Abstract
Behavioral flexibility is a component of executive functioning that allows individuals to adapt to changing environmental conditions. Independent lines of research indicate that the mu opioid receptor (MOR) is an important mediator of behavioral flexibility and responses to psychosocial stress. The current study bridges these two lines of research and tests the extent to which social defeat and MOR affect behavioral flexibility and whether sex moderates these effects in California mice (Peromyscus californicus). Males and females assigned to social defeat or control conditions were tested in a Barnes maze. In males, defeat impaired behavioral flexibility but not acquisition. Female performance was unaffected by defeat. MOR binding in defeated and control mice in the orbitofrontal cortex (OFC), striatum, and hippocampus was examined via autoradiography. Stressed males had reduced MOR binding in the OFC whereas females were unaffected. The MOR antagonist beta-funaltrexamine (1 mg/kg) impaired performance in males naïve to defeat during the reversal phase but had no effect on females. Finally, we examined the effects of the MOR agonist morphine (2.5, 5 mg/kg) on stressed mice. As expected, morphine improved behavioral flexibility in stressed males. The stress-induced deficits in behavioral flexibility in males are consistent with a proactive coping strategy, including previous observations that stressed male California mice exhibit strong social approach and aggression. Our pharmacological data suggest that a down-regulation of MOR signaling in males may contribute to sex differences in behavioral flexibility following stress. This is discussed in the framework of coping strategies for individuals with mood disorders.
Keywords: Sex differences, Social defeat, Barnes maze, Orbitofrontal cortex, Peromyscus californicus
Introduction
Behavioral flexibility is a fundamental process that allows an individual to adapt to changing environmental conditions and can be associated with spatial and/or social strategies that are employed within a given ecological niche (Bond et al. 2007; Tebbich et al. 2010). Coping strategies during times of rapid change can be traced back to an individual’s propensity to demonstrate behavioral flexibility under environmental stress (Coppens et al. 2010). Koolhaas et al. (1999) have identified two types of coping strategies that are employed as a result of environmental change. Proactive individuals are relatively inflexible and continue a rigid routine despite disturbances, whereas reactive individuals demonstrate more flexibility and respond to environmental changes more readily. Interestingly, behavioral flexibility is frequently impaired in individuals diagnosed with stress-induced mental illnesses such as anxiety or depression (Dickstein et al. 2010; Grant et al. 2001). Animal models show that chronic psychosocial stress can induce deficits in several tests of behavioral flexibility (Bondi et al. 2008; Danet et al. 2010; Liston et al. 2006). Furthermore, prenatal stress induces anatomical differences between males and females in cortical areas associated with behavioral flexibility (Murmu et al. 2006), suggesting that flexibility may be contingent upon both stress and sex.
The mu-opioid receptor (MOR) is abundantly expressed in the orbitofrontal cortex (OFC) and dorsal striatum (Mansour et al. 1995), which are important regions that control performance in tasks assessing behavioral flexibility (Boulougouris et al. 2007; Castane et al. 2010). Psychosocial stress can affect MOR expression and trafficking, often in a sex-dependent manner (Gonzales et al. 2011; Milner et al. 2013; Nikulina et al. 1999). Although the effects of stress on MOR function in the OFC have not been studied, activation of MOR improves behavioral flexibility in males (Olson et al. 1979). Hence, we hypothesized that in males, stress would impair behavioral flexibility (consistent with (Bondi et al. 2008; Danet et al. 2010; Liston et al. 2006)) and downregulate MOR in brain areas associated with behavioral flexibility. Alternatively, in females, we expected that stress would not change flexible performance due to the finding that prenatal stress did not alter female OFC dendritic spine density (Murmu et al. 2006). We used the Barnes maze to examine the effects of defeat stress and MOR activity on behavioral flexibility in male and female California mice (Peromyscus californicus). Male and female California mice aggressively defend territories under natural conditions, which allows for the study of defeat stress in females (Trainor et al. 2011; Trainor et al. 2013). An important gap in this literature is that the vast majority of studies have been conducted on males. Many effects of psychosocial stress are sex-dependent (Goel and Bale 2009), suggesting that there could be important sex differences in how stress impacts behavioral flexibility. In our previous studies of California mice, we found that the long term effects of defeat stress are sex-specific and generally consistent with reactive and proactive coping strategies described by Koolhaas and colleagues (Koolhaas et al. 1999). In females, effects of social defeat were more consistent with reactive coping strategies such as social withdrawal (Greenberg et al. 2014), and reduced aggression (Steinman et al. 2015). Behaviors in stressed males were more consistent with proactive coping strategies such as social approach and aggression. In other rodent species and domesticated animals, proactive coping strategies are associated with behavioral inflexibility (Koolhaas et al. 1999). We thus hypothesized that social defeat would impair behavioral flexibility to a greater extent in males.
Materials and Methods
Experiment 1: Effects of Social Defeat Stress on Acquisition and Behavioral Flexibility
Male and female California mice were randomly assigned to three episodes of social defeat or control handling. Mice assigned to defeat were placed in the cage of an aggressive, same-sex breeder California mouse as previously described (Trainor et al. 2013). This protocol exposes males and females to similar levels of aggression by residents and induces rapid short term increases in corticosterone in females but not males (Trainor et al. 2013). In contrast males but not females have elevated levels of baseline corticosterone 2-4 weeks after defeat (Trainor et al. 2011). Mice assigned to control handling were placed in empty cages for 10 minutes, and then placed back in their home cages with their original cagemates. Four weeks following social defeat or control conditions, mice were tested using the extended Barnes Maze protocol (Fig. 1) (see “Barnes Maze” methods below).
