Abstract
Endothelial intercellular adhesion molecule (ICAM) 1 binds neutrophils and facilitates their transmigration into the lung; E-selectin facilitates leukocyte rolling. As neutrophils contribute to tissue destruction in emphysema and chronic obstructive pulmonary disease, we hypothesized that soluble ICAM-1 (sICAM-1) and E-selectin (sE-selectin) would be associated with longitudinal progression of emphysema and lung function decline.
The Multi-Ethnic Study of Atherosclerosis (MESA) enrolled participants 45-84 years old without clinical cardiovascular disease in 2000-02. The MESA Lung Study assessed percent emphysema (<-950 Hounsfield units) on cardiac (2000-07) and full-lung CT scans (2010-12), and spirometry was assessed twice over five years. sICAM-1 and sE-selectin were measured at baseline. Mixed-effect models adjusted for demographics, anthropometry, smoking, C-reactive protein, sphingomyelin and scanner factors.
Among 1,865 MESA Lung participants with measurement of sICAM-1 and percent emphysema the mean log-sICAM-1 was 5.5±0.3 ng/mL and percent emphysema increased 0.73 percentage points (95% CI: 0.34, 1.12; P<0.001) over ten years. A one SD increase in sICAM-1 was associated with an accelerated increase in percent emphysema of 0.23 percentage points over ten years (95% CI: 0.06, 0.39; P=0.007). No significant association was found for sE-selectin, or between any adhesion molecule and lung function.
Higher levels of sICAM-1 were independently associated with progression of percent emphysema in a general population sample.
Keywords: emphysema, CT imaging, endothelium, intercellular adhesion molecule-1
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is defined by spirometric airflow obstruction that does not fully reverse and is the 4th leading cause of death worldwide (1). Emphysema is defined pathologically as the permanent enlargement of airspaces and destruction of alveolar walls (2). Emphysema occurs in the majority of patients with COPD (3) and is not infrequent among smokers without COPD or older never-smokers (4). Emphysema assessed quantitatively on computed tomography (CT) has been associated with increased hospitalizations and mortality in those with and without COPD (5-7). However, the pathogenesis of COPD and emphysema remains incompletely understood and there are no medications targeting emphysema outside of alpha-1 antitrypsin deficiency.
Neutrophils, which are involved in the pathogenesis of emphysema and COPD, migrate into the lung via tight adhesion to intercellular adhesion molecule (ICAM) 1, an adhesion protein on endothelial cells (8, 9). E-selectin, an adhesion molecule expressed on activated endothelial cells, contributes to leukocyte rolling and thereby facilitates tight adhesion (8). ICAM-1 blocking antibodies reduce neutrophilic pulmonary inflammation by two-thirds in animals, suggesting that ICAM-1 may be critical in neutrophil access to the lung (10). Small studies have found altered ICAM-1 and E-selectin in COPD (11, 12), and cross-sectional studies have found inverse associations between soluble ICAM-1 (sICAM-1) and lung function (13, 14). However, no longitudinal study has assessed the relationship between ICAM-1 or E-selectin and the progression of emphysema or decline in lung function.
Since endothelial ICAM-1 correlates with plasma levels of sICAM-1 (15), we tested the a priori hypotheses (16) that sICAM-1 and soluble E-selectin (sE-selectin) are associated with longitudinal increases in the percentage of emphysema-like lung on CT and decline in lung function with the goal to understand subclinical disease progression. We tested this hypothesis in general population sample with mostly subclinical emphysema as it likely represents a biologically-relevant early stage in COPD pathogenesis, and may provide insight into strategies for disease prevention (17). In addition, subjects with clinical disease are more likely to have characteristics that may confound the relationship of interest (e.g., active infection, corticosteroid use). In order to test the specificity of the associations, we also examined soluble vascular cell adhesion molecule (sVCAM)-1, soluble L-selectin (sL-selectin), and soluble P-selectin (sP-selectin), adhesion molecules that increase with inflammation but are less relevant to neutrophil recruitment (8, 9).
METHODS
Multi-Ethnic Study of Atherosclerosis
The Multi-Ethnic Study of Atherosclerosis (MESA) recruited 6,814 participants ages 45 to 84 years and free of clinical cardiovascular disease in 2000-02 from six U.S. communities (18). Exclusion criteria were weight over 300 lbs, pregnancy, and impediments to long-term participation. The MESA Air Pollution Study recruited an additional 257 participants under the same criteria in 2004-07 (19).
The MESA Lung Study enrolled 3,965 MESA participants in 2004-06 who had flow-mediated dilation measured and consented to genetic analyses (20), all MESA Air participants at one site, and an additional 408 MESA participants in 2010-12. The current analysis includes participants in the MESA Lung Study with measurement of sICAM-1 and baseline percent emphysema.
The protocols of MESA and all studies described were approved by the institutional review boards of collaborating institutions and the National Heart, Lung, and Blood Institute. Written informed consent was obtained from all participants.
Measurement of Adhesion Molecules
Plasma sICAM-1 was measured at baseline among 2,621 MESA and the MESA Air participants using an ELISA assay (Parameter Human sICAM-1; R&D Systems, Minneapolis, MN). The coefficient of variation (CV) was 5.0%. Serum sE-selectin was measured at baseline for 998 MESA participants and the MESA Air participants (Parameter Human sE-selectin Immunoassay; R&D Systems; CV 5.7-8.8%).
