Abstract
Objective
Higher serum leptin levels have been associated with a modestly higher incidence of cardiovascular disease in studies involving mostly Caucasian men. We aimed to assess the hypothesis that higher baseline levels of serum leptin are associated with higher risk of future cardiovascular disease in a diverse cohort.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) is a modern, community-based, ethnically-diverse, and sex-balanced prospective cohort study of US adults free from cardiovascular disease. Serum leptin was measured in an ancillary study in 2002-2005. This analysis included 1,905 MESA participants with baseline leptin and incident cardiovascular event data. Leptin levels were modeled as a log-transformed continuous variable and multivariable-adjusted Cox regression was performed for the primary outcome of hard cardiovascular disease, including coronary heart disease and stroke.
Results
The median follow-up was 7.6 years (25th-75th 7.1-8.3) with 7,051 and 6,738 person-years of follow-up in women and men. A hard cardiovascular disease event occurred in 47 women and 63 men. The age- and ethnicity-adjusted hazard ratio estimates for a 1 standard deviation increase in ln(leptin) were 1.16 in women (95% CI 0.78-1.73, p=0.46) and 0.91 (95% CI 0.69-1.20, p=0.51) in men. Pooling sexes, and adjusting for sex in addition to age and ethnicity, estimates were 0.98 (95% CI 0.78-1.23, p=0.89). With additional adjustment for cardiovascular risk factors, the results remained nonsignificant: 0.87 (95% CI 0.68-1.11, p=0.26).
Conclusion
In conclusion, in a modern, US prospective cohort study of multi-ethnic women and men of multi-ethnic backgrounds, leptin levels are not associated with incident cardiovascular events.
Keywords: leptin, obesity, atherosclerosis, cardiovascular disease, heart failure
INTRODUCTION
Leptin is a cytokine with pleiotropic function that is mainly secreted by adipose tissue. It is intricately involved in energy homeostasis, and is potentially implicated in obesity-related cardiovascular disease (CVD).1 Considering more than one-third of adults in the United States are obese,2 and another third are overweight, further understanding the importance of leptin in CVD is of interest. Existing data indicate that leptin signaling could directly contribute to unfavorable levels of CVD risk factors and atherosclerosis, and that higher circulating levels may also indicate a harmful state of leptin resistance.1 However, potentially harmful effects of leptin may be countered by other pleiotropic effects. For example, increased leptin is linked with hypertension3 yet more favorable left ventricular structure and function.4,5
To elucidate the net relevance of leptin signaling to CVD, longitudinal studies of more downstream clinical outcomes are needed. Prospective studies have linked higher baseline leptin levels with the subsequent development of coronary heart disease (CHD),6 CVD,7 and heart failure (HF).8 However, these studies predominantly involved older Caucasian men. Therefore, there has been a call for studies with greater diversity in the ethnicity and sex of participants.9 There is also a need for prospective evaluation of leptin in relation to mortality, and examination of whether factors such as systematic inflammation modify leptin-related CVD risk.1,10
To address these issues and further explore leptin’s possible role in CVD, we conducted an analysis of ancillary study data in the Multi-Ethnic Study of Atherosclerosis (MESA) that tested the hypothesis that higher baseline levels of circulating leptin would be associated with higher CVD risk. Based on prior data,1,10,11 we further hypothesized that risk associations of leptin levels would be modified by other factors; in particular, we expected a strengthening of the risk association in the presence of high C-reactive protein levels.
METHODS
Study Participants
The MESA study is a prospective, population-based cohort study of individuals without clinical cardiovascular disease; its detailed design and organization are available in previous reports.12,13 From July 2000 to September 2002, 6,814 adults were recruited at 6 US field centers (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and St. Paul, Minnesota). This analysis includes a randomly selected sample of 1,905 participants who had leptin measurements and follow-up for CVD events.3,4,14 Each site’s Institutional Review Boards approved the study, and all participants gave written informed consent.
Anthropometric and Adipokine Measurement
At MESA visits 2 or 3, from 2002 to 2005, anthropometric and adipokine measurements were made. Height and weight were measured by a stadiometer and calibrated scale. Body mass index was calculated as weight in kilograms divided by the square of the height in meters. Waist circumference at the umbilicus was measured to the nearest 0.1 centimeters using a steel measuring tape. MESA participants also provided blood samples after a 12 hour fast. At the MESA central laboratory (University of Vermont, Burlington, Vermont), diluted multiplex panels were used for human serum adipokine measurements and validated against gold standard ELISAs. In a neat sample (50 μL EDTA), leptin was measured as part of the human serum adipokine LINCOplex Kit (Linco Research, Inc.; St. Charles, MO). The inter-assay CV for leptin was 10.9% and intra-assay CV was 6.5%. Inter-assay CV's were based on a mean of 4 control samples, with two of the controls from recombinant proteins, one from a single donor EDTA plasma, and one from a pool of high CRP serums. Intra-assay CVs were determined after running a single sample multiple times on a plate. CVs reflect all of the variation in assay steps including pipetting, and variation arising from the assay itself.
