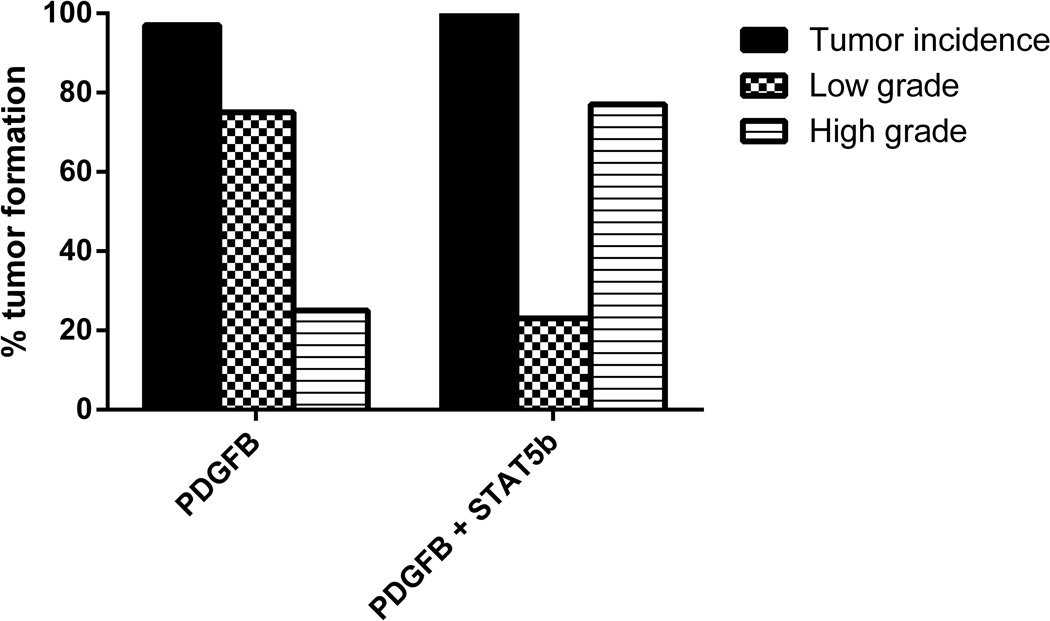Abstract
Signal transducer and activator of transcription 5b (STAT5b) is likely the relevant STAT5 isoform with respect to the process of malignant progression in gliomas. STAT5b is a latent cytoplasmic protein involved in cell signaling through the modulation of growth factors, apoptosis, and angiogenesis. Previous in vitro studies have shown increased STAT5b expression in glioblastomas relative to low-grade tumors and normal brain. We recently demonstrated that phosphorylated STAT5b associates with delta epidermal growth factor receptor in the nucleus and subsequently binds the promoters of downstream effector molecules, including aurora kinase A. Analysis of TCGA dataset reveals that STAT5b is predominantly expressed in proneural (PN) gliomas relative to mesenchymal and neural gliomas. Here, we modeled ectopic expression of STAT5b in vivo using a platelet-derived growth factor subunit B (PDGFB)-dependent mouse model of PN glioma to determine its effect on tumor formation and progression. We showed that co-expression of STAT5b and PDGFB in mice yielded a significantly higher rate of high-grade gliomas than PDGFB expression alone. We also observed shorter survival in the combined expression set. High-grade tumors from the STAT5b+PDGFB expression set were found to have a lower rate of apoptosis than those from PDGFB alone. Furthermore, we showed that increased expression of STAT5b+PDGFB led to increased expression of downstream STAT5b targets, including Bcl-xL, cyclin D1, and aurora kinase A in high-grade tumors when compared to tumors derived from PDGFB alone. Our findings show that STAT5b promotes the malignant transformation of gliomas, particularly the PN subtype, and is a potential therapeutic target.
Keywords: apoptosis, glioma, STAT5b
Introduction
The signal transduction and activator of transcription (STAT) gene family is a group of signal transduction molecules involved in cell signaling, growth factor signaling, angiogenesis, and apoptosis1. One member, STAT3, has been well described as a promoter of malignant progression of gliomas, suppressor of apoptosis, and facilitator of the mesenchymal subtype of high-grade gliomas (HGG)2, 3. Mounting evidence suggests that STAT5b also contributes to the malignant progression of gliomas4, 5. STAT5b, along with STAT5a, are the two isoforms of STAT5. Both are latent cytoplasmic proteins that translocate to the nucleus after phosphorylation and dimerization to modulate DNA transcription6. These two isoforms are highly conserved but demonstrate different functions and differential activation in response to certain kinases such as Src, which only activates STAT5b7–11. STAT5b is known to contribute to tumor progression in human epithelial cancer xenografts, and STAT5b inhibition results in both a lack of tumor progression and decreased levels of downstream STAT5b targets, including B-cell lymphoma extra-large (Bcl-xL) and cyclin D12.
STAT5b may be the pertinent STAT5 isoform contributing to the progression from low-grade glioma (LGG) to HGG. Liang et al. demonstrated that STAT5b expression is higher in glioblastomas (GBM) than in low-grade astrocytomas and normal cortex, and that silencing STAT5b in one GBM cell line caused cell cycle arrest, inhibited cell growth, and inhibited tumor cell invasion13. Silencing STAT5b was also accompanied by a decrease in the downstream signaling molecules Bcl-2, focal adhesion kinase, and vascular endothelial growth factor13. Phosphorylated STAT5b at tyrosine Y699 has been shown to be a downstream target of the mutated, constitutively active delta epidermal growth factor receptor (ΔEGFR), which has been established as an important oncogene for GBM progression and is associated with increased anti-apoptotic Bcl-xL expression14–16. We have recently shown that phosphorylated STAT5b associates with ΔEGFR in the nucleus of glioma cells and, in turn, interacts with DNA promoter sequences, including aurora kinase A—whose expression correlates both with tumor grade and survival in GBM—and Bcl-xL, to effect transcription4, 17. ΔEGFR-mediated phosphorylation of STAT5b proceeds via Src family kinases in glioma cells4. High levels of phosphorylated STAT5b have been shown to be associated with shorter survival and higher Bcl-xL expression4. Gliomas with the ΔEGFR mutation demonstrate resistance to cisplatin-based chemotherapy; this resistance is thought to be due to increased anti-apoptotic Bcl-xL expression from ΔEGFR signaling, and is regulated by phosphorylated STAT5b.
