Abstract
Background
Military personnel and civilians living in areas of armed conflict have increased risk of exposure to blast overpressures that can cause significant hearing loss and/or brain injury. The equipment used to simulate comparable blast overpressures in animal models within laboratory settings is typically very large and prohibitively expensive.
New Method
To overcome the fiscal and space limitations introduced by previously reported blast wave generators, we developed a compact, low-cost blast wave generator to investigate the effects of blast exposures on the auditory system and brain.
Results
The blast wave generator was constructed largely from off the shelf components, and reliably produced blasts with peak sound pressures of up to 198 dB SPL (159.3 kPa) that were qualitatively similar to those produced from muzzle blasts or explosions. Exposure of adult rats to 3 blasts of 188 dB peak SPL (50.4 kPa) resulted in significant loss of cochlear hair cells, reduced outer hair cell function and a decrease in neurogenesis in the hippocampus.
Comparison to existing methods
Existing blast wave generators are typically large, expensive, and are not commercially available. The blast wave generator reported here provides a low-cost method of generating blast waves in a typical laboratory setting.
Conclusions
This compact blast wave generator provides scientists with a low cost device for investigating the biological mechanisms involved in blast wave injury to the rodent cochlea and brain that may model many of the damaging effects sustained by military personnel and civilians exposed to intense blasts.
Keywords: Blast wave generator, low cost, cochlear hair cells, hippocampal neurogenesis, traumatic brain injury, hearing loss
1. INTRODUCTION
Combat personnel and civilians residing in war zones are have increased risk of exposure to intense blast waves in open or closed spaces (Taber et al., 2006). These originate from many sources including muzzle blasts from artillery, exploding bombs and improvised explosive devices (IEDs). Blast wave exposure is a major cause of auditory dysfunction in military personnel. Because the sensory hair cells in the cochlea are especially vulnerable to loud sounds, blast wave exposure often leads to hearing loss and tinnitus. Not surprisingly, noise-induced hearing loss and tinnitus are the most prevalent service connected disabilities costing the Veterans Administration over $1 billion annually (Fausti et al., 2009). In addition to otologic injury, blast waves are a leading cause of traumatic brain injury (TBI) in the military; the number of blast-related TBI cases was estimated to be as high as 320,000 as of June 2011 (Mac Donald et al., 2011). Blast related TBI can result from blast wave overpressure (primary blast exposure) in addition to physical trauma from projectiles accompanying the blast wave (secondary blast exposure) (Wolf et al., 2009). Blast-induced TBI has been associated with a learning deficits, memory loss, depression and post-traumatic stress disorder (Hoge et al., 2008, Nelson et al., 2009, Terrio et al., 2009, Cernak, 2010). The co-morbidity of blast-induced auditory damage and TBI is particularly common with 33%, 43% and 9% of veterans suffering from TBI showing acute, sub-acute and chronic hearing loss, respectively (Hoffer and Balaban, 2011).
Animal models of blast wave exposure have been developed to study blast-induced auditory dysfunction and TBI in order to determine its biological basis in more controlled environments. Blast-induced otologic damage typically results from rupture of the tympanic membrane, damage to the ossicular chain and/or damage to the sensory hair cells in the cochlea that are particularly vulnerable to acoustic overstimulation. Intense blast wave exposures not only damage the sensory hair cells, but also the supporting cells, afferent dendrites and spiral ganglion neurons (Hamernik et al., 1984a, Hamernik et al., 1984b, Roberto et al., 1989, Patterson and Hamernik, 1997, Cho et al., 2013).
Animal models of blast-induced TBI have identified the hippocampus – a brain region important for learning and memory and one of two regions in the adult brain where neurogenesis occurs – as particularly vulnerable to blast exposure (Carbonell and Grady, 1999, Sato et al., 2001). In rodents, blast wave exposure has been shown to alter expression of genes in the hippocampus including the down-regulation of genes involved in neurogenesis (Saljo et al., 2002, Risling et al., 2011). Furthermore, both single and multiple blast exposures produce evidence of hippocampal cell death and alters the level of hippocampal neurogenesis as reflected in doublecortin (DCX) immunolabeling (Kovesdi et al., 2011, Kwon et al., 2011, Kamnaksh et al., 2012). Similar to the learning deficits seen in human TBI patients, blast wave exposure induces cognitive deficits in rodents as measured with the Morris Water Maze (MWM) task, indicative of hippocampal damage (Hamm et al., 1996, Vandevord et al., 2012, Tompkins et al., 2013).
To date, few studies have investigated the co-morbidity of auditory damage and TBI following blast wave exposure. In order to investigate the concurrent effects of blast wave exposure on the auditory system and brain, a method of generating consistent and controllable blast waves is required. The equipment used to simulate blast overpressures in most laboratory settings was typically designed for aerodynamic compressible flow studies and is typically custom-constructed from large, heavy metal tubes (Hamernik et al., 1984a, Chavko, 2009). In addition to being prohibitively expensive, many blast wave generators are extremely large with lengths upward of 19 feet, making them particularly inconvenient to house in biological laboratories. To overcome these space and fiscal constraints, we developed a compact, low cost blast wave generator that can be readily constructed largely from off the shelf components. Here we describe the methods and materials needed to construct the blast wave generator, its performance characteristics and the effectiveness of the blast wave generator to induce cochlear damage and impair neurogenesis in the rodent hippocampus.
2. MATERIALS AND METHODS
2.1 Shock Tube Assembly
The blast wave generator’s major components, the shock tube and the control module, were constructed independently and connected to signal processing equipment and sensors in the laboratory. The fully assembled shock tube and control module are shown in Figure 1. Figure 2 shows an exploded view of the shock tube and its component. The device was primarily constructed from two inch (internal diameter 2.05 inches) Schedule 40 Poly Vinyl Chloride (PVC) pipe and related PVC components. Schedule 80 PVC has a pressure rating of 166 PSIG (1144 kPa gauge pressure), which is well above driver pressures used in the present experiments. The shock tube assembly consists of a high pressure chamber and low (ambient) pressure chamber separated by a thin, brass diaphragm. The 32-inch long high pressure driver section that houses compressed air was connected to the 15-inch low pressure expansion chamber through which the blast wave propagates by way of a 2-inch PVC union. The driver section was enclosed within a 4-inch aluminum tube for safety. The brass diaphragm (Lyon Industries, South Elgin, IL) was held in place by the two mating faces of the union with an extra O-ring groove machined into one of the mating faces. A rubber O-ring (McMaster Carr) in the groove helped to form a tight seal of the brass diaphragm against the wall of the union joint. A hole was drilled in the distal end of the driver section to accommodate a 100 psi (690 kPa) static pressure transducer (Omega Engineering, Stamford, CT) to monitor driver pressure. This end of the driver section was joined to a solenoid assembly (Newark Electronics, Chicago, IL) with a restoring spring. The moving component of the solenoid was attached to a hunting arrow supported within the driver section and parallel to it by a support machined from a Teflon cylinder. The pointed end of the hunting arrow was located behind the diaphragm within the range of motion of the solenoid. This setup allowed the high pressure compressed air in the driver section to be abruptly released by perforating the diaphragm with the solenoid-driven hunting arrow. The shock tube terminates in a PVC cap that was machined to insert a compressed air connection and electrical contacts that provide input to the solenoid. All permanent PVC connections were made using PVC adhesive following priming. A complete list of materials used in construction of the blast tube is given in the Supplementary Materials.
Figure 1.

Fully assembled shock tube (A) and controller (B).
Figure 2.

Exploded view and components of the shock tube.