Risk Factor Assessment
Concomitant to the measures described above, each center also collected updated information on cardiovascular risk factors. Based on their self-report of cigarette use, MESA participants were categorized as current cigarette smokers, former smokers, or persons who had never smoked. With the participant in a seated position, resting blood pressure was measured 3 times using a Dinamap Pro 1000 automated oscillometric sphygmomanometer (Critikon); the average of the last two measurements was used in the analysis. The same central laboratory that measured adipokines also measured lipid profiles, glucose, creatinine, and high-sensitivity C-reactive protein after a 12 hour fast. Diabetes mellitus was defined as either the presence of a fasting plasma glucose level ≥125 mg/dL or a history of treatment with diabetes medications.
Follow-Up
Only events and time-to-event after the measurement of leptin levels were considered; those with CVD prior to exam 2/3 were excluded. MESA investigators tracked study participants who were free of cardiovascular disease at visit 2/3 for subsequent cardiovascular events over a median of 7.6 years. At intervals of 9 to 12 months, trained study personnel contacted participants or family members by telephone and asked them whether any hospital admissions, outpatient diagnoses of cardiovascular disease, or deaths, had occurred. If such an event was reported, then an attempt was made to adjudicate the event by review of the participants’ medical records. For participants who reported hospital admissions, records were obtained 98% of the time, and for participants who reported outpatient cardiovascular diagnoses, records were obtained in 95% of cases. Follow-up telephone interviews were completed in 92% of living participants. For participants who were reported to have died outside of the hospital, the next of kin were interviewed and copies of death certificates were requested. Two physicians on the MESA mortality and morbidity review committee independently classified events; if there was any difference in classification by these physicians, then the full committee made the final decision.
Consistent with the 2013 American College of Cardiology / American Heart Association prevention guidelines,15,16 our primary endpoint for analysis was hard CVD events. In MESA, hard CVD was defined as myocardial infarction, death from CHD, resuscitated cardiac arrest, stroke, or stroke death. As secondary endpoints, we considered components of our primary endpoint, as well as all CVD (hard CVD plus definite angina, probable angina followed by revascularization, other atherosclerotic death, or other CVD death), HF, stroke, and all-cause mortality. Details on MESA follow-up methods and event adjudication are available on the MESA web site at http://www.mesa-nhlbi.org.
Statistical Analysis
Proportions were calculated for categorical variables. Means with standard deviations were calculated for continuous variables with approximately Gaussian distributions. Medians with interquartile ranges were calculated for continuous variables with highly skewed distributions. Based on sexual dimorphism in leptin levels, hormone levels, body fat, and CVD risk,17 as well as statistical evidence of interaction in this study between leptin and sex in relation to the outcome of HF, we stratified by sex. In addition, we examined models in which we pooled sex and adjusted for sex. For our primary analyses, we analyzed leptin as a log-transformed continuous variable: ln(leptin). This maximized power, with estimated >80% power to detect a hazard ratio of >1.2, and is consistent with prior literature.8 As a secondary analysis for hard CVD and all CVD, we analyzed leptin levels by sex-specific quartiles to facilitate comparison with prior analyses.6
We calculated incidence rates per 1000 person-years in the overall study and by leptin quartiles. We used unadjusted Kaplan-Meier plots with the failure function to visualize CVD risk by leptin quartiles; adjusted Kaplan-Meier plots did not provide incremental information. Furthermore, we performed sex-stratified Cox regression in age- and ethnicity-adjusted models. We explored the impact of additional adjustment for traditional CVD risk factors: systolic and diastolic blood pressure, hypertension, LDL-C, HDL-C, cigarette smoking, and diabetes mellitus. These factors were chosen based on a combination of clinical judgment and dataset characteristics. Furthermore, in selected models, we present additional adjustment for body mass index or waist circumference as measures of adiposity for which leptin is a potential mediator of CVD. A drawback of such models is collinearity between leptin and measures of adiposity.