Identifying key molecular signaling molecules that promote the transition from LGG to HGG is critical, as inhibiting their activity may mitigate malignant progression. Although STAT5b levels are higher in GBM than in lower-grade glioma, STAT5b’s ability to promote malignant transformation is not yet clearly understood. Based on our previous work demonstrating the ability of anti-apoptotic signaling to drive glioma progression18, we hypothesized that STAT5b would promote the development of HGG. We used the replication-competent ASLV long terminal repeat with a splice acceptor (RCAS)/Nestin promoter tv-a line (Ntv)a transgenic mouse system to overexpress STAT5b with platelet derived growth factor B (PDGFB) in murine glioneuronal progenitor cells19. Ligand interaction with the PDGF receptor, which is over-expressed in human malignant gliomas results in activation of pro-survival signaling pathways that promote tumor cell growth20, 21. Murine model systems have demonstrated that over-expression of PDGFB in the brain induces predominantly low-grade glioma22 which exhibit proneural characteristics23. Recently, Zhu et al. have demonstrated that co-injection of RCAS viruses expressing Akt along with PDGFB gives rise to HGG with full penetrance in Ntv-a mice24. We have used the RCAS/Ntv-a murine model of glioma to show that BCL-2 (the archetypal inhibitor of apoptosis)18, Survivin (a member of the inhibitor of apoptosis gene family)25, TAZ (Transcriptional coactivator with PDZ-binding motif)26 and STAT3 (a key molecular hub of tumorigenesis)2 when co-expressed with PDGFB causes progression of tumors to HGG. Here we extend our previous in vitro work by demonstrating that STAT5b expression in a PN glioma model promotes the formation of high-grade tumors and decreases tumor-free survival.
Materials and Methods
Dataset analysis
The Memorial Sloan Kettering Cancer Center cBioportal interface (www.cbioportal.org) was used to query the 2008 glioblastoma data set from the Cancer Genome Atlas (TCGA)27, 28. The various phenotypic subtypes of GBM were evaluated by looking at the number of tumors with STAT5b mRNA expression greater than the normalized mean (using the syntax STAT5b: EXP > 0).
Vector constructs
Human STAT5b in conjunction with PDGFB was expressed in mice using the RCAS/Ntv-a transgenic mouse system19. In this system, a gene of interest is cloned into a modified avian retrovirus (RCAS) that is replication defective in mammalian cells. This vector is introduced into a transgenic mouse line (Ntv-a) that expresses TVA (the avian leukosis virus subtype A receptor, the receptor for RCAS) under control of the Nestin promoter. This system makes it possible to transfer and express exogenous genes to Nestin-expressing neural progenitor cells and their progeny29. RCAS- PDGFB was constructed with a hemagglutinin epitope (HA) tag as previously described30. RCAS-STAT5b was constructed by amplifying the STAT5b cDNA coding sequence by polymerase chain reaction, using specialized primers that allow directional cloning into a Gateway entry vector (Life Technologies, Carlsbad, CA). The entry vector was recombined with a previously constructed RCAS destination vector by the proprietary Gateway attL/attR recombination reaction. This generated the RCAS-STAT5b vector construct, which was verified by sequencing.
Transfection of DF-1 cells
DF-1 immortalized chicken fibroblasts were grown in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum (Gibco; Life Technologies, Carlsbad, CA) at 37°C in a humidified atmosphere consisting of 5% CO2 and 95% air. Plasmid versions of the RCAS vectors were transfected into the DF-1 cells using FuGene6 (Roche Diagnostics, Indianapolis, IN), thus producing live virus. The cells were then replicated in culture.
Western blot analysis
To verify STAT5b expression in DF-1 cells after transfection, Western blotting was performed. Whole-cell lysates were made from both untransfected DF-1 cell cultures and DF-1 cells transfected with either RCAS-STAT5b or RCAS-PDGFB. Sodium dodecyl sulfate polyacrylamide gel electrophoresis was used to fractionate 10-µg protein samples using gels with 10% polyacrylamide. Samples were transferred to a polyvinylidene difluoride membrane and probed with anti-influenza hemagglutinin antibody (1:1000; F7; Santa Cruz Biotechnology, Santa Cruz, CA) to verify PDGFB expression and with anti-STAT5b (1:1000, Santa Cruz Biotechnology) to detect total STAT5b expression. Goat anti-rabbit immunoglobulin G (1:2500; Santa Cruz Biotechnology) was used as the secondary antibody. The ECL Plus detection kit (GE Healthcare, Waukesha, WI) was used per the manufacturer’s instructions to develop the blots.
In vivo somatic cell transfer in transgenic mice
The details of the Ntv-a transgenic mouse strain are described elsewhere19. In brief, these mice are a combination of multiple strains: C57BL/6, BALB/c, FVB/N, and cluster differentiation 1. Genes were transferred via the RCAS vector by injecting a defined volume of DF-1 cells (1×104 DF-1 cells in 1–2 µl phosphate-buffered saline) transfected with a given RCAS vector into the frontal lobes of Ntv-a mice, anterior to the coronal suture, using a 10-µl gastight Hamilton syringe (Hamilton Company, Reno, NV). Because the population of Nestin+ cells producing avian tumor receptor virus A is highest 24 to 48 hours after birth, the mice were injected during this time period. The vector constructs used were RCAS-PDGFB (n=29 mice), RCAS-STAT5b (n=30 mice), and RCAS-PDGFB + RCAS-STAT5b (n=34 mice). The mice injected with two RCAS vectors received an equal number of DF-1 cells with each RCAS vector.
The mice were killed when they developed neurological morbidities such as hydrocephalus or neurological symptoms due to the tumor burden or at 90 days after vector injection. The brains were harvested and evaluated for tumor formation. Verification of tumors and histological grading were performed by the study neuropathologist (G.N.F.). Tumors were distinguished as high grade by the presence of brisk mitosis, necrosis, or microvascular proliferation. The animal experiments described in this research were approved by the Institutional Animal Care and Use Committee at The University of Texas MD Anderson Cancer Center (Protocol 08-06-11633).
Immunohistochemical analysis
Harvested mouse brains were embedded in paraffin, and 4-µm sections were used for immunohistochemical analysis. Antigen retrieval was performed with the Thermo Scientific PT Module (Thermo Fisher Scientific, Waltham, MA) with citrate buffer. The Lab Vision Immunohistochemical Autostainer 360 (Thermo Fisher Scientific) was used to perform staining. An avidin-biotin complex technique with diaminobenzidine (Invitrogen, Carlsbad, CA) as the chromogenic substrate and hematoxylin as the counterstain was used to visualize the immunoreactive staining. We obtained monoclonal antibodies against human STAT5b (1:250; Thermo Scientific); Olig2, a PN marker (1:500; Millipore, Billerica, MA); Bcl-xL (1:50; Cell Signaling Technology); cyclin D1 (1:150; Santa Cruz Biotechnology); aurora kinase A (1:200; LifeSpan BioScience, Seattle, WA) cleaved caspase 3 (1:50; Cell Signaling Technology, Beverly, MA) and phosphohistone H3 (1:1000; Millipore) as a marker of cell proliferation31.”