Serum sVCAM-1 and sL-selectin were measured among 2,440 and plasma sP-selectin was measured among 2,572 participants an average of 1.7 years after baseline by ELISA (Parameter Human sVCAM-1, sL-selectin/CD62L, and sP-selectin Quantikine Immunoassays; R&D Systems). The laboratory CVs were 3.4-4.2%, 6.7-7.9%, and 6.7% respectively.
Genotyping
All consenting participants were genotyped with the ITMAT-Broad-CARe (IBC) microarray (Illumina, Inc; San Diego, CA) (21). The genotype of the ICAM-1 single-nucleotide polymorphism (SNP) rs5491 was obtained as it affects binding of sICAM-1 to the ELISA probe used (22). Genotypes for two advanced glycation endproduct-specific receptor (AGER) SNPs (rs2070600 and rs2071288) were also obtained as they have been associated with emphysema (23).
Measurement of Percent Emphysema
All participants underwent cardiac CT scans at baseline following a standardized protocol at full inspiration on electron-beam and multi-detector CT (MDCT) scanners at six centers (24). Participants were coached to total lung capacity and two scans were obtained; lung volume on replicate cardiac CTs were reproducible (r=0.95). The scan with the greatest lung volume was selected for analysis except where image quality differed. Follow-up cardiac CT scans used the same protocol; 45 off-protocol scans were excluded, as were 312 acquired on Aquilion scanners, which do not produce reliable lung density measures. Full-lung scans were performed on 3,204 MESA Lung participants at the ten-year follow-up exam at full inspiration on MDCT scanners following the SPIROMICS protocol (25).
Trained readers performed percent emphysema measurements at a reading center without knowledge of other participant information using modified Pulmonary Analysis Software Suite (PASS) software for cardiac scans and Apollo 1.2 (an updated version of PASS; VIDA Diagnostics, Coralville, IA) for full-lung scans. Percent emphysema was defined as the percentage of lung voxels below −950 Hounsfield units (HU), a threshold chosen based on pathologic comparisons (26), longitudinal study validity (27), and prognostic significance in this cohort (7). The −950 HU threshold on all scans was adjusted for attenuation of air outside the chest to account for scanner variation (28). As cardiac CTs image approximately 66% of lung volume (from the carina to lung bases), the upper-third of full-lung scans was excluded so as to compare the same lung region over time (28). Cardiac CT measures of percent emphysema have been previously validated against full-lung scans in this cohort (r=0.95 on MDCT scanners) (28). Examination of monthly averages of outside air from all scans demonstrated little scanner-drift over more than 11 years, with one exception (Supplement Figure 1). As an alternate measure of emphysema, lung density at the lower 15th percentile was measured as the HU below which 15 percent of all lung voxels have a lower density value.
Spirometry
Spirometry was conducted between 2004-07 and repeated in 2010-12 in accordance with American Thoracic Society-European Respiratory Society guidelines. All participants attempted at least three acceptable maneuvers on the same dry-rolling seal spirometers (Occupational Marketing Inc., Houston, TX) following the MESA Lung protocol; all exams were reviewed by one investigator (29). Airflow obstruction was defined as FEV1/FVC ratio less than 0.70 at the time of participants’ first spirometry measurement.
Smoking and Other Covariates
Age, sex, race/ethnicity, educational attainment, smoking history, alcohol consumption, physical activity, second hand smoke exposure, and recent illness were self-reported. Smoking status was verified with urinary cotinine levels at baseline and ten-year follow-up (20). Height, weight, blood pressure, C-reactive protein, cholesterol, and fasting glucose were measured using standard techniques (30). Plasma sphingomyelin was measured with a validated four-step enzymatic assay (31). Medication-use was assessed by medication inventory (32). Hypertension and diabetes were defined by self-reported physician diagnoses, medication use, or abnormal blood pressure or glucose measurement. Estimates of residential exposure to ambient particulate matter <2.5μm (PM2.5) used spatiotemporal models (19). High attenuation areas (HAAs) on CT were defined as lung regions between −600 and −250 HU, a measure of subclinical parenchymal lung disease (33).
Statistical Analysis
Participants were stratified by quintile of sICAM-1 for descriptive purposes. Mixed linear regression growth-curve models with random intercepts and slopes were used to assess the relationship of sICAM-1 and change in percent emphysema over time. sICAM-1 was log-transformed to improve normality. The initial model included CT scanner model, voxel size, and milliamperes (mAs); the subsequent model adjusted for age, sex, race/ethnicity, height, weight, and education; the final model included cigarettes per day, pack-years, C-reactive protein, and sphingomyelin. Scanner model, voxel size, mAs, height, weight, and cigarettes per day were time-variant. Effect measure modification was assessed for sex, smoking-status, race/ethnicity, age and presence of airflow obstruction. Secondary analyses were performed restricted to participants with AA genotype at rs5491, those with all adhesion molecules measured, scans acquired on Siemens, GE, or MDCT scanners, cardiac CT scans only, scanners with stable outside air attenuation and including scanners that were excluded for technical reasons. Secondary analyses were also performed adjusting for cardiovascular risk factors, relevant medications, alcohol-consumption, physical activity, second-hand smoke, PM2.5 exposure, recent infection, percent HAA, total lung volume on CT and minor alleles at AGER SNPs. Analyses evaluating change in lung density at the lower 15th percentile were also performed. Analyses for lung function and of other adhesion molecules used a similar statistical approach. Cross-sectional analyses used linear regression and were adjusted for baseline measures described above. Statistical significance was defined as two-tailed p-value <0.025, given the hypothesized two adhesion molecules. Analyses were performed using SAS 9.3 (SAS Institute; Cary, NC).
