Abstract
Objective
To determine the effects of the NMDA receptor antagonist, memantine (0, 20, 40 mg/day), upon alcohol drinking and craving in heavy drinkers with or without a family history (FH) of alcoholism, and to explore the modulatory influence of the presence of impulsivity on these outcomes.
Methods
Ninety-two, non-treatment-seeking, heavy drinkers received memantine or placebo for eight days. On the eighth day, they received a priming dose of ethanol followed by a three-hour period of alcohol access.
Results
Memantine at a dose of 20 mg reduced alcohol craving but did not influence alcohol drinking. No effects of FH were observed. In participants with higher baseline levels of impulsivity, 40 mg of memantine reduced alcohol craving but increased alcohol drinking and alcohol-induced stimulation.
Conclusions
NMDA receptor signaling may play divergent roles in mediating alcohol cue-induced craving and alcohol drinking in heavy drinkers. The potential efficacy of memantine as monotherapy for alcohol use disorders may be limited by its tendency to disinhibit drinking in some individuals.
Keywords: Alcohol, Family History, Memantine, Drinking
Introduction
The N-methyl-D-aspartate (NMDA) glutamate receptor is among the highest affinity targets for ethanol in the brain (Grant and Lovinger, 1995). Ethanol-induced modulation of NMDA receptor function is observed in multiple brain regions at doses of ethanol that produce intoxicating behavioral effects in animals (Woodward, 1999; Gass and Olive, 2008). While acute ethanol administration dose-dependently attenuates NMDA receptor function (Grant and Lovinger, 1995; Hoffman et al., 1990), chronic exposure to ethanol enhances glutamatergic synaptic transmission (Nie et al., 1993), and produces cross-tolerance with NMDA receptor antagonists (Grant and Colombo, 1993; Krystal et al., 2003a). These findings are consistent with biochemical and physiological evidence that ethanol dependence is associated with upregulation of NMDA receptors and signaling via these receptors (Acosta et al., 2010; Krystal et al., 2003b).
Considering the above evidence, there is growing interest in the possibility that pharmacologic antagonism of NMDA receptors could play a role in the treatment of alcoholism by attenuating alcohol reward, cue-induced alcohol craving, or alcohol consumption (Holmes et al., 2013). In animals, NMDA receptor antagonists reduce operant responding for alcohol, sensitization of the locomotor response to alcohol, alcohol conditioned place preference, and alcohol self-administration (Holter et al., 1996; Lin and Hubbard, 1995; Vengeliene et al., 2005; Boyce-Rustay and Cunningham, 2004).
The uncompetitive NMDA receptor antagonist memantine, which has been shown to reduce alcohol drinking in animals (Escher and Mittleman, 2006), is a promising agent to test for the treatment of alcoholism. Two laboratory studies in humans (Bisaga and Evans 2004, Krupitksy et al. 2007) found that memantine in the dose range of 15 mg - 40 mg reduced cue-induced alcohol craving. An open-label clinical trial also reported reduction in alcohol drinking and urges to drink (Arias et al., 2007). However, a small 16-week, double-blind, placebo-controlled trial of memantine 40 mg/day dose in alcohol-dependent drinkers did not demonstrate any significant benefits for memantine (Evans et al., 2007). Thus, the potential value of memantine for reducing drinking and craving and the optimal dose for doing so remain open questions.
The purpose of this study was to conduct a controlled laboratory-based study of the dose-related effects of memantine on alcohol consumption, alcohol craving, and subjective response to alcohol in a sample of heavy drinkers. Because family history of alcoholism moderates the response to NMDA receptor antagonists (Narayanan et al., 2013; Jamadar et al., 2012: Petrakis et al., 2004), we evaluated the moderating effect of family history on the effects of memantine. Furthermore, we also explored the modulating influence of impulsivity based on emerging evidence that the NMDA system may be involved in impulsive behaviors, which may influence the decision to drink (Jentsch and Taylor, 1999; Winstanley et al. 2010), and that NMDA antagonists may alter impulsive choice by increasing preference for small immediate rewards (Cottone et al. 2013); while these were planned exploratory analyses we did not have a specific hypothesis about the directionality of the response. We conducted this evaluation using an alcohol drinking paradigm (ADP) that previously has been used to evaluate the effects of many medications on drinking behaviors (e.g., Anton et al., 2004; George et al., 2010; Krishnan-Sarin et al., 2007; McKee et al., 2009; O’Malley et al., 2002; Voronin et al., 2008).
Methods
Participants
We recruited non-treatment-seeking heavy drinkers, who reported drinking alcohol on at least 4 days per week, and consumed 25-70 drinks/week for men and 20-65 drinks for women (determined using Timeline Follow-Back (TFLB) methodology; Sobell and Sobell 1992). Recruited participants were classified as Family history Positive (FHP; a parent and one other first degree relative with alcohol problems) or negative (FHN; no first or second degree relatives with alcohol problems) based on historical criteria determined using the Family History Assessment Module developed by the Collaborative Study on the Genetics of Alcoholism (Rice et al 1995).