Mitotic index
Formalin-fixed, paraffin-embedded tumor tissue was stained with an antibody to phosphohistone H3 to calculate the mitotic index of the tumors in mice injected with RCAS-PDGFB + RCAS-STAT5b (n = 4). Phosphohistone H3staining has been shown to be a reliable method for assessing tumor cell proliferation in glioma31. The total number of cells and number of positive cells were counted in ten non-overlapping, high-powered fields (400× magnification) in the areas of highest tumor density. The mitotic index was calculated as a percentage of positive cells. The median number of cells counted per field was 887 (range, 626–1281).
Apoptotic assay
High-grade tumor sections from mice injected with RCAS-PDGFB + RCAS-STAT5b were stained with antibody to cleaved caspase 3 to detect apoptosis (n = 5)32. Ten non-overlapping, high-powered fields (400×) in areas of high tumor density were identified, and the number of positive cells and the total number of cells were counted. The apoptotic index was calculated as a percentage of positive cells. The median number of cells counted per field was 907 (range, 547–1267).
Phenotypic characterization of tumor
To verify a PN phenotype in tumors generated by injection of RCAS-PDGFB and RCAS-STAT5b vectors in NTV-a mice, formalin-fixed, paraffin-embedded tissue sections of high-grade tumors were stained with an antibody to Olig2, a marker of the PN phenotype (n = 5).
Evaluation of downstream effector molecules
The effect of injection of RCAS vectors on known downstream targets of STAT5b was assessed. Formalin-fixed, paraffin-embedded tumor tissue was stained with antibodies to Bcl-xL, an inhibitor of apoptosis and known target of phosphorylated STAT5b; aurora kinase A, whose promoter is a known target of phosphorylated STAT5b; and cyclin D1, a cell cycle regulation protein and known target of phosphorylated STAT5b. Positively stained cells were identified.
Statistical analysis
Descriptive statistics are reported as percentages and frequencies. The incidence of tumors between injection sets was assessed using the chi-squared test. Non-adjusted time to symptomatic tumor formation was reported by Kaplan-Meier curves. Statistical analysis was carried out using SAS, version 9 and Graphpad Prism, version 5.03 software (San Diego, CA) (http://www.sas.com).
Results
STAT5b expression is increased in PN gliomas
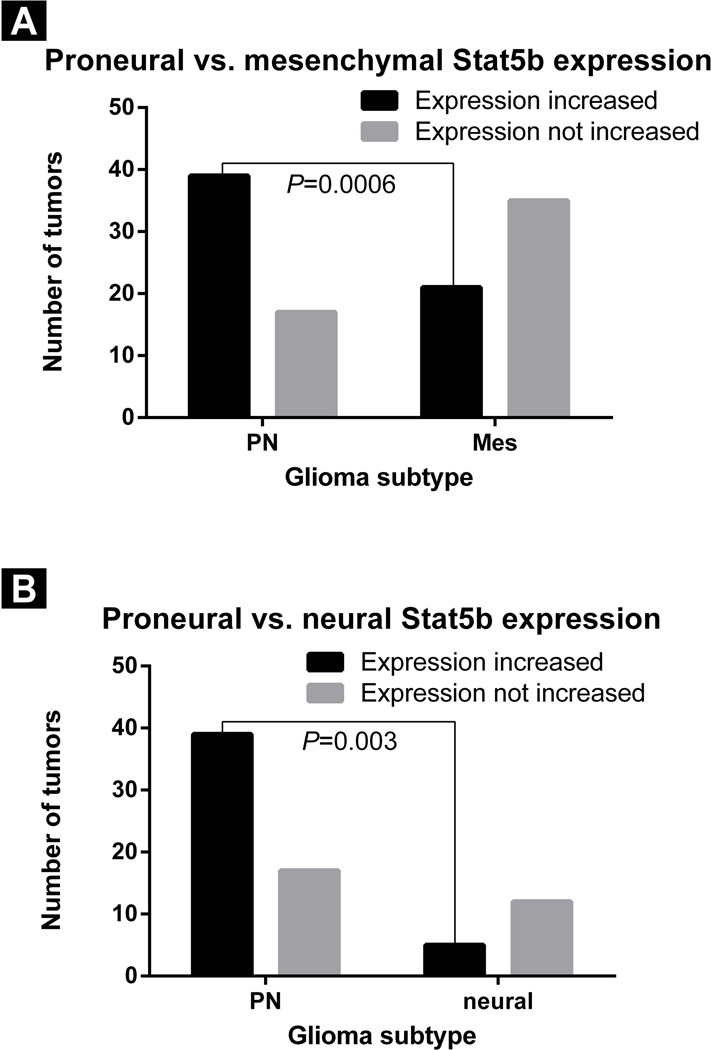
The TCGA 2008 dataset was analyzed to determine whether STAT5b expression was more common in a particular subtype of glioma27, 28. High STAT5b expression was found in a larger percentage of the PN glioma subtype tumors (39/56 [70%]) than in the mesenchymal subtype tumors (21/56 [38%]; chi-squared test; P = 0.0006; 95% confidence interval [CI]: 1.743–8.389; odds ratio: 3.824; Figure 1A). STAT5b expression was also noted to be higher in a larger percentage of the PN glioma subtype than in the neural glioma subtype (5/29 tumors [17%]; chi-squared test; P = 0.003; 95% CI: 1.677–18.08; odds ratio: 5.506; Figure 1B). No difference in the percentage of tumors with high STAT5b expression was seen between PN gliomas and classical gliomas (34/54 tumors [63%]; chi-squared test; P = 0.55; 95% CI: 0.61–2.98; odds ratio:1.35).
Figure 1.
Bar graph demonstrating that high STAT5b expression in the TCGA data set was found in a larger percentage of the PN subtype of GBM relative to A) the mesenchymal subtype (chi-squared test, P = 0.0006) and B) the neural subtype (chi-squared test, P = 0.003).
STAT5b co-expression with PDGFB promotes the development of high-grade tumors
Given the predominance of STAT5b overexpression in the PN subtype of GBM relative to two of the other subtypes, we evaluated the in vivo effect of the overexpression of STAT5b in a PDGFB-dependent model that is known to recapitulate the PN glioma phenotype33. The effect of STAT5b on glioma progression was studied by injecting Ntv-a mice with RCAS-PDGFB alone, RCAS-STAT5b alone, or a combination of RCAS-STAT5b + RCAS-PDGFB. The brains recovered from the mice were examined for evidence of tumor formation. Tumor incidence was 97% (33/34) for the combination injection cohort of RCAS-STAT5b + RCAS-PDGFB and 97% (28/29) for the RCAS-PDGFB alone group (Figure 2). We injected 30 mice with RCAS-STAT5b alone; none of the 30 mice developed neurological morbidity or signs of hydrocephalus during the 90-day observation period, and none had evidence of tumor formation upon examination of brain tissue.
Figure 2.
Bar graph showing increased incidence of HGG formation in the RCAS-PDGFB + RCAS-STAT5b cohort vs. the RCAS-PDGFB cohort.