RESULTS
Study Participants
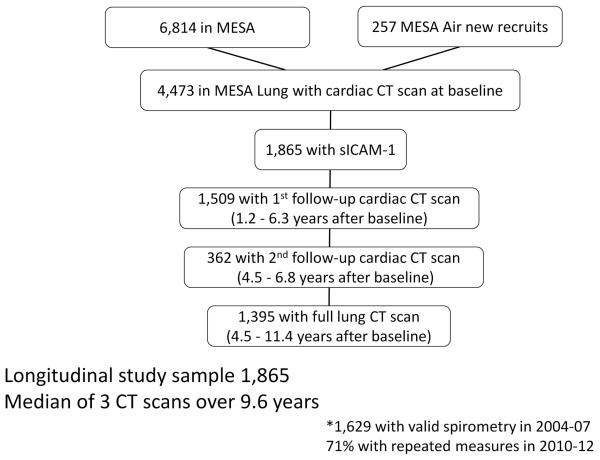
Of 4,473 participants, 1,865 had measures of sICAM-1 and percent emphysema and were included in the current analysis (Figure 1). The included participants did not differ from other MESA participants except they were younger and more likely to be female and white (Supplement Table 1). Eighty-one percent had a follow-up cardiac CT scan and 1,395 (75% of total; 77% of those still living) underwent a full-lung CT at the ten-year follow-up; 95% had at least one follow-up measure of emphysema.
Figure 1.
Description of study sample
Participants were 59±9 years old at baseline, 45% were male, and the race/ethnic distribution was: 47% white, 21% Hispanic, 18% African, and 14% Chinese-Americans. At baseline 15% were current-smokers, 39% former-smokers, and 46% never-smokers. The mean log-sICAM-1 was 5.5±0.3 ng/mL, median percent emphysema was 3.0% (IQR 1.2, 5.8), and mean FEV1/FVC ratio was 0.75±0.08.
Compared to participants with the lowest sICAM-1 measurements, those in the highest quintile were more likely to be female, white or Hispanic, have lower educational attainment, higher BMI and currently smoke (Table 1). There were modest correlations between sICAM-1 and other adhesion molecules (Supplement Table 2).
Table 1.
Selected characteristics of study sample at baseline exam by quintile of sICAM-l
| Quintile of sICAM-1 | |||||
|---|---|---|---|---|---|
|
| |||||
| Q1 (n=373) |
Q2 (n=373) |
Q3 (n=373) |
Q4 (n=373) |
Q5 (n=373) |
|
| sICAM-1 ng/mL (mean, SD) | 170.3 ± 35.3 | 230.8 ± 9.3 | 262.6 ± 9.6 | 296.7 ± 11.5 | 383.7 ± 78.4 |
|
| |||||
| Age (mean ± SD) | 58.1 ± 9.0 | 59.0 ± 9.6 | 59.1 ± 9.5 | 59.7 ± 9.6 | 59.6 ± 9.3 |
| Percent male | 46.6 | 49.9 | 44.8 | 40.8 | 41.8 |
| Race (%) | |||||
| White, non-Hispanic | 28.2 | 51.1 | 54.2 | 53.4 | 49.3 |
| African-American | 32.4 | 12.1 | 13.1 | 15.3 | 17.7 |
| Hispanic/Latino | 13.4 | 14.5 | 21.4 | 24.1 | 29.2 |
| Chinese-American | 26.0 | 22.3 | 11.3 | 7.2 | 3.8 |
| Education (%) | |||||
| Incomplete High School | 14.2 | 9.9 | 11.8 | 16.1 | 19.8 |
| Completed High School | 11.8 | 16.6 | 15.3 | 21.4 | 21.4 |
| Some College | 28.4 | 27.3 | 27.3 | 27.6 | 31.1 |
| Completed College | 23.1 | 20.4 | 17.4 | 14.2 | 15.0 |
| Graduate Degree | 22.3 | 25.5 | 27.9 | 20.7 | 12.7 |
| Height (mean ± SD) cm | 166.9 ± 9.5 | 168.2 ± 10.0 | 167.2 ± 9.9 | 165.8 ± 9.6 | 165.9 ± 9.9 |
| Weight (mean ± SD) lbs | 166.9 ± 39.5 | 166.8 ± 36.8 | 174.2 ± 38.1 | 177.9 ± 39.4 | 180.0 ± 38.2 |
| BMI (mean ± SD) kg/m2 | 27.0 ± 5.2 | 26.6 ± 4.5 | 28.2 ± 5.3 | 29.3 ± 5.9 | 29.7 ± 5.8 |
| Smoking status (%) | |||||
| Never | 51.5 | 53.4 | 48.8 | 43.4 | 32.4 |
| Former | 39.4 | 38.9 | 40.8 | 41.8 | 35.1 |
| Current | 9.1 | 7.8 | 10.5 | 14.7 | 32.4 |
| Pack-years (mean ± SD)* | 19.1 ± 20.8 | 23.9 ± 23.3 | 23.0 ± 22.9 | 23.4 ± 22.3 | 33.0 ± 32.7 |
| Cigarettes/day (mean ± SD)† | 11.4 ± 10.2 | 9.7 ± 7.5 | 8.4 ± 7.8 | 11.6 ± 10.6 | 16.4 ± 12.6 |
| Recent cold or flu, % (No) | 13.2 (49) | 13.0 (48) | 14.5 (54) | 15.5 (58) | 17.4 (64) |
| Oral steroid use, % (No) | 0.54 (2) | 0.54 (2) | 1.9 (7) | 0.27 (1) | 1.1 (4) |
| C-reactive protein (mg/L) | 3.0 ± 5.1 | 2.7 ± 6.0 | 3.0 ± 3.7 | 4.2 ± 5.7 | 5.6 ± 7.1 |
| Sphingomyelin | 43.9 ± 12.7 | 44.4 ± 13.2 | 44.9 ± 18.0 | 43.6 ± 12.3 | 44.9 ± 12.8 |
| sE-selectin, ng/mL (mean, SD) n=928‡ | 40.8 ± 20.5 | 45.1 ± 17.0 | 49.7 ± 21.7 | 52.2 ± 21.3 | 66.3 ± 30.2 |
| sVCAM1, ng/mL (mean, SD) n=1,054‡ | 633.1 ± 150 | 687.7 ± 193 | 728.8 ± 169 | 713.2 ± 194 | 765.5 ± 233 |
| sL-selectin, ng/mL (mean, SD) n=1,054‡ | 894.5 ± 195 | 893.7 ± 186 | 915.1 ± 182 | 950.8 ± 201 | 946.2 ± 196 |
| sP-selectin, ng/mL (mean, SD) n=1,100‡ | 25.9 ± 7.3 | 26.8 ± 9.1 | 28.4 ± 10.6 | 28.9 ± 10.3 | 31.6 ± 10.8 |
for 904 ever-smokers reporting pack-years.