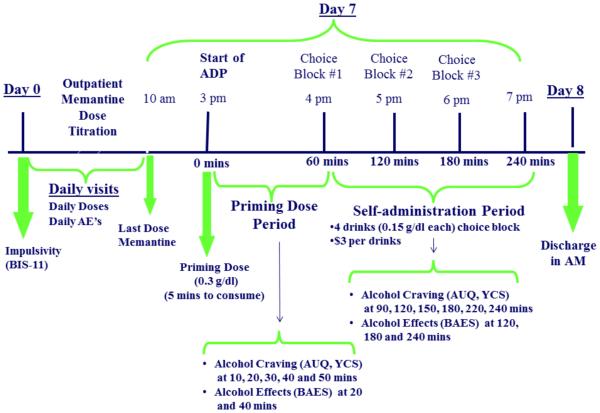
All procedures were approved by the Yale Institutional Review Board. After written informed consent was obtained, a psychiatric evaluation, physical examination, and laboratory assessments including urine toxicology and liver function tests were completed. Individuals were excluded if they 1) were currently taking prescribed or illicit psychotropic medications, 2) had a history of medical contraindications to memantine or alcohol, 3) evidenced significant symptoms of alcohol withdrawal at any intake appointment (defined as Clinical Institute Withdrawal Assessment for Alcohol scale ≥ 8; Sullivan et al., 1989) 4) were abusing or dependent on substances other than alcohol or nicotine, 5) Had any other current Axis-1 diagnoses or were on medications for any Axis-1 disorders, or 6) were pregnant or nursing. The laboratory procedures followed the NIAAA guidelines for administering alcohol in human experimentation (NIAAA 2005). Please see Figure 1 for the study design and timeline of procedures.
Figure 1.
Study Design
Medication
Eligible subjects were stratified by family history status and randomly assigned to receive either 20 mg/day or 40 mg/day of oral memantine or a matching placebo for eight days. Smoking status and gender were balanced within each FH group. Memantine doses were selected based on existing clinical studies (Bisaga and Evans, 2004; Krupitsky et al., 2007). The dose of memantine was titrated up to reduce the incidence of adverse events (20 mg/day group: 5 mg on Day 1, 10 mg on the Day 2 and 20 mg from Days 3-8; 40 mg/day group: 5 mg on Day 1, 10 mg on the Day 2, 20 mg on Day 3 and 30 mg on Day 4 and 40 mg from Days 5-8). Participants arrived between 10 am and 12 pm daily to take their medication and to complete assessments related to craving, drinking, and medication side effects. On the eighth day, participants were admitted to the Yale-New Haven Hospital Research Unit (HRU) at 10 am and received their last dose of medication. Lunch was provided at 12 pm.
Alcohol Drinking Paradigm (ADP)
After completing baseline assessments of alcohol craving and mood, participants consumed a priming dose of alcohol (PD; containing the subject’s preferred alcohol and designed to raise blood alcohol levels to 0.03 g/dl based on calculations of Watson, 1989) followed by a one-hour monitoring period during which additional alcohol was not available and alcohol effects and craving and blood alcohol levels were measured. This was followed by three one-hour self-administration periods during which participants were presented with a tray of 4 alcoholic drinks (each containing the subject’s preferred alcohol and designed to raise blood alcohol levels to 0.015 g/dl) and invited to choose between consuming each of the 4 drinks or receiving $3 per drink. The same procedures were repeated for the second and third self-administration periods. Thus, participants were exposed to the option of drinking up to 12 drinks (0.015 g/dl alcohol in each). Following completion of the session, participants spent the night at the HRU. They were discharged the next morning after receiving an intervention to motivate them to seek treatment that included feedback about their heavy-drinking behavior.
Alcohol Dose
The YNHH Investigational Pharmacy calculated and delivered the dose of alcohol for each participant to the HRU; the doses were designed to raise blood alcohol levels (to 0.03 g/dl for priming drink and 0.015 g/dl for all other drinks) based on the formula specified by Watson (1989) which takes into account gender, weight, and age of the subject. The research assistants mixed each alcohol dose with the participant’s preferred non-caffeinated, non-carbonated mixer in a 1:3 ratio.
Assessments
Drinks Consumed
Number of drinks consumed during each of the three self-administration periods was determined and summed to yield total drinks consumed.
Alcohol Craving
Craving was measured at 30 minutes prior to the priming dose (baseline), and then 10, 20, 30, 40 and 50 min during the priming dose period and every half hour during each self-administration period (i.e. 90, 120, 150, 180, 220 and 240 min) using two scales:
Yale Craving Scale (YCS) (Rojewski et al., under review)
The YCS is a five-item measure that employs a generalized Labeled Magnitude Scale (gLMS) to assess the intensity of craving for alcohol relative to the intensity of the strongest imaginable sensation. The scale includes descriptors of no sensation, barely noticeable, weak, moderate, strong, very strong, and the strongest imaginable. The spacing of these descriptors is quasi-logarithmic. The first item asks participants to rate the intensity of their desire to drink right now and the remaining items ask about craving for alcohol over the past week associated with four specific situations (i.e., strongest desire to drink experienced over the past week; desire to drink when you made an effort to avoid drinking; desire to drink when you found yourself in a stressful situation; desire to drink after having your first drink). Prior to providing craving ratings for alcohol, participants complete a training exercise to ensure that they understand how to use the scale. A psychometric evaluation of the YCS suggests that it evidenced: 1) good internal consistency, 2) scalar measurement invariance which makes it well suited for between group comparisons, and 3) concurrent relationships with drinking outcomes (Rojewski et al., under review). The training exercise and the full 5-item YCS were administered at baseline. During the laboratory sessions, the first item (“desire to drink right now”) was used. To confirm that use of the single item scale was psychometrically justifiable; we conducted a series of bivariate correlations using baseline data (n = 111) to evaluate relationships between the YCS item assessing current alcohol craving and the total YCS score as well as the Alcohol Urge Questionnaire (Bohn et al., 1995). Results of these analyses indicated that the single item craving scale correlated strongly with the YCS total scale score (r=0.78, p<0.001) and the AUQ (r=0.59, p<0.001). We chose to use the YCS as the index of craving over the AUQ based on evidence demonstrating the superior psychometric properties of the YCS relative to the AUQ in measuring alcohol craving in dependent and non-dependent drinkers (Rojewski et al., under review).
