Abstract
Background
Neurochemical monitoring via sampling probes is valuable for deciphering neurotransmission in vivo. Microdialysis is commonly used; however, the spatial resolution is poor.
New Method
Recently push-pull perfusion at low flow rates (50 nL/min) has been proposed as a method for in vivo sampling from the central nervous system. Tissue damage from such probes has not been investigated in detail. In this work, we evaluated acute tissue response to low-flow push-pull perfusion by infusing the nuclear stains Sytox Orange and Hoechst 33342 through probes implanted in the striatum for 200 min, to label damaged and total cells, respectively, in situ.
Results
Using the damaged/total labeled cell ratio as a measure of tissue damage, we found that 33 ± 8% were damaged within the dye region around a microdialysis probe. We found that low-flow push-pull perfusion probes damaged 24 ± 4% of cells in the sampling area. Flow had no effect on the number of damaged cells for low-flow push-pull perfusion. Modeling revealed that shear stress and pressure gradients generated by the flow were lower than thresholds expected to cause damage.
Comparison with existing methods
Push-pull perfusion caused less tissue damage but yielded 1500-fold better spatial resolution.
Conclusions
Push-pull perfusion at low flow rates is a viable method for sampling from the brain with potential for high temporal and spatial resolution. Tissue damage is mostly caused by probe insertion. Smaller probes may yield even lower damage.
Keywords: In vivo sampling, brain tissue damage, push-pull perfusion, microdialysis, cell viability, computational modeling
I. Introduction
In vivo neurochemical monitoring in the brain is an important tool for studying the brain and neural disorders (Robinson et al., 2008; Weiss et al., 2000). Measurements of extracellular neurotransmitter concentrations over time can correlate chemical signaling to behavior, pharmacology, and pathophysiology. Non-invasive in vivo monitoring techniques like positron emission tomography are powerful, but expensive, require subjects to be immobilized, and are limited to a few neurotransmitters, which precludes their use for many basic neuroscience studies ( Lundqvist, 1999). Invasive techniques involving probe insertion into brain tissue are a widely used alternative. Electrochemical probes, for example, offer high temporal and spatial resolution but are limited to a few neurotransmitters. In vivo microdialysis sampling has proven to be a versatile and successful method for neurochemical monitoring in the CNS (Watson et al., 2006).
A weakness of microdialysis sampling is poor spatial resolution. Probes are typically 200-400 μm diameter and 1-4 mm long thus precluding sampling from small brain regions. An alternative sampling method with better spatial resolution is push-pull perfusion (PPP). In this method, the sampling probe consists of two side-by-side or concentric capillaries. Sample is “pulled” from one capillary and artificial cerebrospinal fluid (aCSF) is “pushed” through the other capillary to replace the sampled volume. By sampling just from the tip of the probe, spatial resolution is enhanced relative to microdialysis. Early forms of PPP were conducted at sampling flow rates of ~10 μL/min. These relatively high flow rates were perceived to cause substantial tissue damage (Redgrave, 1977). More recently, highly miniaturized PPP has been reported and used in brain and other tissues (Kottegoda et al., 2002; Thongkhao - On et al., 2004; Lee et al., 2013; Slaney et al., 2012; Slaney et al., 2011). Low-flow PPP uses relatively narrow bore capillaries as the probe and sampling flow rates of just 10-50 nL/min. With the use of smaller bore tubing, the spatial resolution is improved relative to microdialysis or conventional PPP. Indeed, this method has been used to sample from the vitreous humor between lens and retina of the rat eye (Thongkhao - On et al., 2004) and to measure chemical gradients around small brain regions (Slaney et al., 2012). This method has been coupled with segmented flow and microscale analytical techniques to achieve temporal resolution of a few seconds (Slaney et al., 2011). The potential of versatile measurement, high temporal resolution, and high spatial resolution may enable low-flow PPP to become a valuable alternative to sensors and microdialysis for in vivo neurochemical studies.
An important consideration of any invasive technique is the tissue damage caused. Insertion of a device into the brain elicits an immediate injury response due to mechanical disruption (Polikov et al., 2005). Tissue damage associated with microdialysis has been extensively researched. Initial studies found that cerebral blood flow and local glucose metabolism decreased around the probe within 2 h of implantation, but normalized within 24 h (Benveniste et al., 1987). Histological studies of microdialysis probes implanted for 1-3 days have found regions of damaged, degenerating neurons around the probe (Tang et al., 2003; Zhou et al., 2002). A semi-quantitative tissue damage study reported neuronal density decreases up to 400 μm and intercellular disruption up to 1.4 mm from probe implanted for 40 h (Clapp-Lilly et al., 1999). Despite this tissue disruption, microdialysis has successfully been used to monitor brain neurochemistry in many applications (Di Chiara et al., 1996; Torregrossa and Kalivas, 2008).
The tissue response in low-flow PPP has been investigated much less. An initial study reported absence of considerable tissue damage (Kottegoda et al., 2002) based on observation of stained tissue around a probe track; however, no follow up studies have been reported. The absence of comprehensive tissue response data on low-flow PPP is a barrier to its adoption. A potential concern is whether the presence of open flow in the probe would aggravate tissue damage from probe insertion that is observed in conventional PPP.
To further examine the damage associated with low-flow push-pull perfusion, we infused stains for live and damaged cells during acute sampling (200 min) and then imaged brain slices around the sampling tip with confocal microscopy. These experiments allowed us to determine the relative fraction of cells that were damaged around the probe. The results were compared to analogous microdialysis studies. Moreover, computational modeling was used to predict the fluid dynamics at the probe tip to elucidate the role of fluid flow in disturbing brain tissue.