The RCAS-PDGFB cohort of mice developed 21 (75%) LGGs and 7 (25%)HGGs. The cohort of mice injected with both RCAS-STAT5b and RCAS-PDGFB developed 8 (24.2%) LGGs and 25 (75.8%) HGGs. The higher incidence of HGG in mice with overexpression of STAT5b was statistically significant (chi-squared test; P = < 0.0001; 95% CI: 2.91–30.17; odds ratio: 9.38).
Co-expression of STAT5b with PDGFB decreases tumor-free survival
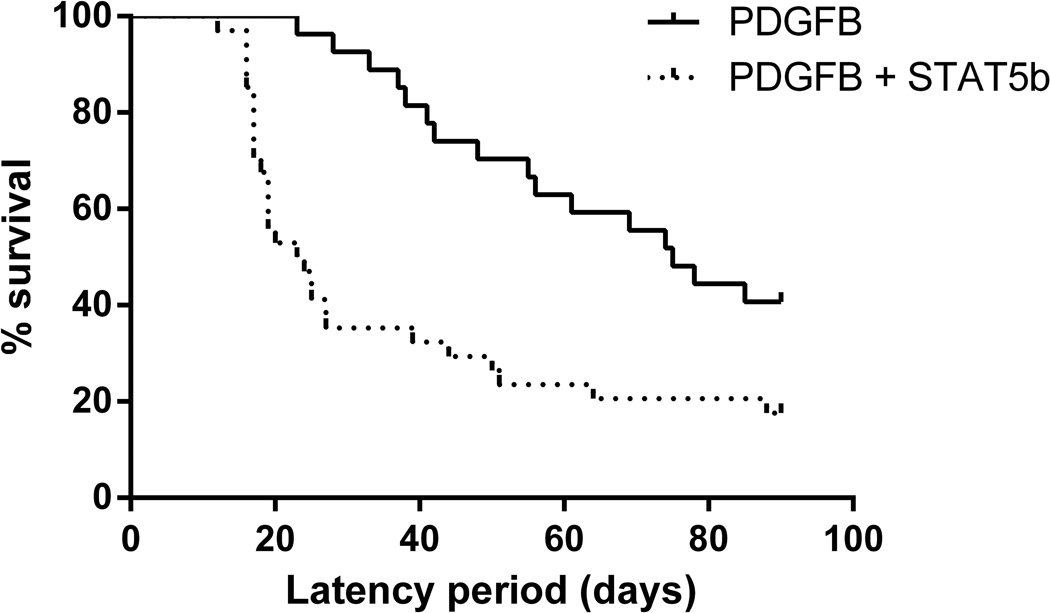
The median time to symptomatic tumor formation for mice injected with RCAS-STAT5b + RCAS-PDGFB was 24 days (range, 12–90), and for the mice injected with RCAS-PDGFB alone was 76.5 days (range, 23–90). Thus, we observed a statistically significant decrease in survival in mice overexpressing STAT5b in conjunction with PDGFB (log rank test, P = 0.0012; 95% CI: 2.65–3.73; Figure 3
Figure 3.
Kaplan-Meier curve demonstrating significantly shorter tumor symptomatic tumor formation period in mice injected with RCAS-PDGFB + RCAS-STAT5b (median, 24 days; range, 12–90) vs. RCAS-PDGFB alone (median, 76.5 days; range, 23–90; log rank test, P = 0.0012; 95% CI: 2.65–3.73)
STAT5b overexpression is associated with a decrease in tumor cell apoptosis without an increase in tumor cell proliferation
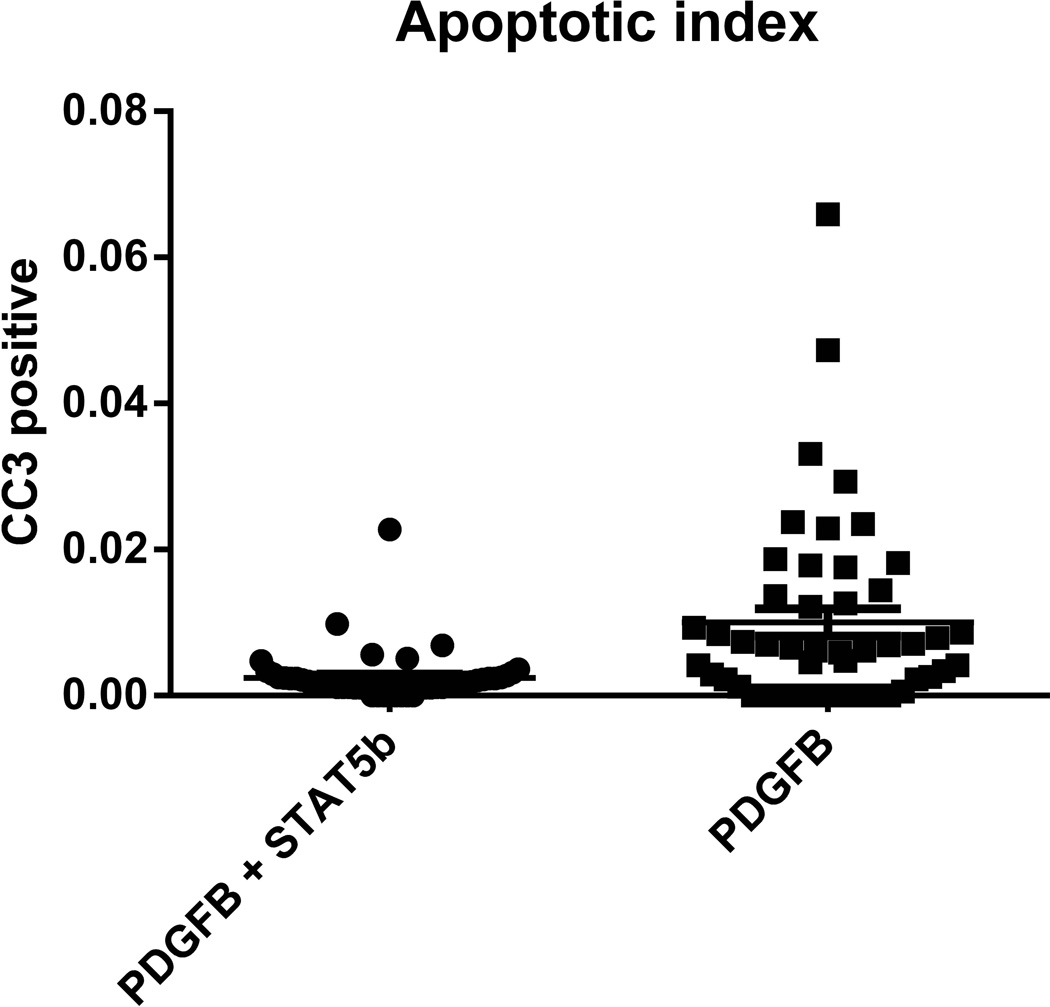
The rate of apoptosis in HGGs in the RCAS-STAT5b + RCAS-PDGFB injection cohort was assessed by staining for cleaved caspase 3 using immunohistochemical methods. The apoptotic index was calculated for HGGs and compared to our previously published values generated by Ntv-a mice injected with RCAS-PDGFB alone2 (Figure 4). There was a statistically significant decrease in apoptosis in the tumors from mice injected with RCAS-STAT5b + RCAS-PDGFB compared to RCAS-PDGFB alone (Student’s t-test; P = 0.0001; 95% CI: 0.004–0.011). We then evaluated the rate of tumor cell proliferation in HGGs by phosphohistone H3 staining. The mitotic index was calculated and compared to our previously reported mitotic indexes for HGGs generated by Ntv-a mice injected with RCAS-PDGFB alone2. The mitotic rate was higher in the RCAS-PDGFB + RCAS-STAT5b injection set (1.6%) than in the RCAS-PDGFB injection set (1.0%), but this was not statistically significant (Student’s t-test; P = 0. 2).