for current smokers, n=278.
sample size includes those in the MESA Lung Study with both adhesion molecules measured.
sICAM-1, sE-selectin, and Longitudinal Change in Percent Emphysema
The 1,865 participants were scanned a median of three times over a median of 9.6 years (IQR 5.0, 10.0), resulting in 5,131 measures of percent emphysema. The mean increase in percent emphysema was 0.73 percentage points over ten years (95% CI: 0.34, 1.12; P<0.001).
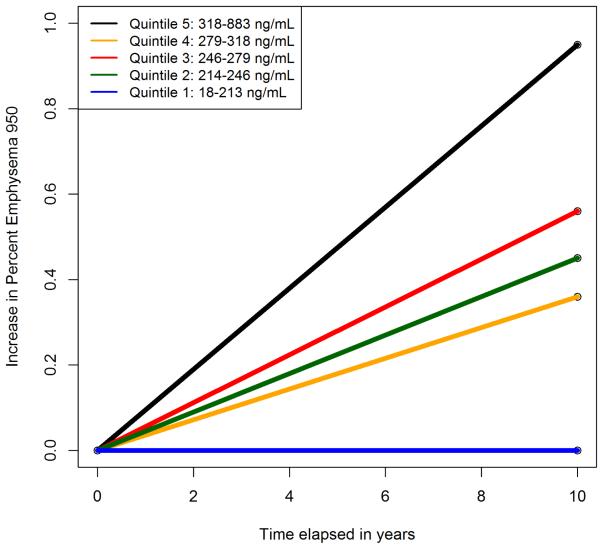
sICAM-1 levels were significantly associated with longitudinal change in percent emphysema in minimally adjusted analyses (change in percent emphysema per SD log-sICAM-1 of 0.24 percentage points over 10 years, 95% CI: 0.08, 0.40; P=0.004). The fully adjusted model yielded very similar results (Table 2 and Figure 2).
Table 2.
Predicted 10 year change in percent emphysema by quintile of sICAM-1 and sE-selectin
| Quintile of adhesion
molecule |
Change over 10 years
per SD log-adhesion molecule (95% CI)* |
P-value* | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |||
| sICAM-1 (n=1,865) | |||||||
| Model 1 | Ref | 0.29 | 0.36 | 0.25 | 0.96 | 0.24 (0.08, 0.40) | 0.004 |
| Model 2 | Ref | 0.45 | 0.54 | 0.37 | 1.07 | 0.27 (0.10, 0.43) | 0.001 |
| Model 3 | Ref | 0.45 | 0.57 | 0.38 | 1.02 | 0.23 (0.06, 0.39) | 0.006 |
| Model 4 | Ref | 0.45 | 0.56 | 0.36 | 0.95 | 0.23 (0.06, 0.39) | 0.007 |
| sE-selectin (n=943) | |||||||
| Model 1 | Ref | −0.14 | −0.02 | 0.21 | 0.66 | 0.21 (−0.03, 0.45) | 0.09 |
| Model 2 | Ref | −0.05 | 0.08 | 0.35 | 0.62 | 0.20 (−0.05, 0.45) | 0.12 |
| Model 3 | Ref | −0.08 | 0.07 | 0.31 | 0.55 | 0.16 (−0.09, 0.40) | 0.22 |
| Model 4 | Ref | −0.13 | 0.03 | 0.23 | 0.49 | 0.16 (−0.09, 0.41) | 0.22 |
Model 1: adjusted for scanner model, voxel size and mAs
Model 2: additionally adjusted for age, sex, sex*time, race, race*time, height, weight and education
Model 3: additionally adjusted for cigarettes per day, pack-years and pack-years*time
Model 4: additionally adjusted for C-reactive protein and sphingomyelin
Effect estimate and P-value derived from multivariate mixed model with log-adhesion molecule as a continuous variable
Figure 2.