2. Materials and methods
All reagents were purchased from Invitrogen, unless otherwise specified. Fused silica capillaries were from Polymicro (Phoenix, AZ). All animal care, housing, and operative procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 85-23, 1985). Rats were housed in a pathogen-free facility at the University of Michigan, given food and water ad libitum, and exposed to a 12-h light/dark cycle. The University Committee on the Use and Care of Animals approved the experimental protocol.
2.1 Probe Fabrication
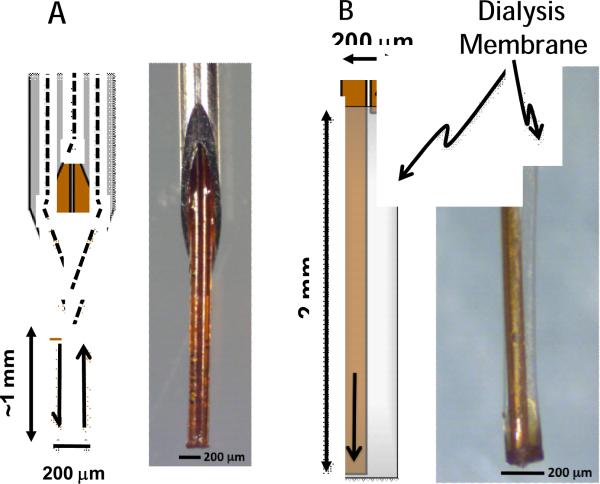
Side-by-side PPP probes were constructed similar to those previously described (Cellar and Kennedy, 2006). Briefly, two 15-cm long 40 μm inner diameter (i.d.) × 100 μm outer diameter (o.d.) capillaries were threaded through a 26-gauge stainless steel needle (BD, Franklin Lakes, NJ). The ends of these capillaries were attached to 2 cm-long 180 μm i.d. × 360 μm o.d. capillaries for connection to 360 μm fittings. The capillary tips extended about 1 mm below the needle (Figure 1A). All probe sections were bonded with cyanoacrylate adhesive (Duro Super Glue, Henkel, Rocky Hill, CT). Side-by-side microdialysis probes were constructed by inserting two 10-cm long × 40 μm i.d. × 100 μm o.d. capillaries into a 200 μm-diameter regenerated cellulose membrane (Parsons and Justice, 1992). The inlet extended past the outlet capillary to form a 2-mm sampling length (Figure 1B). The inlet capillary end was attached to a 2-cm length of 180 μm i.d. × 360 μm o.d. capillary adapter.
Figure 1.
Diagrams and photomicrographs of low-flow PPP (A) and microdialysis (B) probes tested with dye staining experiments. Low-flow PPP probe is oriented with sampling tip down. Arrows illustrate direction of flow.
2.2 Animal Surgery and Neurochemical Sampling
Probes were inserted into male Sprague-Dawley rats weighing 300-400 g anesthetized with isoflurane and mounted in a stereotaxic frame. The probes were placed in the striatum at 1.0 mm anterior to bregma, +2.6 mm lateral to midline, and 4.5 (PPP) or 5.5 (microdialysis) mm ventral to dura (Paxinos and Watson, 2005).
PPP was performed using a syringe pump (Fusion 400, Chemyx, Stafford, TX) that infused aCSF into the rat brain via the “push” line of the probe, and a vacuum pump that withdrew sample via the “pull” line. The push line started with a 25 μL syringe (Gastight, Hamilton Co., Reno, NV), in a syringe pump and continued downstream in the following order: 150 μm i.d. union (ZU1XC, Valco Instruments, Houston, TX), 15-cm long 40 μm i.d. × 360 μm o.d. capillary, 150 μm i.d. union (P-772, Upchurch Scientific, Oak Harbor, WA), inlet capillary of the probe. Similarly, in the direction of flow, the pull line consisted of the outlet capillary of the probe, liquid flow meter (SLG1430-025, Sensirion Inc., Westlake Village, CA), and a 10-cm long 20 μm i.d. × 360 μm o.d. capillary. During insertion, a period of 5-10 s, the probe was operated at 500 nL/min to prevent clogging. Once the probe reached final location, flow was reduced to 50 nL/min. Flow stabilized in < 1 min. The push fluid was aCSF (145 mM NaCl, 2.68 mM KCl, 1.01 mM MgSO4, 1.22 mM CaCl2, 1.55 mM Na2HPO4, 0.45 mM NaH2PO4, pH 7.4). For the flow experiments, flow was maintained at 50 nL/min for 200 min. For no flow, the flow was stopped and probe left in place for the same period. For microdialysis a syringe pump was used to drive aCSF through the probe at 1 μL/min for 200 min in the flow condition and no flow was used for the same period. The microdialysis probe was inserted at the same rate as the PPP probe.
2.3 Cell Viability Dye Infusion
A stain cocktail of 162 mM Hoechst 33342 (H342) and 50 mM Sytox Orange (SO) was prepared by adding 2 μL aliquots of H342 and SO stock solutions to sterile Milli-Q water with glucose to yield 400 μL of isotonic solution. The osmolality of the dye cocktail was adjusted 285-295 mOsm/kg to prevent swelling or shrinking of surrounding cells. After 200 min of neurochemical sampling, the perfusion media was switched from aCSF to the nuclear dye cocktail. In PPP experiments, the dye was infused at 50 nL/min for 20 min. For a comparable staining radius, the dye was infused at 1 μL/min for 30 min in microdialysis experiments.