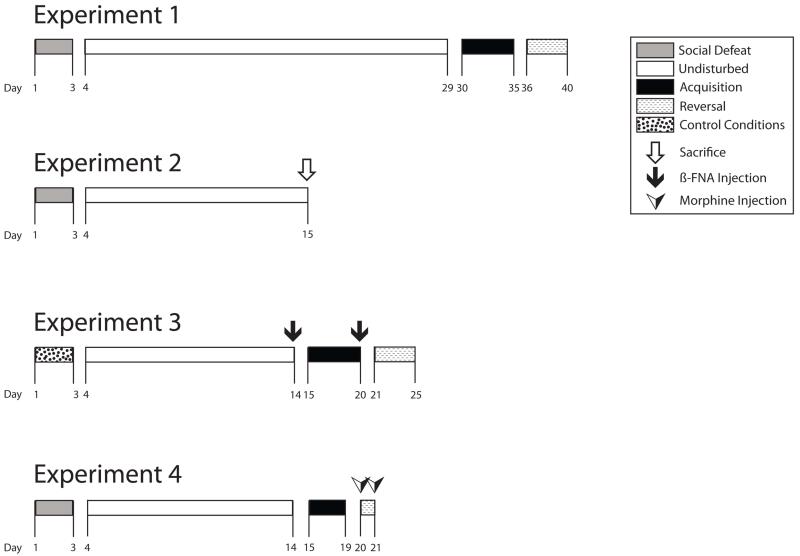
Fig. 1.
Behavioral timelines for experiments 1-4 for all animals. Days are indicated with numbers at the bottom of each timeline. Social defeat is marked with gray bars, the period that mice were undisturbed following defeat or control conditions is marked with white bars, the acquisition phase of the Barnes maze is marked with black bars, the reversal phase of the Barnes maze is marked by dotted line bars, and control conditions are marked with a bar containing circles. Time of sacrifice for experiment 2 is indicated with a white arrow, ß-FNA injections during experiment 3 are marked with black arrows, and morphine injections during experiment 4 are marked with black and white arrows.
Experiment 2: Mu-Opioid Receptor Autoradiography
In previous studies the effects of social defeat on social interaction behavior at two weeks following the last episode of defeat (Trainor et al. 2013) were equivalent to the effects of defeat at four weeks after defeat (Trainor et al. 2011). This suggests that long term changes in brain and behavior detectable at two weeks after defeat remain at four weeks. Mice were randomly assigned to social defeat or control conditions and then euthanized with isoflurane and decapitated two weeks later (Fig. 1). Brains were flash frozen for autoradiography analysis to assess MOR binding. Sections were cut at 20 μm on a cryostat and mounted onto Superfrost Plus Slides (Fisher Scientific, Pittsburgh, PA), and stored at −40°C. Slides were washed in Tris buffer (50mM Tris-HCl, 120mM NaCl, 5mM KCl; pH 7.4). To determine MOR specific binding, slides were incubated for 1 hour at room temperature in a 4.0 nM [3H]DAMGO (PerkinElmer, Waltham, MA) solution containing 400 nM unlabeled U69,593 (Sigma-Aldrich, St. Louis, MO), 400 nM unlabeled DPDPE (Tocris Bioscience, Minneapolis, MN), and Tris buffer. To assess non-specific binding, a separate set of slides were incubated in a 4.0 nM [3H]DAMGO solution and 10 uM naloxone (Tocris Bioscience). All slides were then washed in 4°C Tris buffer, and then ice cold water. Slides were dried and then exposed to Amersham Hyperfilm MP film for 5 months. Films were developed with Kodak GBX Developer and Fixer. Regions of interest were quantified as previously described (Bales et al. 2007) utilizing a Rodbard curve. Optical densities for MOR binding in the lateral orbitofrontal cortex (lOFC), nucleus accumbens (NAc) core and shell, dorsal striatum (CPu), and hippocampal regions CA1, CA3 and the dentate gyrus (DG) were quantified using ImageJ (NIH, Bethesda, MD).
Experiment 3: Effects of β-FNA on Acquisition and Behavioral Flexibility
Based on the results from Experiment 2, we conducted the following behavioral experiments two weeks following defeat because changes in MOR density were established following the two-week time point. Male and female California mice were naïve to defeat and randomly assigned to receive an intraperitoneal (i.p.) injection with either beta-funaltrexamine (β-FNA) (1 mg/kg), an irreversible MOR antagonist (Liu-Chen and Phillips 1987), or saline vehicle (Meilandt et al. 2004). β-FNA is a long-acting drug that can last up to 18 days, losing affinity after day 9 (Martin et al. 1995). β-FNA was administered once twenty-four hours before acquisition testing, and once twenty-four hours before reversal testing immediately following the last acquisition trial (Fig. 1). The second injection was made to 1) ensure that MOR would be blocked throughout the entire reversal phase and 2) make the reversal phase consistent with the acquisition phase (one injection prior to day 1). We did not include stressed males and females in this study because previous studies indicated that MOR signaling increases behavioral flexibility. Thus, if b-FNA were administered to stressed males we would expect a null result because stress males demonstrated a deficit in reversal learning in experiment 1.
Experiment 4: Effects of Morphine on Acquisition and Behavioral Flexibility
All male and female mice experienced social defeat, and were randomly assigned to receive a subcutaneous (s.c.) injection of 2.5 mg/kg morphine, 5.0 mg/kg morphine, or saline vehicle (Farahmandfar et al. 2010) two weeks following social defeat. Since morphine can induce tolerance over repeated, long-term use (Stafford et al. 2001), mice in this experiment were run using the condensed Barnes Maze protocol, and were injected 30 minutes before behavioral testing on days 4 and 5 (reversal trials). Thus mice in this study received only 2 injections (Fig. 1). Similar to experiment 3 we chose not to test the effects of morphine on control males and females. Since we predicted that morphine would improve performance in the reversal phase, it is likely that control animals (which did not show a deficit), would show no significant changes following morphine treatment.
Animals
Male and female California mice were bred in a colony at UC Davis and housed 2-3 per cage in polypropylene cages on either Carefresh (Absorption Corp., Ferndale, WA) or Sanichip bedding (Harlan Teklad, Indianapolis, IN). Food (2016 Harlan Teklad) and water were provided ad libitum. Animals were housed under long day photoperiods (16L:8D). All procedures were approved by the University of California Davis Institutional Laboratory Animal Care and Use Committee, and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Barnes Maze
Experiments 1 and 3 used an extended Barnes Maze protocol (Steinman et al. 2011). Testing occurred during the light phase between 8:00 and 13:00 hours PST. On day 1, each mouse was randomly assigned to a target hole under which was placed an escape container. Each mouse was then placed on the center of the maze and allowed to explore for 5 minutes. If the mouse did not enter the hole after 5 minutes, the experimenter guided it to the target hole. Each mouse was tested in one trial per day for a period of five consecutive days (acquisition days 1-5). Twenty-four hours after the last day of acquisition, each mouse was tested in one reversal trial per day for four days (days 6-9), which is a measure of behavioral flexibility. During reversal trials, the target hole was switched 180° across the maze platform. AnyMaze (Stoelting, Wood Dale, IL, USA) was used to record path length to reach the target hole, number of incorrect holes entered before reaching the target hole (number of errors), and number of entries into the former target hole during reversal. The experimenter was blind to all treatment groups during Barnes Maze testing.