Figure 4.
Scatter plot demonstrating lower cleaved caspase 3 (CC3) expression in the HGG induced by RCAS-STAT5b + RCAS-PDGFB than in the HGG induced by RCAS-PDGFB alone (Student’s t-test; P = 0.0001).
HGGs generated in the STAT5b+ PDGFB injection set maintain a PN phenotype
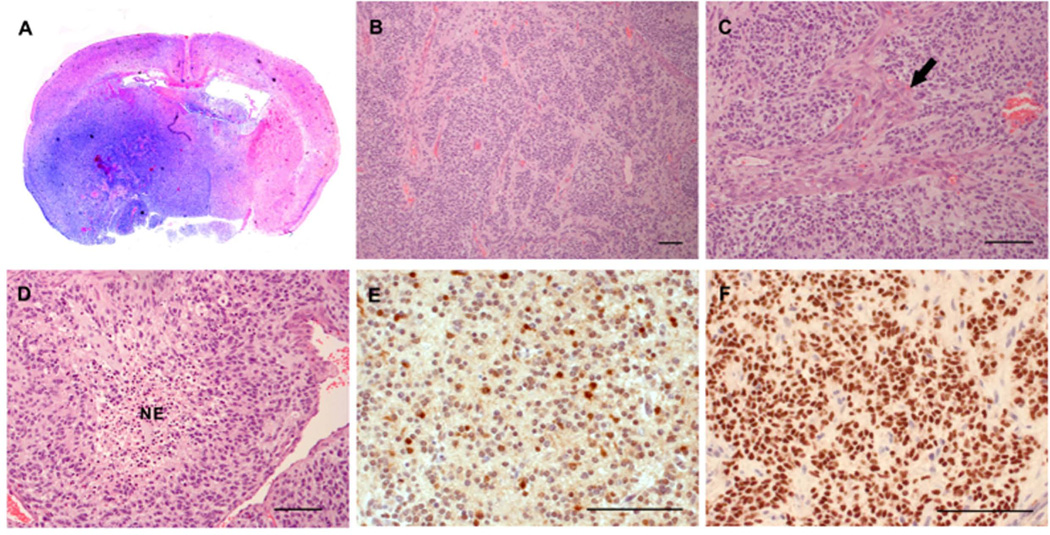
We evaluated the HGGs generated from the RCAS-STAT5b + RCAS-PDGFB injection set (Figure 5A–D) and confirmed STAT5b expression by staining with an antibody specific to human STAT5b. Avid staining of the tumor region was seen, confirming the high STAT5b expression in these HGGs (Figure 5E). Tumors generated by RCAS-PDGFB injection in Ntv-a mice have a PN phenotype, and we have shown previously that tumors arising from this model show homogenously positive staining for Olig2, a well-documented PN marker34–36. In concordance with this, avid staining of tumors induced by RCAS-STAT5b + RCAS-PDGFB with Olig2 was observed, confirming a PN signature (Figure 5F).
Figure 5.
Increased expression of STAT5b and PDGFB induced HGGs. (A) Whole-mount coronal section (hematoxylin and eosin staining) demonstrating tumor formation in the right hemisphere (25× magnification). (B) Hematoxylin and eosin staining showing HGG (100×) characterized by (C) microvascular proliferation indicated by arrow (200×) and (D) pseudopalisading necrosis (indicated by NE; 200×). (E) Avid STAT5b expression was observed intratumorally (400×). (F) The PN phenotype was verified by avid Olig2 staining in the tumor (400×). Scale bar = 100 µm.
STAT5b expression upregulates expression of known STAT5b targets
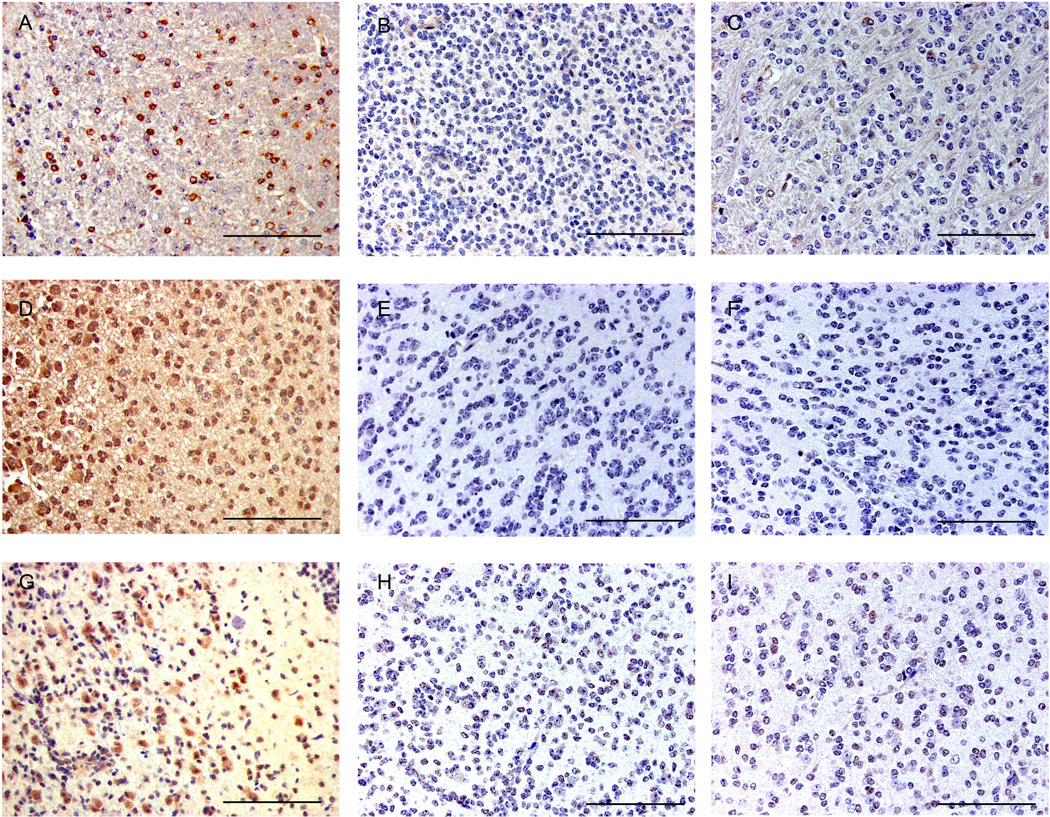
Based on our previous in vitro work identifying STAT5b targets, we further characterized tumors induced by STAT5b. We immunostained a selection of LGGs induced by PDGFB alone and both, LGGs and HGGs induced by PDGFB + STAT5b for expression of aurora kinase A, Bcl-xL4, and cyclin D1, whose promoter has been shown to bind STAT537, 38. Bcl-xL is a known inhibitor of apoptosis and a downstream target of STAT5b. Avid staining for Bcl-xL was seen intratumorally in HGGs from the PDGFB + STAT5b cohort, demonstrating increased expression in the HGGs when compared to the LGGs (Figures 6A–C), where as low expression of Bcl-xL was observed in the PDGFB alone tumors. Aurora kinase A is a serine threonine kinase that controls entry into mitosis as well as centriole pair separation, metaphase alignment of chromosomes, and cytokinesis39, 40. The aurora kinase A promoter is a known target of phosphorylated STAT5b4. A high expression of aurora kinase A within HGGs was demonstrated in the PDGFB+STAT5b group when compared to the LGGs from this group (Figures 6D–F. Cyclin D1 is a cell cycle regulatory protein whose promoter is a target of phosphorylated STAT5b. Prominent, homogenous staining was seen within the tumor, showing increased cyclin D1 expression only in the HGGs from the PDGFB+STAT5b group when compared to the LGGs from the same cohort (Figures 6G–I). Tumors from the PDGFB consistently showed minimal expression of the STAT5b targets (Figure 6) These findings show that in vivo upregulation of STAT5b with PDGFB leads to high expression of known STAT5b targets, including an inhibitor of apoptosis and cell cycle promoters.
Figure 6.
STAT5b overexpression in conjunction with overexpression of PDGFB induces tumors with increased expression of STAT5b downstream effector targets in high-grade tumors relative to low-grade tumors induced by PDGFB alone or PDGFB+STAT5b. Photomicrographs (400× magnification) demonstrating high expression of Bcl-xL in (A) HGG induced by PDGFB+STAT5b but minimal or absent expression in LGG induced bv (B) PDGFB+STAT5b and (C) PDGFB alone. Photomicrographs (400×) of HGG demonstrating high expression of Aurora Kinase A in (D) HGG induced by PDGFB+STAT5b but minimal or absent expression in LGG induced bv (E) PDGFB+STAT5b and (F) PDGFB alone. Photomicrographs (400× magnification) demonstrating high expression of Cyclin D1 in (G) HGG induced by PDGFB+STAT5b but minimal or absent expression in LGG induced bv (H) PDGFB+STAT5b and (I) PDGFB alone. Scale bar = 100 µm. Scale bar = 100 µm.
Discussion