Predicted change in percent emphysema over time by quintile of sICAM-1 among 1,865 participants followed for median of 9.6 years, results are shown for the fully adjusted model
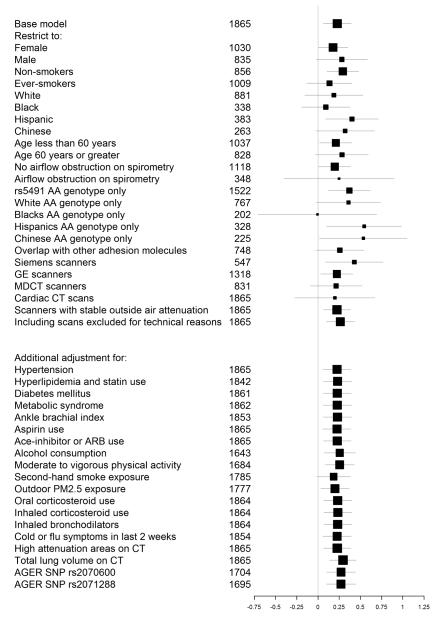
Analyses restricted to 1,522 participants with AA genotype at SNP rs5491 yielded results of greater magnitude (0.37, 95% CI: 0.12, 0.62; P=0.003). There was no evidence for effect modification by sex, smoking status, race/ethnicity, age or presence of airflow obstruction (P-value for interaction 0.88, 0.56, 0.83, 0.65 and 0.88 respectively). The direction and magnitude of the association was similar when analyses were restricted to those with all adhesion molecules measured, scans acquired on Siemens, GE, MDCT scanners, cardiac CT scans or scanners with stable outside air attenuation and with inclusion of scans that were excluded for technical reasons. The results were similar after adjustment for cardiovascular risk factors, relevant medication use, alcohol consumption, physical activity, second hand smoke, PM2.5 exposure, recent infection, percent HAA, total lung volume on CT or the minor alleles of AGER SNPs rs2070600 and rs2071288 (Figure 3). Analyses of the longitudinal change in lung density at the lower 15th percentile showed an association of borderline statistical significance (change per SD log-sICAM-1 of −1.19 HU over 10 years, 95% CI: −2.39, 0.01; P=0.05) in minimally adjusted analyses; with further adjustment results were attenuated and not statistically significant.
Figure 3.
Sensitivity analyses, showing the effect estimates of the change in percent emphysema over 10 years for 1 SD increase in log-sICAM-1
Among 943 participants, sE-selectin was associated with longitudinal change in percent emphysema by a similar magnitude to sICAM-1 in minimally adjusted analyses; however, the relationship did not attain statistical significance and was further attenuated by multivariate adjustment (Table 2). There were no significant associations between sE-selectin and longitudinal change in lung density at the lower 15th percentile.
In cross-sectional analyses, there was a significant negative association between sICAM-1 and percent emphysema (multivariate −0.24 percentage points per SD unit of log-sICAM-1, 95% CI: −0.43, −0.05, P=0.01). Cross-sectional associations were non-significant for sE-selectin (−0.15 percentage points per SD unit of log-sE-selectin, 95% CI: −0.41, 0.11, P=0.25). There was no evidence for effect modification of cross-sectional analyses by the presence of airflow obstruction (P-value for interaction 0.75 for sICAM-1, 0.49 for sE-selectin).
sICAM-1, sE-selectin and Longitudinal Change in Lung Function
Of participants with sICAM-1 measures, 1,629 had valid baseline spirometry and 1,149 completed repeat valid spirometry at a median of 4.7 years (71%; 73% of those living). The mean decline in FEV1 was 23.1 mL/year (95% CI: −24.7, −21.4; P<0.001) and decline in FEV1/FVC ratio was 0.22%/year (95% CI: −0.26, −0.19; P<0.001).
There was no evidence for an association between sICAM-1 or sE-selectin and decline in FEV1 or FEV1/FVC, although results for FEV1/FVC were in the hypothesized direction for both adhesion molecules (Table 3).
Table 3.
Predicted change in FEV1 and FEV1/FVC per year for a 1 SD increase in log-adhesion molecule
| Change per year per SD
log-adhesion molecule (95% CI) |
P-value | |
|---|---|---|
| sICAM-1 | ||
| FEV1, mL (n=1,629) | ||
| Model 1 | −0.51 (−2.97, 1.95) | 0.69 |
| Model 2 | −0.55 (−3.02, 1.93) | 0.66 |
| Model 3 | −0.54 (−3.02, 1.93) | 0.67 |
| FEV1/FVC, % (n=1,605) | ||
| Model 1 | −0.03 (−0.10, 0.03) | 0.27 |
| Model 2 | −0.03 (−0.10, 0.02) | 0.25 |
| Model 3 | −0.03 (−0.10, 0.02) | 0.25 |
|
| ||
| sE-selectin | ||
| FEV1, mL (n=899) | ||
| Model 1 | 0.20 (−3.06, 3.45) | 0.91 |
| Model 2 | 0.10 (−3.17, 3.37) | 0.95 |
| Model 3 | 0.11 (−3.17, 3.38) | 0.95 |
| FEV1/FVC, % (n=888) | ||
| Model 1 | −0.05 (−0.13, 0.03) | 0.23 |
| Model 2 | −0.05 (−0.15, 0.03) | 0.23 |
| Model 3 | −0.05 (−0.15, 0.03) | 0.23 |
Model 1: adjusted for age, sex, race, educational attainment, height and weight (model for FEV1 also adjusted for height2)
Model 2: additionally adjusted for smoking status and pack-years for ever-smokers
Model 3: additionally adjusted for C-reactive protein
Other Adhesion Molecules and Longitudinal Change in Percent Emphysema and Lung Function
Examination of sVCAM-1, sL-selectin, and sP-selectin revealed no evidence for an association with longitudinal change in percent emphysema or lung function (Table 4). Furthermore, 95% confidence intervals for all three of these adhesion molecules excluded an association of the magnitude observed for sICAM-1 and change in percent emphysema.