Alcohol Behavioral Effects
were determined at 10, 20 and 50 minutes during the priming drink period and then every hour at the end of each of the three self-administration periods with the
Biphasic Alcohol Effects Scale
(BAEs; Martin et al., 1993). This 14-item adjective rating scale is used to measure the stimulant and sedative effects of alcohol and has been found to be sensitive to medication effects on alcohol intoxication (Swift et al., 1994). A subset of 6-items comprise the Brief BAES which has been observed to have similar psychometric properties as the original BAES (Rueger & King, 2013). To determine which version of the measure was best suited for use with the current data, we ran a series of confirmatory factor analyses using our data. We evaluated goodness of model fit using the following criteria: CFI > .90, RMSEA < .08 and SRMR < .08. The models evaluating the fit of the Brief BAES evidenced acceptable to excellent fit across all times points (i.e., 20, 50, 110 minutes) with the exception of the RMSEA at 50 minutes (i.e., fit at 20 minutes CFI = .91, RMSEA = .07, SRMR = .06; fit at 50 minutes CFI = .91, 2RMSEA = .12, SRMR = .04; fit at 110 minutes RMSEA = .99, RMSEA = .03, SRMR = .04). The models evaluating the original BAES fit the data poorly (CFI < .90; RMSEA and SRMR > .08). Therefore we chose to use the Brief BAES.
Impulsivity
was determined at the baseline intake session using the
Barratt Impulsiveness Scale, Version 11
(BIS-11; Patton et al., 1995). This self-report 30-item scale is designed to assess a range of impulsive tendencies using a 4-point scale ranging from “rarely/never” to “almost always/always.” The measure is the most widely used impulsivity measure. Based on recent findings of a large factor analytic study (Morean et al., in press) we used two psychometrically sound, four-item subscales to reflect impulsive behaviors (e.g., “I do things without thinking”) and impaired self-regulation (e.g., “I am a careful thinker” [reverse coded]). Both subscales were internally consistent and normally distributed (Behavioral Impulsivity: Cronbach’s α = .79; mean = 9.64, S.D. = 2.69, median = 10.00; Impaired Self-Regulation: Cronbach’s α = .72; mean = 9.42, S.D. = 2.44, median = 10.00). The subscales evidenced moderate overlap (r = .38).
Data Analyses
Baseline demographics, drinking characteristics and adverse events were compared among family history (FH) and medication (MED) conditions using t-tests, ANOVAs, and chi-square tests as appropriate. Drinking during the outpatient treatment period was evaluated using an ANOVA model with FH, MED and time (seven days of outpatient treatment).
There were two primary outcomes of interest based on the original analytic plan: total drinks consumed and craving (YCS), each tested at the α=.05 threshold. For the primary analyses, a series of ANOVA’s were run to examine the interaction of FH status and memantine dose. Specifically, we first analyzed the “Total number of drinks consumed” using an ANOVA model that included MED (0, 20, 40 mg memantine) and FH (negative, positive) as between-subjects factors, with main effects and interactions modeled. Potential confounding factors (sex, age, and baseline drinking) initially were included in the model, but ultimately were dropped if not significant for parsimony. We then used a model mirroring that described above for YCS scores with time (see study time points) added as a within-subjects factor and baseline YCS scores included as a model covariate; main effects, 2-way interactions, and 3-way interactions between MED, FH, and time were modeled. The best-fitting variance-covariance structure was based on the Schwartz-Bayesian Criterion (BIC).
Secondary analyses were considered significant after adjusting the alpha threshold based on the six outcomes tested (Bonferroni-adjusted α=.008). For the model evaluating BAES scores we employed a nonparametric approach for repeated measures data (Brunner and Puri, 2002) due to the fact that BAES data were highly skewed with transformations failing to normalize the data. Thus, BAES data were first ranked, and then fitted using a mixed effects model with an unstructured variance-covariance matrix and p-values adjusted for ANOVA-type statistics (ATS; Brunner and Puri, 2002).
For exploratory analyses examining the interaction of impulsivity with memantine dose, we used ANOVA models mirroring those outlined above, but which included impulsive behavior (in lieu of family history status) and memantine dose as between subjects factors, and time as a within-subjects factors (when appropriate as in earlier models) to examine total drinks consumed, YCS scores, and brief-BAES scores. Treating impulsivity as either continuous or dichotomous (median) produced similar results; thus, to facilitate interpretation the categorical results are presented.