The second major component, the control module, was constructed to regulate, monitor, and transmit the driver tube pressure and ambient temperature to a PC, and discharge the shock tube. Figure 3 shows a circuit diagram of the control module’s electronic components. Its main components include a DPi32-C24 process monitoring device (Omega Engineering, Stamford, CT) and an IP610-X30 electronic air pressure controller (Omega). The process monitoring device is connected to the static pressure transducer in the driver section and displays the driver pressure during tube pressurization. The control module also contains a transistor switch that controls the solenoid that is used to discharge the tube. Input signals used for regulating pressure and firing the shock tube were generated by a real-time signal processor (RP2, Tucker-Davis Technologies, Alachua, FL). The electronics were housed within a commercially available uniframe box (Elma Electronics, Wetzikon, Switzerland). Data collection was controlled by the RP2 at a sampling rate of 100 kHz. A program for the RP2, written using RPvdsEX, was triggered using recorded data by custom MATLAB (MathWorks Inc., Natick, MA) software with ActiveX controls (Tucker-Davis Technologies).
Figure 3.

Circuit diagram of the control module. This component is used to regulate, monitor, and transmit the tube driver pressure, record the ambient temperature, and also discharge the shock tube.
2.2 Shock Tube Discharge
Animal blast exposures were conducted indoors with the blast tube and pressure sensor housed within a sound attenuating booth and air was supplied by shop air up to 85 psig (586 kPa gauge). The air supply provides air filtered to remove traces of oil or particulate matter larger than 40 μm in diameter to prevent damage to the electronic flow control components of the control module. When an experiment is initiated with a user chosen value of driver pressure, a pressure transducer (PX182B-100GI, Model #, Omega Engineering) senses the air pressure in the driver section as it is filled with compressed air flow controlled by the electronic air pressure controller (IP610-X30, Omega Engineering). The driver section pressure is displayed by the process monitor (DPI32C24, Omega Engineering), which communicates with the MATLAB code through a serial port. The driver section is allowed to fill at the maximum flow rate until pressure reaches 75% of the user-provided driver pressure, after which flow is reduced to 75% of the maximum value to prevent premature diaphragm rupture. Once the driver pressure reaches the desired value, MATLAB communicates with the RPvdsEX software to stop air flow with a signal from the RP2 module (TDT) to the electronic air pressure controller. After a programmed delay, another signal is sent to a solenoid assembly attached to the hunting arrow residing within the driver section. The solenoid is energized and the arrow is driven forward, puncturing the diaphragm in a predictable location producing the blast wave, and returns to its original position by force of a spring. The RP2 module simultaneously coordinates data acquisition from the 137A23 pressure sensor (PCB) for a period of 2 seconds at a sampling rate of 100 kHz, capturing important overpressure-related events during blast exposure. Other sensors required by different experimental setups can also be connected to the RP2 for simultaneous data acquisition. The RPvdsEX program allows triggering with adjustable delays, allowing coordinated use of a high speed camera to capture blast-induced events. Buffers from different sensors are stored in the onboard memory of the RP2 during acquisition, after which the MATLAB code retrieves them for data analysis.
For each blast exposure, a high-pressure blast probe (137A23 ICP Pressure Sensor, PCB Piezotronics, Depew, NY) positioned at the mouth of the expansion chamber was used to measure the blast pressure level.
Several diaphragm materials including paper, wax paper, cellophane, polyethylene/aluminum foil/polyester composite and aluminum foil were initially investigated. Later, it was determined that brass foil was the best suited diaphragm material in terms of its ability to maintain a constant pressure without an air leak or spontaneous rupture over a wide range of driver pressures (45–79 psi, 310–545 kPa) while producing a near instantaneous pressure release when the arrow head contacted the diaphragm. Furthermore, a single layer brass diaphragm does not require the tedious assembly of multiple layers of aluminum foil.
2.3 Shock Tube Data Analysis
Blast wave intensity was quantified using the peak overpressure ratio, which is the ratio of pressure in excess of atmospheric pressure. Using the peak overpressure ratio allowed the derivation of a relation between shock tube driver pressure and blast overpressure, and comparison with previously reported data of peak overpressure (Kinney, 1985, Wolf et al., 2009). Consistency of blast waves produced by the blast tube was determined by examining the spread of experimental data at various shock tube driver pressures.
2.4 Animal Subjects
SASCO Sprague-Dawley rats (Charles River Laboratories Inc., Wilmington, MA) were used as subjects (81–123 days of age). All animal procedures were performed in accordance with National Institutes of Health (NIH) guidelines for use and care of laboratory animals and were approved by the University at Buffalo Institutional Animal Care and Use Committee (IACUC).
2.5 Animal Blast Wave Exposure
All blast wave exposures were carried out in a 6 × 6.5 ft double walled sound attenuating booth (Acoustic Systems, Austin, TX). Under ketamine:xylazine anesthesia (50 mg/kg:6 mg/kg; I.P.), rats (n=4) were secured in a wire-mesh (1 cm × 1 cm) cage (20 cm L, 7 cm W, 6 cm H) positioned 2 inches from the mouth of the blast tube expansion chamber (Figure 4). Animals were exposed to three blast waves each separated by approximately 5 minutes. The rats were exposed head on (0° azimuth with respect to the mouth of the blast wave generator). The high-pressure measuring probe positioned at the mouth of the expansion chamber confirmed that the blast waves generated 188 dB SPL (± 0.6 SEM) (50.4 kPa). Healthy age-matched controls (n=4) underwent the same procedures listed above in the absence of detonation of the blast tube.
Figure 4.

Schematic of the positioning of the blast tube, animal, and probe tip during animal blast wave exposures.
2.6 Distortion Product Otoacoustic Emissions (DPOAE)
OHC function was assessed using distortion product otoacoustic emissions (DPOAE) as previously described (Jamesdaniel et al., 2009, Chen et al., 2010). DPOAE were measured in blast wave exposed rats 42 days following exposure (n=4) and in healthy, age-matched controls (n=4). Rats were anesthetized with isoflurane (4% induction, 1.5% maintenance) and placed on a temperature-controlled heating pad to maintain normal body temperature. Prior to testing, rats were examined with an otoscope to exclude tympanic membrane perforation, middle ear infection and debris in the external auditory canal. DPOAE recordings were performed in a sound attenuating chamber. The earpiece containing an ER10B+ microphone (Etymotic Research Inc., Elk Grove Village, IL) and two sound delivery tubes was inserted into the ear canal. Two IHS-3738 high frequency transducers (Intelligent Hearing System, Miami, FL) were used to deliver the primary tones, F1 and F2, to the ear canal via flexible tubes connected to the earpiece. The F2/F1 ratio was set at 1.2. The output of the microphone was fed to the input of the DPOAE system, digitized and evaluated using the system software. DPOAEs were recorded at 2F1–F2 using a Smart Distortion Product Otoacoustic Emission System (Intelligent Hearing System) (Chen et al., 2010). DPOAE input/output functions were measured at F2 frequencies of 8 and 16 kHz. The intensity of F1 was varied from 25 to 70 dB SPL in 5-dB steps and the intensity of F2 was 10 dB lower than that of F1. The output of the microphone was sampled at 40 kHz over a period of 204 ms; the spectrum of each sweep was computed and averaged over 32 non-rejected sweeps. Sweeps with an average noise level 10 dB above the initially measured noise floor were rejected. The noise floor was measured in a 24 Hz band surrounding 2.F1–F2. DPOAE results from the left ear were plotted using GraphPad Prism (Version 5; GraphPad Software, La Jolla, CA), and two-way analyses of variance between groups (ANOVA) (group × sound intensity) were used to statistically compare the results at each F2 (8 and 16 kHz).