Experiment 4 followed a condensed Barnes Maze protocol previously used on Peromyscus (Jasarevic et al. 2012). In this protocol, each mouse was tested in two trials per day with a 20 minute inter-trial interval (ITI) for three consecutive days (acquisition days 1-3). One day after the last day of acquisition, each mouse was tested in two trials of reversal per day (days 4 and 5, 20 minute ITI).
Statistical Analyses
Longitudinal mixed-model analyses were used to assess the rate of learning for latency to complete the maze, path length, and number of errors. This model was chosen to analyze the rate of learning because it estimates individual trajectories over time as well as differences in such trajectories across all subjects (Laird and Ware 1982; Raudenbush and Bryk 2002). In particular, we estimated intercepts (day 1 or day 6) and slopes (rate of learning), and allowed them to vary across individuals (McArdle and Anderson 1990). We used Repeated Measure (RM) ANOVA to test for main effects of stress and sex, and a sex*stress interaction on path length, number of errors, and number of entries into the former target hole between subjects. Path length was square root or log transformed and number of errors was log transformed for ANOVA analyses due to heterogeneity of variance. MOR receptor binding was analyzed using Univariate ANOVA for each region of interest and was log transformed due to heterogeneity of variance.
Results
Experiment 1: Effects of Social Defeat on Acquisition and Behavioral Flexibility
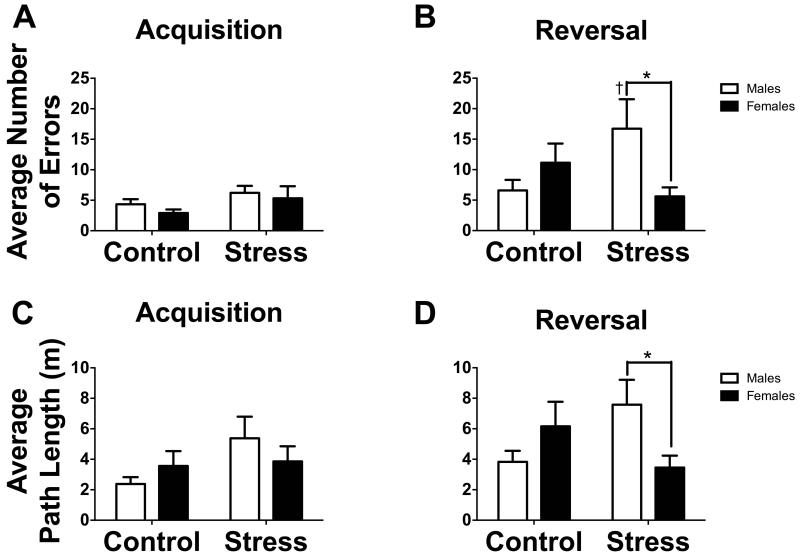
Consistent with Jasarevic et al. (2012), both males and females improved performance across the acquisition phase with no sex differences in performance (Table 1). Both males and females also improved performance across reversal (Table 1). During reversal, there was a significant sex*stress interaction between subjects for number of errors (Fig. 2B, F1,36 = 5.22, p < 0.05) and path length (Fig. 2D, F1,37 = 6.225, p < 0.05). Stressed males made more errors on average as compared to control males (F1,36 = 4.969, p < 0.05) and stressed females (F1,36 = 5.291, p < 0.05). There was a non-significant trend towards a greater average path length to reach the target hole for stressed males as compared to control males (F1,37 = 3.835, p = 0.058). Stressed males demonstrated a significantly longer path length to reach the target hole as compared to stressed females (F1,37 = 4.908, p < 0.05). No treatment or group differences were detected with RM ANOVA in acquisition (Fig. 2A & C, all p’s > 0.05).
Table 1. Rate of Learning: Performance Across Barnes Maze Acquisition and Reversal Phases.
Table representing the mixed effects model during acquisition and reversal for latency, path length, and number of errors. The table represents t- and p-values for the estimated slopes (rate of learning) across acquisition (days 1-5) and reversal (days 6-9) for all animals.
| Acquisition | Reversal | |||
|---|---|---|---|---|
|
| ||||
| t-value | p-value | t-value | p-value | |
| Experiment 1 | ||||
|
| ||||
| Latency | −2.29 | 0.023* | −2.55 | 0.012* |
| Path Length | −3.71 | 0.0003** | −2.15 | 0.033* |
| Errors | −3.42 | 0.0008** | −2.60 | 0.01* |
|
| ||||
| Experiment 2 | ||||
|
| ||||
| Latency | −2.09 | 0.038* | −0.39 | 0.70 |
| Path Length | −0.65 | 0.52 | 0.20 | 0.84 |
| Errors | −0.39 | 0.70 | 1.61 | 0.11 |
|
| ||||
| Experiment 3 | ||||
|
| ||||
| Latency | −3.12 | 0.0021** | −2.89 | 0.0046** |
| Path Length | −5.45 | <0.0001** | −1.99 | 0.0485** |
| Errors | −3.37 | 0.0009** | −2.64 | 0.0094** |
p<0.05
p<0.01
Fig. 2.
