Abstract
In the present study, we investigated the effect of medial temporal lobe (MTL) damage on human decision making in the context of reward-based intertemporal choice. During intertemporal choice, humans typically devalue (or discount) a future reward to account for its delayed arrival (e.g., preferring $30 now over $42 in 2 months), but this effect is attenuated when participants engage in episodic future thinking, i.e., project themselves into the future to imagine a specific event. We hypothesized that this attenuation would be selectively impaired in amnesic patients, who have deficits in episodic future thinking. Replicating previous work, in a standard intertemporal choice task, amnesic patients showed temporal discounting indices similar to healthy controls. Consistent with our hypothesis, while healthy controls demonstrated attenuated temporal discounting in a condition that required participants first to engage in episodic future thinking (e.g., to imagine spending $42 at a theatre in 2 months), amnesic patients failed to demonstrate this effect. Moreover, as expected, amnesic patients’ narratives were less episodically rich than those of controls. These findings extend the range of tasks that are shown to be MTL dependent to include not only memory-based decision-making tasks but also future-oriented ones.
Keywords: hippocampus, amnesia, temporal discounting, delay discounting, mental time travel
Introduction
The medial temporal lobe (MTL), traditionally known for its role in memory, has recently been implicated in a broader range of cognitive processes. Emerging evidence suggests that damage to the MTL impairs performance on decision-making tasks when choice is influenced by representations of previous experiences (e.g., Iowa Gambling Task; Gupta et al., 2009; Gutbrod et al., 2006; also see Yee et al., 2014), suggesting that MTL-based memory processes play a role in shaping decisions. In another body of research, it has been demonstrated that patients with MTL lesions are impaired at episodic future thinking (Kwan et al., 2010; Race et al., 2011; Tulving, 1985; but see Squire et al., 2010), which refers to the act of projecting oneself into the future to experience an event that has not yet occurred (e.g., a walk along the beach on your next vacation; Atance et al., 2001). What is currently unknown is whether these episodic future-thinking deficits also have functional consequences for decision making. Such evidence would suggest that, akin to memory, MTL-based episodic future thinking capacities influence choice.
One decision-making task that requires future-oriented thought is the intertemporal choice task. In this task, participants often devalue a future reward to account for its delayed arrival, a phenomenon referred to as temporal discounting (e.g., preferring $30 now over $42 in 2 months). In light of the requirement to consider a future reward, it might be expected that MTL lesions would alter temporal discounting. But contrary to this expectation, patients with amnesia show discounting indices in the range of healthy controls (Kwan et al., 2012; 2013). However, it is possible that controls do not ordinarily engage in episodic future thinking in this task. Consistent with this notion, MTL activity is not typically observed in intertemporal choice (Ballard et al., 2009; Kable et al., 2007; Peters et al., 2009).
Importantly, explicit instructions to engage in future thinking can influence participants’ performance in intertemporal choice: When participants are asked first to “imagine” the delayed reward (e.g., imagine spending $42 at a theatre in 2 months), temporal discounting is attenuated (Benoit, Gilbert, & Burgess, 2011; Peters & Büchel, 2010), such that participants are more likely to wait for the later, larger reward. The magnitude of this attenuation is correlated with the vividness or emotional intensity of imagined episodes (Benoit, Gilbert, & Burgess, 2011; Peters & Büchel, 2010), providing evidence that episodic future thinking is influencing this shift in intertemporal choice. The magnitude of this effect is also correlated with the extent to which the hippocampus is functionally coupled with midline prefrontal regions typically associated with reward-based processing (Benoit et al., 2011; Peters et al., 2010). These imaging findings suggest a role for the MTL in the observed attenuation of temporal discounting. However, only lesion data can provide definitive evidence that this attenuation is causally linked to the MTL.
In the present study, we explored the causal role of the MTL and episodic future thinking in decision making by examining temporal discounting in MTL amnesia in (a) a standard intertemporal choice paradigm and (b) an intertemporal choice paradigm in which episodic future thinking counteracts the inclination to choose immediate rewards in healthy individuals (Benoit et al., 2011; Peters et al., 2010). We predict that amnesic patients will discount rewards similarly to controls in the standard task, in line with previous findings (Kwan et al., 2012; 2013). In contrast, while we expect that controls will show attenuated temporal discounting when asked first to imagine spending the reward in a future scenario, we predict that patients will fail to show this change in temporal discounting.
Materials and Methods
Participants
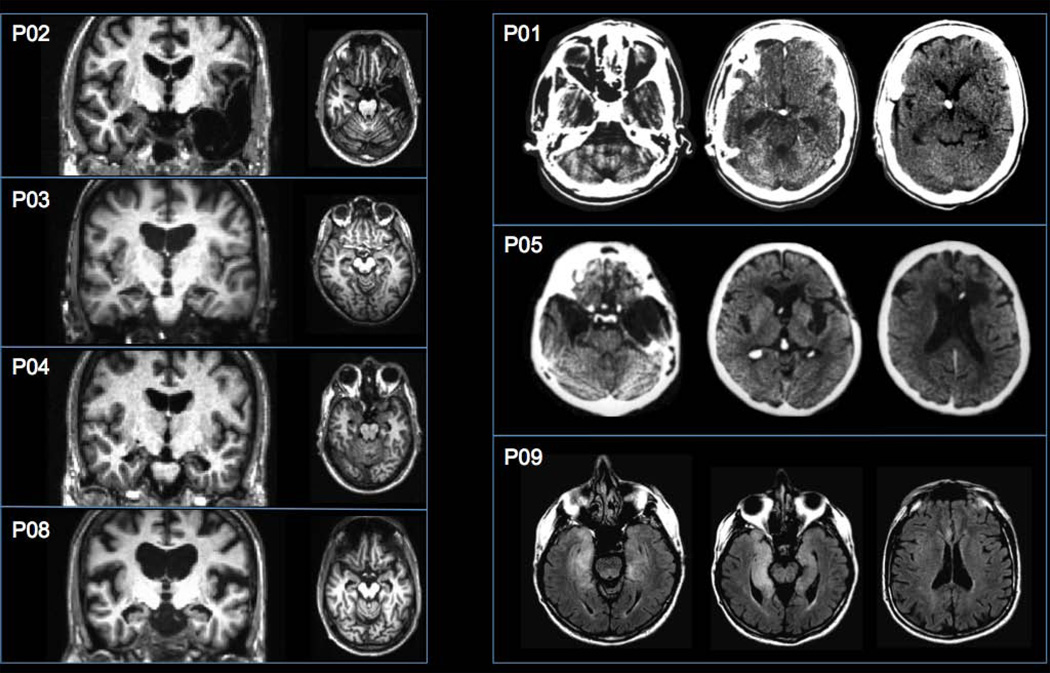
Nine patients with amnesia (3 women) participated in the study (see Table 1 for demographic and neuropsychological data). Each patient’s neuropsychological profile indicated severe impairment limited to the domain of memory. Etiology of amnesia included ischemia or anoxia (6 patients), encephalitis (2 patients), and status epilepticus followed by temporal lobectomy (1 patient). Three patients (P03, P04, P08) had lesions restricted to the hippocampus (confirmed with volumetrics in two patients; see Table 1), one patient (P01) had a lesion that included the hippocampus and MTL cortices, and two patients (P02, P05) had lesions that extended beyond the MTL into anterolateral temporal neocortex. For one of the encephalitis patients (P09), MRI was acquired in the acute phase of the illness and no visible lesions were observed on T1-weighted images. However, T2-flair images showed bilateral hyperintensities in the hippocampus and MTL cortices and anterior insula. Patients’ lesions are presented in Figure 1. Two patients (P06, P07), who had suffered from cardiac arrest, could not be scanned due to medical contraindications and are thus not included in the figure. MTL pathology for these patients was inferred based on etiology and neuropsychological profile. As shown in Table 1, volumetric data for the hippocampus and MTL cortices was available for 3 patients (P02, P03, P04), using methodology reported elsewhere (Kan et al., 2007).