2.7 Tissue Preparation
At 42 days post-exposure, the blast-exposed rats (n= 4) and age matched control rats (n=4) were deeply anesthetized with 86 mg/kg i.p. of Fatal-Plus (Vortech Pharmaceuticals Ltd., Dearborn, MI) and perfused through the heart with 0.1 M phosphate buffered saline (PBS) followed by 10% phosphate buffered formalin (Fischer Scientific, Pittsburgh, PA). Following the perfusion, brains and cochlea were removed. Cochleae were stored in 10% phosphate buffered formalin for several weeks until processing. The brains were post-fixed in formalin overnight at 4° C. As described in our earlier publications, brain tissue containing the left or right hippocampus was cryoprotected in 30% sucrose (Sigma-Aldrich, St. Louis, MI) in 0.1 M PBS pH 7.4 (Sigma-Aldrich) overnight at 4° C until the tissue sank (Kraus et al., 2010). The following day, the brain tissue was cut on a cryostat into 40 μm thick coronal sections that were collected into storage solution (30% ethylene glycol, 30% glycerol in 0.1M PBS) and stored at −20° C until processing.
2.8 Cochleograms
Our procedures for preparing cochleograms have been described previously (Hofstetter et al., 1997, Reyes et al., 2001). Harvested cochleae were infused with Harris’ hematoxylin staining solution for 5 minutes. The cochlear basilar membrane was dissected out and mounted as a flat surface preparation in glycerin on a glass slide. For sensory hair cell counting, specimens were examined under a light microscope (400x) and the number of missing inner hair cells (IHCs) and outer hair cells (OHCs) were counted over 0.24 mm intervals along the entire length of the cochlea. A cochleogram was constructed by plotting the percent IHC and OHC loss as a function of the percent distance from the apex of the cochlea. For illustrative purposes, individual cochleograms (from the left ear) were grouped to generate a mean cochleogram for the blast wave exposed rats using custom software. Cochlear position was related to frequency using a cochlear frequency-place map (Muller et al., 2005).
2.9 Hippocampus and Doublecortin (DCX) Immunolabeling
Immunolabeling for the microtubule associated protein doublecortin (DCX) was carried out on free-floating tissue sections as described previously (Kraus et al, 2010). Tissue sections were initially removed from the cryoprotectant and rinsed in 0.1 M phosphate buffered saline, pH 7.4 (PBS). Tissue sections were washed in 0.1 M PBS for 30 minutes at room temperature between each of the following incubation steps. For deactivation of endogenous peroxidase, sections were treated with 0.3% H2O2 (Fischer Scientific) in distilled water for 15 minutes. Next, the sections were pre-treated with blocking solution containing 10% normal horse serum (Vector Laboratories, Burlingame, CA) and 0.05% Triton X-100 (Fischer Scientific) in 0.1 M PBS (PBS-Triton) for 30 minutes. Sections were then incubated with primary antibody against DCX (polyclonal; raised in goat; 2 μg/ml; sc-8066; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) in PBS-Triton with 1.0% normal horse serum for 2 hours at room temperature. Subsequent incubation with biotinylated secondary antibody (anti-goat IgG, Vector Laboratories) in PBS-Triton with 1.5% normal horse serum was done for 60 minutes at room temperature. Sections were processed using Vectastain ABC kits (Vector Laboratories) and labeling was visualized using the glucose oxidase modification of the diaminobenzidine (DAB) method (Van der Gucht et al, 2006, Manohar et al, 2012). Immunolabeled sections were mounted on Fisher Superfrost polarized slides and dried overnight. Slides were then dehydrated in increasing alcohol, cleared in xylene and sealed using DPX (Fisher Scientific).
For both the blast wave exposed rats and age-matched controls, DCX-positive cells were identified in coronal sections throughout the rostral-caudal axis of the hippocampus. DCX-positive cell bodies in the subgranular zone of the dentate gyrus of the hippocampus were counted at high magnification (400x) under brightfield illumination (Axioskop, Carl Zeiss MicroImaging Inc., Thornwood, NY) by the same experimenter who was blind to the treatment condition. A DCX-positive cell was counted when the cell body was clearly recognizable by its morphology (Kraus et al., 2010). For each coronal section, the number of counted cells was divided by the length of the sub-granular zone (SGZ) to obtain a measure of cell density (#/mm). Average cell densities were compared between blast wave exposed rats and the age-matched controls (two-tailed Student’s t-test, P<0.05). For illustrative purposes, photomicrographs of the hippocampus were taken using a digital camera (SPOT Insight; Diagnostic Instruments Inc., Sterling Heights, MI) and processed with imaging software (SPOT Software, version 4.6). Figures were assembled using Adobe Photoshop (Adobe Systems, Inc., San Jose, CA).
3. RESULTS
3.1 Blast Tube Assessment
We constructed a low-cost blast wave generator that can be used to assess auditory and brain injury in animal models. The compressed air driven device is fired by puncturing a diaphragm separating a driver section from a low pressure expansion section using a hunting arrow and a solenoid assembly. Detailed performance of the device has been previously described in our publications (Newman, 2010, Newman and Mollendorf, 2010). The blasts produced by the blast wave generator at different driver pressures were characterized using an empirical model and tested for consistency. Blast waves with expected overpressures were consistently produced up to around 200 dB SPL (200.6 kPa), and the waveforms of the blasts reflected those seen in literature (Wolf et al., 2009).
The waveforms of blasts produced by the device were plotted on a voltage vs. time graph using pressure sensor calibration data and time varying voltage information (Figure 5). When tested in an open space, blast waveforms showed characteristics similar to typical Friedlander curves for blasts in open spaces (see Figure 5 from Newman and Mollendorf, 2010). When tested in the sound-attenuating booth, the waveforms obtained were qualitatively comparable to waveforms seen in blast tests conducted in closed spaces (Fig 5, compare A to B) (Wolf et al., 2009). In the closed space explosion, after an initial spike in overpressure due to the blast front, the overpressure signature showed one or two oscillations due to minor reflections from the walls of the acoustic chamber in which the experiment was conducted, while simultaneously decaying in magnitude as expected. The number and magnitude of these oscillations were low because of the acoustic insulation provided by the walls of the chamber. The signature also showed a small negative pressure phase that was significantly less than expected based on an idealized Friedlander blast waveform. Qualitative and quantitative measurements of the characteristics of the blast wave signature when the tube was used in open air conditions can also be found in our other publications (Newman, 2010, Newman and Mollendorf, 2010). The signature obtained in these tests was also found to be qualitatively comparable to the expected blast wave signature in an open field with a significant negative phase. The difference in the waveforms lies in the oscillations of pressure seen in the decay phase after the initial pressure spike as there are no reflections from surrounding objects or surfaces in the open.
Figure 5.

(A) Typical overpressure vs. time plot for blast waves produced by the shock tube in a closed space. (B) Idealized closed space blast waveform of IEDs. Comparison of A and B show qualitative similarities such as the lack of a negative phase and oscillations produced by internal reflections in the closed space. (B, adapted from Wolf et al 2009, with permission).
The performance of the blast tube was also characterized by analyzing the relationship between the driver pressure and overpressure of the blast wave in dB SPL. The tube was fired using several different driver pressures between 30 and 80 psi (206.8 and 551.6 kPa), and the resulting spread of overpressure values can be seen in Figure 6. The data point (14.7,0) (in psi) was used to account for a lack of blast overpressure if the driver section was at atmospheric pressure. As the tube is expected to be used to produce a relatively high range of blast overpressures for this application, data points between driver pressures of 45 and 79 psi (310.3 and 544.7 kPa) were used to produce a fit to determine the driver pressure vs overpressure relationship. A linear fit, dB SPL = 0.3666 × + 166.51 (x = driver pressure in psi), was found to describe the performance of the tube with a coefficient of determination (R2) of 0.96. This relationship illustrates that the blast wave generator can be used to produce reliable blast overpressures by controlling the pressure to which the driver section is filled with air. However, diaphragm slip was found to cause premature pressure release, leading to lower overpressure values. Diaphragm slip was observed to be an issue as driver pressures rose beyond 79 psi (545 kPa) due to the limited sealing provided by the PVC union and O-ring assembly, establishing the corresponding overpressure of around 200 dB SPL (200.6 kPa) as the current shock tube’s maximum capability.
