorized the distribution of values using quartiles62), a median split54, 67, 68), or various threshold values53, 64).
Study findings
Findings of the included studies indicated associations of increased allostatic load with work-related loads including effort-reward-imbalance, low work safety, low decision latitude, and low job control, as well as with health consequences like exhaustion, burnout, and low self-rated health. Details are presented in Table 2. One study61) reported no findings related to allostatic load.
Quality assessment
Six studies (38%) failed the minimum hurdle (item 4) and therefore were scored as being a study of poor quality. The mean of the summary quality score (Table 4) was 76% (range: 40–100%), but only six studies (38%) were judged to be of good or very good quality according to our definition. Twelve out of 16 studies provided a clearly described hypothesis. Studies in our sample varied in terms of size, setting and participant composition with the exception of the two studies of Juster et al. that used the same 30 participants to explore different hypotheses53, 54). Although two studies did not account for potential confounders in the analysis61, 67), the majority incorporated a wide number and type of potentially confounding characteristics such as age, gender, smoking, alcohol, educational or marital status, physical exercise, children at home, type A personality, burnout, working time, occupational status, and self-rated health. The most frequently used confounder was age (data not shown).
Table 4. Quality assessment of studies in the sample (N=16).
| Methodological items | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author | 1 hypothesis | 2 population | 3 sampling | 4 mediators & outcomes | 5 ALI calculation | 6 measurement | 7 confounding | 8 associations | 9 cut-offs | 10 multivariate analysis | Method score | Quality | ||
| n | % | |||||||||||||
| Articles | Bellingrath | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 7/10 | 70 | fair |
| De Castro | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 6/10 | 60 | fair | |
| Fischer | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 9/10 | 90 | good | |
| Hasson | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9/10 | 90 | good | |
| Johansson | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 4/10 | (40) | poor | |
| Juster (2011) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 7/10 | 70 | fair | |
| Juster (2012) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 8/10 | 80 | good | |
| Juster (2013) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9/10 | 90 | good | |
| Langelaan | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 8/10 | (80) | poor | |
| Li (English) | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 8/10 | (80) | poor | |
| Li (Chinese) | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 7/10 | (70) | poor | |
| Lipowicz | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7/10 | (70) | poor | |
| Näswall | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7/10 | (70) | poor | |
| Schnorpfeil | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10/10 | 100 | very good | |
| Sun | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9/10 | 90 | good | |
| Von Thiele | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7/10 | 70 | fair | |
Studies failing item 4 were rated as being of poor quality (shown by %-score in brackets).
Discussion
This systematic review summarizes current knowledge about measuring allostatic load in the workforce. We identified substantial heterogeneity in terms of the extent to which the concept of allostasis was fully represented by operational measures and the range of biomarkers used to quantify allostatic load. Furthermore we found varying methodological quality.
Our data illustrate the numerous ways in which the representation of the allostatic load concept has diverged from the original description. Newer biomarkers in diverse combinations have been introduced. No single set of biomarkers has been developed to standardly calculate an index of allostatic load. This may be a reason for a large number of biomarkers identified in our sample. Although use of standardized definitions, specific thresholds indicating higher risk and methods for calculating other risk indices such as Metabolic Syndrome71), Framingham Risk Score72, 73), SCORE74), or Prospective Cardiovascular Muenster score (PROCAM)75) is common, there appears to be relatively little uniformity in defining and measuring allostatic load. We observed, that slightly less than two thirds of the studies in our sample included neuroendocrine and immunological biomarkers together, although there is greater explanatory power by doing so. For example Näswall et al. did not include any primary mediators in their Allostatic Load Index, although salivary cortisol was measured65). Failing this elementary conceptual rule might increase the potential for falsely negative findings. Although some authors have stated that the diversity of biomarkers used for allostatic load is not as problematic as expected56, 76), a minimum standard should be defined to benefit from a multisystemic based formulation. For example men tend to exhibit dysregulation in cardiovascular parameters, while women are more likely to exhibit high levels of neuroendocrine parameters76). Additionally, empirical evidence for mortality supports the advantages of measuring multisystem functioning as well. Goldman showed that inclusion of neuroendocrine biomarkers (epinephrine, IL-6) increased explanatory power for 3 yr mortality over a model restricted to clinical measures like blood pressure and metabolic biomarkers in 927 elderly Taiwanese45). Karlamangla described better prediction of mortality and declining physical functioning by the original allostatic load compared to the Metabolic Syndrome and primary mediators alone in 729 elderly retired Americans44).