Table 1.
Demographic and neuropsychological characteristics of amnesic patients
| Patients | Etiology | Age | Edu | WAIS III | WMS III | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| VIQ | WMI | GM | VD | AD | Hippo Vol Loss (%) |
Subhipp Vol Loss (%) |
||||
| P01 | Anoxia/Ischemia | 63 | 12 | 83 | 84 | 52 | 56 | 55 | N/A | |
| P02 | Anoxia + Left Temporal Lobectomy | 50 | 16 | 86 | 84 | 49 | 53 | 52 | 63% | 60%a |
| P03 | Anoxia | 55 | 14 | 90 | 99 | 45 | 53 | 52 | 70% | - |
| P04 | CO poisoning | 57 | 14 | 111 | 117 | 59 | 72 | 52 | 22% | - |
| P05 | Encephalitis | 85 | 18 | 133 | 133 | 45 | 53 | 58 | N/A | |
| P06 | Cardiac arrest | 61 | 17 | 134 | 126 | 86 | 78 | 86 | N/A | |
| P07 | Cardiac arrest | 64 | 16 | 110 | 92 | 86 | 78 | 83 | N/A | |
| P08 | Anoxia/Ischemia | 45 | 12 | 103 | 95 | 59 | 68 | 55 | N/A | |
| P09 | Encephalitis | 71 | 13 | 99 | 104 | 49 | 56 | 58 | N/A | |
Note: Age, age in years; Edu, education in years; WAIS-III, Wechsler Adult Intelligence Scale-III (Wechsler, 1997a); WMS-III, Wechsler Memory Scale-III (Wechsler, 1997b); VIQ, verbal IQ; WMI, Working Memory Index; GM, General Memory; VD, Visual Delayed; AD, Auditory Delayed; CO, carbon monoxide; Hipp Vol Loss = bilateral hippocampal volume loss; Subhipp Vol Loss = bilateral parahippocampal gyrus volume loss.
Volume loss in left anterior parahippocampal gyrus (i.e., entorhinal cortex, medial aspect of the temporal pole, and the medial portion of perirhinal cortex; see Kan et al., 2007 for methodology).
Figure 1.
Structural CT and MRI scans depicting medial temporal lobe lesions for seven of the nine amnesic patients (see Methods). The left side of the brain is displayed on the right side of the image. T1-weighted MRI images show lesion locations for P02, P03, P04, and P08 in the coronal and axial plane, CT scans show lesion locations for P01 and P05 in the axial plane, and T2-flair images shows lesion location for P09 in the axial plane.
Thirteen healthy control participants (7 women) were matched to the patient group in age (65 ± 9.5 years), education (15 ± 2.2 years) and verbal IQ (109.9 ± 16.9), which was assessed with the Wechsler Adult Intelligence Scale-III (WAIS-III; Wechsler, 1997). All participants provided informed consent in accordance with the Institutional Review Boards at Boston University and the VA Boston Healthcare System.
Materials and Procedure
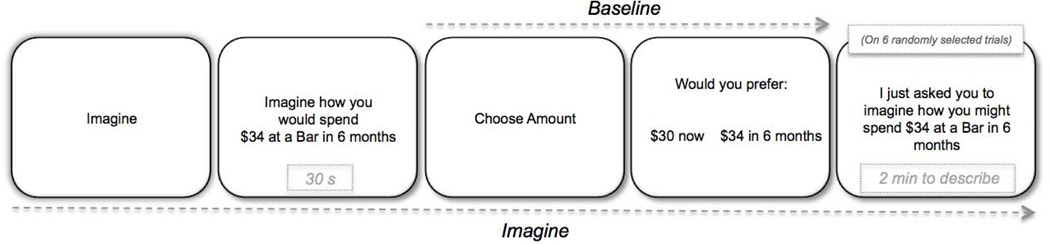
Participants considered hypothetical monetary rewards in a task that was modified from Benoit, Gilbert and Burgess (2011) and Peters and Büchel (2010). Previous research indicates that hypothetical rewards yield similar temporal discounting patterns to that of real rewards (i.e., a subset of randomly selected trials are rewarded; e.g., Johnson et al., 2002) and therefore, we used hypothetical rewards. There were two conditions: baseline and imagine. In the baseline condition, participants were asked to indicate their preference for a hypothetical sum of money in the future (e.g., $34 in 6 months) or a smaller, immediate reward (held constant at $30 dollars). To avoid the use of a strictly economic strategy (i.e., consideration of inflation and/or interest rates), participants were told that the money could not be saved but had to be spent at the time of receipt. The magnitude of the future reward and the delay of delivery varied across 6 rewards ($34, $38, $42, $48, $54 and $58) and 6 delays (2 mo, 4 mo, 6 mo, 9 mo, 1 yr, and 2 yr). These parameters were selected based on pilot data to elicit a delayed choice on approximately half of the trials. Complete crossing of reward and delay yielded a total of 36 trials, which were presented in random order. All stimuli were presented on a computer screen, one stimulus at a time, using E-prime software. Participants were first given 4 practice trials to familiarize themselves with the materials and procedure. Trials began with a self-paced cue (i.e., “Choose Amount”). The cue was provided to prepare participants for the task ahead and to provide reminders of instructions when needed (see Figure 2). Participants were given as much time as they needed to make their response. Stimuli were read aloud by the experimenter. All responses were self-paced and keyed in by the experimenter.