Figure 6.
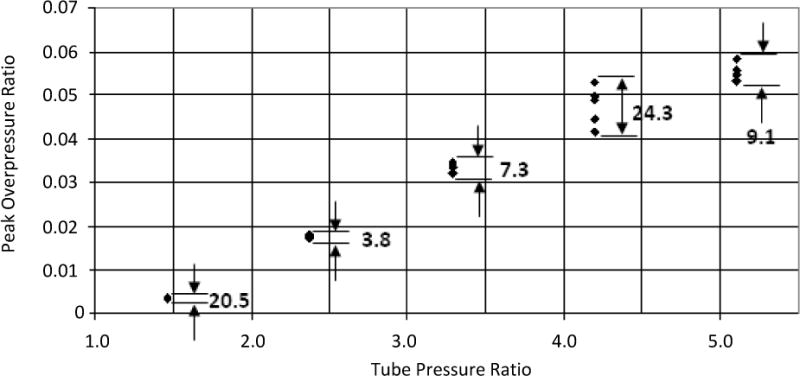
The distribution of blast overpressures is plotted as a graph of peak overpressure ratio against tube pressure ratio. The peak overpressure ratio is calculated as the difference between the peak measured pressure and atmospheric pressure, divided by atmospheric pressure. The tube pressure ratio is the ratio of driver section pressure to atmospheric pressure. Variation in the data is shown in percent of mean value. The average variation across all data points was ~13%.
3.2 DPOAE Post Blast Exposure
We compared the mean DPOAE input/output functions in the blast wave exposed rats versus age-matched controls at two different frequencies (F2 = 8 and 16 kHz). As shown in Figure 7A, blast wave exposure caused a significant reduction in DPOAE amplitude, a physiological indicator of impaired OHC function (significant main effect for ‘group’ in two-way ANOVA; P<0.0001 for 8 kHz, P<0.03 for 16 kHz). Maximum DPOAE-losses of 19 dB and 15 dB were observed for the 8 kHz and 16 kHz input/output functions, respectively.
Figure 7.

Effects of blast exposure on the inner ear. (A) Mean input/output functions of distortion product otoacoustic emissions (DPOAE) in the blast wave exposed rats and age-matched controls at 8 kHz and 16 kHz. At both frequencies, there was a significant reduction in DPOAE amplitude (significant main effect for ‘group’ in two-way ANOVA; P<0.0001 for 8 kHz, P<0.03 for 16 kHz). Closed circles represent control rats, whereas closed triangles represent blast wave rats 42 days after exposure. Vertical bars represent SEM. N = 4 rats in each group; left ear only. (B) Mean cochleogram showing degree of inner- (IHC) and outer hair cell (OHC) loss as a function of percent distance from the apex of the cochlea in four rats measured 42 days following blast wave exposure. Extensive hair cell loss was observed in the high-frequency (basal) portion of the cochlea. At all positions along the basilar membrane, OHC loss (dashed line) was greater than IHC loss (solid line). Vertical dotted lines and associated labels at 20–80% distance from apex relate the cochlear position to frequency (in kHz).
3.3 Blast Wave Induced Hair Cell Loss
Little or no hair cell loss was seen in the control rats (cochleograms not shown). The mean (n=4) cochleogram for the blast exposed rats showed extensive loss (Fig 7B). There was nearly total loss of OHC and IHC near the basal, high frequency end of the cochlea (90–100% distance). IHC and OHC losses gradually declined toward the low-frequency portion of the cochlea; hair cell losses were less than 20% at the apical end (0–20% distance). While the loss of OHC was greater than IHC, the differences were relatively small over most of the cochlea.
3.4 Blast Wave Exposure Suppresses Hippocampal Neurogenesis
To determine the effect of blast wave exposure on hippocampal neurogenesis, we compared the average density of newly born neurons located in the subgranular zone of the dentate gyrus using DCX immunolabeling. As shown in Figure 8 (A and C), in a representative control rat, DCX-positive cells could be seen forming a near continuous band along the subgranular zone with vertically extending dendrites. In contrast, 42 days following blast wave exposure, the number of DCX-positive cells was greatly reduced (Figure 8; B and D). A comparison of the average density of DCX-positive cells (Figure 8, E) revealed that the population of neuronal precursor cells was significantly reduced by ~41% in the rats exposed to blast waves 42 days earlier (21.3±1.1, mean ± SEM) compared to age-matched controls (36.1±2.1, mean ± SEM; two-tailed Student’s t-test, P<0.003) indicative of a persistent, long-term suppression of neurogenesis following blast wave exposure.
Figure 8.

Blast exposure reduces hippocampal neurogenesis. Representative photomicrographs showing neuronal precursor cells immunolabeled for doublecortin (DCX) in the subgranular zone (SGZ) in the dentate gyrus (DG) of the hippocampus in a control rat (A and C) and an age-matched rat measured 42 days following blast wave exposure (B and D). Compared to the control, the blast wave exposed rat showed a reduced number of darkly-labeled DCX-positive cell bodies in the SGZ. Magnification in the top and bottom panels was 100x and 400x, respectively. (E) Blast wave exposure in adult rats caused a long-term suppression of hippocampal neurogenesis, as measured by the average density of DCX-labeled neuronal precursor cells in the subgranular zone (SGZ). Compared to control rats, the average density of DCX-positive cells in the SGZ was significantly reduced in age-matched rats that survived for 42 days following blast wave exposure (*P<0.003; two-tailed Student’s t-test), indicative of suppressed hippocampal neurogenesis. Values shown represent mean ± SEM. N=4 for both groups.
4. DISCUSSION
We developed a compact, low cost blast wave generator that can be constructed largely from readily available commercial materials and can be easily housed in a laboratory environment. Tests of the device indicate that it can cause significant damage to the inner ear and impair neurogenesis in the brain.
4.1 Blast tube features
Several types of blast wave generation systems have been used to study blast-related traumatic brain injury (TBI) in brain-simulant or animal models, and for a variety of other experiments. Devices previously reported in the literature vary in their method of blast wave production, as well as their cost and size. Some shock tubes are driven by explosives, such as the one described by D.A. Freiwald (Freiwald, 1972). In this shock tube, the primary source of blast wave energy was a fast burning cylinder of plastic bonded explosive. In general, explosive-charge driven shock tubes result in a change in the chemical environment in the driven or expansion section, making them unsuitable for use with animal models. Compressed gas driven shock tubes are the most commonly used devices for blast injury pathophysiology research. Many shock tubes have a standard layout consisting of a high and low pressure section separated by a diaphragm. Some devices use compressed low-molecular weight gases such as helium or a combination of gases with air in the driver section to prevent the physical effects of density differences between the driver gas and air in the driven section (Cernak et al., 2011, Garman et al., 2011). Other pneumatic devices use compressed air alone instead of a combination of gases, and blast wave production is controlled by the material properties of the diaphragm. Mylar membranes of different thicknesses or multiple layers have been used to obtain different blast overpressures (Long et al., 2009, Ahlers et al., 2012). In one study, a multi-mode stock tube used oxohydrogen and cyclotrimethylenetrinitramine as explosives to generate a blast wave, and was also capable of generating shock waves using compressed-air and compressed-helium driven membrane rupture (Reneer et al., 2011). Other methods such as electric sparks or mechanical impact can also be used to produce lower dB SPL pulses (Birch et al., 2003).