Table 4.
Predicted change in percent emphysema, FEV1 and FEV1/FVC by levels of sVCAM-1, sL-selectin and sP-selectin
| Change per SD-log adhesion molecule (95% CI) |
P-value | |
|---|---|---|
| Percent emphysema, change per 10 years*† | ||
| sVCAM-1 (n=1,932) | −0.024 (−0.18, 0.13) | 0.77 |
| sL-selectin (n=1,932) | 0.001 (−0.16, 0.16) | 0.99 |
| sP-selectin (n=2,025) | −0.001 (−0.15, 0.14) | 0.99 |
| FEV1 (mL), change per year*‡ | ||
| sVCAM-1 (n=1,771) | −0.33 (−2.80, 2.14) | 0.79 |
| sL-selectin (n=1,771) | −1.92 (−4.25, 0.41) | 0.11 |
| sP-selectin (n=1,858) | −0.52 (−2.78, 1.75) | 0.66 |
| FEV1/FVC (%), change per year* | ||
| sVCAM-1 (n=1,749) | 0.02 (−0.04, 0.07) | 0.56 |
| sL-selectin (n=1,749) | 0.01 (−0.04, 0.06) | 0.76 |
| sP-selectin (n=1,834) | −0.02 (−0.06, 0.03) | 0.52 |
The models shown are adjusted for age, sex, race, education, height, weight, cigarettes per day, pack-years and C-reactive protein
The model for percent emphysema is also adjusted for scanner model, voxel size, mAs, sex*time, race*time, pack-years*time and sphingomyelin
The model for FEV1 is also adjusted for height2
DISCUSSION
Higher levels of sICAM-1 were associated with accelerated progression of percent emphysema over ten years in this general population sample. The findings were consistent across multiple subgroups and robust to additional adjustment for a large number of possible confounders. Associations for sE-selectin were of similar magnitude in minimally adjusted analyses but did not attain statistical significance. In contrast, there was no evidence for association of other adhesion molecules with change in percent emphysema, or between any adhesion molecule and lung function. These findings suggest that ICAM-1, and associated neutrophil recruitment into the lung, may play a role in the progression of subclinical emphysema.
This is the first study of which we are aware to show an association between sICAM-1 levels and longitudinal progression of percent emphysema. These findings are consistent with small studies showing that circulating sICAM-1 is increased in patients with COPD (11, 12). The result for sE-selectin was of similar magnitude to sICAM-1 in minimally adjusted analyses, but was non-significant, in part due to the smaller sample size, and was further attenuated after full adjustment. Circulating sE-selectin has also been found to be increased in COPD in some (12), but not all prior studies (11).
ICAM-1 is critical to transendothelial migration of neutrophils into multiple organs including the lung (8, 9). ICAM-1 expression in the lung is upregulated in the setting of inflammation, and ICAM-1 blocking antibodies can significantly reduce neutrophilic pulmonary inflammation in animal studies (10, 34). Furthermore, ICAM-1 and sE-selectin are upregulated in endothelial perturbation and dysfunction (35), which is directly linked to emphysema in mouse models (36) and has been observed in COPD (37, 38).
In contrast to results for sICAM-1 and sE-selectin, there was no evidence for association of other soluble adhesion molecules with progression of percent emphysema. VCAM-1 is important in transendothelial migration of basophils and eosinophils (9), leukocytes less relevant to the pathogenesis of emphysema. P-selectin and L-selectin are not affected by endothelial perturbation (8); in addition, selectins facilitate leukocyte rolling, which may be less relevant in the lung, given the small pulmonary capillaries and long neutrophil transit times (39). Alternative explanations for the specificity of the association of sICAM-1 appear less likely: all adhesion molecules were measured with similar precision; sVCAM-1, sL-selectin, and sP-selectin were measured one to two years after baseline, an interval that was short compared to follow-up; and sensitivity analyses limited to participants with measures of all adhesion molecules showed consistent results for sICAM-1.
The lack of an association of sICAM-1 with change in lung function may have several explanations. First, change in percent emphysema and a detectable decline in lung function may represent distinct processes. Findings from many recent biomarker and genetic studies support this contention (3, 40-43). Second, ICAM-1 expression on epithelial cells may have more relation to airflow obstruction and be less likely to be reflected in circulating sICAM-1 levels. Finally, the smaller sample size and shorter follow-up for lung function analyses raise the possibility of a false negative result; however, the confidence intervals suggest this was unlikely.
The major strengths of this study include repeated CT scans over ten years, large sample-size and the multi-ethnic, population-based sample. However, there are limitations that should be discussed. Adhesion molecules were measured in plasma or serum rather than membrane-bound on pulmonary endothelium. The latter was clearly infeasible in a study of this size and duration, and there is in vivo evidence that soluble adhesion molecules, including sICAM-1, relate to their endothelial expression (15). It is important to note that there are short term variations in plasma levels of sICAM-1 and sE-selectin in healthy individuals (variation coefficients of 7.9% and 11.3%, respectively) (44), although this would be expected to weaken the observed associations.
The magnitude of the association of sICAM-1 and progression of percent emphysema was modest; however, the progression for each SD of sICAM-1 represented nearly one-third of the average progression in this general population sample with mostly subclinical emphysema. In this cohort, percent emphysema has been associated with reduced LV filling, dyspnea and increased all-cause mortality (7, 45, 46). As such, the progression of subclinical emphysema likely represents an early stage in disease pathogenesis, and associated pathways may be relevant to disease prevention (17).