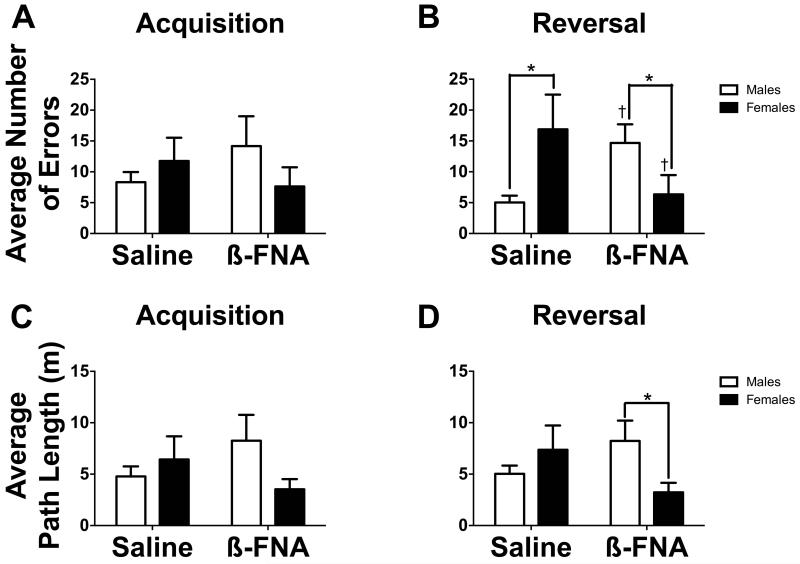
(A and B) Bar graphs representing the average number of errors before finding the target hole in (A) acquisition and (B) reversal phases for control and socially defeated mice. (C and D) Bar graphs representing the average path length before finding the target hole in (C) acquisition and (D) reversal phases for control and socially defeated mice. White bars represent males and black bars represent females (n = 9-11 mice).
*p < 0.05 between sex comparison
†p < 0.05 within sex comparison
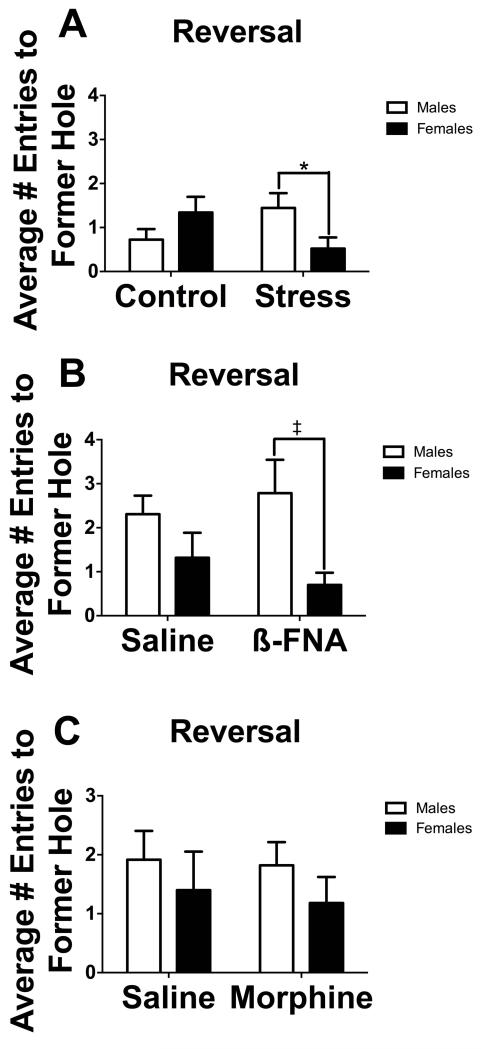
To determine if mice showed perseveration during reversal trials, we analyzed the number of entries into the former acquisition hole. There was a significant sex*stress interaction for the number of former target hole entries during reversal (Fig. 3A, F1,37 = 6.45, p < 0.05). Stressed males entered the former target hole more frequently than stressed females, (F1,37 = 4.51, p < 0.05). There was also a non-significant trend towards stressed females entering the former target hole less often than control females (F1,37 = 3.95, p = 0.05).
Fig. 3.
Bar graphs representing the average number of entries into the former acquisition hole during the reversal phase of the Barnes Maze in (A) control and socially defeated mice (n = 9-11 mice), (B) mice receiving saline or ß-FNA (n = 8-10 mice), or (C) mice receiving saline (n = 5-9 mice) or morphine (n = 11-14 mice). White bars represent males and black bars represent females.
*p < 0.05
‡p = 0.05
Experiment 2: Mu-Opioid Receptor Autoradiography
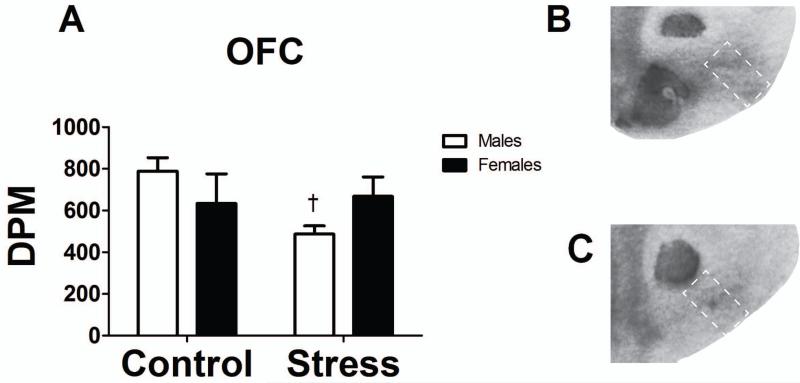
MOR binding was significantly reduced in the lOFC of stressed males as compared to males experiencing control conditions (Fig. 4, p < 0.05), while no differences were detected in females (p > 0.05). No differences were observed in the nucleus accumbens (NAc) core or shell, caudate putamen (CPu), or hippocampus (all p’s > 0.05).
Fig. 4.
(A) Bar graph representing the disintegrations per minute (DPM) for MOR binding in the lOFC in socially defeated or control mice. White bars represent males and black bars represent females (n = 4-5 mice). (B) A representative image of the lOFC in a control male depicting MOR binding following the autoradiography assay. (C) A representative image of the lOFC in a defeated male depicting MOR binding following the autoradiography assay. White dotted boxes represent the region of interest (lOFC).
†p < 0.05 within sex comparison
Experiment 3: Effects of ß-FNA on Acquisition and Behavioral Flexibility
All experimental groups improved performance over the course of the acquisition phase, but not reversal (Table 1). During reversal, RM analyses revealed a significant sex*drug interaction between subjects for number of errors (Fig. 5B, F1,32 = 11.70, p < 0.05) and path length (Fig. 5D, F1,32 = 4.45, p < 0.05). Males receiving saline made significantly fewer errors than males receiving β-FNA (F1,32 = 6.99, p < 0.05) and females receiving saline (F1,32 = 6.54, p < 0.05), but did not differ in path length as compared to males receiving β-FNA (F1,32 = 1.72, p > 0.05). Females receiving β-FNA demonstrated fewer errors (F1,32 = 5.24, p < 0.05) and a shorter path length to reach the target hole (F1,32 = 4.80, p < 0.05) as compared to males receiving β-FNA, but showed no differences in path length as compared to females receiving saline (F1,32 = 2.78, p > 0.05). Females receiving β-FNA also made significantly fewer errors as compared to females receiving saline (F1,32 = 4.87, p < 0.05). No treatment or group differences were detected with RM ANOVA in the acquisition phase (Fig 5A & C, all p’s > 0.05).