Most shock tubes used to study blast wave exposures in animals are custom made and difficult to set up and operate. They often require expensive materials for construction and fixtures, occupy a significant amount of space, and are very large in comparison to the size of the subject or experimental setup. Some studies used a metallic blast tube 17.5 ft long with a 1 ft diameter (Long et al., 2009, Dalle Lucca et al., 2012). Other blast tubes are also of significant sizes, and have diameters from 6 to 18 inches (Cernak et al., 2011). Different sections are also made of thick metal, adding to the inconvenience of setting up a blast tube. As a result, setting up and maintaining such a device can be relatively difficult and expensive.
4.2 Blast tube utility
The blast wave generator developed for this study has several characteristics that make it suitable for assessment of the effects of blast waves in animal models. Our aims for its construction included simplicity, ability to use commonly available commercial hardware and software components for construction, accurate control of blast wave overpressure and time of firing, and ability to use the shock tube without significant training or knowledge of construction. The small dimensions make it easy to house within a limited amount of space. The simple union arrangement to hold the diaphragm in place allows quick replacement of the diaphragm between blast wave experiments. Lightweight PVC construction allows easy handling, adjustment and relocation, while the additional metal shield on the driven section improves safety.
Studies exploring the effects of blast waves in animal models can benefit from accurate control of blast wave overpressure and time of generation. The use of a solenoid driven arrow to initiate the blast wave reduces dependence of the time of blast wave generation on the material properties of the diaphragm. We hypothesize that the rupture of one diaphragm layer produces more consistent wave front characteristics at different overpressures than multiple layers, although such variations require documentation. Such a set-up also allows smaller increments in blast wave overpressure when compared to the step change resulting from addition of another diaphragm layer.
The streamlined control of blast wave generation using a single command provides significant benefits for the user. After selecting a desired overpressure, the pressurization of the driven section and generation of the shock wave occur successively without the need for additional control. Data collection is also easier because an experiment-specific layout of sensors and measurement devices can be triggered simultaneously to capture various experimental parameters. These features make the presented blast wave generator design useful to study blast wave related injury in animal models.
Importantly, the blast waves produced by the generator have overpressure and positive peak duration characteristics appropriate for small animal models. The blast overpressures used in this study had a peak gage pressure of 190 dB SPL (63.4 kPa) and a positive phase duration of 4–6 ms. These were below the parameters required for 50% survival at 24 hours after blast wave exposure in rats (White and Richmond, 1959, Bass et al., 2008), representing a range in which brain and auditory injury studies would be significant. Higher blast wave overpressures and positive phase durations that cause greater fatality are less relevant because of the low chance of individual survival due to pulmonary damage. The maximum overpressure produced by the tube in all tests was 200 dB SPL (~29 psig, 53.7 psia) with a positive phase duration of 7 ms. These parameters were above the curve representing 50% survival at 24 hours for small animals. In essence, the blast wave generator is capable of consistently producing a range of useful overpressures and positive phase durations for the study of auditory and brain injury in small animal models.
The shock tube produced a typical blast waveform consisting of positive and negative phases in a low overpressure test conducted in an open space ((Newman, 2010, Newman and Mollendorf, 2010).). The waveform is qualitatively similar to the idealized pressure vs time signature from Kinney & Graham (Kinney, 1985). The deviations seen from blast wave characteristics predicted by the Friedlander waveform (Kinney, 1985, Bass et al., 2008, Varas, 2011) at higher overpressures can be attributed to the effects of blast wave reflection, diaphragm rupture, interactions between the expanding air and the arrow inside the driven section and minor variations in the internal diameter of the union and the expansion section.
Varas et al. 2011 presented results from the Bass lethality model (Bass et al., 2008) showing that the average IED blast has an overpressure between 10 and 200 kPa (1.45 and 29.00 psi), and a positive phase duration of 4 to 10 ms. A driver pressure of 65 psi in our shock tube produced a blast wave with a peak overpressure of 14.28 psig and a positive phase duration of 6–7 ms, which replicates the loading conditions produced by an average IED blast with respect to these two parameters only. Overpressure and positive phase duration are considered to be the major contributors to significant anatomical and physiological effects of blast waves (Varas, 2011). However, the blast waveforms obtained in this study differ significantly in positive phase duration and oscillation amplitude from the IED blast waveform in a (enclosed space) presented by Gupta and Przekwas 2013. IED blast waves take on many forms based on the settings, and our blast profile presents one possible scenario for blast waveforms in closed spaces.
There are certain drawbacks with a low cost PVC based shock tube. As driver pressures increase beyond 80 psi, the union is unable to prevent the diaphragm from slipping as the driver section fills with air, resulting in premature detonation at lower pressures. However, this is also often a result of experimental error due to insufficient force used to tighten the diaphragm prior to use. The rotation of the mating faces of the union with the diaphragm in between can also result in mechanical stresses of the diaphragm that can cause a change in rupture characteristics. In our tests, this wasn’t seen to be a significant factor because the overpressures produced were very consistent if diaphragm slip did not occur. Another drawback results from the size of the PVC tubes used to construct the tube. In order to place objects within the expansion section, they had to be less than 2 inches across. However, using a gently expanding expansion section can overcome this problem while preventing a major change in the wave front characteristics that can be caused due to rapid expansion.
4.3 Biological Effects of Blast Exposure
To assess the effectiveness our blast wave generator, anesthetized rats were individually exposed to 3 blast waves at 5-minute intervals and were then allowed to recover for 42 days. We purposely used blast exposures with peak SPL values less than 190 dB since higher peak pressures rupture the tympanic membrane thereby reducing the acoustic energy entering the cochlea (Roberto et al., 1989). During preliminary testing of the blast tube using the same exposures as in the present study, we did not observe tympanic membrane perforations following blast exposure. Moreover, we did not observe damage to the tympanic membrane or ossicles when the cochleas were harvested and processed for histological analysis. Prior studies investigating the effects of blast-wave exposure on the auditory system and brain have utilized either single or multiple blast exposures (Long et al., 2009, Kamnaksh et al., 2012). Given that exposure to multiple blasts is common amongst military personnel in active war zones, we sought to characterize the effects of multiple blast exposures utilizing our blast tube in the current study (Hoge et al., 2008, Elder et al., 2010). The effect of blast wave exposure on the inner ear was quantified by investigating cochlear hair cell loss and OHC function while the effects of blast wave exposure on hippocampal neurogenesis was analyzed using immunohistochemical labeling for DCX to quantify the number and distribution of immature neurons.
Because of the sensitivity of the ear to acoustic stimulation, we expected the blast wave exposure to cause significant cochlear damage. The mean cochleograms for the blast exposed animals demonstrated a significant loss of both OHC and IHC in the cochlea. These results are consistent with previous reports of blast wave induced cochlear damage. Patterson and Hamernik reported that the predominant cause of blast-related injury was the disruption of sensory cell attachment to the basilar membrane (Patterson and Hamernik, 1997). In extreme cases of mechanical trauma, long sections of sensory cells are lost. Hair cells experience loss of function due to intermixing of cochlear fluids following the disruption of tight junctions between Hensen cells. Damage to IHC and OHC coupled with damage to the oval or round window has also been reported in blast exposed humans (Fausti et al., 2009); however, we did not observe any damage to these structures presumably because we limited our exposures to less than 190 dB pSPL.