Figure 2.
Trial overview for the experimental conditions.
The imagine condition was similar to the baseline condition, except that participants were first asked to pre-experience what they could purchase in a given scenario (e.g., “imagine how you would spend $34 at a street fair in 6 months”). Participants were instructed to imagine the scenario in as much detail as possible, including the spatial layout, what happens, who is present, and their thoughts and feelings. As shown in Figure 2, trials began with a self-paced cue (i.e., “Imagine”). Next, the scenario was presented (e.g., “imagine how you would spend $34 at a street fair in 6 months”); participants imagined each offer for 30 s, in silence. Participants next indicated their preference for the future reward that they had just considered (e.g., $34 in 6 months) or for the immediate $30 reward. Participants were additionally told that the decision was independent of the preceding imagined situation, such that the amount chosen did not have to be spent in the scenario that was just considered (Benoit et al., 2011).
For six randomly selected trials in the imagine condition, immediately after making their decision about the reward, participants were asked to describe what they thought about during the imagination task; participants were told to speak about the scenario extemporaneously (e.g., “I just asked you to imagine how you would spend $34 at a bar in 6 months. Can you think about that event again but this time describe it to me in as much detail as possible so I can really picture it”) until it was evident that they had reached a natural ending point or until 2 minutes passed. This question served to determine the vividness (see below) of their imagined experiences. Participants were provided with additional prompts (e.g., “is there anything else you are imagining for this event?”) if the description was impoverished, semanticized or merely a description of a past event. If participants were able to provide additional details, they continued with their narrative. Responses were audio recorded for later scoring. Only a subset of trials were sampled in order to reduce the impact of fatigue on performance, which is particularly likely to occur in patients.
To avoid confusion with task instructions, the two conditions (baseline, imagine) were presented in blocked format on different days, separated by a minimum of 1 week. The imagine session was always presented in the second session to avoid carryover effects of pre-experiencing, which would likely contaminate the baseline condition.
To avoid potential group differences in familiarity and preference, in a pre-session (which occurred immediately following the baseline condition, on the same day), participants were presented with 28 events (e.g., a pub, an amusement park, a concert). For each scenario, participants indicated their familiarity (yes-familiar; no-unfamiliar) and, if the event was deemed familiar, participants also rated how much they would enjoy being at that event, using a 5-point Likert scale (1 - dislike a lot; 5 - enjoy a lot). There were no group differences in the percentage of scenarios with which participants were familiar (patients: 87%; controls: 96%; t11.84 = 1.66, p = .12) or which they perceived enjoyment for (patients: 77%; controls: 74%; t20 = −.62, p = .54). Only events that participants rated as both familiar and enjoyable (i.e., a rating higher than 3) were selected for the experimental session. Within these constraints, twelve randomly selected events were chosen for each participant. Perceived enjoyment for these twelve selected events did not differ between patients and controls (mean rating: 4.49 vs. 4.64, respectively; t18.30 = 1.67, p = .11).
To ensure that the scenarios were paired with realistic amounts, the original 28 events were binned, a priori, into “low-cost” categories (events that typically involve spending less than $42; e.g., a street fair) and “high-cost” categories (events that typically involve spending more than $48; e.g., a food and wine exposition). For each participant, six unique events were chosen from each cost category. Each event was repeated three times to allow for pairing of each event with each of the three dollar-amounts in that cost category (low: $34, $38, $42; high: $48, $54 and $58). The resulting 18 low-cost and 18 high-cost event trials were pseudorandomly assigned to one of the six delays such that each delay occurred three times across the 18 trials in each category. No identical combination of event, delayed amount, and delay was generated, thereby creating 36 unique trials. The order of trials was pseudo-randomized such that no event repeated in immediate succession.
Scoring
Intertemporal Choice
Following Benoit et al. (2011), two intertemporal choice (i.e., temporal discounting) dependent measures were analyzed, a “reward index” and a “choice index”. The “reward index” reflects the extent to which the accumulated reward exceeds the amount that would be obtained by always choosing the immediate award. It was calculated as the difference between a participant’s actual accumulated reward and the minimum accumulated reward possible (i.e., constant selection of the smaller immediate reward), divided by the difference between the maximum accumulated reward possible (i.e., constant selection of the larger reward) and the minimum accumulated reward possible, yielding the formula: [actual −minimum] / [maximum − minimum]. By this calculation, the value of the reward index ranged from 0.0 to 1.0, with consistent selection of the smaller, immediate reward yielding a reward index of 0.0, and consistent selection of the larger, later reward yielding a reward index of 1.0. (n.b. Benoit and colleagues calculated the “reward index” as the proportion of accumulated reward [total reward obtained / maximum possible reward]. As this does not incorporate the minimum accumulated reward possible, it obscures the functional minimum value of this index. For comparison purposes we reanalyzed our data using this metric and the same pattern of results was observed). The “choice index” is the proportion of delayed options chosen (delay trials / total trials). Since both indices yielded similar results, only the reward index is reported here.