Immediately following dye infusion, probes were removed and animals were transcardially perfused with 200 mL phosphate buffered saline followed by 200 mL of 4% paraformaldehyde fixative using a constant pressure system (Bjornsson et al., 2008). Brains were removed and post-fixed for 24 h. Horizontal 100 μm tissue sections (perpendicular to the probe tract) were collected using a vibratome (VT1000, Leica, Buffalo Grove, IL). A series of 50-60 tissue slices were collected from each animal, reaching a depth of 4.5 (PPP) / 5.5 (microdialysis) mm from the dorsal surface of the brain. Alternating tissue slices (every other 100 μm) within the staining range were mounted in ProLong Gold for confocal imaging.
2.4 Confocal Imaging and Cell Count Analysis
Brain sections were imaged within 48 h of dye infusion to prevent loss of fluorescent labeling through diffusion. Three dimension (3D) data sets of 512 × 512 pixel resolution, from each H342 and SO labeled tissue slice, were acquired on a laser-scanning confocal microscope with a 10x objective (0.4 NA) (FluoView 500, Olympus, Center Valley, PA) at 405 nm and 543 nm excitation. Images were imported into Imaris (Bitplane, South Windsor, CT) for quantification of H342 and SO labeling.
The “count spot” module was selected and filters for cell size and background intensity were set to attain stained-cell count. Because the dyes labeled all cell nuclei, including microglia, astrocytes, and neurons, we set the upper limit of nuclei width to 10 μm, an approximation based on reported values of average neuronal nucleus widths (3 to 18 μm) and visually matching labeled spot size to perceived average nuclei size (West et al., 1991). The percentage of damaged cells (SO-labeled) per total cells (H342-labeled) within reach of the dye cocktail was calculated. MetaMorph software (Molecular Devices, Sunnyvale, CA, USA) was used for the concentric ring analysis of damaged/total cell ratio.
2.5 Computational Modeling of PPP
COMSOL (COMSOL, Burlington, MA) was used to model PPP sampling and estimate velocity, pressure, and shear stress gradients experienced by the tissue during sampling. For the model, the brain was drawn as a 15 × 15 cm square, an area several orders of magnitude greater than the sampling area. Two 4.5 cm side-by-side 100 μm width capillaries with 40 μm width channels were drawn, extending ventrally from the horizontal upper boundary, or surface, of the brain. The brain was modeled as an isotropic porous medium (Caicedo et al., 2010; Mace et al., 2010) with density = 1000 kg/m3, dynamic viscosity = 0.001 Pa·s, permeability = 1 × 10−11 m2, porosity = 0.4, elastic modulus = 7 kPa, and shear modulus = 12 kPa. For Dirichlet boundary conditions, the concentration of NaCl, the solute with the highest concentration in aCSF (145 mM), was entered. The density and dynamic viscosity of aCSF, with properties near identical to water, were input as 1 kg/m3 and 8.9 × 10−4 Pa·s, respectively. Because fluid flow in PPP does not cause enough pressure to deform brain tissue, as calculated mathematically, a diffusion-based flow model was appropriate. The “convection-diffusion equation” module was chosen to simulate the movement of fluid due to diffusion. All probe walls except for the 40 μm lengths of inlet (and outlet in PPP) were designated with zero flux.
The “laminar flow” module was selected to incorporate aCSF properties and flow rate. Laminar inflow was input as 50 nL/min. Laminar outflow for PPP was also 50 nL/min and was set as “pressure, no viscous stress”. A time-dependent simulation from 0 to 200 min was run to create velocity, pressure, and shear stress maps. We graphed streamlines that ran tangent along the entire vector field in our PPP COMSOL models. A particle with zero diffusivity was introduced into the flow field to verify that these lines defined the sampling area. This particle was hypothesized to follow the path drawn by the streamline, thus defining the limits of neurochemical flow. The streamlines were assumed to connect the outer horizontal limits of the inlet and outlet.
3. Results
3.1 Cell Viability Staining
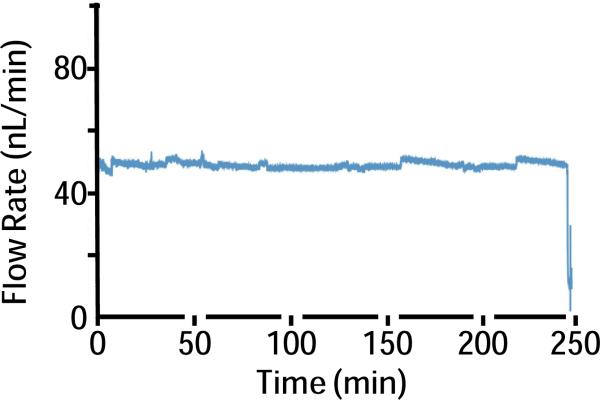
We examined acute tissue effects of low-flow PPP and microdialysis sampling probes (Figure 1) with and without neurochemical sampling flow (n = 4 for each condition; total n = 16). The sampling flow rate of 50 nL/min for low-flow PPP was verified by measurement using an in-line flow meter (Figure 2). Sampling tips had a cross sectional area of ~0.016 mm2 (two 100 μm side-by-side capillaries) and ~0.031 mm2 (200 μm diameter dialysis membrane) for PPP and microdialysis, respectively. To evaluate acute tissue damage, H342/SO dye cocktail was infused through the probes after sampling. H342 is a membrane permeant dye that stains the nucleus of all cells. SO is a membrane impermeable dye that can only stain cells with compromised membranes. Therefore SO staining indicates a damaged cell. By comparing the number of cells that had been stained by the two dyes, it was possible to determine the fraction of cells damaged around the different probes.
Figure 2.