Results
Baseline Characteristics
305 heavy drinking, potential participants gave written informed consent. Of these, 111 were randomly assigned to receive 0, 20 or 40 mg/day of memantine. Among those randomized, 5 subjects never started the study, 7 people dropped out due to side effects prior to laboratory test days, 4 individuals were excluded from the study due to the repeated presence of illicit drugs in their urine, and 5 participants withdrew from the study due to personal or family conflicts. The 90 study completers were mostly male (n=64, 71%) with a diverse racial distribution (61 Caucasian, 23 African American, 6 Other) and an average age of 30.9 (SD=8.5). The sample comprised an equal number of FHN and FHP individuals (n=45); of the 90 completers, 12 (13%) met DSM-IV criteria for alcohol abuse and 78 (87%) for alcohol dependence (determined using SCID-IV; First et al., 1996). During the 30 days prior to the laboratory session, FHN participants consumed 162 (SD=57) drinks and FHP consumed 169 (SD=53) drinks. No differences in baseline demographic characteristics or alcohol consumption were observed based on FH status or medication condition (see Table 1). Similarly, no differences in alcohol consumption were noted during the seven-day outpatient memantine treatment period
Table 1.
Demographic information on participants. All comparisons of MED groups within each FH condition were non-significant.
| Familly History Negative (n=45) | Family History Positive (n=45) | |||||
|---|---|---|---|---|---|---|
| Placebo (n=16) |
20 mg (n=15) |
40 mg (n=14) |
Placebo (n=15) |
20 mg (n=16) |
40 mg (n=14) |
|
| Male, n (%) | 12 (75%) | 10 (67%) | 11 (79%) | 10 (67%) | 11 (69%) | 10 (71%) |
| Smokers, n (%) | 6 (38%) | 9 (40%) | 9 (36%) | 9 (60%) | 7 (44%) | 8 (57%) |
| Age (SE) | 28.2 (1.7) | 31.1 (2.7) | 32.1(2.4) | 28.2 (1.6) | 34.3 (2.1) | 31.9 (2.3) |
| Total # drinksa, mean (SE) |
182.1 (18.1) |
139.5 (11.1) |
162.9 (11.4) | 166.9 (15.4) |
168.7 (13.5) |
173 (12.5) |
| Drinks/drinking daya, mean (SE) |
7.9 (0.7) | 5.8 (0.4) | 6.4 (0.5) | 7.1 (0.7) | 7.1 (0.5) | 6.9 (0.6) |
Drinking data is based on the 90-day baseline period obtained with the Time-Line Follow-back Interview.
Memantine Adverse Events
There was a statistically significant effect of memantine dose in the number of individuals reporting adverse effects, with the 40 mg dose producing greater side effects than the 20 mg or placebo. Across groups, the predominant adverse events included headache (11.6%), dizziness (5.6%), nausea (6.9%), fatigue (6.0%) and nervousness (5.35%). However, we did not observe specific relationships between rates of drop out and memantine dose. The frequency of adverse events did not differ by FH status.
Primary Outcomes
Drinking
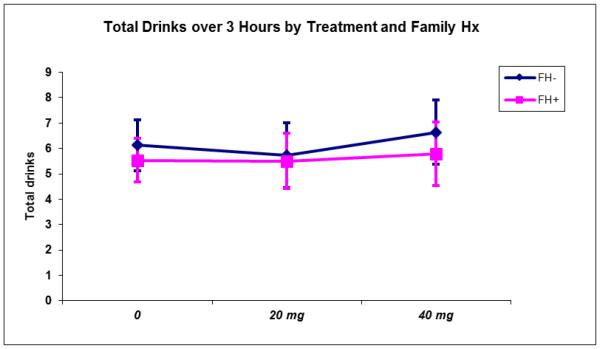
There were no main or interactive effects of FH or MED status on total number of drinks consumed during the ad-lib drinking sessions (see Figure 2).
Figure 2.
Total number of drinks consumed during the three-hour self-administration period by FH status and MEM dose.
Alcohol Craving
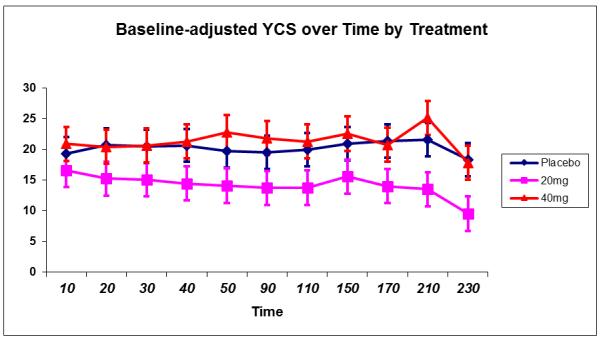
A main effect of MED (F(2,79)=3.1, p=.05) was observed on baseline-adjusted YCS scores where craving was reduced overall among those receiving 20 mg MEM compared to both placebo (p=.05) and 40 mg MEM (p=.02). A main effect of time (F(10,780)=3.5, p=.0002) was observed due to lower craving scores at the end of the SA period, particularly among the 20 mg MEM group. No interactive effects with MEM were observed (all p>.61). Memantine effects on craving are depicted in Figure 3.
Figure 3.
Alcohol Craving (Yale Craving Scale) during the priming dose and self-administration periods by MEM dose.