The allostatic load concept includes measurement of primary mediators and secondary outcomes as described. These parameters reflect subclinical symptoms while tertiary outcomes are the clinical diagnoses although this is not always selective (e.g. hypertension). Nevertheless there is no agreement on how to best measure primary mediators like neuroendocrine biomarkers, whether in urine, or saliva, or blood specimen. While blood samples are easy to take, urine or saliva collections for the time of 12 or 24 h as well as repeated measurements is difficult and highly complex77) and impractical. For daily usage however there is need for practical techniques to collect the right parameters cost-effectively. Additional inclusion of the vagal activity measured by heart rate variability might be such an easy assessable primary mediator for example78,79,80,81,82) as it was used by Juster83), although it has been defined as a cardiovascular secondary outcome and not as primary mediator by the author. Apart from the problem to assess primary mediators over time, their levels may also be related to such factors as allergies, sleep deprivation, or infections misleading the existing allostatic state84, 85).
An important aspect of quantifying allostatic load is the method used in summarizing its potential effects. Scoring algorithms in the reviewed articles were congruent by scoring high risk values as “1”, but several reports used thresholds that varied depending on the distribution of the biomarkers in the group under study. Although all studies in our sample used the highest risk quartile for calculation of an Allostatic Load Index, we found an unusually broad range of thresholds especially for blood lipids and fasting blood glucose. That brings us to the point that the allostatic load model would benefit from standardized definitions for subclinical threshold values of secondary outcomes, possibly stratified by age- and gender. This approach was used by two studies included to this review68, 83). The use of primary mediators like neuroendocrine biomarkers with circadian changes77, 86) would benefit from a clinical threshold approach as well. An index calculation based on risk quartiles could be impractical for use by a company physician as it requires statistical knowledge, resources, and a defined population for screening. It would not be possible to explore one individual at a time. Hampson coherently argued for the use of linear z-scores that increase statistical power87) but this recommendation may share similar limitations.
Different approaches to measure allostatic load have been developed. On one side, simple count-based formulation like the so-called Group Allostatic Load Index based on sample’s distribution of biomarker values or the Norm Allostatic Load Index based on normative biomarker values are a crude measurement of cumulative risk profiles55). The latter is still pending due to unestablished biomarker norms56). On the other side, more complex scoring algorithms allowing unequal weighting of the different biomarkers showed stronger relationships to health outcomes88). The use of clinical cut-offs rather than extreme sample quartiles however may represent an unrealistic threshold for healthy employees, because people at work are healthier than employees on sick-leave or early retirement which are usually not explored (healthy worker effect)55). Even if the usage is impractical, the advantage of using risk quartiles is that subclinical values for biomarkers are taken into account. A subclinical state of health reflects the conceptual framework of allostatic load much better than clinical manifestations. Earlier measurement may enable earlier intervention that alters development of multiple tertiary outcomes. Tertiary outcomes of allostatic overload are normally time delayed. Among 22,000 participants of the NHANES study (National Health and Nutrition Examination Survey) allostatic load steadily increased among people aged 20–60 yr and then plateaued during the period of greatest mortality risk up to the age of 9089) even without inclusion of primary mediators. On the one hand this illustrates the effects of aging, on the other hand it shows a 40 yr “window of opportunity” for intervention56) and highlights the importance of examination of psychosocial stress and allostatic load at younger ages and in apparently healthy employees. Would it be possible to counteract the effects of aging and decreasing flexibility of our bodily systems? For example, a common used approach to measure employee health for prevention matters is the work-ability index90, 91). This index was successfully designed to keep apparently healthy employees fit at work in order to prevent early retirement and productivity loss due to employee’s health and resources92, 93).