Sample trace of pull flow rate during low-flow PPP in vivo. Flow rate was recorded by a flow meter as vacuum was used to pull flow. Push flow rate in PPP was maintained at 50 nL/min by a syringe pump to match pull flow rate.
3.2 Confocal Fluorescence Microscopy
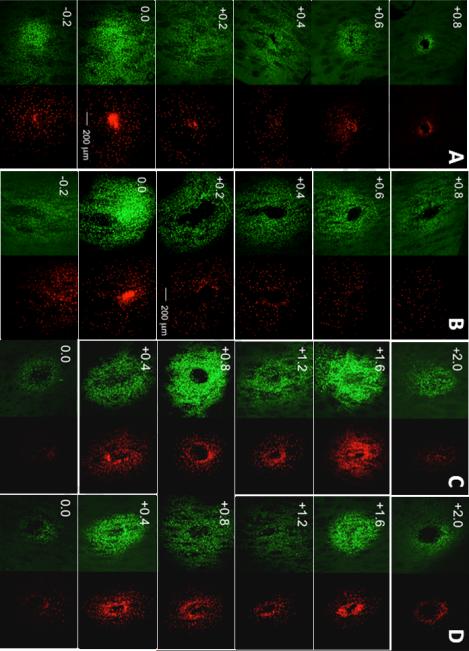
Figure 3 shows samples of maximum intensity projections of 3-D confocal stacks separated into H342 (pseudo-colored green) and SO (pseudo-colored red) channels, displaying total cells and damaged cells, respectively, for PPP and microdialysis. For PPP, the section with the largest number of stained cells was near the sampling tip (0.0). The Z-range of staining was asymmetric, extending 0.2 below the tip to +0.8 mm above the tip. The asymmetry is attributed to tissue disruption along the probe track allowing lower flow resistance in that direction. The fluorescent signal gradually decreased moving away ventrally/dorsally from the probe tips. In microdialysis, the staining area coincided with the active length of the dialysis membrane going from 0.0 mm (the probe tip) to +2.0 mm (position dorsal of the tip). Compared to PPP, the fluorescent signal in microdialysis remained relatively consistent throughout the dye-stained area with no vertical asymmetry.
Figure 3.
Representative fluorescent confocal microscopy images (voxel size 2.5 × 2.5 × 3.2 μm) of cell viability-stained 100 μm horizontal sections in PPP with no flow (A), PPP with sampling flow of 50 nL/min (B), microdialysis no flow (C), and microdialysis flow (D). Left column for each probe condition shows H342 (pseudo-colored green) staining and the right column shows SO (pseudo-colored red) staining. Stains were infused together at the end of the sampling period to gauge extent of damaged cells (stained by SO) relative to total cells stained (H342). Sections are labeled according to their relative vertical distance from the sampling tip (0.0) in mm with negative values indicating more ventral. All photomicrographs have identical scaling (scale bar = 200 μm).
3.3 Quantification of Staining
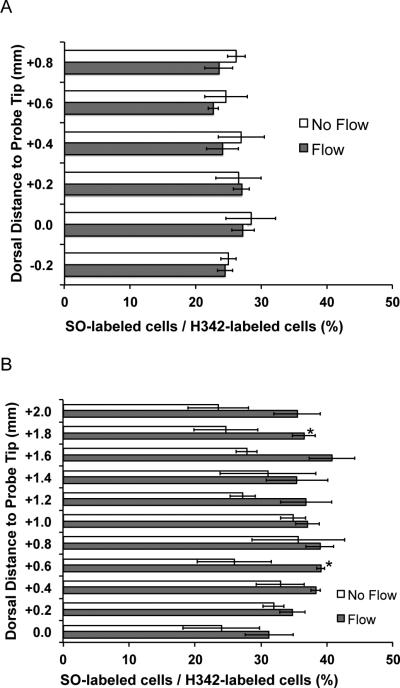
To quantify tissue response, Imaris image analysis software was used to count cells labeled by H342 and SO within reach of the dye at each section (Figure 4). For PPP, the percentage of damaged cells was between 23% and 28% along the probe track length. In the region closest to actual sampling points in PPP (0.0 to 0.2 mm ventral to the probe tip), we found that the percentage of dead cells was near 27% for PPP. Interestingly, relatively little effect of sampling flow was observed on the percent of damaged cells. This result suggests that the flow used for sampling in low-flow PPP is not especially damaging. This is an important point for PPP, as exposure of cells to flow is an obvious potential source of tissue damage. A caveat is that in all our experiments some flow was present as the probe was inserted (decreased from 500 down to 50 nL/min over 1 min) and flow is required to deliver dye (for 20 min at the end of experiment). Therefore these experiments evaluate the effect of short bursts of flow relative to continuous sampling flow for 3 h. Damaged cell percentage in microdialysis was between 25% and 40% all along the probe. Overall, across all sections analyzed, PPP (24 ± 4%) damaged fewer cells than microdialysis (33 ± 8%) (p < 0.05, one-way ANOVA, Tukey test). Interestingly, a slightly higher percentage of SO-labeled cells was found with flow during microdialysis. This effect however was only statistically significant at two points along the probe track (1.8 and 0.6 mm dorsal of the probe tip, p < 0.05).
Figure 4.
Fraction of stained cells that were stained with Sytox Orange (SO), a membrane impermeable dye for PPP (A) and microdialysis (B) for flow and no flow conditions. The number of cells stained with H342, a membrane permeable dye, was counted as the total number of stained cells to calculate the fraction. SO labeled cells are considered to be damaged. Sections with significant differences (p < 0.05) in damaged/total cell ratio between flow and non- flow are labeled with an asterisk. Error bars are SEM (n = 4).