There were no violations of the proportional hazards assumption as tested by log-log plots, Schoenfeld residuals, and interaction testing of leptin quartiles with a binary variable for time (< or ≥ median amount of follow-up time). To evaluate for effect modification, we tested interaction terms of ln(leptin) (continuous) crossed with biologically plausible covariates, including age (< or ≥ 65 years), ethnicity (white/non-white), body mass index (< or ≥ 25), hypertension (yes/no), diabetes (yes/no), and C-reactive protein (< or ≥ upper quartile).
All analyses were performed with Stata software version 11.0 (StataCorp, College Station, Texas). We considered a two-tailed p value of <0.05 nominally significant. We did not adjust for multiple testing in this exploratory analysis and thereby view significant results as hypothesis generating.
RESULTS
Characteristics of Study Sample
Table 1 displays baseline characteristics at visit 2/3 stratified by sex and leptin quartiles. The sample was split evenly by sex and the mean age was 64.5 years. Forty-percent of participants were White, 21% Black, 13% Chinese, and 26% Hispanic. Median leptin levels were more than three times higher in women (25.0 ng/mL, 25th-75th 12.8-42.3) compared to men (7.1 ng/mL, 25th-75th 3.2-14.3). Across sex-specific quartiles of leptin, there was a general pattern of a more unfavorable cardiovascular risk factor profile and the strongest associations were with obesity-related variables.
Table 1.
Baseline Study Sample Characteristics Stratified by Sex and Leptin Quartiles
| All (N=1,905) |
Women (N = 961) |
Men (N = 944) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Leptin Quartile (ng/mL) | Leptin Quartile (ng/mL) | |||||||||
| Q1 (0.9 to <12.8) |
Q2 (12.8 to <25.0) |
Q3 (25.0 to <42.3) |
Q4 (42.3 to 224.9) |
Q1 (0.03 to <3.2) |
Q2 (3.2 to <7.1) |
Q3 (7.1 to <14.3) |
Q4 (14.3 to 150.0) |
|||
| Age, mean (SD), y | 64.5 (9.6) | 65.1 (9.5) |
65.1 (9.6) |
65.2 (9.4) |
65.1 (9.0) |
64.1 (10.1) |
63.8 (10.2) |
64.2 (9.9) |
63.6 (9.3) |
|
| Ethnicity, No. (%) | ||||||||||
| White | 764 (40.1) | 121 (50.2) |
88 (36.8) |
84 (35.0) |
73 (30.3) |
94 (39.8) |
105 (44.5) |
108 (45.8) |
91 (38.6) |
|
| Black | 399 (20.9) | 29 (12.0) |
46 (19.3) |
53 (22.1) |
98 (40.7) |
30 (12.7) |
30 (12.7) |
44 (18.6) |
69 (29.2) |
|
| Chinese | 249 (13.1) | 60 (24.9) |
29 (12.1) |
23 (9.6) |
8 (3.3) |
59 (25.0) |
32 (13.6) |
27 (11.4) |
11 (4.7) |
|
| Hispanic | 493 (25.9) | 31 (12.9) |
76 (31.8) |
80 (33.3) |
62 (25.7) |
53 (22.5) |
69 (29.2) |
57 (24.2) |
65 (27.5) |
|
| Body mass index | 28.2 (5.2) | 23.3 (3.0) |
26.8 (4.2) |
29.3 (4.3) |