Fig. 5.
(A and B) Bar graphs representing the average number of errors before finding the target hole in (A) acquisition and (B) reversal phases for mice receiving saline or ß-FNA. (C and D) Bar graphs representing the average path length before finding the target hole in (C) acquisition and (D) reversal phases for mice receiving saline or ß-FNA. White bars represent males and black bars represent females (n = 8-10 mice).
*p < 0.05 between sex comparison
†p < 0.05 within sex comparison
There was no main effect of sex or drug for the number of former target hole entries, nor was there a significant sex*drug interaction (all p’s > 0.05) (Fig 2B). Males receiving β-FNA showed a non-significant trend towards entering the former hole more often than females receiving β-FNA (F1,36 = 4.06, p = 0.05). There were no effects of β-FNA on locomotor activity (Table 2).
Table 2. Locomotor Behavior.
Table representing locomotor activity for male and female mice receiving ß-FNA (days 1 and 6) or morphine (day 7). Distance traveled is reported as mean ± S.E.
| Study | Treatment | Sex | Distance (m) |
|---|---|---|---|
| β-FNA | |||
| Path Length Day 1 | Saline | Male | 10.361 ± 2.950 |
| Female | 7.363 ± 3.109 | ||
| β-FNA | Male | 6.717 ± 3.109 | |
| Female | 5.611 ± 3.298 | ||
| Path Length Day 6 | Saline | Male | 7.324 ± 2.098 |
| Female | 7.301 ± 2.211 | ||
| β-FNA | Male | 6.132 ± 2.211 | |
| Female | 4.128 ± 2.346 | ||
|
| |||
| Morphine | |||
| Path Length Day 7 | Saline | Male | 9.086 ± 2.463 |
| Female | 6.516 ± 3.305 | ||
| Morphine | Male | 9.344 ± 1.975 | |
| Female | 6.783 ± 2.228 | ||
Mean ± S.E.
Experiment 4: Effects of Morphine on Acquisition and Behavioral Flexibility
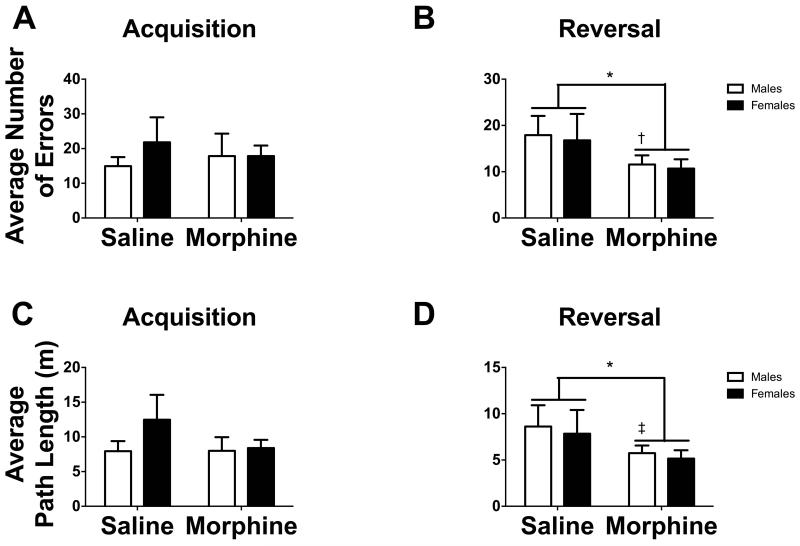
All experimental groups improved performance over the course of acquisition and reversal phases (Table 1). There were no significant differences between 2.5 mg/kg or 5.0 mg/kg of morphine, so morphine doses were combined for all of the following analyses. There was a significant main effect of drug treatment in the reversal phase such that mice receiving morphine made significantly fewer errors (Fig. 6B, F1,35 = 5.859, p < 0.05) and demonstrated significantly shorter path lengths to find the target hole (Fig. 6D, F1,35 = 4.562, p < 0.05). Unlike previous studies there was no significant sex x treatment interaction. The lack of an interaction was driven by the unexpected poor performance in saline treated females. We hypothesize that this may be due to handling from injections given 30 min before testing. We previously observed that handling, such as vaginal lavage, can impact behavior of female California mice (Silva et al. 2010). Although the predicted interaction was not observed, morphine specifically improved male performance in reversal as predicted (F1,35 = 4.946, p < 0.05).
Fig. 6.
(A and B) Bar graphs representing the average number of errors before finding the target hole in (A) acquisition and (B) reversal phases for mice assigned to receive (A) or receiving (B) saline or morphine. (C and D) Bar graphs representing the average path length before finding the target hole in (C) acquisition and (D) reversal phases for mice assigned to receive (C) or receiving (D) saline (n = 5-9 mice) or morphine (n = 11-14 mice). White bars represent males and black bars represent females.
*p < 0.05
†p < 0.05 within sex comparison
‡p < 0.1 within sex comparison
No treatment (mice assigned to receive saline versus morphine in reversal) or group differences were detected with RM ANOVA in the acquisition phase (Fig. 6A & C, all p’s > 0.05) or with Univariate ANOVA in the number of former hole entries (Fig. 2C, all p’s > 0.05). There were no effects of morphine on locomotor activity (Table 2).
Discussion
We demonstrated that social defeat impaired behavioral flexibility in males exposed to defeat stress, while females were resilient to these deficits. Inhibition of MOR in males but not females naive to defeat induced deficits in behavioral flexibility on average, while activation of MOR ameliorated deficits in behavioral flexibility in mice exposed to defeat. In the MOR antagonist study, mice were treated during both the acquisition and reversal learning stage. However, no effects of b-FNA were observed during acquisition phase, which further strengthens the conclusion that MOR impacts behavioral flexibility rather than learning per se. We also observed that males but not females exposed to defeat had reduced MOR binding in the lOFC, a brain region that is critical for the expression of flexible behaviors (Bissonette et al. 2008; McAlonan and Brown 2003). The present studies are consistent with recent data showing that a reversal task is more sensitive to neurological insult in men versus women (Niemeier et al. 2007; Schopp et al. 2001), and that activation of MOR rescues impairments in flexible behaviors (Quednow et al. 2008).