3.4 Concentric Ring Cell Count and Dead/Total Cell Ratio
Damage radiating outward from the probes was also evaluated by performing damaged/total cell counts within concentric circles at the vertical placements 0.0 and +1.0 mm from the probe tip in PPP and microdialysis, respectively. These vertical placements were chosen because they are centered on the sampling region (i.e., near the tip of the PPP probe and at the center of the MD probe). H342-labeled cells extended as far as approximately 700 μm from the probe center. The fraction of damaged cells was higher near the probe and decreased gradually moving away from the probe (Figure 5); however, some SO-labeled cells were observed in some sections as far as 700 μm away.
Figure 5.
The gradient of SO-labeled/H342-labeled cells with respect to the horizontal probe hole center in PPP and microdialysis for flow conditions (n = 4 for each group). Cell count was performed within concentric rings of increasing radii at peak cell count sections: at the probe tip (0.0) in PPP, and +1.0 mm from the probe tip in microdialysis. Significant differences (p < 0.05) between PPP flow and microdialysis flow only existed within 250 μm of the probe hole center. Error bars are SEM. Inset: example of concentric rings used to calculate the gradient of dead/total cell ratio in the transverse plane. The image shown is from a PPP sample.
Because sampling probes were removed from the brain immediately after sampling, the probe hole retracted and cells were partially restored to their original spatial distribution in many sections. Thus, concentric rings represent roughly analogous cell count regions in PPP and microdialysis. No significant differences existed between PPP (flow and no flow) and microdialysis beyond 250 μm away from the probe centers (p > 0.05, one-way ANOVA, Tukey test, data not shown).
3.5 Computational Models of Fluid Flow
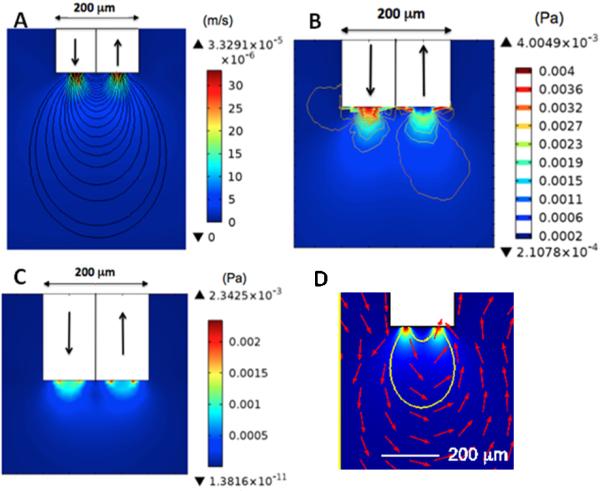
The experimental results suggest that sampling flow did not cause excess cell damage in low-flow PPP despite the presence of direct tissue contact to the flow. To better understand this result, we modeled velocity, pressure, and shear stress due to flow at the sampling tips of a low- flow PPP probe (Figure 6). The effects of flow during insertion (500 nL/min for 10 s) and sampling (50 nL/min for 200 min) were both modeled. Flow during insertion (not shown) and during sampling yielded vector fields that spanned similar areas and displayed comparable gradient patterns; however, values for velocity, pressure, and shear stress were lower during sampling PPP due to the lower flow rates. Pressure fields extended approximately 100 μm below the probe tip and shear stress fields were observed around 50 μm ventrally from the probe tip. In contrast, staining was observed at least 200 μm from the tip in low flow PPP.
Figure 6.
Numerical modeling of low flow PPP at 50 nL/min. In all drawings, the white area represents the sampling capillary tip and arrows indicate direction of flow. (A) Calculated velocity map. (B) Calculated pressure map. (C) Calculated shear stress map. (D) Close up of data from A with streamlines (in red) indicating direction of flow around probe tip.
Flow velocity was downward/outward flow at the inlet and upward/inward at the outlet (Figure 6A, D). The maximal velocities were at the inlet and outlet. During the insertion phase, the maximum velocity was 3.3 × 10−4 m/s and during sampling it was 3.3 × 10−5 m/s. Pressure contour lines closely matched velocity gradients and also illustrated the highest pressure at the probe inlet (Figure 6B). The maximum pressure was 44 mPa during insertion and 4 mPa during sampling. These pressures are much lower than 5 MPa, the lower threshold reported to cause cell death (Frey et al., 2008).
The shear stress map in the PPP models showed slightly larger overall area of shear stress at the inlet, but a greater area of maximum shear at the perimeter of the outlet (Figure 6C). The maximum shear stress values for 500 nL/min and 50 nL/min were 25 mPa and 2.3 mPa, respectively. Shear stress greater than 5 mPa have been shown to be detrimental to cell viability (Milan et al., 2009). This threshold was not reached during sampling flow rates but was exceeded for the flow rates when the probe was inserted.
As expected, streamlines started at the limits of the inlet/outlet and connect to the outer limits of the outlet/inlet. A solute particle with zero diffusivity followed the path drawn by the streamline. The volume where flow exits the push line and enters the pull line is approximately 0.003 mm3. This flow pattern may be considered to be a sampling volume.
4. Discussion
4.1 Cell Viability during PPP and Microdialysis
The results comparing cell viability following microdialysis and low-flow PPP provide insights into the potential utility of low-flow PPP and routes to improve it. Microdialysis supplanted conventional PPP performed at microliter/min flow rates for several reasons including the perception that by preventing exposure of tissue to flow, it would cause less tissue damage. Given the widespread acceptance and success of microdialysis, studies comparing tissue damage from microdialysis to low-flow PPP could be valuable in establishing the viability of low-flow PPP.