34.3 (5.4) |
24.2 (2.8) |
26.9 (2.7) |
28.3 (3.0) |
32.3 (4.5) |
|
| Waist circumference, cm | 98.3 (14.2) | 84.1 (9.5) |
94.0 (11.8) |
99.1 (12.3) |
111.9 (15.3) |
88.7 (8.1) |
96.9 (7.6) |
100.6 (8.3) |
110.8 (11.4) |
|
| Hypertension, treateda | 886 (46.9) | 89 (37.2) |
109 (45.8) |
125 (53.0) |
158 (65.6) |
61 (26.0) |
94 (40.3) |
119 (50.6) |
131 (56.5) |
|
| SBP, mmHg | 124 (21) | 121 (23) |
123 (21) |
127 (23) |
131 (23) |
119 (19) |
122 (18) |
125 (18) |
125 (18) |
|
| DBP, mmHg | 70 (10) | 66 (10) | 67 (9) | 68 (10) | 69 (10) | 72 (9) | 73 (9) | 74 (9) | 74 (10) | |
| Hyperlipidemia, treatedb | 452 (24.4) | 39 (16.6) |
68 (29.1) |
62 (27.1) |
64 (27.2) |
45 (19.5) |
49 (21.3) |
69 (29.7) |
56 (24.4) |
|
| Non-HDL cholesterol, mg/dLc |
139 (35) | 132 (33) |
145 (37) |
142 (35) |
144 (37) |
132 (34) |
139 (34) |
139 (35) |
136 (35) |
|
| LDL cholesterol, mg/dLd | 113 (31) | 110 (29) |
117 (32) |
115 (33) |
117 (32) |
111 (30) |
111 (29) |
112 (31) |
107 (33) |
|
| HDL cholesterol, mg/dLc | 52 (15) | 64 (19) |
55 (15) |
54 (14) |
54 (13) |
51 (13) |
46 (13) |
45 (11) |
43 (12) |
|
| Diabetes mellitusc | 265 (13.9) | 23 (9.5) | 24 (10.0) |
33 (13.8) |
39 (16.3) |
22 (9.3) |
31 (13.1) |
39 (16.6) |
54 (22.9) |
|
| Current smokere | 215 (11.4) | 28 (11.6) |
20 (8.4) |
21 (8.8) |
25 (10.4) |
36 (15.4) |
31 (13.3) |
26 (11.1) |
28 (12.0) |
|
Missing data for
16;
49;
2;
28;
11 participants
SBP = systolic blood pressure; DBP = diastolic blood pressure; HDL = high-density lipoprotein cholesterol
Hard CVD Events
From visit 2 or 3 (as appropriate), the median years of follow-up was 7.6 (7.1-8.3) with a total analysis time of 13,789 person-years (7,051 person-years in women, 6,738 person-years in men). A hard CVD event occurred in 110 participants, of which 47 occurred among women and 63 among men. The overall hard CVD event rate per 1,000 person-years in women was 6.7 (95% CI 5.0-8.9) and in men 9.4 (95% CI 7.3-12.0).
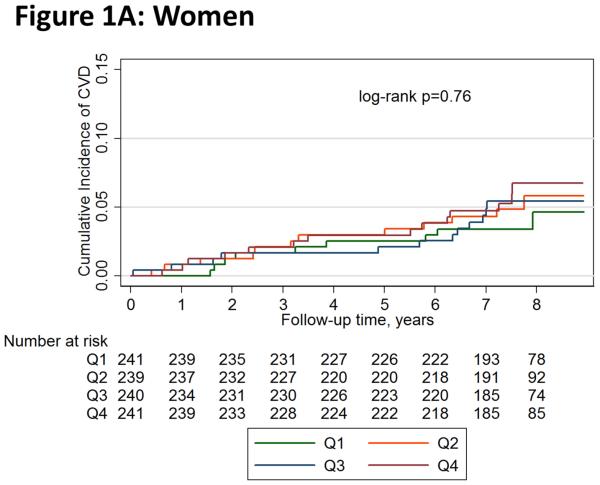
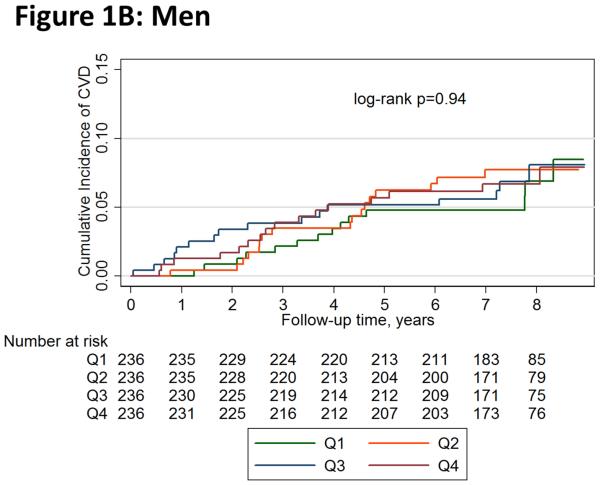
Figure 1 shows unadjusted Kaplan-Meier failure function plots of hard CVD by leptin quartiles in women and men. Hard CVD event rates per 1,000 person-years in women were 5.1 (95% CI 2.6-9.7) for quartile 1, 6.8 (95% CI 3.9-12.0) for quartile 2, 6.9 (95% CI 3.9-12.1) for quartile 3, and 8.0 (95% CI 4.7-13.4) for quartile 4; the numeric increase across quartiles was not statistically significant (log-rank p=0.76). The respective rates in men were also not significantly different across quartiles (log-rank p=0.94): 8.1 (95% CI 4.8-13.7), 10.2 (95% CI 6.3-16.4), 9.5 (95% CI 5.8-15.5), and 9.6 (95% CI 5.9-15.7).