Our results should be interpreted within the context of several limitations. First, the reported findings have limited generalizability given the small number of eligible studies under review. Nevertheless, a review about biomarkers of chronic stress and the impact on health showed similar results56). Second, the included articles were characterized by substantial heterogeneity regarding study methods and quality. Only six of the included 16 studies (38%) were judged to be of good or very good quality. Thus, well-designed studies on allostatic load in the workforce are warranted in future. However, our study remains valuable in describing the areas in which heterogeneity was found. Third, we acknowledge the possibility of verification bias. Our results could have been affected by the type and sequence of tests used to define the presence of allostatic overload. Fourth, the observational nature of studies in our sample precludes inferences of causality in the instances in which allostatic load occurred. So far, longitudinal studies for allostatic load exist for mainly elderly people28, 94, 95). Further research should include longitudinal study designs for working populations as well. Although it was not the objective of this review to explore associations of work-related stressors and allostatic load, we have presented numerous findings of workplace conditions and health outcomes linked to allostatic load doing justice to the literature. These findings described in Table 2 seem to be obvious, but an additional review or meta-analysis focused on the association of work-related stress and allostatic load should be undertaken to explore present evidence.
Conclusions
There is a limited number of studies measuring allostatic load in the workforce and these can be further characterized by a high degree of methodological heterogeneity; in addition, there is limited evidence and consensus for a defined set of biomarkers using defined thresholds to calculate an Allostatic Load Index. Therefore, in the near future, recommendations of standardized approaches to conceptualize and measure allostatic load are urgently needed for advancing knowledge in this area. Particularly, measurement should consider a multi-organ system approach including a set of defined primary mediators and secondary outcomes, as well as the definition of thresholds for all variables regarding the usability by occupational health practitioners. Differences in age, gender, and socioeconomic status should be discussed for these thresholds. This approach could keep the general concept, but include a limited number of biomarkers with one parameter of neurophysiologic pathways (i.e. hypothalamic pituitary adrenal axis, autonomic nervous system) and one biomarker that has strong predictive power for future disease events (i.e. myocardial infarction). As a gold standard this set of variables should always be included when measuring allostatic load. The variables should be easy and reliable to measure with a potential threshold within a subclinical range. In addition to this core definition of variables other parameters could also be added to ensure a broader approach as flexible definition without omitting the gold standard. A longitudinal study should test the predictive value of ALI in comparison to single biomarkers or other well-known risk scores.
Acknowledgments
We gratefully acknowledge Prof. David Litaker from Case Western Reserve University for his valuable comments during the preparation of this manuscript. We also thank Catherin Bosle for her support.
Appendix
Search terms (period until 31 December 2013)
allostase OR allostasic OR allostasic’ OR allostasie OR allostasis OR allostasis/acclimation OR allostasis/allostatic OR allostasis/genetics OR allostasis/immunology OR allostasis/physiology OR allostasis’ OR allostastic OR allostatic OR allostatic/homeostatic OR allostatin OR allostatins OR allostatique allostatic load OR allostatic
References
- 1.Head J, Kivimäki M, Siegrist J, Ferrie JE, Vahtera J, Shipley MJ, Marmot MG. (2007) Effort-reward imbalance and relational injustice at work predict sickness absence: the Whitehall II study. J Psychosom Res 63, 433–40. [DOI] [PubMed] [Google Scholar]
- 2.Schreuder JAH, Roelen CAM, Koopmans PC, Moen BE, Groothoff JW. (2010) Effort-reward imbalance is associated with the frequency of sickness absence among female hospital nurses: a cross-sectional study. Int J Nurs Stud 47, 569–76. [DOI] [PubMed] [Google Scholar]
- 3.Chiang YM, Chang Y. (2012) Stress, depression, and intention to leave among nurses in different medical units: implications for healthcare management/nursing practice. Health Policy 108, 149–57. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Fu H, Hu Y, Shang L, Wu Y, Kristensen TS, Mueller BH, Hasselhorn HM. (2010) Psychosocial work environment and intention to leave the nursing profession: results from the longitudinal Chinese NEXT study. Scand J Public Health 38 Suppl, 69–80. [DOI] [PubMed] [Google Scholar]
- 5.Elliehausen H, Donker L, Fritzsche A, Konerding J, Pavlovsky B, Schott S, Seidel D. (2002) Stress und Arbeitsunfall. Zeitschrift für betriebliche Prävention und Unfallversicherung (12), 614–9.