The seminal report on low-flow PPP found no clear region of damage around the probe based on visual examination of micrographs stained with cresyl violet (Kottegoda et al., 2002). Live-damaged cell staining provides a more detailed and quantitative assessment of tissue effects (Retterer et al., 2008). A study assessing the tissue damage caused by push-pull electroosmotic sampling used propidium iodide damaged-cell staining to show that approximately 10% of cells were damaged by that sampling method in ex vivo tissue (Hamsher et al., 2010). Using a similar strategy, we found that low-flow PPP in vivo sampling damaged approximately 20-30% of cells immediately around the probe. The tissue damage is centered on the area closest to probe shaft and decreases radially away. Interestingly, our results showed that sampling flow caused relatively minor effects. Thus, the open flow of a low-flow PPP probe is not detrimental to cell viability. Because sampling flow had no effect, our results suggest that most of the damage observed is due to insertion or possibly the brief periods of flow during insertion and dye loading.
We found that about 33% of the cells within the diffusion zone from a dialysis membrane were damaged. The damage is fairly uniform along the length of the probe and decreases radially away from the probe. The results seem to agree with prior studies of tissue damage in microdialysis. A semi-quantitative study on microdialysis illustrated ultrastructural disturbances as far as 400 μm to 1.4 μm from the probe tract (Clapp-Lilly et al., 1999). Similarly, we observed about 10% of the cells were damaged about 700 μm from the horizontal probe hole center. Interestingly, we observed a trend towards increased damage when flow was applied during microdialysis. The effect of flow may relate to some outflow from the probe due to ultrafiltration effects. Indeed, preliminary modeling of microdialysis membranes (not shown) suggested the potential for a low flow out of the probe membrane under these conditions. Another possibility is that a component of the aCSF, which would be delivered at a greater concentration with flow, might accelerate cell damage.
In comparing low-flow PPP to microdialysis we find a lower percentage of damaged cells (24 versus 33%) in the diffusion or staining zone. A likely reason for this difference is the size of the probes. The dialysis probes present a cross-sectional area that is approximately twice that of the push-pull probes due to the presence of the circular membrane. As a result, more tissue is displaced. The direct quantitative comparison of PPP and microdialysis tissue damage however, should be considered with caution. Comparisons could only be made in the regions were dyes penetrated. Differences in the delivery of dye (across membrane versus direct injection) will undoubtedly affect these results in ways that are difficult to discern. We tried to account for these differences by normalizing the fraction damaged cells to total cells. Furthermore, these comparisons were only made at a single time point and it is possible that over time different results would emerge due to differences in tissue response and recovery from the two techniques. These data represent tissue responses observed during acute measurements. In some cases, probes are left in for at least 24 h before measurements are made and therefore a different pattern may emerge.
Although microdialysis damages cells in the sampling area, as expected for an invasive technique, it has been routinely used for successful measurements in vivo and has been widely accepted (Di Chiara et al., 1996). The demonstration that low-flow PPP can cause lower percentage of dead cells in the sampling area than microdialysis argues favorably for its use in in vivo studies. While further and more detailed studies will be required, both in terms of demonstrating neurochemical measurements and tissue effects, these initial results support the idea that this method of sampling can be viable. Further improvements may also be achieved. In view of the effect that larger probes cause more tissue displacement and therefore damage, the fabrication of smaller probes may prove useful (Lee et al., 2013). Once the optimal operational parameters for such microfabricated probes are identified, a comparable tissue damage study will be performed. Other possibilities have been reported that may be applied to PPP as well. Anti-inflammatory reagents delivered through the probe could counteract the immune response to the foreign body probe (Córcoles and Boutelle, 2013; Jaquins-Gerstl et al., 2011; Nesbitt et al., 2013). Another strategy to minimize tissue damage is to coat or make the probe out of more biocompatible materials like titanium.
4.2 Insights from Computational Modeling
The lower tissue damage of PPP relative to microdialysis may be surprising in view of the exposure of tissue to flow. The modeling results shed light on these observations. Calculated pressure values in PPP were several orders of magnitude lower than the cell-death threshold value (LaPlaca et al., 1997), showing that pressure generated by fluid flow is not a contributing factor to tissue damage. During sampling, the shear stress was also well below tissue damage thresholds (LaPlaca et al., 1997). During the brief preparatory phase of PPP where flow rates were 500 nL/min, the shear stress values exceeded the values that cause tissue damage. Spatially, this critical shear stress is observed no more than 20 μm ventrally from the probe tip in PPP. Also, the shear stress field in PPP does not cross the vertical plane of the probe. Temporally, this critical shear stress is only in effect for <60 s in PPP. These spatial and temporal factors may mitigate damage associated with shear stress from elevated flow. Nevertheless, one potential route to lessen tissue damage is to use lower flow rates throughout the insertion process. Higher flow was used because it is presumed to help prevent clogging as the probe is inserted.
Numerical models for PPP showed pressure and shear stress effects restricted to a smaller area than the area of cell damage as revealed by the stains. Although pressure was determined not to play a major role in tissue damage, it covered the most area out of all the modeled parameters. In PPP, substantial pressure extended approximately 50 μm below the probe tip. From cell viability data, tissue damage was observed at least 200 μm from the tip in low flow PPP. This result is consistent with the idea that tissue damage observed was primarily due to tissue displacement and disruption during implantation with minor effects of flow.