Figure 1.
Unadjusted Kaplan-Meier Failure Function of Hard Cardiovascular Disease Events by Leptin Quartiles in (A) Women and (B) Men
Table 2 shows the main results. When analyzing leptin as a continuous, log-transformed variable, there was not a statistically significant association of leptin levels with hard CVD. In sex-stratified analysis, adjusted for age and ethnicity, the hard CVD hazard ratio estimates for a 1 standard deviation increase in ln(leptin) were 1.16 in women (95% CI 0.78-1.73, p=0.46) and 0.91 (95% CI 0.69-1.20, p=0.51) in men. Pooling sexes, and adjusting for sex in addition to age and ethnicity, estimates were 0.98 (95% CI 0.78-1.23, p=0.89). With additional adjustment for cardiovascular risk factors, the results remained nonsignificant: 0.87 (95% CI 0.68-1.11, p=0.26). This was also the case with additional adjustment for body mass index (0.84, 95% CI 0.62-1.14, p=0.26) or waist circumference (0.80, 95% CI 0.59-1.08, p=0.14).
Table 2.
Hazard Ratios per 1 Standard Deviation Increase in ln(Leptin)
| Hazard Ratio (95% CI), P-value |
||||||
|---|---|---|---|---|---|---|
| Sex Pooled (N = 1905) |
Women (N = 961) |
Men (N = 944) |
||||
| Model Adjustment |
Age, sex, ethnicity | Age, sex, ethnicity, risk factorsa |
Age, ethnicity | Age, ethnicity, risk factorsa |
Age, ethnicity | Age, ethnicity, risk factorsa |
| Primary Endpoint | ||||||
| Hard CVD (110 events) |
0.98 (0.78-1.23), 0.89 |
0.87 (0.68-1.11), 0.26 |
1.16 (0.78-1.73), 0.46 |
1.01 (0.67-1.52), 0.95 |
0.91 (0.69-1.20), 0.51 |
0.80 (0.59-1.08), 0.15 |
| Secondary Endpoints | ||||||
| All CVD (152 events) |
1.02 (0.84-1.23), 0.86 |
0.90 (0.74-1.10), 0.31 |
1.33 (0.90-1.96), 0.15 |
1.12 (0.75-1.67), 0.58 |
0.93 (0.75-1.16), 0.54 |
0.84 (0.66-1.06), 0.15 |
| Hard CHD (68 events) |
1.16 (0.86-1.55), 0.33 |
1.01 (0.74-1.38), 0.95 |
1.58 (0.89-2.78), 0.12 |
1.42 (0.81-2.50), 0.22 |
1.02 (0.72-1.45), 0.89 |
0.88 (0.60-1.28), 0.50 |
| Stroke (44 events) |
0.83 (0.59-1.18), 0.31 |
0.76 (0.52-1.10), 0.15 |
0.93 (0.53-1.65), 0.81 |
0.79 (0.43-1.46), 0.45 |
0.77 (0.49-1.20), 0.25 |
0.70 (0.43-1.14), 0.16 |
| HF (52 events) |
* | * | 2.06 (1.04-4.06), 0.04 |
1.85 (0.93-3.68), 0.08 |
0.95 (0.64-1.43), 0.82 |
0.89 (0.57-1.38), 0.61 |
| Mortality (146 events) |
0.99 (0.81-1.21), 0.93 |
0.99 (0.80-1.22), 0.92 |
0.95 (0.67-1.35), 0.77 |
0.83 (0.57-1.21), 0.33 |
1.02 (0.80-1.29), 0.89 |
1.09 (0.84-1.42), 0.53 |
CVD = cardiovascular disease; CHD = coronary heart disease; HF = heart failure
P-value on sex-leptin interaction term: 0.045; therefore, pooled result not appropriate
Systolic blood pressure, diastolic blood pressure, hypertension, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, cigarette smoking, diabetes mellitus; given missing risk factor data, N = 1,840 (941 women and 908 men)
Table 3 (top) shows the quartile-based analysis. Women had hazard ratios for hard CVD events that were ~30% higher for leptin quartiles 2 and 3 relative to quartile 1, and ~60% higher for quartile 4, however the confidence intervals were wide and crossed 1. In men, point estimates for the hazard ratios approximated 1 for comparisons of higher leptin quartiles with quartile 1.
Table 3.