Distortion product otoacoustic emission (DPOAE) testing is a noninvasive tool that can be used to study cochlear function by analyzing low intensity sounds produced by the inner ear in response to acoustic stimulation. DPOAEs are believed to be produced by the electromotile properties of the cochlear OHC in response to changes in transmembrane voltage (Liberman et al., 2002, Ashmore, 2008). The transmembrane potential is determined by the resting potential of the OHC along with the +80 mV extracellular endolymphatic potential above the apical pole of the OHC (Mills and Rubel, 1994, Li et al., 2011). DPOAEs can be measured in response to different frequencies in order to assess OHC function along the tonotopic axis of the cochlea. Comparison of DPOAEs in blast-exposed rats versus age-matched controls allows for the analysis of blast-induced cochlear damage at specific points along the cochlear partition. As shown in Figure 7A, blast wave exposure caused a significant reduction in DPOAE amplitude suggesting damage to the cochlear OHC. Prior studies in the chinchilla suggest that every 10% loss of OHC results in a reduction of DPOAE amplitude of 3–4 dB (Hofstetter et al., 1997). Inspection of the cochleograms (Figure 7B) at cochlear locations corresponding to the stimulus frequencies used to elicit the DPOAE reveal OHC losses on the order of 25–30%. Based on the results from chinchilla studies, we would expect to see DPOAE amplitude reductions that range between 8–12 dB when testing at 70 dB SPL. Our data in rat show amplitude reduction on the order of 10 and 13 dB at 8 kHz and 16 kHz respectively, very close to the predicted values. Thus, the DPOAE amplitude reductions match reasonably well with the size of OHC lesions.
The hippocampus plays and important role in memory formation and spatial navigation (Barnes, 1979, Mishkin, 1982, Goodman et al., 2010). The subgranular zone of the dentate gyrus is one of the few regions in which neurogenesis persists in the adult brain (Gould and McEwen, 1993, Jin et al., 2001, Gould, 2007). Neurogenesis can be quantified by analyzing the expression of the microtubule binding protein doublecortin (DCX) because of the transient nature of protein expression in newborn and migrating neurons (Francis et al., 1999, Gleeson et al., 1999). DCX immunoreactivity is seen in proliferating cells, and drops to below detectable levels after cells begin expressing mature neuronal markers (Brown et al., 2003). Since the hippocampus in one of the more vulnerable regions of the brain (Umile et al., 2002) and since unilateral noise induced hearing loss suppresses neurogenesis (Kraus et al., 2010), we expected that our blast wave exposure would suppress neurogenesis. A comparison of neurogenesis in control and 42 day post-blast wave exposed animals showed that the population of newly born neurons was reduced by 41%, consistent with our earlier study of noise-induce hearing loss (Kraus et al., 2010). Because neurogenesis was quantified in animals 42 days post-blast wave exposure, these results are consistent with a long-term reduction in neurogenesis. Our results differ from previous studies that suggested an increase in hippocampal neurogenesis after single and multiple blasts (Kovesdi et al., 2011, Kwon et al., 2011, Kamnaksh et al., 2012); however, these studies were based on qualitative inspection of immunolabeling and failed to objectively quantify the changes in neurogenesis. Other potential explanations for the observed differences could be the post-exposure survival times and differences in the blast exposure conditions. Regardless of the specific outcome, there is growing evidence that noise exposure, either from blast wave or continuous high intensity noise, can have an effect on hippocampal neurogenesis.
Previous studies have demonstrated that blast exposure has a significant impact on memory in rodents (Cernak et al., 2001, Ahlers et al., 2012) and cognitive functioning in humans (Cernak et al., 1999, Martin et al., 2008, Cernak, 2010). Our result of a long-term reduction in neurogenesis in the hippocampus following blast exposure may underlie these findings given the implicated involvement of hippocampal neurogenesis in memory and cognitive functioning (Deng et al., 2010). Additionally, others have reported evidence of additional forms of damage in the central nervous system following blast exposure including neural degeneration (Svetlov et al., 2010, Garman et al., 2011, Sajja et al., 2012). We have not observed evidence of cell death in the hippocampus after the 190 dB pSPL blasts (data not shown) which may be due to the low level blast exposure that we used compared to other studies. The reduction in neurogenesis observed in the hippocampus, as measured by expression DCX, may be a result of the hearing loss generated by blast exposure instead of direct blast wave induced damage in the brain.
4.4 Conclusions
We constructed a low-cost blast wave generator that can be used to assess auditory and brain injury in animal models. The blast wave generator was constructed from off the shelf components and reliably produced blasts up to around 200 dB SPL. The blast waveforms obtained using our experimental setup within a closed acoustic chamber qualitatively resembled experimentally derived waveforms of blasts in closed spaces (Wolf et al., 2009), and represent one type of IED blast. The blast wave generator itself is capable of producing waves with overpressure profiles similar to those seen in a stereotypical Friedlander curve for blasts in open spaces, as seen during initial testing of the device in an open space. Unlike many existing blast wave generators that are typically large, expensive, and are not commercially available, the blast wave generator reported here provides a low-cost method of generating blast waves in a typical laboratory setting. Exposure of adult rats to 3 blasts of ~190 dB peak SPL with this shock tube resulted in significant loss of cochlear hair cells, reduced cochlear outer hair cell function, and reduced neurogenesis in the hippocampus. These results demonstrate the ability of the shock tube to produce both auditory and brain injury. This compact blast wave generator provides neuroscientists with a low cost device for investigating the biological mechanisms involved in blast wave injury to the rodent cochlea and brain that may model many of the damaging effects sustained by military personnel and civilians exposed to intense blasts.
Supplementary Material
Highlights.
Many devices used in animal models of blast wave exposure are large and expensive
We developed a low cost blast wave generator easily housed in laboratory settings
The generator reliably produced blasts with peak pressures of up to 198 dB SPL (159.4 kPa)
Exposure of rats to blast waves caused damage to the inner ear and hippocampus
This low cost device is useful for studying blast induced ear and brain injury
Acknowledgments
Research supported in part by part by NIH National Institute on Deafness and Other Communicative Disorders (R01DC011808, RS) and the Office of Naval Research (N000141210731, RS).
Abbreviations
- OHC
outer hair cell
- IHC
inner hair cell
- DPOAE
distortion product otoacoustic emission
- IED
improvised explosive device
- TBI
traumatic brain injury
- PVC
poly vinyl chloride
- PBS
phosphate buffered saline
- DCX
doublecortin
- MWM
Morris water maze
- PCB
printed circuit board
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Andrew J. Newman, Email: j.newman@ttu.edu.
Sarah H. Hayes, Email: shhayes@buffalo.edu.
Abhiram S. Rao, Email: abhirams@buffalo.edu.
Brian L. Allman, Email: brian.allman@schulich.uwo.ca.
Senthilvelan Manohar, Email: smanohar@buffalo.edu.
Dalian Ding, Email: dding@buffalo.edu.
Daniel Stolzberg, Email: dstolzbe@uwo.ca.
Edward Lobarinas, Email: elobarinas@ufl.edu.
Joseph C. Mollendorf, Email: molendrf@buffalo.edu.
Richard Salvi, Email: salvi@buffalo.edu.