STAT5b expression is associated with the development of gliomas, but its role in the malignant progression of gliomas remains unclear. Our group has recently shown that STAT5b contributes to tumor cell survival by suppressing apoptosis and that apoptotic suppression (mediated by another STAT family member, STAT3) is a key contributor to the degeneration of LGGs to HGGs2. Here, we show that co-expression of STAT5b with PDGFB promotes malignant progression in a PN model of glioma. We also show that increased STAT5b expression leads to shorter survival in mice. Further, we show in vivo that STAT5b decreases apoptosis in HGGs and is associated with increased expression of Bcl-xL. These results confirm and extend our previous work correlating STAT5b with a more aggressive tumor phenotype.
We have recently demonstrated that another member of the STAT family, STAT3, may induce the mesenchymal phenotype of HGG when overexpressed in glioma2. The different subtypes of HGG can be defined by variations in the signaling cascades prominent in each subtype41. These signal transduction pathways correspond to the phenotypic subtypes of HGG36. Our analysis of TCGA expression data shows increased expression of STAT5b in the PN subtype of HGGs. It has been suggested that the phenotypic subtypes of HGG exist on a continuum, ranging from the relatively well-differentiated PN phenotype with a better prognosis and extending to the more aggressive mesenchymal phenotype with a poorer prognosis35. Secondary HGGs are most likely to be of the PN phenotype, whereas recurrent HGGs tend to shift to a mesenchymal phenotype35, 36. The HGGs induced by the co-expression of STAT5b and PDGFB maintained the PN phenotype, consistent with our analysis of TCGA expression data.
We have previously shown that STAT5b binds and increases the activity of the aurora kinase A promoter4. Previous studies have demonstrated that Aurora kinase A expression increases as tumor grade increases17. Moreover, inhibition of Aurora kinase A was synergistic with radiation in glioblastoma42. Accordingly, we found high expression of Aurora kinase A intratumorally in HGGs generated by the co-expression of STAT5b with PDGFB. This is consistent with STAT5b expression, increasing across the glioma spectrum from LGG to HGG and reaching a maximal expression in PN GBM.
We found a direct correlation between STAT5b overexpression in vivo and shorter survival in Ntv-a mice. This is consistent with our previous work demonstrating that human tumor samples with high STAT5b expression were associated with shorter survival4 where in, we have shown that STAT5b can be activated by ΔEGFR and then associate together in the nucleus to affect gene transcription by directly binding to regulatory DNA regions4. Our findings in this paper support this conclusion, as we demonstrated high expression of the known downstream targets of STAT5b cyclin D1, aurora kinase A, and Bcl-xL43, 44. Although this would predict that STAT5b overexpression would drive tumor cell proliferation, we were unable to show a statistical increase in mitotic activity in tumors induced by STAT5b + PDGFB relative to those induced by PDGFB alone. This suggests that STAT5b promotes tumor proliferation primarily by inhibiting apoptosis. This differs from results shown by Xi et al., who found that silencing of STAT5b decreased cell division but did not increase apoptosis, despite a decrease in both cyclin D1 and Bcl-xL expression12. This, however, was demonstrated in a xenograft model of squamous cell carcinoma of the head and neck, rather than a glial tumor12. Liang et al. also performed silencing experiments in a GBM cell line and similarly found that silencing STAT5b induced cell cycle arrest in the G1 to S phase and decreased cell division. They also found that levels of the anti-apoptotic molecule Bcl-2 were decreased in cell lines treated with STAT5b silencing13. Our current results are consistent with our previous studies showing that apoptotic suppression strongly drives tumor progression18, 25. Conversely, others have shown that the loss of signaling programs that result in the induction of apoptosis increases the survival of tumor bearing Ntv-a mice24. These results underscore the importance of apoptotic suppression on tumor progression.