Secondary Outcomes and Analyses
Alcohol-Induced Stimulation and Sedation by Family History
For stimulation, there was a significant MED by time interaction (num df=7.2, ATS=3.2, p=.002) explained by linear increases in stimulation irrespective of FH status over the course of the drinking session among the 40 mg group (p=.001) but not among those receiving placebo (p=.23) or 20 mg memantine (p=.62). Remaining model effects were not significant. The unadjusted significant effects presented for stimulation remain significant at the adjusted α=008 threshold.
For sedation, no significant effects were observed.
Exploratory Analyses of Impulsivity Status in lieu of Family History
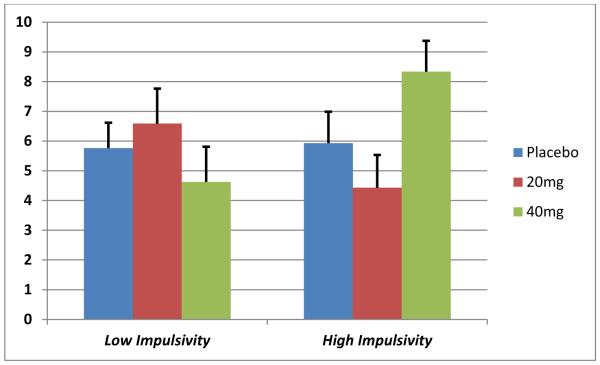
For total drinks consumed during the ad-lib session, a MED by impulsivity interaction was observed (F(2,84)=3.5, p=.03). Post-hoc tests showed subjects with heightened impulsivity administered 40 mg MEM consumed more drinks compared to both their less impulsive counterparts receiving 40 mg MEM (p=.02) and compared to subjects with elevated impulsivity receiving 20 mg MEM (p=.02). These effects are depicted in Figure 4.
Figure 4.
Total number of drinks consumed during the three-hour self-administration period by Impulsivity status and MEM dose.
In terms of craving, a significant MED by impulsivity by time interaction was observed for YCS scores (F(20,79)=3.1, p=.02). Post-hoc testing, however, revealed no discernable MED differences across levels of impulsivity and time.
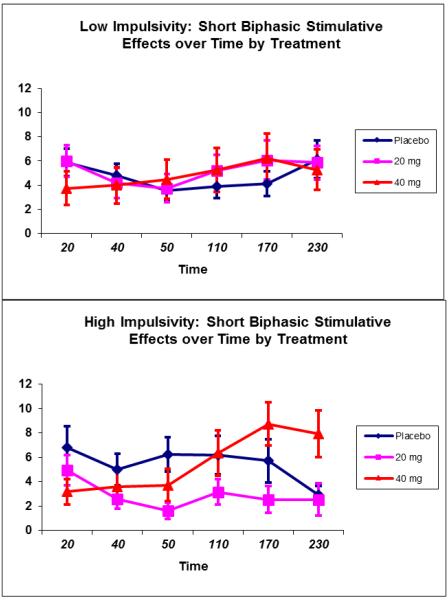
For stimulation, a trend for a MED by impulsivity by time interaction (p=.06) was observed (see Figure 5) primarily due to increased stimulation at the end of the ad-lib drinking session (170 and 230 min) among high impulsive subjects treated with 40 mg MEM compared to subjects on 20 mg MEM (p=.02 and .01, respectively). No discernable main or interactive effects of sedation were observed.
Figure 5.
Alcohol-induced stimulation (Short-Biphasic Alcohol Effects Scale) by Impulsivity status and MEM dose
Discussion
The main finding from this study was that the NMDA glutamate receptor antagonist memantine, at the 20 mg dose reduced craving for alcohol in this drinking paradigm in alcohol dependent heavy drinkers. Having reduced the subjective urge to drink, one might have expected that memantine would have reduced alcohol consumption as well. However, this was not the case. Thus, this study raises the possibility that memantine disrupts the common associations between reward-related aspects of alcohol and consumption presumably by blocking NMDA glutamate receptors.
The current findings are consistent with earlier observations that memantine reduces craving in alcohol-consuming humans; Bisaga and Evans (2004) observed that, memantine reduced craving prior to alcohol consumption in “light-moderate” (10-30 drinks/week) drinkers but failed to suppress the craving that emerged following alcohol consumption, while Krupitsky and colleagues (2007) observed that memantine produced a dose-related reduction in craving elicited by an alcohol cue (holding a drink) in abstinent alcohol dependent inpatients. In the current study, we observed that memantine produced reductions in alcohol craving following exposure to the priming dose of alcohol in drinkers who had more habitual and heavy drinking patterns. However, the fact that the lower, but not the higher dose of memantine decreased alcohol craving was unexpected given earlier observed dose dependent effects on craving (Krupitsky et al ., 2007). The discrepant findings may be due to differences in design features and study populations between the studies. For example, Krupitsky and colleagues studied abstinent alcoholics and found that memantine had dose dependent ethanol-like effects independent of exposure to alcohol related cues. In contrast, our study examined responses following exposure to alcohol and alcohol cues among heavy drinkers.
Our results also suggest that higher doses of memantine increase the stimulatory effects of ethanol, which is consistent with prior reports of NMDA receptor antagonist-ethanol interactions in animals (Meyer and Phillips 2003). Stimulation from alcohol is known to be reinforcing and associated with drinking (Crabbe et al., 2010; King et al., 2011; 2014). Therefore, the observed increase in stimulation in our study could explain why the high dose of memantine did not reduce craving or drinking. Thus, it may be the case that using lower doses of antagonists like memantine, which do not appear to enhance stimulation, or other methods of modulating the NMDA system may be a better approach to lowering alcohol craving in habitual heavy drinkers.