References
- Ahlers ST, Vasserman-Stokes E, Shaughness MC, Hall AA, Shear DA, Chavko M, McCarron RM, Stone JR. Assessment of the effects of acute and repeated exposure to blast overpressure in rodents: toward a greater understanding of blast and the potential ramifications for injury in humans exposed to blast. Frontiers in neurology. 2012;3:32. doi: 10.3389/fneur.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. Cochlear outer hair cell motility. Physiological reviews. 2008;88:173–210. doi: 10.1152/physrev.00044.2006. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Bass CR, Rafaels KA, Salzar RS. Pulmonary injury risk assessment for short-duration blasts. Journal of Trauma-Injury Infection and Critical Care. 2008;65:604–615. doi: 10.1097/TA.0b013e3181454ab4. [DOI] [PubMed] [Google Scholar]
- Birch RS, Gerges SN, Vergara EF. Design of a pulse generator and shock tube for measuring hearing protector attenuation of high-amplitude impulsive noise. Appl Acoust. 2003;64:269–286. [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. The Journal of comparative neurology. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Carbonell WS, Grady MS. Regional and temporal characterization of neuronal, glial, and axonal response after traumatic brain injury in the mouse. Acta neuropathologica. 1999;98:396–406. doi: 10.1007/s004010051100. [DOI] [PubMed] [Google Scholar]
- Cernak I. The importance of systemic response in the pathobiology of blast-induced neurotrauma. Frontiers in neurology. 2010;1:151. doi: 10.3389/fneur.2010.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak I, Merkle AC, Koliatsos VE, Bilik JM, Luong QT, Mahota TM, Xu LY, Slack N, Windle D, Ahmed FA. The pathobiology of blast injuries and blast-induced neurotrauma as identified using a new experimental model of injury in mice. Neurobiol Dis. 2011;41:538–551. doi: 10.1016/j.nbd.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Cernak I, Savic J, Ignjatovic D, Jevtic M. Blast injury from explosive munitions. The Journal of trauma. 1999;47:96–103. doi: 10.1097/00005373-199907000-00021. discussion 103–104. [DOI] [PubMed] [Google Scholar]
- Cernak I, Wang Z, Jiang J, Bian X, Savic J. Cognitive deficits following blast injury-induced neurotrauma: possible involvement of nitric oxide. Brain injury: [BI] 2001;15:593–612. doi: 10.1080/02699050010009559. [DOI] [PubMed] [Google Scholar]
- Chavko M. In: Telephone conversation. Newman J, editor. 2009. [Google Scholar]
- Chen GD, Kermany MH, D’Elia A, Ralli M, Tanaka C, Bielefeld EC, Ding D, Henderson D, Salvi R. Too much of a good thing: long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hearing research. 2010;265:63–69. doi: 10.1016/j.heares.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SI, Gao SS, Xia A, Wang R, Salles FT, Raphael PD, Abaya H, Wachtel J, Baek J, Jacobs D, Rasband MN, Oghalai JS. Mechanisms of hearing loss after blast injury to the ear. PloS one. 2013;8:e67618. doi: 10.1371/journal.pone.0067618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle Lucca JJ, Chavko M, Dubick MA, Adeeb S, Falabella MJ, Slack JL, McCarron R, Li Y. Blast-induced moderate neurotrauma (BINT) elicits early complement activation and tumor necrosis factor alpha (TNFalpha) release in a rat brain. J Neurol Sci. 2012;318:146–154. doi: 10.1016/j.jns.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GA, Mitsis EM, Ahlers ST, Cristian A. Blast-induced mild traumatic brain injury. The Psychiatric clinics of North America. 2010;33:757–781. doi: 10.1016/j.psc.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Wilmington DJ, Gallun FJ, Myers PJ, Henry JA. Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. J Rehabil Res Dev. 2009;46:797–809. doi: 10.1682/jrrd.2008.09.0118. [DOI] [PubMed] [Google Scholar]
- Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- Freiwald DA. Approximate Blast Wave Theory and Experimental-Data for Shock Trajectories in Linear Explosive-Driven Shock-Tubes. J Appl Phys. 1972;43:2224–&. [Google Scholar]
- Garman RH, Jenkins LW, Switzer RC, 3rd, Bauman RA, Tong LC, Swauger PV, Parks SA, Ritzel DV, Dixon CE, Clark RS, Bayir H, Kagan V, Jackson EK, Kochanek PM. Blast exposure in rats with body shielding is characterized primarily by diffuse axonal injury. J Neurotrauma. 2011;28:947–959. doi: 10.1089/neu.2010.1540. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Goodman T, Trouche S, Massou I, Verret L, Zerwas M, Roullet P, Rampon C. Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience. 2010;171:769–778. doi: 10.1016/j.neuroscience.2010.09.047. [DOI] [PubMed] [Google Scholar]
- Gould E. Opinion – How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS. Neuronal birth and death. Current opinion in neurobiology. 1993;3:676–682. doi: 10.1016/0959-4388(93)90138-o. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Przekwas A. Mathematical Models of Blast-Induced TBI: Current Status, Challenges, and Prospects. Frontiers in neurology. 2013;4:59. doi: 10.3389/fneur.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamernik RP, Turrentine G, Roberto M, Salvi R, Henderson D. Anatomical correlates of impulse noise-induced mechanical damage in the cochlea. Hearing research. 1984a;13:229–247. doi: 10.1016/0378-5955(84)90077-7. [DOI] [PubMed] [Google Scholar]
- Hamernik RP, Turrentine G, Wright CG. Surface morphology of the inner sulcus and related epithelial cells of the cochlea following acoustic trauma. Hearing Res. 1984b;16:143–160. doi: 10.1016/0378-5955(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Temple MD, Pike BR, ODell DM, Buck DL, Lyeth BG. Working memory deficits following traumatic brain injury in the rat. J Neurotraum. 1996;13:317–323. doi: 10.1089/neu.1996.13.317. [DOI] [PubMed] [Google Scholar]
- Hoffer ME, Balaban CD. Vestibular Rehabilitation: Ready for the Mainstream. Neurorehabilitation. 2011;29:125–125. doi: 10.3233/NRE-2011-0686. [DOI] [PubMed] [Google Scholar]
- Hofstetter P, Ding D, Salvi R. Magnitude and pattern of inner and outer hair cell loss in chinchilla as a function of carboplatin dose. Audiology. 1997;36:301–311. doi: 10.3109/00206099709071981. [DOI] [PubMed] [Google Scholar]
- Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. Soldiers returning from Iraq. The New England journal of medicine. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- Jamesdaniel S, Ding D, Kermany MH, Jiang H, Salvi R, Coling D. Analysis of cochlear protein profiles of Wistar, Sprague-Dawley, and Fischer 344 rats with normal hearing function. J Proteome Res. 2009;8:3520–3528. doi: 10.1021/pr900222c. [DOI] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamnaksh A, Kwon SK, Kovesdi E, Ahmed F, Barry ES, Grunberg NE, Long J, Agoston D. Neurobehavioral, cellular, and molecular consequences of single and multiple mild blast exposure. Electrophoresis. 2012;33:3680–3692. doi: 10.1002/elps.201200319. [DOI] [PubMed] [Google Scholar]
- Kinney GFaKJG. Explosive shocks in air. New York: Springer-Verlag; 1985. [Google Scholar]