Social organization can have a major impact on sex differences in cognitive function. Previous studies in other species of polygynous rodents have reported that males outperform females in acquiring spatial memories (Gaulin and Fitzgerald 1986; 1989; Jonasson 2005). Unlike most species of rodents, California mice are monogamous (Ribble 1991). Consistent with previous reports in California mice (Jasarevic et al. 2012), we observed no sex differences in the acquisition of spatial memories in California mice. Similarly, no sex differences in the acquisition of spatial learning were observed in monogamous pine voles (Microtus pinetorum) (Gaulin and Fitzgerald 1986). Thus studying sex differences in behavioral flexibility in a monogamous species is ideal because the absence of differences during acquisition ensures that any differences during reversal are not an artifact of differences in acquisition learning. Spatial learning and memory is thought to be influenced by sexual selection (Sherry et al. 1992) and has been hypothesized to be more vulnerable to insults that affect steroid hormone signaling pathways (Jasarevic et al. 2011). Behavioral flexibility relies on different neural circuits and neurochemical pathways than does acquisition learning (Bissonette and Powell 2012; Ghods-Sharifi et al. 2008). Effects of defeat stress on Barnes maze performance were limited to reversal, and primarily males. This suggests that the signaling pathways mediating behavioral flexibility are more vulnerable to stress in males as compared to females.
Our results suggest that reduced MOR signaling in males may play an important role in mediating this deficit. Interestingly, the OFC was the only brain region in which MOR binding was affected by defeat stress. A study of healthy male combat veterans showed that combat exposure increased MOR binding in the OFC as compared to a control population (Liberzon et al. 2007). However, veterans diagnosed with post-traumatic stress disorder had significantly lower MOR binding than unaffected veterans. There were no women in this study. In general, sex differences in MOR action are poorly understood. The analgesic effects of MOR have been reported to be stronger in females as compared to males (Cook et al. 2000; Kavaliers and Innes 1987), an effect thought to be mediated in part by the periaquductal gray (Bernal et al. 2007). Our results highlight the need for further investigation of sex differences in MOR function in cognitive processes.
The impact of stress on flexible behavior could potentially extend beyond spatial performance. Deficits in executive function, including behavioral flexibility, are commonly observed in stress-induced psychiatric disorders such as depression and post-traumatic stress disorder (Channon 1996; Kanagaratnam and Asbjornsen 2007). Few studies have been sufficiently powered to examine sex differences in behavioral flexibility. One study investigating patients with major depressive disorder found that men who ruminated on depressive symptoms demonstrated increased perseverative behaviors, a measure of cognitive inflexibility, as compared to women who ruminated on depressive symptoms (Davis and Nolen-Hoeksema 2000). Similarly, emerging data suggest that there are sex differences in behavioral flexibility following traumatic brain injury (TBI). Men who experienced TBI performed more poorly on tests of behavioral flexibility as compared to women who experienced TBI, regardless of the site of injury (Niemeier et al. 2007; Schopp et al. 2001). It is useful to revisit the idea that behavioral flexibility can be associated with different coping strategies following environmental perturbation (Coppens et al. 2010), especially in the context of TBI and major depressive disorder. Individuals who are relatively inflexible in assimilating to environmental change are said to follow a proactive coping strategy (Koolhaas et al. 1999). Interestingly, cognitive behavioral therapy (CBT), which is a clinical approach for treating several forms of mental illnesses (Butler et al. 2006; DeRubeis et al. 1999; Hofmann and Smits 2008; Taylor 1996) appears to depend in part on engaging behavioral flexibility. There is some evidence that deficiencies in behavioral flexibility, which could be stress induced, may impair the effectiveness of CBT (Garety et al. 1997). Our data suggest that future studies need to more closely examine behavioral flexibility in both male and female patients, and consider the potential impact of sex differences in the effects of stress on circuits mediating behavioral flexibility.
Gonadal hormones are a logical mechanism for mediating sex differences in behavior and brain function. In a previous study, we considered the effect of gonadectomy on stress hormones and behavior (Trainor et al. 2013). Castration exaggerated corticosterone responses during defeat, and ovariectomy blunted these responses. Gonadectomy, however, had no effect on long term social withdrawal responses. In contrast, females raised on corncob bedding (which contains estrogen-like components) had blunted social withdrawal responses, suggesting that the developmental effects of estrogens may be more important for mediating sex differences in behavioral responses to stress. A related possibility is that social housing conditions could impact the effects of defeat on behavioral flexibility. All of our mice were pair housed, which has been found to blunt the neurobiological effects of defeat stress (Isovich et al. 2001). It is possible that single housing could have sex-specific effects on behavior, and is an interesting avenue for future study. Effects of stress on flexible behaviors could also be modulated by kappa opioid receptor (KOR) signaling, as several forms of stress induce release of dynorphin and activation of KOR (Bruchas et al. 2007). Although KOR knockout was not found to affect spatial learning in radial mazes or water mazes (Jamot et al. 2003), behavioral flexibility has not been tested.
Our results in California mice show that there are important sex differences in how defeat stress and MOR signaling impact behavioral flexibility. Our results demonstrated impaired male performance during reversal on average following stress. These results are consistent with recent findings linking altered MOR expression with stress-induced psychiatric disorders (Liberzon et al. 2007) and male-biased impairments in flexibility following brain injury (Niemeier et al. 2007; Schopp et al. 2001). These results suggest that clinical studies targeting behavioral flexibility as a therapeutic mechanism to treat psychiatric disorders will need to be sufficiently powered to examine men and women independently. Our conclusions highlight the importance of studying sex differences in biomedical research (Cahill 2006), specifically focusing on the necessity of including females in models of psychiatric disease due to prominent sex differences in behavior following psychosocial stress.
Acknowledgements
The authors thank Karen Bales, Robert Berman, Simona Ghetti, and Charles Heyser for thoughtful comments on the manuscript, Andrea Silva for technical assistance, and Cindy Clayton for animal care. This research was funded by a Graduate Research Fellowship awarded by the NSF to S.A.L., an NIH National Research Service Award F31 MH095253-01 from NIMH to M.Q.S., and R01MH085069 to B.C.T.