As indicated earlier, our results suggest that there may be dissociation between the ability of memantine to reduce alcohol craving and its failure to suppress alcohol consumption. In this regard, this study provides an important addition to the literature by suggesting that alcohol consumption may be driven, only in part, by goal-directed processes over which people are aware and exert voluntary control. At least two processes may have contributed to the persistence of drinking in the face of reduced alcohol craving. The first is that NMDA antagonism with memantine may have compromised the executive control over impulsive behaviors like excessive drinking. Blockade of NMDA receptors has been shown to increase impulsive responding in humans and animals (Floresco et al. 2008), suggesting that decreased glutamate/NMDA function may contribute to diminished inhibition of behavior in psychiatric disorders (Amitai and Markou 2010; Pattij and Vanderschuren 2008). Prior studies from our group showed that memantine impaired the recruitment of executive control networks, as evaluated with fMRI (Jamadar et al., 2012). Exploratory analyses in the current study indicate that baseline levels of impulsivity were associated with memantine-related changes in alcohol consumption; specifically, those who were more impulsive had increases in alcohol consumption at the higher dose suggesting that memantine may have further disinhibited drinking in these participants. Interestingly, these increases in drinking were accompanied by reductions in craving, supporting the idea that memantine treatment leads to a dissociation between drinking and craving in the heavy drinkers. These exploratory findings require replication in future studies.
Another consideration is that the heavy drinkers we studied are probably habitual drinkers who drink in a rather automatic fashion in response to alcohol cues (i.e., in the absence of goal-directed choice) and continue drinking even in the absence of alcohol reward. The persistence of drinking despite the reduction in the urge to drink following memantine is consistent with the presence of habitual drinking. Interestingly, the effects of memantine contrasted with those of naltrexone in an earlier laboratory study (Krishnan-Sarin et al., 2007) where we observed no effects on alcohol craving (unpublished data) but reduced alcohol drinking. When considering the naltrexone and memantine findings in concert, maximum potential therapeutic benefit may be achieved by combining these two medications which affect different alcohol related processes.
It is important to note that this study has some limitations. First, we studied NMDA antagonism with memantine which blocks not only NMDA receptors but also alpha-7 nicotinic receptors and 5-HT3 receptors with lower affinity (Aracava et al., 2005; Rammes et al., 2001). The relationship between memantine occupancy at these other receptors and the current findings is not known. Our study was also not designed to examine longer term outcomes. For example, it is possible that the observed reduction in craving with memantine might be of benefit to promote continued abstinence among individuals who have quit drinking; future studies should use different models to examine this issue.
In summary, a low dose (20 mg/day) of the NMDA receptor antagonist memantine reduced alcohol craving primed by the consumption of an alcohol drink. However, it failed to reduce alcohol consumption. Thus, our results add to the current understanding of the complex relationship between alcohol craving and drinking and highlight the equally complicated role of NMDA glutamate receptors in both processes. In so doing, the current study supports the importance of NMDA receptor systems in mediating alcohol craving but also raises cautionary notes regarding the promise of NMDA receptor antagonism as a therapeutic approach for alcoholism. Future studies may need to examine more nuanced approaches of modulating the NMDA system, through other receptor or second messenger systems, which may reduce alcohol craving and drinking without increasing positive alcohol effects or impulsive responding. Mapping the biology of alcohol reward and consumption in humans appears to be an important step in translating preclinical advances into future therapeutics.
Acknowledgements
This research was supported by the National Institute on Alcohol Abuse and Alcoholism Center Grant P50AA12870, M01RR00125. Dr. Krystal has served as a consultant to the following pharmaceutical companies: Atlas Venture, Aventis Pharmaceuticals, Biomedisyn Corporation, Bristol-Meyers Squibb, Centre de Recherche Pierre Fabre, Cypress Bioscience, Inc., Eli Lilly and Co., Fidelity Biosciences, Forest Laboratories, Glaxo-SmithKline, Janssen Research Foundation, Merz Pharmaceuticals, Organon International, Inc (CNS Advisory Board), Pfizer Pharmaceuticals, Shire Pharmaceuticals, Sumitomo Pharmaceuticals America, Ltd., Takeda Industries, UCB Pharma and US Micron. Dr. O'Malley has received research support through contracts (principally for company sponsored trials) or donated clinical supplies from Alkermes, Lipha Pharmaceuticals, GlaxoSmithKline, Forest Laboratories, Dupont, OrthoMcNeil, Bristol-Myers Squib, Sanofi-Aventis, and Mallinckrodt. She has consulted to Alkermes, Pfizer, Johnson&Johnson/OrthoMcNeil, GlaxoSmithKline, Eli Lilly and Forest Laboratories and received travel reimbursement from Alkermes.