- 6.Halkos G, Bousinakis D. (2010) The effect of stress and satisfaction on productivity. Int J Prod Perform Manag 59, 415–31. [Google Scholar]
- 7.Houtman I, Jettinghoff K, Cedillo L .(2007) Protecting Workers’ Health Series No. 6 Raising awareness of stress at work in developing countries (ISBN 92 4 159165 X). [Google Scholar]
- 8.González ER, Irastorza WCX .(2010) ESENER—European Survey of Enterprises on New and Emerging Risks. European Risk Observatory Report. Managing safety and health at work. http://osha.europa.eu.
- 9.Backé EM, Seidler A, Latza U, Rossnagel K, Schumann B. (2012) The role of psychosocial stress at work for the development of cardiovascular diseases: a systematic review. Int Arch Occup Environ Health 85, 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu W, Zhao Y, Guo L, Guo Y, Gao W. (2009) Job stress and coronary heart disease: a case-control study using a Chinese population. J Occup Health 51, 107–13. [DOI] [PubMed] [Google Scholar]
- 11.Marmot MG, Smith GD, Stansfeld S, Patel C, North F, Head J, White I, Brunner E, Feeney A. (1991) Health inequalities among British civil servants: the Whitehall II study. Lancet 337, 1387–93. [DOI] [PubMed] [Google Scholar]
- 12.Xu W, Hang J, Gao W, Zhao Y, Li W, Wang X, Li Z, Guo L. (2012) Association between effort-reward imbalance and glycosylated hemoglobin (HbA1c) among Chinese workers: results from SHISO study. Int Arch Occup Environ Health 85, 215–20. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Jarczok MN, Loerbroks A, Schöllgen I, Siegrist J, Bosch JA, Wilson MG, Mauss D, Fischer JE. (2013) Work stress is associated with diabetes and prediabetes: cross-sectional results from the MIPH Industrial Cohort Studies. Int J Behav Med 20, 495–503. [DOI] [PubMed] [Google Scholar]
- 14.Loerbroks A, Gadinger MC, Bosch JA, Stürmer T, Amelang M. (2010) Work-related stress, inability to relax after work and risk of adult asthma: a population-based cohort study. Allergy 65, 1298–305. [DOI] [PubMed] [Google Scholar]
- 15.Rugulies R, Norborg M, Sørensen TS, Knudsen LE, Burr H. (2009) Effort-reward imbalance at work and risk of sleep disturbances. Cross-sectional and prospective results from the Danish Work Environment Cohort Study. J Psychosom Res 66, 75–83. [DOI] [PubMed] [Google Scholar]
- 16.Siegrist J. (2008) Chronic psychosocial stress at work and risk of depression: evidence from prospective studies. Eur Arch Psychiatry Clin Neurosci 258 Suppl 5, 115–9. [DOI] [PubMed] [Google Scholar]
- 17.Maslach C, Schaufeli WB, Leiter MP. (2001) Job burnout. Annu Rev Psychol 52, 397–422. [DOI] [PubMed] [Google Scholar]
- 18.Chennoufi L, Ellouze F, Cherif W, Mersni M, M’rad MF. (2012) Stress et épuisement professionnel des enseignants tunisiens. Encephale 38, 480–7. [DOI] [PubMed] [Google Scholar]
- 19.Finney C, Stergiopoulos E, Hensel J, Bonato S, Dewa CS. (2013) Organizational stressors associated with job stress and burnout in correctional officers: a systematic review. BMC Public Health 13, 82.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elfering A, Grebner S, Gerber H, Semmer NK. (2008) Workplace observation of work stressors, catecholamines and musculoskeletal pain among male employees. Scand J Work Environ Health 34, 337–44. [DOI] [PubMed] [Google Scholar]
- 21.Schulte P, Vainio H. (2010) Well-being at work—overview and perspective. Scand J Work Environ Health 36, 422–9. [DOI] [PubMed] [Google Scholar]
- 22.Kirsten W. (2010) Making the link between health and productivity at the workplace—a global perspective. Ind Health 48, 251–5. [DOI] [PubMed] [Google Scholar]
- 23.European Trade Union Confederation (2004) Framework agreement on work-related stress. http://www.etuc.org/IMG/pdf_Framework_agreement_on_work-related_stress_EN.pdf.