Episodic Future Thinking
The six randomly selected narratives from the imagine condition for each participant were scored using an adaptation of the Autobiographical Interview (AI; Levine et al., 2002) that has been modified for scoring future narratives (Race et al., 2011). Due to a technical error, one control participant’s audio file was corrupted, thus the analysis did not include this participant’s narratives. Briefly, the narratives were segmented into informational bits that were classified as either “internal” or “semantic”. Details were considered internal if they were directly related to the main event described, were specific to time and place, and conveyed a sense of episodic pre-experiencing (i.e., episodic future thinking). Internal details were subdivided into five specific categories: (1) event (happenings), (2) place (spatial location), (3) time (temporal information), (4) perceptual (sensory details), and (5) thought/emotional (emotional states). By contrast, semantic details pertained to general and personal facts or extended events that did not require pre-experiencing of a specific time and place. A third category called “other” was used to code additional details tangential or unrelated to the main event, including repetitions, metacognitive (‘I can’t remember’), or editorial statements (‘It was the best of times’).
Interrater reliability was calculated based on approximately 20% of the narratives, with an equal number of narratives selected from each group. Prior to coding these narratives, two raters practiced coding narratives that were not included in the present study (both patients and controls), which were obtained from other studies in our laboratory. Once agreement between the two raters was achieved, the two raters proceeded to code the narratives from the present sample. As in prior studies (Race et al., 2011; Hassabis et al., 2007), the primary scorer was not blind to subjects’ group membership, but the second trained scorer was blind to group status. Interrater reliability was high for internal detail categories (event, place, time, perceptual, and thought/emotional; Cronbach’s = 0.83–.96) as well as for semantic (Cronbach’s = 0.90) and “other” details Cronbach’s = 0.95).
Results
Intertemporal Choice
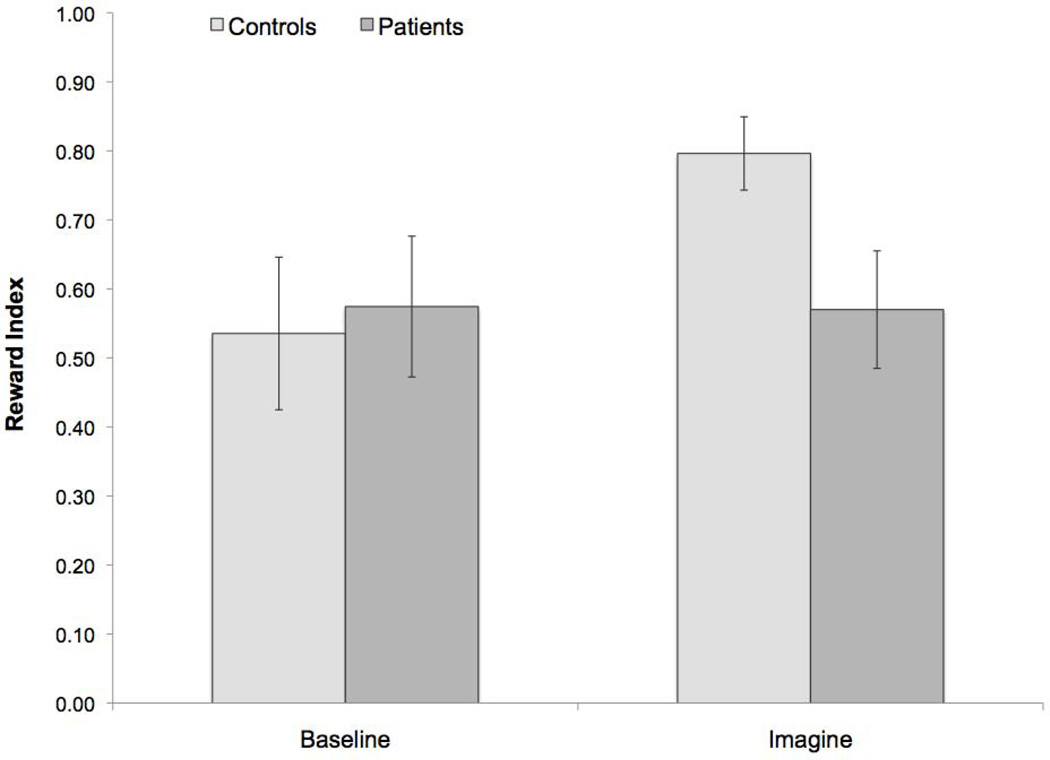
Intertemporal choice data (Figure 3) were assessed with mixed-design ANOVAs, with factors of group (patient vs. control) and condition (baseline vs. imagine). There was a significant main effect of condition (F1,20 = 6.53, p = .019) and a significant interaction between group and condition (F1,20 = 6.99, p = .016). Patients and controls did not significantly differ in the reward index at baseline (t20 = −.25, p = .81). By contrast, patients’ reward index scores were lower than those of controls in the imagine condition (t20 = 2.38, p = .03). Paired-sample t-tests showed that the reward index was higher for the imagine condition relative to the baseline condition in controls (t12 = −3.65, p = .003) but not in patients (t8 = .07, p = .944). To investigate whether isolated hippocampal damage was sufficient to produce these effects, we compared the control group to the patients with volumetrically confirmed hippocampal-only lesions (n = 2). This produced a similar pattern of findings: patients did not significantly differ from controls in the reward index at baseline (t12.26 = −.92 p = .38), but their reward index scores were lower than controls in the imagine condition (t12.00 = 3.32, p = .006). Mean difference scores (imagine minus baseline) for patients with hippocampal lesions (−0.03) were comparable to those of the remaining patients who had more extensive lesions (0.02).
Figure 3.
Mean reward index for healthy controls and amnesic patients. Error bars indicate SEM.