Age- and Ethnicity-Adjusted Hazard Ratios for Cardiovascular Disease Events and All-Cause Mortality Associated with Leptin Quartiles, Stratified by Sex
| Women (N = 961) HR (95% CI), P-value |
Men (N = 944) HR (95% CI), P-value |
|||||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |
| Primary Endpoint | ||||||||
| Hard CVD (110 events) |
Ref | 1.35 (0.56- 3.26), 0.50 |
1.32 (0.55- 3.21), 0.54 |
1.61 (0.67- 3.88), 0.29 |
Ref | 1.14 (0.56- 2.31), 0.72 |
1.05 (0.51- 2.17), 0.89 |
0.99 (0.48- 2.05), 0.98 |
| Secondary Endpoints* | ||||||||
| All CVD (152 events) |
Ref | 1.50 (0.63- 3.56), 0.36 |
1.47 (0.62- 3.51), 0.38 |
2.07 (0.89- 4.81), 0.09 |
Ref | 1.50 (0.86- 2.59), 0.15 |
0.94 (0.51- 1.72), 0.84 |
1.12 (0.62- 2.01), 0.71 |
| Mortality (146 events) |
Ref | 0.57 (0.27- 1.21), 0.14 |
0.57 (0.26- 1.23), 0.15 |
0.78 (0.38- 1.61), 0.50 |
Ref | 1.35 (0.75- 2.43), 0.32 |
1.04 (0.56- 1.95), 0.89 |
1.10 (0.59- 2.04), 0.77 |
CVD = cardiovascular disease; Ref = reference group; HR = hazard ratio; CI = confidence interval
Insufficient number of events for secondary endpoints aside from all CVD and mortality to generate quartile-based estimates
Secondary CVD Events and All-Cause Mortality
When pooling sexes, and adjusting for age, sex, and ethnicity (Table 2), we did not observe any statistically significant associations of log-transformed leptin levels with secondary endpoints. In women, but not men (p-value for interaction 0.045), there was an association of higher leptin levels with HF (HR per 1 standard deviation increase in ln(leptin) 2.06, 95% CI 1.04-4.06, p=0.04) (Table 2, middle column). With further adjustment for CVD risk factors, the point estimate for the hazard ratio was reduced by 10% and the association was no longer significant (HR 1.85, 95% CI 0.93-3.68, p=0.08). Adding adjustment for body mass index reduced the hazard ratio point estimate by an additional 14% to 1.60 (95% CI 0.65-3.94, p=0.31).
In the quartile-based analysis for all CVD and mortality (Table 3, bottom), results were consistent with the continuous analysis in that no significant relationships were observed. Women had hazard ratio point estimates for all CVD events that were ~50% higher for leptin quartiles 2 and 3 relative to quartile 1, and ~100% higher for quartile 4, however the confidence intervals were wide and crossed 1. In men, comparisons were also not significant and we did not observe any clear pattern across progressive quartiles.
Evaluation for Effect Modification
In unadjusted Cox models of hard CVD events, we did not detect any interaction between leptin levels and age, ethnicity, body mass index, hypertension, diabetes, or C-reactive protein (p>0.10 for all).
DISCUSSION
In a prospective, population-based, multi-ethnic US cohort study of women and men, contrary to our hypothesis, circulating baseline leptin levels were not significantly associated with risk for incident hard CVD. Exploratory analyses of other CVD endpoints were mostly unrevealing, aside from a signal for higher risk of HF in women with higher leptin levels. However, this association was borderline and lost significance after adjustment for CVD risk factors. For all of the endpoints, interaction testing of leptin did not suggest effect modification by systemic inflammation, as captured in C-reactive protein levels, or by age, ethnicity, body mass index, hypertension, or diabetes.
We were surprised by the lack of a clear and consistent association of leptin levels with CVD events in MESA. The findings highlight the importance of studying diverse cohorts and considering multiple clinically important endpoints rather than only selected ones.18 In line with our results, a prospective cohort study of African American women and men did not show an association of leptin with incident CHD or stroke.19 Because leptin is involved in a complex network of homeostatic mechanisms, it may be that the net effect of leptin on CVD is generally neutral. However, our single study must be taken in the context of prior and future studies, and alone does not provide an adequate test of such a hypothesis.