- 24.McEwen BS, Stellar E. (1993) Stress and the individual. Mechanisms leading to disease. Arch Intern Med 153, 2093–101. [PubMed] [Google Scholar]
- 25.Kopp MS, Thege BK, Balog P, Stauder A, Salavecz G, Rózsa S, Purebl G, Adám S. (2010) Measures of stress in epidemiological research. J Psychosom Res 69, 211–25. [DOI] [PubMed] [Google Scholar]
- 26.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. (1997) Price of adaptation—allostatic load and its health consequences. MacArthur studies of successful aging. Arch Intern Med 157, 2259–68. [PubMed] [Google Scholar]
- 27.Borrell LN, Dallo FJ, Nguyen N. (2010) Racial/ethnic disparities in all-cause mortality in U.S. adults: the effect of allostatic load. Public Health Rep 125, 810–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seeman TE, McEwen BS, Rowe JW, Singer BH. (2001) Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci USA 98, 4770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH. (2006) Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci USA 103, 14158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabbah W, Watt RG, Sheiham A, Tsakos G. (2008) Effects of allostatic load on the social gradient in ischaemic heart disease and periodontal disease: evidence from the Third National Health and Nutrition Examination Survey. J Epidemiol Community Health 62, 415–20. [DOI] [PubMed] [Google Scholar]
- 31.McEwen BS. (2003) Mood disorders and allostatic load. Biol Psychiatry 54, 200–7. [DOI] [PubMed] [Google Scholar]
- 32.Borsook D, Maleki N, Becerra L, McEwen B. (2012) Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron 73, 219–34. [DOI] [PubMed] [Google Scholar]
- 33.Borrell LN, Crawford ND. (2011) Social disparities in periodontitis among US adults: the effect of allostatic load. J Epidemiol Community Health 65, 144–9. [DOI] [PubMed] [Google Scholar]
- 34.Bernard C .(1878) Lecons sur les phenomenes de la vie communs aux animaux et aux vegetaux, Librairie J.-B.-Bailliere et Fils, Paris. [Google Scholar]
- 35.Cannon WB .(1932) The wisdom of the body, W.W. Norton & Company, New York. [Google Scholar]
- 36.Sterling P, Eyer J .(1988) Handbook of Life Stress, Cognition and Health. Allostasis: A new paradigm to explain arousal pathology, John Wiley & Sons, New York. [Google Scholar]
- 37.McEwen B, Nasveld P, Palmer MAR .(2012) Allostatic load. A review of the literature 2012. http://www.dva.gov.au/health_and_wellbeing/research/Documents/allostatic.pdf.