Episodic Future Thinking
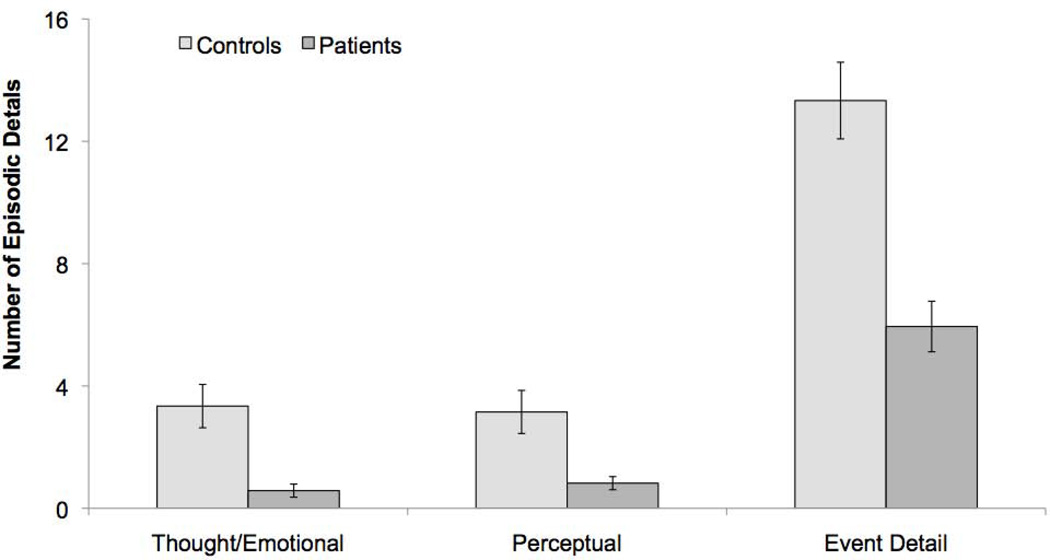
Separate analysis of the data before the general prompting (see methods) yielded similar results to that obtained after prompting, hence only the latter are presented. Given the violation of normality for the AI data, we used non-parametric statistics (Mann-Whitney U). As expected, patients generated fewer internal details relative to controls (mean 8.27 vs. 22.18, respectively; mean rank 5.22 vs. 15.33, respectively; U = 2.00, p < .0001) but they generated an equivalent number of semantic and “other” details (p = .64; p = .59, respectively). To explore the nature of the internal detail deficit in more detail, we examined thought/emotional, perceptual and event detail subcategories; time and place details were excluded because of floor effects in both groups (mean number of time and place details were .11 and .80, respectively, in amnesics and .38 and 1.98, respectively, in controls). These floor effects were likely due to the specificity of the probes (e.g., “imagine how you would spend $34 at a street fair in 6 months”), which already localize the event in time and place. The analysis for the remaining detail types revealed that patients generated fewer details than did controls in all three event subcategories (in patients and controls, respectively, mean thought/emotional details: .58 vs. 3.34; perceptual details: .82 vs. 3.15; event details: 5.94 vs. 13.33; mean rank thought/emotional details: 6.22 vs. 14.58; perceptual details: 6.89 vs. 14.08, event details: 5.56 vs. 15.08; all Us > 4.00, ps < .009; see Figure 4).
Figure 4.
Mean number of episodic future details produced in healthy controls and amnesic patients for thought/emotional, perceptual and event detail subtypes. Error bars indicate SEM. Data were analyzed non-parametrically (see main text for descriptive statistics).
Relationship between Intertemporal Choice and Episodic Future Thinking
Non-parametric correlational analyses (i.e., Spearman) were used to examine the relationship between change in reward index scores (i.e., [imagine – baseline]) and each detail type (thought/emotional, perceptual and event details) separately. Examination of the scatter plots between change scores and detail types for each group revealed two bivariate outliers (one in controls and one in patients), who were thus removed from the correlational analyses. (Removal of these outliers from the episodic future thinking analyses reported above did not change the pattern of results observed).
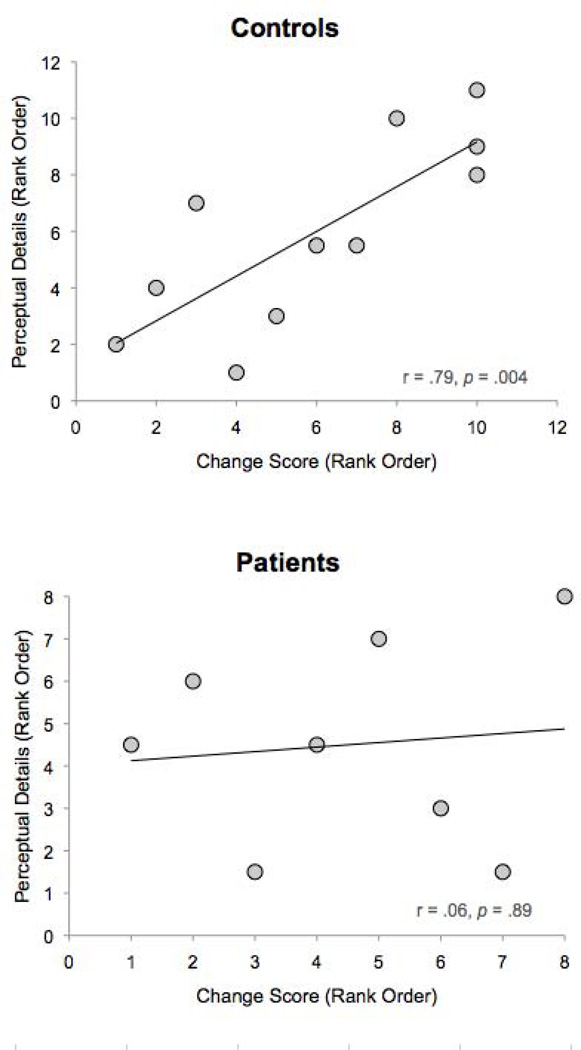
In controls, there was a marginal positive correlation between change scores and perceptual details generated before experimenter probing (r = .55, p = .08), and this relationship was statistically significant when including details generated after probing (r = .79, p = .004; see Figure 5). No significant correlations were observed before or after probing between change scores and thought/emotional details (p = .31; p = .33, respectively) or event details (p = .41; p = .14, respectively). These findings reveal that control participants who produced the most perceptual details also showed the greatest effect of episodic future thinking on intertemporal choice. No significant correlations between change scores and details generated were observed in the patients before (all ps > .11) or after experimenter probing (all ps > .52), likely due to low number of details and restricted range for patients, particularly for perceptual and thought/emotional detail types (also see Figure 5).
Figure 5.
Correlation (Spearman Rank-Order) between change in reward index scores (i.e., [imagine – baseline]) and number of perceptual future details produced in healthy controls and in patients.