The cross-sectional association between percent emphysema and sICAM-1 was significant; however, in situations with divergent findings the longitudinal relationship is generally preferred (47).
Due to the long-term study, unequal loss to follow-up due may result in bias; however, a high proportion were rescanned at ten years. Confounding is also a concern, as it is in any observational study. The few studies on determinants of emphysema progression show that in addition to sex, BMI and active smoking, emphysema progression is associated with surfactant protein D (SPD), soluble receptor for advanced glycation endproducts (sRAGE) and sphingomyelin (3, 23, 48). Confounding by these factors is unlikely as adjustment for sphingomyelin and two AGER SNPs, which affect sRAGE levels, had little impact on the results and SPD is only weakly correlated with inflammatory markers, which relate to sICAM-1 levels (49). Conditions associated with sICAM-1 include cardiac risk factors, smoking, alcohol intake, rheumatoid arthritis, cancer and acute inflammatory conditions (50). However, MESA measured most of these factors precisely and additional adjustment had little effect on the results.
The sICAM-1 assay used does not reliably measure sICAM-1 in carriers of the T allele at rs5491 (22). This SNP is not uncommon among African-Americans and Hispanics. However, analyses restricted to AA genotype showed consistent results.
Percent emphysema was assessed on the lower 66% of the lungs and hence the lung apices were not included; however, percent emphysema measures from this region of the lung correlate well with those from full-lung scans in MESA (28). Due to the long follow-up time, there were inevitably changes in CT scanner models for each participant, as well as technological advances in image acquisition and processing that contribute to variation in quantitative emphysema measures. However, attenuation of air outside the body was remarkably stable over more than a decade and sensitivity analyses of scans acquired on similar scanners showed consistent results. True volume correction could not be performed as lung apices were not included, however lung volumes on repeat cardiac CT scans were highly reproducible. In addition, correction for lung volume may obscure relevant findings as CT lung volume may be a reflection of either improved inspiratory effort or disease-related hyperinflation. Analyses of the change in lung density at the lower 15th percentile yielded results for sICAM-1 that were in the expected direction but not statistically significant. This lung density measure has been used and recommended for assessing longitudinal change in emphysema in diseased populations (3, 27, 51), but has not previously been studied in a population sample with largely subclinical emphysema.
In conclusion, sICAM-1 levels were associated with accelerated progression of percent emphysema on CT scan over ten years in this general population sample. These findings suggest that ICAM-1 may be important in the pathogenesis of emphysema, and further studies of this pathway in COPD and emphysema are warranted.
Supplementary Material
Highlights.
We measured plasma intercellular adhesion molecule (ICAM) 1 in a population sample
We examined change in percent emphysema and lung function in mixed-effects models
Percent emphysema was assessed on cardiac CT (2000-07) and full lung CT (2010-12)
Higher soluble ICAM-1 was associated with faster progression of percent emphysema
ICAM-1, involved in neutrophil migration in the lung may be important in emphysema
ACKNOWLEDGEMENTS
This research was supported by contracts N01-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-RR-024156 and UL1-RR-025005 from NCRR. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
This publication was also developed under a STAR research assistance agreement, No. RD831697 (MESA Air), awarded by the U.S Environmental Protection Agency. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication.
FUNDING
NIH R01-HL077612, RC1-HL100543, R01-HL093081, N01-HC-95159 through N01-HC-95169, UL1-TR000040, R01-HL098077, EPA RD83169701
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.WHO: the top 10 causes of death. 2013 Jul; [cited 2014 Mar]. Available from: http://www.who.int/mediacentre/factsheets/fs310/en/
- 2.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS, GOLD Scientific Committee Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO global initiative for chronic obstructive lung disease (GOLD) workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 3.Coxson HO, Dirksen A, Edwards LD, et al. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE Study. Lancet Respir Med. 2013;1:129–136. doi: 10.1016/S2213-2600(13)70006-7. [DOI] [PubMed] [Google Scholar]
- 4.Omori H, Nakashima R, Otsuka N, et al. Emphysema detected by lung cancer screening with low-dose spiral CT: prevalence, and correlation with smoking habits and pulmonary function in Japanese male subjects. Respirology. 2006;11:205–210. doi: 10.1111/j.1440-1843.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- 5.McAllister DA, Ahmed FS, Austin JH, et al. Emphysema predicts hospitalisation and incident airflow obstruction among older smokers: a prospective cohort study. PloS One. 2014;9:e93221. doi: 10.1371/journal.pone.0093221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johannessen A, Skorge TD, Bottai M, et al. Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med. 2013;187:602–608. doi: 10.1164/rccm.201209-1722OC. [DOI] [PubMed] [Google Scholar]
- 7.Oelsner EC, Hoffman EA, Folsom AR, et al. Association of emphysema-like lung on cardiac computed tomography and mortality in persons without airflow obstruction: the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Ann Intern Med. 2014 doi: 10.7326/M13-2570. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly M, Hwang JM, Kubes P. Modulating leukocyte recruitment in inflammation. The J Allergy Clin Immunology. 2007;120:3–10. doi: 10.1016/j.jaci.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Bochner BS, Luscinskas FW, Gimbrone MA, et al. Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J Exp Med. 1991;173:1553–1557. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorkness RL, Mehta H, Kaplan MR, Miyasaka M, Hefle SL, Lemanske RF., Jr Effect of ICAM-1 blockade on lung inflammation and physiology during acute viral bronchiolitis in rats. Pediatr Res. 2000;47:819–824. doi: 10.1203/00006450-200006000-00023. [DOI] [PubMed] [Google Scholar]
- 11.Blidberg K, Palmberg L, James A, et al. Adhesion molecules in subjects with COPD and healthy non-smokers: a cross sectional parallel group study. Respir Res. 2013;14:47. doi: 10.1186/1465-9921-14-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aldonyte R, Eriksson S, Piitulainen E, Wallmark A, Janciauskiene S. Analysis of systemic biomarkers in COPD patients. COPD. 2004;1:155–164. doi: 10.1081/copd-120030828. [DOI] [PubMed] [Google Scholar]
- 13.Oelsner EC, Pottinger TD, Burkart KM, et al. Adhesion molecules, endothelin-1 and lung function in seven population-based cohorts. Biomarkers. 2013;18:196–203. doi: 10.3109/1354750X.2012.762805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thyagarajan B, Smith LJ, Barr RG, et al. Association of circulating adhesion molecules with lung function: the CARDIA Study. Chest. 2009;135:1481–1487. doi: 10.1378/chest.08-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laudes IJ, Guo RF, Riedemann NC, et al. Disturbed homeostasis of lung intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 during sepsis. Am J Path. 2004;164:1435–1445. doi: 10.1016/S0002-9440(10)63230-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barr RG. Endothelial dysfunction, biomarkers and lung function. 2003 [cited 2014 Feb 20]. Available from: http://www.agingportfolio.org/projects/project/5R01HL077612-02.