- 38.Schulkin J. (2011) Social allostasis: anticipatory regulation of the internal milieu. Front Evol Neurosci 2, 111.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McEwen BS, Gianaros PJ. (2010) Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Ann N Y Acad Sci 1186, 190–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McEwen BS, Wingfield JC. (2003) The concept of allostasis in biology and biomedicine. Horm Behav 43, 2–15. [DOI] [PubMed] [Google Scholar]
- 41.McEwen BS. (2006) Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci 8, 367–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Picard M, Juster RP, McEwen BS. (2014) Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat Rev Endocrinol 10, 303–10. [DOI] [PubMed] [Google Scholar]
- 43.McEwen BS. (2000) Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology 22, 108–24. [DOI] [PubMed] [Google Scholar]
- 44.Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. (2002) Allostatic load as a predictor of functional decline. MacArthur studies of successful aging. J Clin Epidemiol 55, 696–710. [DOI] [PubMed] [Google Scholar]
- 45.Goldman N, Turra CM, Glei DA, Seplaki CL, Lin YH, Weinstein M. (2006) Predicting mortality from clinical and nonclinical biomarkers. J Gerontol A Biol Sci Med Sci 61, 1070–4. [DOI] [PubMed] [Google Scholar]
- 46.Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, Berkman LF, Reuben DB. (2004) Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Soc Sci Med 58, 1985–97. [DOI] [PubMed] [Google Scholar]
- 47.McEwen BS. (1998) Protective and damaging effects of stress mediators. N Engl J Med 338, 171–9. [DOI] [PubMed] [Google Scholar]
- 48.Ryan R, Prictor M, McKenzie J.(2011) Cochrane Consumers and Communication Review Group. Study Quality Guide. http://www.latrobe.edu.au/chcp/cochrane/resources.html.
- 49.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Reprint—preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther 89, 873–80. [PubMed] [Google Scholar]
- 50.Li W, Zhang J, Wang S. (2007) Relationship between job stress and allostatic load. China Occupational Medicine (03).
- 51.Li Wea (2007) Job stress related to glyco-lipid allostatic load, adiponectin and visfatin. Stress and Health 23, 257−66. [Google Scholar]
- 52.Sun J, Wang S, Zhang J, Li W. (2007) Assessing the cumulative effects of stress: The association between job stress and allostatic load in a large sample of Chinese employees. Work & Stress 21, 333–47. [Google Scholar]
- 53.Juster RP, Sindi S, Marin MF, Perna A, Hashemi A, Pruessner JC, Lupien SJ. (2011) A clinical allostatic load index is associated with burnout symptoms and hypocortisolemic profiles in healthy workers. Psychoneuroendocrinology 36, 797–805. [DOI] [PubMed] [Google Scholar]
- 54.Juster RP, Lupien S. (2012) A sex- and gender-based analysis of allostatic load and physical complaints. Gend Med 9, 511–23 [DOI] [PubMed] [Google Scholar]
- 55.Beckie TM. (2012) A systematic review of allostatic load, health, and health disparities. Biol Res Nurs 14, 311–46. [DOI] [PubMed] [Google Scholar]
- 56.Juster RP, McEwen BS, Lupien SJ. (2010) Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev 35, 2–16. [DOI] [PubMed] [Google Scholar]
- 57.McEwen BS. (2003) Interacting mediators of allostasis and allostatic load: towards an understanding of resilience in aging. Metabolism 52, Suppl 2, 10–6. [DOI] [PubMed] [Google Scholar]
- 58.Ariëns GA, van Mechelen W, Bongers PM, Bouter LM, van der Wal G. (2000) Physical risk factors for neck pain. Scand J Work Environ Health 26, 7–19. [DOI] [PubMed] [Google Scholar]
- 59.Hoogendoorn WE, van Poppel MN, Bongers PM, Koes BW, Bouter LM. (1999) Physical load during work and leisure time as risk factors for back pain. Scand J Work Environ Health 25, 387–403. [DOI] [PubMed] [Google Scholar]
- 60.Higgins JP, Green S .(2011) Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. www.cochrane-handbook.org.