4.3 Consistent sampling flow and spatial resolution.
The experimental results and modeling provided other insights into low-flow PPP. Our flow rate data, the first reported for PPP, show that stable flows of 50 nL/min could be routinely achieved for in vivo sampling. As low, stable flow with open sampling probes is not obviously achievable due to potential clogging and bubbles, this finding is encouraging for routine quantitative sampling. These modeling results provided a measure of the spatial resolution for sampling (Figure 6D). The streamlines can provide an estimate of how far into the tissue flow, and therefore sampling, occurs. We estimate this to be about 0.003 mm3, in agreement with a previous experimental estimate of 0.004 mm3 (Slaney, et al. 2012). The sampling area of the tip was 0.04 mm2 in PPP. This is about 1,500 times smaller than the sampling area of a 2 mm long microdialysis probe. These comparisons highlight the significant improvement in spatial resolution that is possible with low-flow PPP.
4.4 Effect of anesthesia
These experiments were recorded on anesthetized animals; but, it is clear that low-flow PPP will have to be adapted to awake animals to provide broadest utility. Fortunately, no technical barriers exist to prevent such advances. The main requirement is to develop a secure holder that will keep the probes in place and to improve fraction collection at low flow rates. In this regard, we caution that tissue damage may be affected by anesthesia; however, it is reasonable to expect that the main conclusions, i.e. that low-flow PPP causes comparable or less tissue damage than microdialysis, are correct with or without anesthesia.
5. Conclusions
This study shows that low-flow push-pull perfusion causes less tissue damage than microdialysis as measured by the ratio of damaged cells to total cells labeled by diffusible dyes. The lower damage is likely due to the smaller overall size of the probe; however, some uncertainties remain in direct quantitative comparisions because of the differences in delivery of the dyes; although this is mitigated by the use of a normalization in evaluation. The data indicate that flow during low-flow PPP does not contribute to damage. Numerical models show that this lack of flow effect is due to the low shear stress and pressure caused by the low flow rates used. Undoubtedly, incorporation of low-flow in PPP has dramatically reduced the tissue damage caused by the higher flow rates in earlier iterations of PPP. These findings support the concept of low-flow PPP as a relatively biocompatible, high spatial resolution tool for neurochemical monitoring. The work also shows the potential utility of modeling for evaluating probes. Future work could involve optimizing models and testing a variety of conditions. 3D models, with additional mechanical conditions like strain, will provide more accurate information on sampling-induced brain injury. Moreover, the solid mechanics of probe insertion can be computed with more advanced calculations.
Highlights for “Experimental Evaluation and Computational Modeling of Tissue Damage from Low-Flow Push-Pull Perfusion Sampling In Vivo”.
Push-pull perfusion at 50 nL/min was evaluated for tissue damage by infusing stains
Push-pull damaged 24% of cells, less than the 33% observed with microdialysis
Modeling and data reveal that flow did not contribute to damage
Low-flow push-pull perfusion provides high spatial resolution for in vivo sampling
Acknowledgments
The work was supported by NIH R37 EB003320. The project used the Morphology and Image Analysis Core of the Michigan Diabetes Research Center that is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK020572). We thank Daryl Kipke and William Shain for helpful discussions and access to some equipment used in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benveniste H, Drejer J, Schousboe A, Diemer NH. Regional cerebral glucose phosphorylation and blood-flow after insertion of a microdialysis fiber through the dorsal hippocampus in the rat. Journal of Neurochemistry. 1987;49:729–34. doi: 10.1111/j.1471-4159.1987.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Bjornsson CS, Lin G, Al-Kofahi Y, Narayanaswamy A, Smith KL, Shain W, Roysam B. Associative image analysis: A method for automated quantification of 3D multi-parameter images of brain tissue. Journal of Neuroscience Methods. 2008;170:165–78. doi: 10.1016/j.jneumeth.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo HH, Hernandez M, Fall CP, Eddington DT. Multiphysics simulation of a microfluidic perfusion chamber for brain slice physiology. Biomedical Microdevices. 2010;12:761–7. doi: 10.1007/s10544-010-9430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellar NA, Kennedy RT. A capillary-PDMS hybrid chip for separations-based sensing of neurotransmitters in vivo. Lab on a Chip. 2006;6:1205–12. doi: 10.1039/b603561b. [DOI] [PubMed] [Google Scholar]
- Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL. An ultrastructural analysis of tissue surrounding a microdialysis probe. Journal of Neuroscience Methods. 1999;90:129–42. doi: 10.1016/s0165-0270(99)00064-3. [DOI] [PubMed] [Google Scholar]
- Córcoles EP, Boutelle MG. Biosensors and Invasive Monitoring in Clinical Applications. Springer; 2013. Microdialysis. pp. 45–7. [Google Scholar]
- Di Chiara G, Tanda G, Carboni E. Estimation of in-vivo neurotransmitter release by brain microdialysis: the issue of validity. Behavioural pharmacology. 1996;7:640–57. [PubMed] [Google Scholar]
- Frey B, Janko C, Ebel N, Meister S, Schlucker E, Meyer-Pittroff R, Fietkau R, Herrmann M, Gaipl US. Cells Under Pressure - Treatment of Eukaryotic Cells with High Hydrostatic Pressure, from Physiologic Aspects to Pressure Induced Cell Death. Current Medicinal Chemistry. 2008;15:2329–36. doi: 10.2174/092986708785909166. [DOI] [PubMed] [Google Scholar]