References
- Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS. Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressinreceptors. Neuroscience. 2007;144:38–45. doi: 10.1016/j.neuroscience.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal SA, Morgan MM, Craft RM. PAG mu opioid receptor activation underlies sex differences in morphine antinociception. Behavioural brain research. 2007;177:126–33. doi: 10.1016/j.bbr.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:11124–30. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Powell EM. Reversal learning and attentional set-shifting in mice. Neuropharmacology. 2012;62:1168–74. doi: 10.1016/j.neuropharm.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond AB, Kamil AC, Balda RP. Serial reversal learning and the evolution of behavioral flexibility in three species of North American corvids (Gymnorhinus cyanocephalus, Nucifraga columbiana, Aphelocoma californica) J Comp Psychol. 2007;121:372–379. doi: 10.1037/0735-7036.121.4.372. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:320–31. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behavioural brain research. 2007;179:219–28. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:11614–23. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clinical psychology review. 2006;26:17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nature reviews Neuroscience. 2006;7:477–84. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Castane A, Theobald DE, Robbins TW. Selective lesions of the dorsomedial striatum impair serial spatial reversal learning in rats. Behavioural brain research. 2010;210:74–83. doi: 10.1016/j.bbr.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon S. Executive dysfunction in depression: the Wisconsin Card Sorting Test. Journal of affective disorders. 1996;39:107–14. doi: 10.1016/0165-0327(96)00027-4. [DOI] [PubMed] [Google Scholar]
- Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ. Sex-related differences in the antinociceptive effects of opioids: importance of rat genotype, nociceptive stimulus intensity, and efficacy at the mu opioid receptor. Psychopharmacology. 2000;150:430–42. doi: 10.1007/s002130000453. [DOI] [PubMed] [Google Scholar]
- Coppens CM, de Boer SF, Koolhaas JM. Coping styles and behavioural flexibility: towards underlying mechanisms. Philos T R Soc B. 2010;365:4021–4028. doi: 10.1098/rstb.2010.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danet M, Lapiz-Bluhm S, Morilak DA. A cognitive deficit induced in rats by chronic intermittent cold stress is reversed by chronic antidepressant treatment. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2010;13:997–1009. doi: 10.1017/S1461145710000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RN, Nolen-Hoeksema S. Cognitive inflexibility among ruminators and nonruminators. Cognitive Ther Res. 2000;24:699–711. [Google Scholar]
- DeRubeis RJ, Gelfand LA, Tang TZ, Simons AD. Meidcations versus cognitive behavior therapy for severely depressed outpatients: mega-analysis of four randomized comparisons. Am J Psychiatry. 1999;156:1007–1013. doi: 10.1176/ajp.156.7.1007. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Finger EC, Brotman MA, Rich BA, Pine DS, Blair JR, Leibenluft E. Impaired probabilistic reversal learning in youths with mood and anxiety disorders. Psychol Med. 2010;40:1089–1100. doi: 10.1017/S0033291709991462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahmandfar M, Karimian SM, Naghdi N, Zarrindast MR, Kadivar M. Morphine-induced impairment of spatial memory acquisition reversed by morphine sensitization in rats. Behavioural brain research. 2010;211:156–63. doi: 10.1016/j.bbr.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Garety P, Fowler D, Kuipers E, Freeman D, Dunn G, Bebbington P, Hadley C, Jones S. London-East Anglia randomised controlled trial of cognitive-behavioural therapy for psychosis. II: Predictors of outcome. The British journal of psychiatry: the journal of mental science. 1997;171:420–6. doi: 10.1192/bjp.171.5.420. [DOI] [PubMed] [Google Scholar]
- Gaulin SJC, Fitzgerald RW. Sex-Differences in Spatial Ability - an Evolutionary Hypothesis and Test. Am Nat. 1986;127:74–88. [Google Scholar]
- Gaulin SJC, Fitzgerald RW. Sexual Selection for Spatial-Learning Ability. Animal behaviour. 1989;37:322–331. [Google Scholar]
- Ghods-Sharifi S, Haluk DM, Floresco SB. Differential effects of inactivation of the orbitofrontal cortex on strategy set-shifting and reversal learning. Neurobiology of learning and memory. 2008;89:567–73. doi: 10.1016/j.nlm.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Goel N, Bale TL. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. Journal of neuroendocrinology. 2009;21:415–20. doi: 10.1111/j.1365-2826.2009.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales KL, Chapleau JD, Pierce JP, Kelter DT, Williams TJ, Torres-Reveron A, McEwen BS, Waters EM, Milner TA. The influences of reproductive status and acute stress on the levels of phosphorylated mu opioid receptor immunoreactivity in rat hippocampus. Frontiers in endocrinology. 2011:2. doi: 10.3389/fendo.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MM, Thase ME, Sweeney JA. Cognitive disturbance in outpatient depressed younger adults: Evidence of modest impairment. Biological psychiatry. 2001;50:35–43. doi: 10.1016/s0006-3223(00)01072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg GD, Laman-Maharg A, Campi KL, Voigt H, Orr VN, Schaal L, Trainor BC. Sex differences in stress-induced social withdrawal: role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Frontiers in behavioral neuroscience. 2014;7:223. doi: 10.3389/fnbeh.2013.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Smits JA. Cognitive-behavioral therapy for adult anxiety disorders: a meta-analysis of randomized placebo-controlled trials. The Journal of clinical psychiatry. 2008;69:621–32. doi: 10.4088/jcp.v69n0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isovich E, Engelmann M, Landgraf R, Fuchs E. Social isolation after a single defeat reduces striatal dopamine transporter binding in rats. The European journal of neuroscience. 2001;13:1254–6. doi: 10.1046/j.0953-816x.2001.01492.x. [DOI] [PubMed] [Google Scholar]