- 61.Johansson G, Huang Q, Lindfors P. (2007) A life-span perspective on women’s careers, health, and well-being. Soc Sci Med 65, 685–97. [DOI] [PubMed] [Google Scholar]
- 62.von Thiele U, Lindfors P, Lundberg U. (2006) Self-rated recovery from work stress and allostatic load in women. J Psychosom Res 61, 237–42. [DOI] [PubMed] [Google Scholar]
- 63.Bellingrath S, Weigl T, Kudielka BM. (2009) Chronic work stress and exhaustion is associated with higher allostastic load in female school teachers. Stress 12, 37–48. [DOI] [PubMed] [Google Scholar]
- 64.Hasson D, Von Thiele Schwarz U, Lindfors P. (2009) Self-rated health and allostatic load in women working in two occupational sectors. J Health Psychol 14, 568–77. [DOI] [PubMed] [Google Scholar]
- 65.Näswall K, Lindfors P, Sverke M. (2012) Job insecurity as a predictor of physiological indicators of health in healthy working women: an extension of previous research. Stress Health 28, 255–63. [DOI] [PubMed] [Google Scholar]
- 66.Langelaan S, Bakker AB, Schaufeli WB, van Rhenen W, van Doornen LJP. (2007) Is burnout related to allostatic load? Int J Behav Med 14, 213–21. [DOI] [PubMed] [Google Scholar]
- 67.de Castro AB, Voss JG, Ruppin A, Dominguez CF, Seixas NS. (2010) Stressors among Latino day laborers. A pilot study examining allostatic load. AAOHN J 58, 185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lipowicz A, Szklarska A, Malina RM. (2014) Allostatic load and socioeconomic status in Polish adult men. J Biosoc Sci 46, 155–67. [DOI] [PubMed] [Google Scholar]
- 69.Schnorpfeil P, Noll A, Schulze R, Ehlert U, Frey K, Fischer JE. (2003) Allostatic load and work conditions. Soc Sci Med 57, 647–56. [DOI] [PubMed] [Google Scholar]
- 70.Fischer JC, Kudielka BM, von Känel R, Siegrist J, Thayer JF, Fischer JE. (2009) Bone-marrow derived progenitor cells are associated with psychosocial determinants of health after controlling for classical biological and behavioral cardiovascular risk factors. Brain Behav Immun 23, 419–26. [DOI] [PubMed] [Google Scholar]
- 71.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WPT, Loria CM, Smith SC., JrHational Heart, Lung, and Blood InstituteAmerican Heart AssociationWorld Heart FederationInternational Atherosclerosis SocietyInternational Association for the Study of Obesity (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–5. [DOI] [PubMed] [Google Scholar]
- 72.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. (1998) Prediction of coronary heart disease using risk factor categories. Circulation 97, 1837–47. [DOI] [PubMed] [Google Scholar]
- 73.Grundy SM, Balady GJ, Criqui MH, Fletcher G, Greenland P, Hiratzka LF, Houston-Miller N, Kris-Etherton P, Krumholz HM, LaRosa J, Ockene IS, Pearson TA, Reed J, Washington R,, Smith SC., Jr (1998) Primary prevention of coronary heart disease: guidance from Framingham: a statement for healthcare professionals from the AHA Task Force on Risk Reduction. Circulation 97, 1876–87. [DOI] [PubMed] [Google Scholar]
- 74.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvänne M, Scholte OP, Reimer WJ, Vrints C, Wood D, Zamorano JL,, Zannad F, European Association for Cardiovascular Prevention & Rehabilitation (EACPR)ESC Committee for Practice Guidelines (CPG) (2012) European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J 33, 1635–701. [DOI] [PubMed] [Google Scholar]
- 75.Assmann G, Cullen P, Schulte H. (2002) Simple scoring scheme for calculating the risk of acute coronary events based on the 10-year follow-up of the prospective cardiovascular Münster (PROCAM) study. Circulation 105, 310–5. [DOI] [PubMed] [Google Scholar]
- 76.Seeman TE, Singer BH, Ryff CD, Dienberg Love G, Levy-Storms L. (2002) Social relationships, gender, and allostatic load across two age cohorts. Psychosom Med 64, 395–406. [DOI] [PubMed] [Google Scholar]
- 77.Loucks EB, Juster R, Pruessner JC. (2008) Neuroendocrine biomarkers, allostatic load, and the challenge of measurement. A commentary on Gersten. Soc Sci Med 66, 525–30. [Google Scholar]