- Kovesdi E, Gyorgy AB, Kwon SK, Wingo DL, Kamnaksh A, Long JB, Kasper CE, Agoston DV. The effect of enriched environment on the outcome of traumatic brain injury; a behavioral, proteomics, and histological study. Frontiers in neuroscience. 2011;5:42. doi: 10.3389/fnins.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus KS, Mitra S, Jimenez Z, Hinduja S, Ding D, Jiang H, Gray L, Lobarinas E, Sun W, Salvi RJ. Noise Trauma Impairs Neurogenesis in the Rat Hippocampus. Neuroscience. 2010;167:1216–1226. doi: 10.1016/j.neuroscience.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SK, Kovesdi E, Gyorgy AB, Wingo D, Kamnaksh A, Walker J, Long JB, Agoston DV. Stress and traumatic brain injury: a behavioral, proteomics, and histological study. Frontiers in neurology. 2011;2:12. doi: 10.3389/fneur.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Ding D, Jiang H, Fu Y, Salvi R. Co-administration of cisplatin and furosemide causes rapid and massive loss of cochlear hair cells in mice. Neurotox Res. 2011;20:307–319. doi: 10.1007/s12640-011-9244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- Long JB, Bentley TL, Wessner KA, Cerone C, Sweeney S, Bauman RA. Blast overpressure in rats: recreating a battlefield injury in the laboratory. J Neurotrauma. 2009;26:827–840. doi: 10.1089/neu.2008.0748. [DOI] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, Snyder AZ, Raichle ME, Witherow JR, Fang R, Flaherty SF, Brody DL. Detection of Blast-Related Traumatic Brain Injury in U.S. Military Personnel. New Engl J Med. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar S, Paolone NA, Bleichfeld M, Hayes SH, Salvi RJ, Baizer JS. Expression of doublecortin, a neuronal migration protein, in unipolar brush cells of the vestibulocerebellum and dorsal cochlear nucleus of the adult rat. Neuroscience. 2012;202:169–183. doi: 10.1016/j.neuroscience.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Lu WC, Helmick K, French L, Warden DL. Traumatic brain injuries sustained in the Afghanistan and Iraq wars. The American journal of nursing. 2008;108:40–47. doi: 10.1097/01.NAJ.0000315260.92070.3f. quiz 47–48. [DOI] [PubMed] [Google Scholar]
- Mills DM, Rubel EW. Variation of distortion product otoacoustic emissions with furosemide injection. Hearing Res. 1994;77:183–199. doi: 10.1016/0378-5955(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Mishkin M. A memory system in the monkey. Philosophical transactions of the Royal Society of London. 1982;298:83–95. doi: 10.1098/rstb.1982.0074. [DOI] [PubMed] [Google Scholar]
- Muller M, von Hunerbein K, Hoidis S, Smolders JW. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hearing research. 2005;202:63–73. doi: 10.1016/j.heares.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Nelson LA, Yoash-Gantz RE, Pickett TC, Campbell TA. Relationship Between Processing Speed and Executive Functioning Performance Among OEF/OIF Veterans: Implications for Postdeployment Rehabilitation. J Head Trauma Rehab. 2009;24:32–40. doi: 10.1097/HTR.0b013e3181957016. [DOI] [PubMed] [Google Scholar]
- Newman AJ. Department of Mechanical and Aerospace Engineering, vol Master of Science. University at Buffalo, State University of New York; 2010. The Peak Overpressure Field Resulting from the Discharge of an Open-ended Shock Tube. [Google Scholar]
- Newman AJ, Mollendorf JC. The Peak Overpressure Field Resulting From Shocks Emerging From Circular Shock Tubes. J Fluid Eng-T Asme. 2010;132 [Google Scholar]
- Patterson JH, Hamernik RP. Blast overpressure induced structural and functional changes in the auditory system. Toxicology. 1997;121:29–40. doi: 10.1016/s0300-483x(97)03653-6. [DOI] [PubMed] [Google Scholar]
- Reneer DV, Hisel RD, Hoffman JM, Kryscio RJ, Lusk BT, Geddes JW. A Multi-Mode Shock Tube for Investigation of Blast-Induced Traumatic Brain Injury. J Neurotraum. 2011;28:95–104. doi: 10.1089/neu.2010.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes S, Ding DL, Sun W, Salvi R. Effect of inner and outer hair cell lesions on electrically evoked otoacoustic emissions. Hearing Res. 2001;158:139–150. doi: 10.1016/s0378-5955(01)00309-4. [DOI] [PubMed] [Google Scholar]
- Risling M, Plantman S, Angeria M, Rostami E, Bellander BM, Kirkegaard M, Arborelius U, Davidsson J. Mechanisms of blast induced brain injuries, experimental studies in rats. Neuroimage. 2011;54:S89–S97. doi: 10.1016/j.neuroimage.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Roberto M, Hamernik RP, Turrentine GA. Damage of the auditory system associated with acute blast trauma. Annals of Otology, Rhinology, & Laryngology – Supplement. 1989;140:23–34. doi: 10.1177/00034894890980s506. [DOI] [PubMed] [Google Scholar]
- Sajja VSSS, Galloway MP, Ghoddoussi F, Thiruthalinathan D, Kepsel A, Hay K, Bir CA, VandeVord PJ. Blast-induced neurotrauma leads to neurochemical changes and neuronal degeneration in the rat hippocampus. Nmr Biomed. 2012;25:1331–1339. doi: 10.1002/nbm.2805. [DOI] [PubMed] [Google Scholar]
- Saljo A, Bao F, Shi J, Hamberger A, Hansson HA, Haglid KG. Expression of c-Fos and c-Myc and deposition of beta-APP in neurons in the adult rat brain as a result of exposure to short-lasting impulse noise. J Neurotrauma. 2002;19:379–385. doi: 10.1089/089771502753594945. [DOI] [PubMed] [Google Scholar]
- Sato M, Chang E, Igarashi T, Noble LJ. Neuronal injury and loss after traumatic brain injury: time course and regional variability. Brain research. 2001;917:45–54. doi: 10.1016/s0006-8993(01)02905-5. [DOI] [PubMed] [Google Scholar]
- Svetlov SI, Prima V, Kirk DR, Gutierrez H, Curley KC, Hayes RL, Wang KK. Morphologic and biochemical characterization of brain injury in a model of controlled blast overpressure exposure. The Journal of trauma. 2010;69:795–804. doi: 10.1097/TA.0b013e3181bbd885. [DOI] [PubMed] [Google Scholar]
- Taber KH, Warden DL, Hurley RA. Blast-related traumatic brain injury: What is known? J Neuropsych Clin N. 2006;18:141–145. doi: 10.1176/jnp.2006.18.2.141. [DOI] [PubMed] [Google Scholar]
- Terrio H, Brenner LA, Ivins BJ, Cho JM, Helmick K, Schwab K, Scally K, Bretthauer R, Warden D. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. The Journal of head trauma rehabilitation. 2009;24:14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- Tompkins P, Tesiram Y, Lerner M, Gonzalez LP, Lightfoot S, Rabb CH, Brackett DJ. Brain injury: neuroinflammation, cognitive deficit, and magnetic resonance imaging in a model of blast induced traumatic brain injury. J Neurotrauma. 2013;30:1888–1897. doi: 10.1089/neu.2012.2674. [DOI] [PubMed] [Google Scholar]
- Umile EM, Sandel ME, Alavi A, Terry CM, Plotkin RC. Dynamic imaging in mild traumatic brain injury: Support for the theory of medial temporal vulnerability. Arch Phys Med Rehab. 2002;83:1506–1513. doi: 10.1053/apmr.2002.35092. [DOI] [PubMed] [Google Scholar]
- Van der Gucht E, Youakim M, Arckens L, Hof PR, Baizer JS. Variations in the structure of the prelunate gyrus in Old World monkeys. Anat Rec Part A. 2006;288A:753–775. doi: 10.1002/ar.a.20350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevord PJ, Bolander R, Sajja VS, Hay K, Bir CA. Mild neurotrauma indicates a range-specific pressure response to low level shock wave exposure. Annals of biomedical engineering. 2012;40:227–236. doi: 10.1007/s10439-011-0420-4. [DOI] [PubMed] [Google Scholar]
- Varas JM, Philippens M, Meijer SR, van den Berg AC, Sibma PC, van Bree JLMJ, de Vries DVWM. Physics of IED blast shock tube simulations for mTBI research. Frontiers in neurology. 2011;2:1–14. doi: 10.3389/fneur.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CS, Richmond DR. ORNL US Atomic Energy Commission TID-5764. 1959. Blast biology; pp. 1–89. [PubMed] [Google Scholar]
- Wolf SJ, Bebarta VS, Bonnett CJ, Pons PT, Cantrill SV. Blast injuries. Lancet. 2009;374:405–415. doi: 10.1016/S0140-6736(09)60257-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