REFERENCES
- Acosta G, Hasenkamp W, Daunais JB, Friedman DP, Grant KA, Hemby SE. Ethanol self-administration modulation of NMDA receptor subunit and related synaptic protein mRNA expression in prefrontal cortical fields in cynomolgus monkeys. Brain Res. 2010;1318:144–54. doi: 10.1016/j.brainres.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai N, Markou A. Disruption of Performance in the Five-Choice Serial Reaction Time Task Induced By Administration of N-Methyl-D-Aspartate Receptor Antagonists: Relevance to Cognitive Dysfunction in Schizophrenia. Biol Psychiat. 2010;68(1):5–16. doi: 10.1016/j.biopsych.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, Drobes DJ, Voronin K, Durazo-Azizv R, Moak D. Naltrexone effects on alcohol consumption in a clinical lab paradigm: temporal effects of drinking. Psychopharmacology. 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Aracava Y, Pereira EF, Maelicke A, Albuquerque EX. Memantine blocks alpha7*nicotinic acetylcholine receptors more potently than n-methyl-D-aspartate receptors in rat hippocampal neurons. J Pharmacol Exp Ther. 2005;312(3):1195–205. doi: 10.1124/jpet.104.077172. [DOI] [PubMed] [Google Scholar]
- Arias AJ, Feinn R, Covault J, Kranzler HR. Memantine for Alcohol Dependence: An Open-label Pilot Study. Addict Disord Their Treat. 2007;6:77–83. [Google Scholar]
- Bisaga A, Evans SM. Acute effects of memantine in combination with alcohol in moderate drinkers. Psychopharmacology. 2004;172(1):16–24. doi: 10.1007/s00213-003-1617-5. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cunningham CL. The Role of NMDA Receptor Binding Sites in Ethanol Place Conditioning. Behav Neurosci. 2004;118(4):822–834. doi: 10.1037/0735-7044.118.4.822. [DOI] [PubMed] [Google Scholar]
- Brunner E, Puri ML. A class of rank-score tests in factorial designs. J Stat Plan Infer. 2002;103(1):331–360. [Google Scholar]
- Cottone P, lemolo A, Narayan AR, Kwak J, Momaney D, Sabino V. The uncompetitive NMDA receptor antagonists ketamine and memantine preferentially increase the choice for a small, immediate reward in low-impulsive rats. Psychopharmacology. 2013;226(1):127–138. doi: 10.1007/s00213-012-2898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Belknap JK. Belknap The complexity of alcohol drinking: Studies in rodent genetic models. Behav Genet. 2010;40:737–750. doi: 10.1007/s10519-010-9371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escher T, Mittleman G. Schedule-induced alcohol drinking: non-selective effects of acamprosate and naltrexone. Addict Biol. 2006;11:55–63. doi: 10.1111/j.1369-1600.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- Evans SM, Levin FR, Brooks DJ, Garawi F. A pilot double-blind treatment trial of memantine for alcohol dependence. Alcohol Clin Exp Res. 2007;31:775–782. doi: 10.1111/j.1530-0277.2007.00360.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams Janet BW. Structured clinical interview for DSM-IV—clinical version (SCID-CV) (User's Guide and Interview) American Psychiatric Press, Inc; Washington, D.C.: 1996. [Google Scholar]
- Floresco SB, Maric TL, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort-and delay-based decision making. Neuropsychopharmacol. 2008;33(8):1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DT, Herion DW, Jones CL, Phillips MJ, Hersh J, Hill D, Heilig M, Ramchandani VA, Geyer C, Spero DE, Singley ED, O'Malley SS, Bishai R, Rawlings RR, Kunos G. Rimonabant (SR141716) has no effect on alcohol self-administration or endocrine measures in non-treatment-seeking heavy alcohol drinkers. Psychopharmacology (Berl) 2010;208(1):37–44. doi: 10.1007/s00213-009-1704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Colombo G. Discriminative stimulus effects of ethanol: effect of training dose on the substitution of N-methyl-D-aspartate antagonists. J Pharm Exp Ther. 1993;264(3):1241–1247. [PubMed] [Google Scholar]
- Grant KA, Lovinger DM. Cellular and behavioral neurobiology of alcohol: receptor-mediated neuronal processes. Clin Neurosci. 1995;3:155–164. [PubMed] [Google Scholar]
- Hoffman PL, Rabe CS, Grant KA, Valverius P, Hudspith M, Tabakoff B. Ethanol and the NMDA receptor. Alcohol. 1990;7:229–231. doi: 10.1016/0741-8329(90)90010-a. [DOI] [PubMed] [Google Scholar]
- Holmes A, Spanagel R, Krystal JH. Glutamatergic targets for new alcohol medications. Psychopharmacology (Berl) 2013;229(3):539–554. doi: 10.1007/s00213-013-3226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holter SM, Danysz W, Spanagel R. Evidence for alcohol antl-craving properties of memantine. Eur J Pharmacol. 1996;314:RI-2. doi: 10.1016/s0014-2999(96)00670-x. [DOI] [PubMed] [Google Scholar]
- Jamadar S, DeVito EE, Jiantonio RE, Meda SA, Stevens MC, Potenza MN, Krystal JH, Pearlson GD. Memantine, an NMDA receptor antagonist, differentially influences Go/No-Go performance and fMRI activity in individuals with and without a family history of alcoholism. Psychopharmacology (Berl) 2012;222(1):129–40. doi: 10.1007/s00213-011-2628-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146(4):373–90. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Arch Gen Psychiat. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, Cao D. Alcohol Challenge Responses Predict Future Alcohol Use Disorder Symptoms: A 6-Year Prospective Study. Biol psychiat. 2014;75(10):798–806. doi: 10.1016/j.biopsych.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Krystal JH, Shi J, Pittman B, O’Malley SS. Family history of alcoholism influences naltrexone-induced reduction in alcohol drinking. Biol psychiat. 