Several potential limitations of our study may serve as explanations for our null results. Considering measurement of our exposure, we relied on single measurements of leptin from multiplex panels in a fasting state. It is possible that repeated measurements, use of a gold standard ELISA assay, or measurement in a nonfasting state each could have strengthened risk associations. Even then, circulating leptin levels cannot be expected to fully capture leptin signaling at the cellular level, which is thought to be tissue-specific.1 With regards to CVD events, power was an issue in this analysis with relatively small numbers of events in women and men who were included in this ancillary study. With a greater number of events, it is possible that some of the hazard ratios would have remained stable and become statistically significant as the confidence intervals tightened. A greater number of events would have also allowed for survival analyses stratified by ethnicity in addition to sex, as well as adjustment for other covariates. While MESA participants and their physicians were informed of coronary artery calcium score results, which could modify use of preventive therapies, these were not associated with leptin levels in MESA,20 in contrast to prior studies.10,21,22
In a prior study of 550 men with fatal CHD or nonfatal myocardial infarction and 1,184 controls nested within a prospective study of British men, the population was younger with a mean age of ~53 years.6 Compared with MESA, there was a much higher prevalence of smoking (~50%) and more poorly controlled systolic blood pressure (~150 mmHg). Those in the top tertile of baseline leptin levels, compared with the bottom tertile, had an age- and town-adjusted odds ratio for CHD of 1.25 (95% CI 0.96 to 1.62), decreasing to 0.98 (95% CI 0.72 to 1.34) after adjustment for body mass index. The authors then conducted a systematic review, including seven prospective reports with heterogeneous findings (I(2) = 60%, 13% to 82%), and the pooled adjusted risk was 1.44 (95% CI 0.95 to 2.16). Our study neither confirms nor disconfirms these prior results.
In a prospective study of 4,080 older men followed for HF,8 participants were more similar to MESA with a mean age of ~70 years and smoking prevalence of <20%, although systolic blood pressure was poorly controlled at ~150 mmHg. The median leptin levels of 9-12 ng/mL were higher than men in MESA. With 228 cases of incident HF over a mean follow-up period of 9 years, increased circulating leptin levels were associated with an increased risk of HF in men without pre-existing CHD, but not those with pre-existing CHD. The association with HF was independent of body mass index and other covariates (adjusted HR for a 1 standard deviation increase in log(leptin): 1.30, 95% CI 1.06 to 1.61, p = 0.01), and an association between body mass index and HF was lost after adjustment for leptin levels. While we could not reproduce this association in men, the upper end of our confidence interval is not incongruent with this prior study.
Although the association of higher levels of leptin with HF risk in women became nonsignificant upon adjustment for multiple cardiovascular risk factors, the increase in risk expressed by the odds ratio point estimate is novel and of interest. This result is biologically plausible because women have higher leptin levels than men, and those with the highest leptin levels may be more likely to have leptin resistance.1 One key feature of leptin resistance appears to be insulin resistance and a prior MESA study found a consistent association of leptin levels with insulin resistance by HOMA-IR across ethnic groups.23 The transition from a leptin sensitive to a leptin resistant state could explain our observation. At this time, leptin resistance remains a conceptual framework with high leptin levels serving as a marker; there is not a standardized clinical definition.
Conclusion
In conclusion, in a prospective cohort study of multi-ethnic women and men from the general community in the US, we did not observe a significant relationship of leptin levels with hard CVD. Considering other CVD endpoints and all-cause mortality, there was not a clear and consistent association of leptin levels with higher risk. Further study appears to be warranted as, notwithstanding lack of significance upon multiple adjustment, in women the point estimate of the odds ratio suggested an association between leptin and HF.
Research Highlights.
Prior studies of leptin and CVD risk have involved primarily older Caucasian men.
We examined a modern prospective cohort study of multi-ethnic US women and men.
Leptin was not significantly associated with future risk of CVD or all-cause mortality.
Acknowledgements
All authors had full access to the data and jointly decided to submit the manuscript for publication. This research was supported by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040 and UL1-TR-001079 from NCRR. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. NIH sponsored MESA committees reviewed and approved the proposal, abstract, and manuscript from the present study. All authors had full access to the data and jointly decided to submit the manuscript for publication. SSM is supported by the Pollin Cardiovascular Prevention Fellowship, the Marie-Josée and Henry R. Kravis endowed fellowship, and a National Institutes of Health training grant (T32HL07024).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: the authors have no relevant disclosures.