Discussion
In the present study, we demonstrate that the influence of episodic future thinking on intertemporal choice depends on the MTL. First, in accordance with previous research (Benoit et al., 2011; Peters et al., 2010), we found that healthy individuals opted for the future reward more often when they imagined consuming it (i.e., episodic future thinking) prior to making intertemporal choices, accruing 26% more financial reward relative to a standard baseline condition that did not require episodic future thinking (see Figure 3). However, we extend previous findings by providing novel evidence that the MTL is necessary for this effect: Episodic future thinking failed to induce a shift toward more future-oriented choices in patients with MTL amnesia, who are impaired in vividly imagining novel future experiences (e.g., Kwan et al., 2010; Race et al., 2011; Tulving, 1985; but see Squire et al., 2010).
Our results are consistent with recent proposals that suggest that MTL-based episodic future thinking has adaptive utility for humans (Boyer, 2008; Klein et al., 2010; Peters et al., 2011). In particular, Boyer (2008) argues that imagining future episodes is crucial for decision making because it provides a means to experience hypothetical future outcomes that override current goals; future imagining allows the consequences of a choice to be experienced prior to a decision. Consistent with this idea, a previous study has shown that the impact of episodic future thinking on temporal discounting is larger for participants who imagined future events as more vivid (Peters et al., 2010), while another study showed that participants were more likely to choose the delayed amount for episodes that were experienced with greater emotional intensity (Benoit et al., 2011). Critically, since patients with MTL damage are impaired at pre-experiencing future events, their reduced preference for delayed rewards in the imagine condition in comparison to controls likely reflects an impaired ability to richly represent future outcomes of actions.
There are at least two ways in which episodic future thinking may influence intertemporal choice decisions. First, it is possible that by imagining a potential future reward in a specific context (e.g., $34 at a bar in 1 month), the future is perceived as more concrete, which may aid an individual in assigning it a subjective value. Kurth-Nelson et al. (2012) argue that this effect is the result of a search process by which one vicariously samples future outcomes to determine the subjective value of the reward (also see e.g., Johnson et al., 2007). According to the authors, such a search is necessary even in situations where information about the future reward is explicitly presented (i.e., you are offered $34 to be received in 1 month): In order to determine the utility of the delayed reward, one needs to determine what circumstances may apply at that time (Kurth-Nelson et al., 2012). Future imagining may facilitate this search process. A second possibility, which is not mutually exclusive, is that imagining a future outcome brings this outcome closer in subjective time to the present (Boyer, 2008), which may reduce the perceived temporal delay (i.e., the future feels less far away in time). It is an open question which of these mechanisms underlies the observed influence of future thinking on intertemporal choice behavior in controls in the present study, and by extension, which of these mechanisms is compromised following MTL lesions in amnesia.
It is also important to determine more precisely what aspect of episodic future thinking is critical in shaping choices. Imagined future events involve multimodal representations (temporal, spatial, affective, etc.), and each of these types of information could potentially influence intertemporal choice. We observed that the effect of episodic future thinking on intertemporal choice was largest for participants who produced the most perceptual details, but was unrelated to other episodic detail types. This effect was evident only in the healthy controls, likely due to low detail production across multiple episodic categories in patients (also see Race et al., 2011). This finding is in accordance with previous work by Peters et al. (2010) who observed that those participants who showed the largest effect of episodic future thinking on intertemporal choice were those who imagined the episodes with the greatest vividness, a construct that likely maps onto perceptual phenomenology. Yet, other theorists have emphasized the affective component as the critical motivational force in decision making because emotions mediate the subjective experience of hypothetical rewards and punishments (Boyer, 2008; D'Argembeau et al., 2007). In support of this idea, Benoit et al. (2011) showed that participants were more likely to choose the delayed amount for episodes that were experienced with greater emotional intensity. While we did not find evidence to support a putative role of emotion in the effects observed in the present study (i.e., there was no relationship between change in intertemporal choice and emotional detail generation), our method of probing this question differed from that of Benoit and colleagues. We tallied the number of emotional details subjects produced in their narratives, while Benoit and colleagues used a subjective rating scale that probed the degree of emotional intensity felt while performing the task, a construct that may not map onto the number of emotional details generated per se. Moreover, Benoit et al. employed a within-subject analysis (which was not possible in the present study, given that only 6 narratives were probed), which may be a more sensitive measure. More recent studies on the role of emotion in mediating the effects of episodic future thinking on intertemporal choice are equivocal. Liu and colleagues (2013) showed that the attenuation in temporal discounting associated with episodic future thinking was specific to positive events, while neutral events had no effect on intertemporal choice, and negative events increased temporal discounting (i.e., participants were more inclined to choose the present reward). By contrast, Lin and Epstein (2014) found that the attenuation in temporal discounting associated with episodic future thinking did not differ as a function of whether the imagined future events were positive or neutral.
Our paradigm also leaves open the question of whether episodic future thinking influences intertemporal choice behavior by virtue of being episodic or by virtue of being future-oriented. Given that amnesic patients are impaired in imagining episodes regardless of whether those episodes are atemporal or set in the future (Hassabis et al., 2007), the question is difficult to address in this population. However, Lin and colleagues (2014) recently showed that episodic future thinking had a greater attenuating effect on temporal discounting than did episodic present thinking, suggesting that mental time travel (i.e., future thinking) does play a role in shifting choice behavior.
More broadly, the finding that patients discount hypothetical rewards normally at baseline (also see Kwan et al., 2012; 2013 for similar findings) but are impaired when the task draws on episodic future thinking suggests that decision making can be accomplished in multiple ways, not all of which require the MTL (Bornstein et al., 2013; Daw et al., 2005). Indeed, during standard intertemporal choice (baseline) participants report a number of strategies, including making choices based on intuition (i.e., a ‘gut feeling’), or consideration of economic factors (e.g., interest, inflation), with the latter likely drawing on well-established semantic knowledge (also see Kwan et al 2012). While our “imagine” condition encouraged the use of an additional strategy, namely episodic future thinking, our findings suggest that the tendency to use episodic future thinking spontaneously during standard intertemporal choice conditions is likely minimal.