- Jamot L, Matthes HW, Simonin F, Kieffer BL, Roder JC. Differential involvement of the mu and kappa opioid receptors in spatial learning. Genes, brain, and behavior. 2003;2:80–92. doi: 10.1034/j.1601-183x.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- Jasarevic E, Sieli PT, Twellman EE, Welsh TH, Schachtman TR, Roberts RM, Geary DC, Rosenfeld CS. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. P Natl Acad Sci USA. 2011;108:11715–11720. doi: 10.1073/pnas.1107958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasarevic E, Williams SA, Roberts RM, Geary DC, Rosenfeld CS. Spatial Navigation Strategies in Peromyscus: a Comparative Study. Animal behaviour. 2012;84:1141–1149. doi: 10.1016/j.anbehav.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci Biobehav R. 2005;28:811–825. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kanagaratnam P, Asbjornsen AE. Executive deficits in chronic PTSD related to political violence. Journal of anxiety disorders. 2007;21:510–25. doi: 10.1016/j.janxdis.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Innes DG. Sex and day-night differences in opiate-induced responses of insular wild deer mice, Peromyscus maniculatus triangularis. Pharmacology, biochemistry, and behavior. 1987;27:477–82. doi: 10.1016/0091-3057(87)90351-0. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MA, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–35. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- Liberzon I, Taylor SF, Phan KL, Britton JC, Fig LM, Bueller JA, Koeppe RA, Zubieta JK. Altered central micro-opioid receptor binding after psychological trauma. Biological psychiatry. 2007;61:1030–8. doi: 10.1016/j.biopsych.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:7870–4. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Chen LY, Phillips CA. Covalent labeling of mu opioid binding site by [3H]beta-funaltrexamine. Molecular pharmacology. 1987;32:321–9. [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–9. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Dworkin SI, Smith JE. Alkylation of mu opioid receptors by beta-funaltrexamine in vivo: comparison of the effects on in situ binding and heroin self-administration in rats. The Journal of pharmacology and experimental therapeutics. 1995;272:1135–40. [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioural brain research. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Anderson E. Latent variable growth models for research on aging. In: Birren JE, Schaie KW, editors. Handbook of the Psychology of Aging. Academic Press; San Diego, CA, USA: 1990. [Google Scholar]
- Meilandt WJ, Barea-Rodriguez E, Harvey SA, Martinez JL., Jr. Role of hippocampal CA3 mu-opioid receptors in spatial learning and memory. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:2953–62. doi: 10.1523/JNEUROSCI.5569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Burstein SR, Marrone GF, Khalid S, Gonzalez AD, Williams TJ, Schierberl KC, Torres-Reveron A, Gonzales KL, McEwen BS, Waters EM. Stress differentially alters mu opioid receptor density and trafficking in parvalbumin-containing interneurons in the female and male rat hippocampus. Synapse. 2013;67:757–772. doi: 10.1002/syn.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. The European journal of neuroscience. 2006;24:1477–87. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- Niemeier JP, Marwitz JH, Lesher K, Walker WC, Bushnik T. Gender differences in executive functions following traumatic brain injury. Neuropsychol Rehabil. 2007;17:293–313. doi: 10.1080/09602010600814729. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Hammer RP, Jr., Miczek KA, Kream RM. Social defeat stress increases expression of mu-opioid receptor mRNA in rat ventral tegmental area. Neuroreport. 1999;10:3015–9. doi: 10.1097/00001756-199909290-00026. [DOI] [PubMed] [Google Scholar]
- Olson GA, Olson RD, Kastin AJ, Green MT, Roig-Smith R, Hill CW, Coy DH. Effects of an enkephalin analog on complex learning in the rhesus monkey. Pharmacology, biochemistry, and behavior. 1979;11:341–5. doi: 10.1016/0091-3057(79)90146-1. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Csomor PA, Chmiel J, Beck T, Vollenweider FX. Sensorimotor gating and attentional set-shifting are improved by the mu-opioid receptor agonist morphine in healthy human volunteers. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2008;11:655–69. doi: 10.1017/S1461145707008322. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd edn. Sage; Thousand Oaks, CA: 2002. [Google Scholar]
- Ribble DO. The Monogamous Mating System of Peromyscus-Californicus as Revealed by DNA Fingerprinting. Behav Ecol Sociobiol. 1991;29:161–166. [Google Scholar]
- Schopp LH, Shigaki CL, Johnstone B, Kirkpatrick HA. Gender differences in cognitive and emotional adjustment to traumatic brain injury. J Clin Psychol Med S. 2001;8:181–188. [Google Scholar]
- Sherry DF, Jacobs LF, Gaulin SJC. Spatial Memory and Adaptive Specialization of the Hippocampus. Trends Neurosci. 1992;15:298–303. doi: 10.1016/0166-2236(92)90080-r. [DOI] [PubMed] [Google Scholar]
- Silva AL, Fry WH, Sweeney C, Trainor BC. Effects of photoperiod and experience on aggressive behavior in female California mice. Behavioural brain research. 2010;208:528–34. doi: 10.1016/j.bbr.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford K, Gomes AB, Shen J, Yoburn BC. mu-Opioid receptor downregulation contributes to opioid tolerance in vivo. Pharmacology, biochemistry, and behavior. 2001;69:233–7. doi: 10.1016/s0091-3057(01)00525-1. [DOI] [PubMed] [Google Scholar]
- Steinman MQ, Crean KK, Trainor BC. Photoperiod interacts with food restriction in performance in the Barnes maze in female California mice. The European journal of neuroscience. 2011;33:361–70. doi: 10.1111/j.1460-9568.2010.07528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman MQ, Laredo SA, Lopez EM, Manning CE, Hao RC, Doig IE, Campi KL, Flowers AE, Knight JK, Trainor BC. Hypothalamic vasopressin systems are more sensitive to the long term effects of social defeat in males versus females. Psychoneuroendocrinology. 2015;51C:122–134. doi: 10.1016/j.psyneuen.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. Meta-analysis of cognitive-behavioral treatments for social phobia. Journal of behavior therapy and experimental psychiatry. 1996;27:1–9. doi: 10.1016/0005-7916(95)00058-5. [DOI] [PubMed] [Google Scholar]
- Tebbich S, Sterelny K, Teschke I. The tale of the finch: adaptive radiation and behavioural flexibility. Philos T R Soc B. 2010;365:1099–1109. doi: 10.1098/rstb.2009.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Pride MC, Villalon Landeros R, Knoblauch NW, Takahashi EY, Silva AL, Crean KK. Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus) PloS one. 2011;6:e17405. doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor BC, Takahashi EY, Campi KL, Florez SA, Greenberg GD, Laman-Maharg A, Laredo SA, Orr VN, Silva AL, Steinman MQ. Sex differences in stress-induced social withdrawal: independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Hormones and behavior. 2013;63:543–50. doi: 10.1016/j.yhbeh.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]