REFERENCES
- 1.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52:1201–1210. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison MA, Ix JH, Morgan C, et al. Higher leptin is associated with hypertension: the Multi-Ethnic Study of Atherosclerosis. J Hum Hypertens. 2013;27:617–622. doi: 10.1038/jhh.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allison MA, Bluemke DA, McClelland R, et al. Relation of leptin to left ventricular hypertrophy (from the Multi-Ethnic Study of Atherosclerosis) Am J Cardiol. 2013;112:726–730. doi: 10.1016/j.amjcard.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieb W, Sullivan LM, Aragam J, et al. Relation of serum leptin with cardiac mass and left atrial dimension in individuals >70 years of age. Am J Cardiol. 2009;104:602–605. doi: 10.1016/j.amjcard.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sattar N, Wannamethee G, Sarwar N, et al. Leptin and coronary heart disease: prospective study and systematic review. J Am Coll Cardiol. 2009;53:167–175. doi: 10.1016/j.jacc.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 7.Kappelle PJ, Dullaart RP, van Beek AP, Hillege HL, Wolffenbuttel BH. The plasma leptin/adiponectin ratio predicts first cardiovascular event in men: a prospective nested case-control study. Eur J Intern Med. 2012;23:755–759. doi: 10.1016/j.ejim.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Obesity and risk of incident heart failure in older men with and without pre-existing coronary heart disease: does leptin have a role? J Am Coll Cardiol. 2011;58:1870–1877. doi: 10.1016/j.jacc.2011.06.057. [DOI] [PubMed] [Google Scholar]
- 9.Deswal A. Obesity, leptin, and incident heart failure. J Am Coll Cardiol. 2011;58:1878–1880. doi: 10.1016/j.jacc.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 10.Martin SS, Qasim AN, Rader DJ, Reilly MP. C-reactive protein modifies the association of plasma leptin with coronary calcium in asymptomatic overweight individuals. Obesity. 2012;20:856–861. doi: 10.1038/oby.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ble A, Windham BG, Bandinelli S, et al. Relation of plasma leptin to C-reactive protein in older adults (from the Invecchiare nel Chianti study) Am J Cardiol. 2005;96:991–995. doi: 10.1016/j.amjcard.2005.05.058. [DOI] [PubMed] [Google Scholar]
- 12.Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 13.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 14.Allison MA, Jensky NE, Marshall SJ, Bertoni AG, Cushman M. Sedentary behavior and adiposity-associated inflammation: the Multi-Ethnic Study of Atherosclerosis. Am J Prev Med. 2012;42:8–13. doi: 10.1016/j.amepre.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goff DC, Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 16.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 17.Nedungadi TP, Clegg DJ. Sexual dimorphism in body fat distribution and risk for cardiovascular diseases. J Cardiovasc Transl Res. 2009;2:321–327. doi: 10.1007/s12265-009-9101-1. [DOI] [PubMed] [Google Scholar]
- 18.Czarny MJ, Martin SS, Kohli P, Metkus T, Blumenthal RS. Nonfatal outcomes in the primary prevention of atherosclerotic cardiovascular disease: Is all-cause mortality really all that matters? Circ Cardiovasc Qual Outcomes. 2014;7:481–485. doi: 10.1161/CIRCOUTCOMES.114.000871. [DOI] [PubMed] [Google Scholar]
- 19.Bidulescu A, Liu J, Chen Z, et al. Associations of adiponectin and leptin with incident coronary heart disease and ischemic stroke in African Americans: The Jackson Heart Study. Front Public Health. 2013;1:e16. doi: 10.3389/fpubh.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes-Austin JM, Wassel CL, Jimenez J, et al. The relationship between adiposity-associated inflammation and coronary artery and abdominal aortic calcium differs by strata of central adiposity: The Multi-Ethnic Study of Atherosclerosis (MESA) Vasc Med. 2014;19:264–271. doi: 10.1177/1358863X14537545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reilly MP, Iqbal N, Schutta M, et al. Plasma leptin levels are associated with coronary atherosclerosis in type 2 diabetes. J Clin Endocrinol Metab. 2004;89:3872–3878. doi: 10.1210/jc.2003-031676. [DOI] [PubMed] [Google Scholar]
- 22.Qasim A, Mehta NN, Tadesse MG, et al. Adipokines, insulin resistance, and coronary artery calcification. J Am Coll Cardiol. 2008;52:231–236. doi: 10.1016/j.jacc.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen-Torvik LJ, Wassel CL, Ding J, et al. Associations of body mass index and insulin resistance with leptin, adiponectin, and the leptin-to-adiponectin ratio across ethnic groups: the Multi-Ethnic Study of Atherosclerosis (MESA) Ann Epidemiol. 2012;22:705–709. doi: 10.1016/j.annepidem.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]