The finding that amnesic patients perform normally in standard intertemporal choice is consistent with fMRI studies showing that performance in this task is not typically associated with MTL activation. Instead, these studies most consistently demonstrate involvement of midline prefrontal and striatal regions, which code for the subjective value of rewards (Ballard et al., 2009; Kable et al., 2007; Peters et al., 2009). By contrast, imaging studies have implicated the MTL when intertemporal choice draws on episodic future thinking: Attenuated temporal discounting following episodic future thinking is associated with increased midline prefrontal-MTL coupling (Benoit et al., 2011; Peters et al., 2010). One hypothesis that stems from these observations is that signals from the MTL, which code for representations of future scenarios, add weight to the value of future rewards represented by medial prefrontal regions (Benoit et al., 2011). Our lesion data, coupled with these imaging findings, suggest that the neural substrates supporting intertemporal choice depend on the strategy employed, with decisions supported by episodic future thinking drawing on MTL processes. It should be noted, however, that the MTL is part of a larger network of brain regions that support episodic future thinking (Schacter et al., 2012). Thus, it is likely that lesions to other parts of this network may also disrupt influences of episodic future thinking on decision making.
The observation that amnesic patients are unimpaired on standard temporal discounting paradigms is in stark contrast to rodent studies, which consistently demonstrate that hippocampal lesions produce steeper temporal discounting (Abela et al., 2013; Cheung et al., 2005; Labudda et al., 2009; Mariano et al., 2009; McHugh et al., 2008; Rawlins et al., 1985). However, there are a number of differences between intertemporal choice paradigms used in human and rodent studies. While human experiments involve the use of questionnaires that probe hypothetical reward options (i.e., rewards and time delays that are not actually experienced within the experiment), rodent studies involve experiencing the delay and receipt of rewards in real time. It is interesting to speculate whether the experiential quality of the rodent studies promotes a more episodic-like strategy, whereby future responses are adjusted based on recently encoded experiences (i.e., the experience of the delay or consumption of the reward on recent trials). An important goal for future research in amnesia is to model discounting in a manner that is more analogous to these rodent studies.
Another question that has not yet been addressed is whether, aside from episodic future thinking, other strategies can also attenuate temporal discounting. Some clues can be derived from a comparison of our study to that of Benoit et al. (2011). Unlike the present study, in which the baseline task was a standard intertemporal choice paradigm, the baseline task used by Benoit et al. (2011) required participants to list what items could be purchased with the money in the given future scenario, a task that involves semantic consideration of the future. Notably, the attenuation of temporal discounting (imagine minus baseline) was smaller in Benoit et al. (2011) than in our study (difference score was 5%, in Benoit et al., 2011 versus 9% in the present study when the reward index was calculated in an analogous manner to that of Benoit et al., 2011; see results). Although the differences between these studies may be explained by task-related factors (e.g., relative magnitude or time-delay differences), it is also possible that the reduced attenuation in Benoit et al. (2011) reflects more future-oriented choices in their baseline task relative to ours, choices that may have been influenced by semantic future thinking. In light of this possibility, it would be of interest to determine if amnesic patients, who are capable of engaging in at least some rudimentary aspects of semantic future thinking (Race et al., 2013), could use such processes to support more future-oriented decisions.
An important caveat in interpreting the present findings is that our approach deviates from those used in the economics literature that employ model-based analyses to investigate the shape of the discounting curve (i.e., exponential versus hyperbolic; Green et al., 2004). Given the limitations inherent in studies with neurological patients, we opted instead for a paradigm used in neuroimaging studies (e.g., see Benoit et al., 2011) that is more feasible for use with patients because it includes far fewer trials. The disadvantage of this approach is that it precludes analyses to determine the nature of the discounting function, and therefore limits the conclusions we can draw about the role of episodic future thinking in discounting patterns per se.
It is important to consider the possibility that because our patients have a brain injury they may have been less motivated to engage in episodic future thinking (i.e., a health problem may elicit feelings of uncertainty about the future). Evidence weighing against this possibility comes from the finding that patients were as inclined as healthy controls to select the future reward in our baseline task (also see Kwan et al., 2012; 2013), suggesting that they did incorporate the future in their decision making. Moreover, patients did not differ from healthy controls in how often they predicted enjoying the probe events (e.g., baseball game, bowling alley etc.), suggesting that they were just as enthusiastic about participating in various events in the future.
In summary, the results of the present study demonstrate that the role of the MTL in decision making is not limited to choices that draw on memory (e.g., Gupta et al., 2009; Gutbrod et al., 2006; Wimmer et al., 2012; Yee et al., 2014), but extends to choices that benefit from future thinking as well. Moreover, our findings add to a growing body of evidence that future-thinking processes subserved by the MTL have adaptive value for humans (Benoit et al., 2011; Peters et al., 2010).
Acknowledgements
This research was supported by grant MH093431 from the National Institutes of Mental Health, grant IO1 CX000925 from the Clinical Science Research and Development Service of the Department of Veterans Affairs, and a post-doctoral fellowship from the Canadian Institutes of Health Research awarded to D.J.P. All authors contributed to the study design and interpretation of the results. D.J.P. collected and analyzed the data and wrote the manuscript with input from M.M.K. and M.V. The authors thank Keely Burke for research assistance. The authors also thank Dr. Matthew D. Grilli for comments on an early draft of this manuscript.
Footnotes
The authors declare no competing financial interests or other conflicts of interest.