While cyclin D1 activity is necessary for cell division, increasing its expression past physiological levels in isolation may not necessarily promote tumor cell proliferation. Similarly, we suggest that while increasing levels of anti-apoptotic molecules, including Bcl-xL and Bcl-2, is sufficient to decrease apoptosis, their absence alone is not sufficient to induce apoptosis. Further, tumor cell proliferation driven by PDGFB may be stronger than the proliferative effect being engendered by STAT5b. Nonetheless, our data suggest a relevant mechanism for tumorigenesis in vivo in which STAT5b effects tumor proliferation primarily by inhibiting apoptosis through activation of Bcl-xL as opposed to increasing cell division. This is consistent with our earlier work demonstrating that increased BCL-2 expression promotes tumor progression primarily by disabling apoptosis18
Conclusion
In this study, we demonstrate that overexpression of STAT5b PDGFB drives malignant progression in a PDGFB-dependent murine model of PN glioma primarily by inhibiting apoptosis in tumor cells. We also show that STAT5b overexpression is associated with shorter survival in tumor-bearing mice. Our results suggest that STAT5b represents an important therapeutic target.
Acknowledgments
This work was supported by the National Institutes of Health Grant Number K08 NS070928 (G.R.); and the Marnie Rose Foundation (GR). The authors wish to thank Luanne Jorewicz for editorial assistance with the manuscript.
Footnotes
Novelty: We hypothesized that STAT5b may drive progression from low- to high-grade glioma. We analyzed TCGA data and found that STAT5b expression correlates strongly with proneural glioblastoma (GBM). We then modeled expression of STAT5b with PDGFB in a proneural model of murine glioma. We find that STAT5b strongly promotes malignant degeneration to high-grade glioma in this model and decreases tumor-free survival. We show that this effect is mediated through the anti-apoptotic effect of STAT5b.
The authors have no conflicts of interest to declare.
References
- 1.Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doucette TA, Kong LY, Yang Y, Ferguson SD, Yang J, Wei J, Qiao W, Fuller GN, Bhat KP, Aldape K, Priebe W, Bogler O, et al. Signal transducer and activator of transcription 3 promotes angiogenesis and drives malignant progression in glioma. Neuro Oncol. 2012;14:1136–1145. doi: 10.1093/neuonc/nos139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carro MS, Lim WK, Alvarez MJ, Bollo RJ, Zhao X, Snyder EY, Sulman EP, Anne SL, Doetsch F, Colman H, Lasorella A, Aldape K, et al. The transcriptional network for mesenchymal transformation of brain tumours. Nature. 2010;463:318–325. doi: 10.1038/nature08712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latha K, Li M, Chumbalkar V, Gururaj A, Hwang Y, Dakeng S, Sawaya R, Aldape K, Cavenee WK, Bogler O, Furnari FB. Nuclear EGFRvIII-STAT5b complex contributes to glioblastoma cell survival by direct activation of the Bcl-XL promoter. International journal of cancer Journal international du cancer. 2013;132:509–520. doi: 10.1002/ijc.27690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang QC, Xiong H, Zhao ZW, Jia D, Li WX, Qin HZ, Deng JP, Gao L, Zhang H, Gao GD. Inhibition of transcription factor STAT5b suppresses proliferation, induces G1 cell cycle arrest and reduces tumor cell invasion in human glioblastoma multiforme cells. Cancer Lett. 2009;273:164–171. doi: 10.1016/j.canlet.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Robinson GW, Gouilleux F, Groner B, Hennighausen L. Cloning and expression of Stat5 and an additional homologue (Stat5b) involved in prolactin signal transduction in mouse mammary tissue. Proc Natl Acad Sci U S A. 1995;92:8831–8835. doi: 10.1073/pnas.92.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotarla I, Ren S, Zhang Y, Gehan E, Singh B, Furth PA. Stat5a is tyrosine phosphorylated and nuclear localized in a high proportion of human breast cancers. International journal of cancer Journal international du cancer. 2004;108:665–671. doi: 10.1002/ijc.11619. [DOI] [PubMed] [Google Scholar]
- 8.Kazansky AV, Kabotyanski EB, Wyszomierski SL, Mancini MA, Rosen JM. Differential effects of prolactin and src/abl kinases on the nuclear translocation of STAT5B and STAT5A. J Biol Chem. 1999;274:22484–22492. doi: 10.1074/jbc.274.32.22484. [DOI] [PubMed] [Google Scholar]
- 9.Nevalainen MT, Xie J, Torhorst J, Bubendorf L, Haas P, Kononen J, Sauter G, Rui H. Signal transducer and activator of transcription-5 activation and breast cancer prognosis. J Clin Oncol. 2004;22:2053–2060. doi: 10.1200/JCO.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 10.Park SH, Liu X, Hennighausen L, Davey HW, Waxman DJ. Distinctive roles of STAT5a and STAT5b in sexual dimorphism of hepatic P450 gene expression. Impact of STAT5a gene disruption. J Biol Chem. 1999;274:7421–7430. doi: 10.1074/jbc.274.11.7421. [DOI] [PubMed] [Google Scholar]
- 11.Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- 12.Xi S, Zhang Q, Gooding WE, Smithgall TE, Grandis JR. Constitutive activation of Stat5b contributes to carcinogenesis in vivo. Cancer Res. 2003;63:6763–6771. [PubMed] [Google Scholar]
- 13.Liang Y, Diehn M, Watson N, Bollen AW, Aldape KD, Nicholas MK, Lamborn KR, Berger MS, Botstein D, Brown PO, Israel MA. Gene expression profiling reveals molecularly and clinically distinct subtypes of glioblastoma multiforme. Proc Natl Acad Sci U S A. 2005;102:5814–5819. doi: 10.1073/pnas.0402870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chumbalkar V, Latha K, Hwang Y, Maywald R, Hawley L, Sawaya R, Diao L, Baggerly K, Cavenee WK, Furnari FB, Bogler O. Analysis of phosphotyrosine signaling in glioblastoma identifies STAT5 as a novel downstream target of DeltaEGFR. J Proteome Res. 2011;10:1343–1352. doi: 10.1021/pr101075e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heimberger AB, Hlatky R, Suki D, Yang D, Weinberg J, Gilbert M, Sawaya R, Aldape K. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11:1462–1466. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 16.Nagane M, Coufal F, Lin H, Bogler O, Cavenee WK, Huang HJ. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56:5079–5086. [PubMed] [Google Scholar]