2007;62(6):694–697. doi: 10.1016/j.biopsych.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Limoncelli D, Webb E, Gueorguieva R, D'Souza DC, Boutros NN, Trevisan L, Charney DS. Altered NMDA glutamate receptor antagonist response in recovering ethanol-dependent patients. Neuropsychopharmacol. 2003a;28(11):2020–2028. doi: 10.1038/sj.npp.1300252. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Mason G, Trevisan L, D'Souza DC. N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther. 2003b;99(1):79–94. doi: 10.1016/s0163-7258(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Neznanova O, Masalov D, Burakov AM, Didenko T, Romanova T, Tsoy M, Bespalov A, Slavina TY, Grinenko AA, Petrakis IL, Pittman B, Gueorguieva R, Zvartau EE, Krystal JH. Effect of memantine on cue-induced alcohol craving in recovering alcohol-dependent patients. Am J Psychiat. 2007;164:519–523. doi: 10.1176/ajp.2007.164.3.519. [DOI] [PubMed] [Google Scholar]
- Lin N, Hubbard JI. An NMDA receptor antagonist reduces ethanol preference in untrained but not trained rats. Brain Res Bull. 1995;36:421–424. doi: 10.1016/0361-9230(94)00215-m. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- McKee SA, Harrison ELR, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto M, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy drinking smokers. Biol psychiat. 2009;66:185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Phillips TJ. Sensitivity to ketamine, alone or in combination with ethanol, is altered in mice selectively bred for sensitivity to ethanol's locomotor effects. Alcohol Clin Exp Res. 2003;27(11):1701–1709. doi: 10.1097/01.ALC.0000093602.00193.39. [DOI] [PubMed] [Google Scholar]
- Morean ME, DeMartini KS, Leeman RF, Pearlson GD, Anticevic A, Krishnan-Sarin S, Krystal JH, O'Malley SS. Psychometrically Improved, Abbreviated Versions of Three Classic Measures of Impulsivity and Self-Control. Psychol Assessment. 2014;26(3):1003–1020. doi: 10.1037/pas0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan B, Stevens MC, Jiantonio RE, Krystal JH, Pearlson GD. Effects of memantine on event-related potential, oscillations, and complexity in individuals with and without family histories of alcoholism. J Stud Alcohol Drugs. 2013;74(2):245–257. doi: 10.15288/jsad.2013.74.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Advisory Council on Alcohol Abuse and Alcoholism Recommended Council Guidelines on Ethyl Alcohol Administration in Human Experimentation. 2005 doi: 10.1111/j.1530-0277.2009.00988.x. Revised May 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Yuan X, Madamba SG, Siggins GR. Ethanol decreases glutamatergic synaptic transmission in rat nucleus accumbens in vitro: naloxone reversal. J Pharm Exp Ther. 1993;266:1705–1712. [PubMed] [Google Scholar]
- O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek J. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. TrendsPharmacol Sci. 2008;29(4):192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, Trevisan L, Gelernter J, Krystal JH. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiat. 2004;161(10):1776–82. doi: 10.1176/ajp.161.10.1776. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Rammes G, Rupprecht R, Ferrari U, Zieglgänsberger W, Parsons CG. "The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner". Neurosci Lett. 2001;306(1-2):81–84. doi: 10.1016/s0304-3940(01)01872-9. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history of diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Rojewski AM, Morean ME, Toll BA, McKee SA, Krishnan-Sarin S, Green BG, Bartoshuk LM, O’Malley SS. The Yale Craving Scale: Development and Psychometric Properties. Psychol Assessment; (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueger SY, King AC. Validation of the Brief Biphasic Alcohol Effects Scale (B-BAES) Alcohol Clin Exp Res. 2013;37:470–476. doi: 10.1111/j.1530-0277.2012.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press, Inc.; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: The revised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar) Brit J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Swift RM, Whelihan W, Kuznetsov O, Buongiorno G, Hsuing H. Naltrexone-induced alterations in human ethanol intoxication. Am J Psychiat. 1994;151:1463–1467. doi: 10.1176/ajp.151.10.1463. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bachteler D, Danysz W, Spanagel R. The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacology. 2005;48:822–829. doi: 10.1016/j.neuropharm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Voronin K, Randall P, Myrick H, Anton R. Aripiprazole Effects on Alcohol Consumption and Subjective Reports in a Clinical Laboratory Paradigm-Possible Influence of Self-Control. Alcohol Clin Exp Res. 2008;32(11):1954–1961. doi: 10.1111/j.1530-0277.2008.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson TE. Total body water and alcohol levels: updating the fundamentals. In: Krow KE, Batt RD, editors. Human metabolism of alcohol. CRC Press; FL: 1989. pp. 41–66. [Google Scholar]
- Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight Into the Relationship Between Impulsivity and Substance Abuse From Studies Using Animal Models. Alcohol Clin Exp Res. 2010;34:1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JJ. Ionotropic glutamate receptors as sites of action for ethanol in the brain. Neurochem Int. 1999;35:107–113. [PubMed] [Google Scholar]