- Hamsher AE, Xu H, Guy Y, Sandberg M, Weber SG. Minimizing Tissue Damage in Electroosmotic Sampling. Analytical Chemistry. 2010;82:6370–6. doi: 10.1021/ac101271r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist H, Lubberink M, Tolmachev V, Lövqvist A, Sundin A, Beshara S, Bruskin A, Carlsson J, Westlin JE. Positron Emission Tomography and Radioimmunotargeting: General Aspects. Acta Oncologica. 1999;38:335–41. doi: 10.1080/028418699431410. [DOI] [PubMed] [Google Scholar]
- Jaquins-Gerstl A, Shu Z, Zhang J, Liu Y, Weber SG, Michael AC. Effect of Dexamethasone on Gliosis, Ischemia, and Dopamine Extraction during Microdialysis Sampling in Brain Tissue. Analytical Chemistry. 2011;83:7662–7. doi: 10.1021/ac200782h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottegoda S, Shaik I, Shippy SA. Demonstration of low flow push-pull perfusion. Journal of Neuroscience Methods. 2002;121:93–101. doi: 10.1016/s0165-0270(02)00245-5. [DOI] [PubMed] [Google Scholar]
- LaPlaca MC, Lee VMY, Thibault LE. An in vitro model of traumatic neuronal injury: loading rate-dependent changes in acute cytosolic calcium and lactate dehydrogenase release. Journal of neurotrauma. 1997;14:355–68. doi: 10.1089/neu.1997.14.355. [DOI] [PubMed] [Google Scholar]
- Lee WH, Slaney TR, Hower RW, Kennedy RT. Microfabricated sampling probes for in vivo monitoring of neurotransmitters. Analytical chemistry. 2013;85:3828–31. doi: 10.1021/ac400579x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace E, Cohen I, Martin A, Montaldo G, Fink M, Tavitian B, Tanter M. In vivo brain elasticity mapping in small animals using ultrasound and its application to cerebral ischemia. Biomedical Imaging: From Nano to Macro. IEEE International Symposium on. 2010;2010:245–8. [Google Scholar]
- Milan JL, Planell JA, Lacroix D. Computational modelling of the mechanical environment of osteogenesis within a polylactic acid-calcium phosphate glass scaffold. Biomaterials. 2009;30:4219–26. doi: 10.1016/j.biomaterials.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Nesbitt KM, Jaquins-Gerstl A, Skoda EM, Wipf P, Michael AC. Pharmacological Mitigation of Tissue Damage during Brain Microdialysis. Analytical chemistry. 2013;85:8173–9. doi: 10.1021/ac401201x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Justice JB. Extracellular Concentration and In Vivo Recovery of Dopamine in the Nucleus Accumbens Using Microdialysis. Journal of Neurochemistry. 1992;58:212–8. doi: 10.1111/j.1471-4159.1992.tb09298.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic Press; Amsterdam ; Boston: 2005. [Google Scholar]
- Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. Journal of Neuroscience Methods. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Redgrave P. A modified push-pull system for the localised perfusion of brain tissue. Pharmacology Biochemistry and Behavior. 1977;6:471–4. doi: 10.1016/0091-3057(77)90187-3. [DOI] [PubMed] [Google Scholar]
- Retterer ST, Smith KL, Bjornsson CS, Turner JN, Isaacson MS, Shain W. Constant pressure fluid infusion into rat neocortex from implantable microfluidic devices. Journal of Neural Engineering. 2008;5:385–91. doi: 10.1088/1741-2560/5/4/003. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Hermans A, Seipel AT, Wightman RM. Monitoring Rapid Chemical Communication in the Brain. Chemical Reviews. 2008;108:2554–84. doi: 10.1021/cr068081q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaney TR, Mabrouk OS, Porter-Stransky KA, Aragona BJ, Kennedy RT. Chemical gradients within brain extracellular space measured using low flow push–pull perfusion sampling in vivo. ACS chemical neuroscience. 2012;4:321–9. doi: 10.1021/cn300158p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaney TR, Nie J, Hershey ND, Thwar PK, Linderman J, Burns MA, Kennedy RT. Push-Pull Perfusion Sampling with Segmented Flow for High Temporal and Spatial Resolution in Vivo Chemical Monitoring. Analytical Chemistry. 2011;83:5207–13. doi: 10.1021/ac2003938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A, Bungay PM, Gonzales RA. Characterization of probe and tissue factors that influence interpretation of quantitative microdialysis experiments for dopamine. Journal of Neuroscience Methods. 2003;126:1–11. doi: 10.1016/s0165-0270(03)00003-7. [DOI] [PubMed] [Google Scholar]
- Thongkhao-On K, Kottegoda S, Pulido JS, Shippy SA. Determination of amino acids in rat vitreous perfusates by capillary electrophoresis. Electrophoresis. 2004;25:2978–84. doi: 10.1002/elps.200405941. [DOI] [PubMed] [Google Scholar]
- Torregrossa MM, Kalivas PW. Microdialysis and the neurochemistry of addiction. Pharmacology Biochemistry and Behavior. 2008;90:261–72. doi: 10.1016/j.pbb.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Venton BJ, Kennedy RT. In vivo measurements of neurotransmitters by microdialysis sampling. Analytical Chemistry. 2006;78:1391–9. doi: 10.1021/ac0693722. [DOI] [PubMed] [Google Scholar]
- Weiss DJ, Lunte CE, Lunte SM. In vivo microdialysis as a tool for monitoring pharmacokinetics. TrAC Trends in Analytical Chemistry. 2000;19:606–16. [Google Scholar]
- West M, Slomianka L, Gundersen HJG. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. The Anatomical Record. 1991;231:482–97. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Zhou F, Braddock JF, Hu Y, Zhu X, Castellani RJ, Smith MA, Drew KL. Microbial origin of glutamate, hibernation and tissue trauma: an in vivo microdialysis study. Journal of Neuroscience Methods. 2002;119:121–8. doi: 10.1016/s0165-0270(02)00177-2. [DOI] [PubMed] [Google Scholar]