References
- Abela AR, Chudasama Y. Dissociable contributions of the ventral hippocampus and orbitofrontal cortex to decision-making with a delayed or uncertain outcome. Eur J Neurosci. 2013;37(4):640–647. doi: 10.1111/ejn.12071. [DOI] [PubMed] [Google Scholar]
- Atance CM, O'Neill DK. Episodic future thinking. Trends Cogn Sci. 2001;5(12):533–539. doi: 10.1016/s1364-6613(00)01804-0. [DOI] [PubMed] [Google Scholar]
- Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. Neuroimage. 2009;45(1):143–150. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Burgess PW. A neural mechanism mediating the impact of episodic prospection on farsighted decisions. J Neurosci. 2011;31(18):6771–6779. doi: 10.1523/JNEUROSCI.6559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein AM, Daw ND. Cortical and hippocampal correlates of deliberation during model-based decisions for rewards in humans. PLoS Comput Biol. 2013;9(12):e1003387. doi: 10.1371/journal.pcbi.1003387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer P. Evolutionary economics of mental time travel? Trends Cogn Sci. 2008;12(6):219–224. doi: 10.1016/j.tics.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Cheung TH, Cardinal RN. Hippocampal lesions facilitate instrumental learning with delayed reinforcement but induce impulsive choice in rats. BMC Neurosci. 2005;6:36. doi: 10.1186/1471-2202-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argembeau A, Van der Linden M. Emotional aspects of mental time travel. Behavioral and Brain Sciences. 2007;30:220–221. [Google Scholar]
- Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci. 2005;8(12):1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull. 2004;130(5):769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Duff MC, Denburg NL, Cohen NJ, Bechara A, Tranel D. Declarative memory is critical for sustained advantageous complex decision-making. Neuropsychologia. 2009;47(7):1686–1693. doi: 10.1016/j.neuropsychologia.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutbrod K, Krouzel C, Hofer H, Muri R, Perrig W, Ptak R. Decision-making in amnesia: do advantageous decisions require conscious knowledge of previous behavioural choices? Neuropsychologia. 2006;44(8):1315–1324. doi: 10.1016/j.neuropsychologia.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci U S A. 2007;104(5):1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, van der Meer MA, Redish AD. Integrating hippocampus and striatum in decision-making. Curr Opin Neurobiol. 2007;17(6):692–697. doi: 10.1016/j.conb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 2002;77(2):129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan IP, Giovanello KS, Schnyer DM, Makris N, Verfaellie M. Role of the medial temporal lobes in relational memory: neuropsychological evidence from a cued recognition paradigm. Neuropsychologia. 2007;45(11):2589–2597. doi: 10.1016/j.neuropsychologia.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SB, Robertson TE, Delton AW. Facing the future: memory as an evolved system for planning future acts. Mem Cognit. 2010;38(1):13–22. doi: 10.3758/MC.38.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth-Nelson Z, Bickel W, Redish AD. A theoretical account of cognitive effects in delay discounting. Eur J Neurosci. 2012;35(7):1052–1064. doi: 10.1111/j.1460-9568.2012.08058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan D, Carson N, Addis DR, Rosenbaum RS. Deficits in past remembering extend to future imagining in a case of developmental amnesia. Neuropsychologia. 2010;48(11):3179–3186. doi: 10.1016/j.neuropsychologia.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Kwan D, Craver CF, Green L, Myerson J, Boyer P, Rosenbaum RS. Future decision-making without episodic mental time travel. Hippocampus. 2012;22(6):1215–1219. doi: 10.1002/hipo.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan D, Craver CF, Green L, Myerson J, Rosenbaum RS. Dissociations in Future Thinking Following Hippocampal Damage: Evidence From Discounting and Time Perspective in Episodic Amnesia. J Exp Psychol Gen. 2013;142(4) doi: 10.1037/a0034001. [DOI] [PubMed] [Google Scholar]
- Labudda K, Frigge K, Horstmann S, Aengenendt J, Woermann FG, Ebner A, Markowitsch HJ, Brand M. Decision making in patients with temporal lobe epilepsy. Neuropsychologia. 2009;47(1):50–58. doi: 10.1016/j.neuropsychologia.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: dissociating episodic from semantic retrieval. Psychol Aging. 2002;17(4):677–689. [PubMed] [Google Scholar]
- Lin H, Epstein LH. Living in the moment: Effects of time perspective and emotional valence of episodic thinking on delay discounting. Behav Neurosci. 2014;128(1):12–19. doi: 10.1037/a0035705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Feng T, Chen J, Li H. The value of emotion: how does episodic prospection modulate delay discounting? PLoS One. 2013;8(11):e81717. doi: 10.1371/journal.pone.0081717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariano TY, Bannerman DM, McHugh SB, Preston TJ, Rudebeck PH, Rudebeck SR, Rawlins JN, Walton ME, Rushworth MF, Baxter MG, et al. Impulsive choice in hippocampal but not orbitofrontal cortex-lesioned rats on a nonspatial decision-making maze task. Eur J Neurosci. 2009;30(3):472–484. doi: 10.1111/j.1460-9568.2009.06837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh SB, Campbell TG, Taylor AM, Rawlins JN, Bannerman DM. A role for dorsal and ventral hippocampus in inter-temporal choice cost-benefit decision making. Behav Neurosci. 2008;122(1):1–8. doi: 10.1037/0735-7044.122.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Büchel C. Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. J Neurosci. 2009;29(50):15727–15734. doi: 10.1523/JNEUROSCI.3489-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66(1):138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Peters J, Büchel C. The neural mechanisms of inter-temporal decision-making: understanding variability. Trends Cogn Sci. 2011;15(5):227–239. doi: 10.1016/j.tics.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Race E, Keane MM, Verfaellie M. Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. J Neurosci. 2011;31(28):10262–10269. doi: 10.1523/JNEUROSCI.1145-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Race E, Keane MM, Verfaellie M. Losing sight of the future: Impaired semantic prospection following medial temporal lobe lesions. Hippocampus. 2013;23(4):268–277. doi: 10.1002/hipo.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins JN, Feldon J, Butt S. The effects of delaying reward on choice preference in rats with hippocampal or selective septal lesions. Behav Brain Res. 1985;15(3):191–203. doi: 10.1016/0166-4328(85)90174-3. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, Szpunar KK. The future of memory: remembering, imagining, and the brain. Neuron. 2012;76(4):677–694. doi: 10.1016/j.neuron.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, van der Horst AS, McDuff SG, Frascino JC, Hopkins RO, Mauldin KN. Role of the hippocampus in remembering the past and imagining the future. Proc Natl Acad Sci U S A. 2010;107(44):19044–19048. doi: 10.1073/pnas.1014391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Memory and Consciousness. Canadian Psychology. 1985;26(1):1–12. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Third Edition (WAIS-III) administration and scoring manual. San Antonio, TX: Harcourt Assessment; 1997. [Google Scholar]
- Wimmer GE, Shohamy D. Preference by association: how memory mechanisms in the hippocampus bias decisions. Science. 2012;338(6104):270–273. doi: 10.1126/science.1223252. [DOI] [PubMed] [Google Scholar]
- Yee LT, Warren DE, Voss JL, Duff MC, Tranel D, Cohen NJ. The hippocampus uses information just encountered to guide efficient ongoing behavior. Hippocampus. 2014;24(2):154–164. doi: 10.1002/hipo.22211. [DOI] [PMC free article] [PubMed] [Google Scholar]