- 17.Lehman NL, O'Donnell JP, Whiteley LJ, Stapp RT, Lehman TD, Roszka KM, Schultz LR, Williams CJ, Mikkelsen T, Brown SL, Ecsedy JA, Poisson LM. Aurora A is differentially expressed in gliomas, is associated with patient survival in glioblastoma and is a potential chemotherapeutic target in gliomas. Cell Cycle. 2012;11:489–502. doi: 10.4161/cc.11.3.18996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doucette T, Yang Y, Zhang W, Fuller GN, Suki D, Fults DW, Rao G. Bcl-2 promotes malignant progression in a PDGF-B-dependent murine model of oligodendroglioma. Int J Cancer. 2011;129:2093–2103. doi: 10.1002/ijc.25869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12:3675–3685. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potapova O, Fakhrai H, Baird S, Mercola D. Platelet-derived growth factor-B/v-sis confers a tumorigenic and metastatic phenotype to human T98G glioblastoma cells. Cancer Res. 1996;56:280–286. [PubMed] [Google Scholar]
- 21.Potapova O, Fakhrai H, Mercola D. Growth factor PDGF-B/v-sis confers a tumorigenic phenotype to human tumor cells bearing PDGF receptors but not to cells devoid of receptors: evidence for an autocrine, but not a paracrine, mechanism. Int J Cancer. 1996;66:669–677. doi: 10.1002/(SICI)1097-0215(19960529)66:5<669::AID-IJC15>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Lindberg N, Kastemar M, Olofsson T, Smits A, Uhrbom L. Oligodendrocyte progenitor cells can act as cell of origin for experimental glioma. Oncogene. 2009;28:2266–2275. doi: 10.1038/onc.2009.76. [DOI] [PubMed] [Google Scholar]
- 23.Lei L, Sonabend AM, Guarnieri P, Soderquist C, Ludwig T, Rosenfeld S, Bruce JN, Canoll P. Glioblastoma models reveal the connection between adult glial progenitors and the proneural phenotype. PLoS One. 2011;6:e20041. doi: 10.1371/journal.pone.0020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Z, Khan MA, Weiler M, Blaes J, Jestaedt L, Geibert M, Zou P, Gronych J, Bernhardt O, Korshunov A, Bugner V, Lichter P, et al. Targeting Self-Renewal in High-Grade Brain Tumors Leads to Loss of Brain Tumor Stem Cells and Prolonged Survival. Cell stem cell. 2014 doi: 10.1016/j.stem.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Doucette T, Latha K, Yang Y, Fuller GN, Rao A, Rao G. Survivin transcript variant 2 drives angiogenesis and malignant progression in proneural gliomas. Neuro Oncol. 2014 doi: 10.1093/neuonc/nou034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhat KP, Salazar KL, Balasubramaniyan V, Wani K, Heathcock L, Hollingsworth F, James JD, Gumin J, Diefes KL, Kim SH, Turski A, Azodi Y, et al. The transcriptional coactivator TAZ regulates mesenchymal differentiation in malignant glioma. Genes & development. 2011;25:2594–2609. doi: 10.1101/gad.176800.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc Natl Acad Sci U S A. 1998;95:1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15:1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colman H, Giannini C, Huang L, Gonzalez J, Hess K, Bruner J, Fuller G, Langford L, Pelloski C, Aaron J, Burger P, Aldape K. Assessment and prognostic significance of mitotic index using the mitosis marker phospho-histone H3 in low and intermediate-grade infiltrating astrocytomas. Am J Surg Pathol. 2006;30:657–664. doi: 10.1097/01.pas.0000202048.28203.25. [DOI] [PubMed] [Google Scholar]
- 32.Arai M, Sasaki A, Saito N, Nakazato Y. Immunohistochemical analysis of cleaved caspase-3 detects high level of apoptosis frequently in diffuse large B-cell lymphomas of the central nervous system. Pathol Int. 2005;55:122–129. doi: 10.1111/j.1440-1827.2005.01808.x. [DOI] [PubMed] [Google Scholar]
- 33.Hukkelhoven E, Liu Y, Yeh N, Ciznadija D, Blain SW, Koff A. Tyrosine phosphorylation of the p21 cyclin-dependent kinase inhibitor facilitates the development of proneural glioma. The Journal of biological chemistry. 2012;287:38523–38530. doi: 10.1074/jbc.M112.366542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ligon KL, Alberta JA, Kho AT, Weiss J, Kwaan MR, Nutt CL, Louis DN, Stiles CD, Rowitch DH. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. Journal of neuropathology and experimental neurology. 2004;63:499–509. doi: 10.1093/jnen/63.5.499. [DOI] [PubMed] [Google Scholar]
- 35.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 36.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Groot RP, Raaijmakers JA, Lammers JW, Koenderman L. STAT5-Dependent CyclinD1 and Bcl-xL expression in Bcr-Abl-transformed cells. Mol Cell Biol Res Commun. 2000;3:299–305. doi: 10.1006/mcbr.2000.0231. [DOI] [PubMed] [Google Scholar]
- 38.Matsumura I, Kitamura T, Wakao H, Tanaka H, Hashimoto K, Albanese C, Downward J, Pestell RG, Kanakura Y. Transcriptional regulation of the cyclin D1 promoter by STAT5: its involvement in cytokine-dependent growth of hematopoietic cells. Embo J. 1999;18:1367–1377. doi: 10.1093/emboj/18.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marumoto T, Honda S, Hara T, Nitta M, Hirota T, Kohmura E, Saya H. Aurora-A kinase maintains the fidelity of early and late mitotic events in HeLa cells. J Biol Chem. 2003;278:51786–51795. doi: 10.1074/jbc.M306275200. [DOI] [PubMed] [Google Scholar]
- 40.Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320:1655–1658. doi: 10.1126/science.1157425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brennan C, Momota H, Hambardzumyan D, Ozawa T, Tandon A, Pedraza A, Holland E. Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS One. 2009;4:e7752. doi: 10.1371/journal.pone.0007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barton VN, Foreman NK, Donson AM, Birks DK, Handler MH, Vibhakar R. Aurora kinase A as a rational target for therapy in glioblastoma. J Neurosurg Pediatr. 2010;6:98–105. doi: 10.3171/2010.3.PEDS10120. [DOI] [PubMed] [Google Scholar]
- 43.Debierre-Grockiego F. Anti-apoptotic role of STAT5 in haematopoietic cells and in the pathogenesis of malignancies. Apoptosis. 2004;9:717–728. doi: 10.1023/B:APPT.0000045785.65546.a2. [DOI] [PubMed] [Google Scholar]
- 44.Kalita A, Gupta S, Singh P, Surolia A, Banerjee K. IGF-1 stimulated upregulation of cyclin D1 is mediated via STAT5 signaling pathway in neuronal cells. IUBMB Life. 2013;65:462–471. doi: 10.1002/iub.1152. [DOI] [PubMed] [Google Scholar]