- 78.Jarczok MN, Jarczok M, Mauss D, Koenig J, Li J, Herr RM, Thayer JF. (2013) Autonomic nervous system activity and workplace stressors—a systematic review. Neurosci Biobehav Rev 37, 1810–23. [DOI] [PubMed] [Google Scholar]
- 79.Thayer JF, Sternberg E. (2006) Beyond heart rate variability: vagal regulation of allostatic systems. Ann N Y Acad Sci 1088, 361–72. [DOI] [PubMed] [Google Scholar]
- 80.Weber CS, Thayer JF, Rudat M, Wirtz PH, Zimmermann-Viehoff F, Thomas A, Perschel FH, Arck PC, Deter HC. (2010) Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. Eur J Appl Physiol 109, 201–11. [DOI] [PubMed] [Google Scholar]
- 81.Fischer JE, Thayer JF. (2006) Invited commentary: tapping the tip of the iceberg. Am J Epidemiol 163, 888–90, discussion 891–2. [DOI] [PubMed] [Google Scholar]
- 82.McCaffery JM, Marsland AL, Strohacker K, Muldoon MF, Manuck SB. (2012) Factor structure underlying components of allostatic load. PLoS ONE 7, e47246.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Juster RP, Moskowitz DS, Lavoie J, D’Antono B. (2013) Sex-specific interaction effects of age, occupational status, and workplace stress on psychiatric symptoms and allostatic load among healthy Montreal workers. Stress 16, 616–29. [DOI] [PubMed] [Google Scholar]
- 84.Borish L, Schmaling K, DiClementi JD, Streib J, Negri J, Jones JF. (1998) Chronic fatigue syndrome: identification of distinct subgroups on the basis of allergy and psychologic variables. J Allergy Clin Immunol 102, 222–30. [DOI] [PubMed] [Google Scholar]
- 85.Kavelaars A, Kuis W, Knook L, Sinnema G, Heijnen CJ. (2000) Disturbed neuroendocrine-immune interactions in chronic fatigue syndrome. J Clin Endocrinol Metab 85, 692–6. [DOI] [PubMed] [Google Scholar]
- 86.Gersten O. (2008) Neuroendocrine biomarkers, social relations, and the cumulative costs of stress in Taiwan. Soc Sci Med 66, 507–19, discussion 520–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hampson SE, Goldberg LR, Vogt TM, Hillier TA, Dubanoski JP. (2009) Using physiological dysregulation to assess global health status: associations with self-rated health and health behaviors. J Health Psychol 14, 232–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seeman T, Merkin SS, Crimmins E, Koretz B, Charette S, Karlamangla A. (2008) Education, income and ethnic differences in cumulative biological risk profiles in a national sample of US adults: NHANES III (1988-1994). Soc Sci Med 66, 72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Crimmins EM, Johnston M, Hayward M, Seeman T. (2003) Age differences in allostatic load: an index of physiological dysregulation. Exp Gerontol 38, 731–4. [DOI] [PubMed] [Google Scholar]
- 90.de Zwart BCH, Frings-Dresen MH, van Duivenbooden JC. (2002) Test-retest reliability of the Work Ability Index questionnaire. Occup Med (Lond) 52, 177–81. [DOI] [PubMed] [Google Scholar]
- 91.Radkiewicz P, Widerszal-Bazyl M. (2005) Psychometric properties of Work Ability Index in the light of comparative survey study. Int Congr Ser 1280, 304–9. [Google Scholar]
- 92.van den Berg TI, Robroek SJ, Plat JF, Koopmanschap MA, Burdorf A. (2011) The importance of job control for workers with decreased work ability to remain productive at work. Int Arch Occup Environ Health 84, 705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ilmarinen J, Tuomi K, Klockars M. (1997) Changes in the work ability of active employees over an 11-year period. Scand J Work Environ Health 23 Suppl 1, 49–57. [PubMed] [Google Scholar]
- 94.Clark MS, Bond MJ, Hecker JR. (2007) Environmental stress, psychological stress and allostatic load. Psychol Health Med 12, 18–30. [DOI] [PubMed] [Google Scholar]
- 95.Seeman T, Glei D, Goldman N, Weinstein M, Singer B, Lin YH. (2004) Social relationships and allostatic load in Taiwanese elderly and near elderly. Soc Sci Med 59, 2245–57. [DOI] [PubMed] [Google Scholar]