- 17.Drummond MB, Buist AS, Crapo JD, Wise RA, Rennard SI. Chronic obstructive pulmonary disease: NHLBI workshop on the primary prevention of chronic lung diseases. Ann Am Thorac Soc. 2014;11(Suppl 3):S154–160. doi: 10.1513/AnnalsATS.201312-432LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: Objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman JD, Adar SD, Allen RW, et al. Prospective study of particulate air pollution exposures, subclinical atherosclerosis, and clinical cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Am J Epidemiol. 2012;176:825–837. doi: 10.1093/aje/kws169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez J, Jiang R, Johnson WC, MacKenzie BA, Smith LJ, Barr RG. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med. 2010;152:20. doi: 10.1059/0003-4819-152-4-201002160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musunuru K, Lettre G, Young T, et al. Candidate gene association resource (CARe): design, methods, and proof of concept. Circ Cardiovasc Genet. 2010;3:267–275. doi: 10.1161/CIRCGENETICS.109.882696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Register TC, Burdon KP, Lenchik L, et al. Variability of serum soluble intercellular adhesion molecule-1 measurements attributable to a common polymorphism. Clin Chem. 2004;50:2185–2187. doi: 10.1373/clinchem.2004.036806. [DOI] [PubMed] [Google Scholar]
- 23.Cheng DT, Kim DK, Cockayne DA, et al. Systemic soluble receptor for advanced glycation endproducts is a biomarker of emphysema and associated with AGER genetic variants in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:948–957. doi: 10.1164/rccm.201302-0247OC. [DOI] [PubMed] [Google Scholar]
- 24.Carr JJ, Nelson JC, Wong ND, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) Study. Radiology. 2005;234:35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 25.Couper D, Lavange LM, Han M, et al. Design of the SubPopulations and InteRmediate OutcoMes In COPD Study (SPIROMICS) Thorax. 2014;69:492–5. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gevenois PA, De Vuyst P, de Maertelaer V, et al. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1996;154:187–192. doi: 10.1164/ajrccm.154.1.8680679. [DOI] [PubMed] [Google Scholar]
- 27.Parr DG, Stoel BC, Stolk J, Stockley RA. Validation of computed tomographic lung densitometry for monitoring emphysema in alpha1-antitrypsin deficiency. Thorax. 2006;61:485–490. doi: 10.1136/thx.2005.054890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman EA, Jiang R, Baumhauer H, et al. Reproducibility and validity of lung density measures from cardiac CT scans – the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16:689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hankinson JL, Kawut SM, Shahar E, Smith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults: the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Chest. 2010;137:138–145. doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MESA manual of operations: field center and laboratory procedures. University of Washington; Seattle: 2008. [cited 2014 Feb]. Available from: http://www.mesa-nhlbi.org/publicDocs/MesaMop/MesaMop1-5-01.doc. [Google Scholar]
- 31.Jiang XC, Paultre F, Pearson TA, et al. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol. 2000;20:2614–2618. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 32.Smith NL, Psaty BM, Heckbert SR, Tracy RP, Cornell ES. The reliability of medication inventory methods compared to serum levels of cardiovascular drugs in the elderly. J Clin Epidemiol. 1999;52:143–146. doi: 10.1016/s0895-4356(98)00141-3. [DOI] [PubMed] [Google Scholar]
- 33.Lederer DJ, Enright PL, Kawut SM, et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Am J Respir Crit Care Med. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burns AR, Takei F, Doerschuk CM. Quantitation of ICAM-1 expression in mouse lung during pneumonia. J Immunol. 1994;153:3189–3198. [PubMed] [Google Scholar]
- 35.Liao JK. Linking endothelial dysfunction with endothelial cell activation. J Clin Invest. 2013;123:540–541. doi: 10.1172/JCI66843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrache I, Natarajan V, Zhen L, et al. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11:491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peinado VI, Barbera JA, Ramirez J, et al. Endothelial dysfunction in pulmonary arteries of patients with mild COPD. Am J Physiol. 1998;274:L908–913. doi: 10.1152/ajplung.1998.274.6.L908. [DOI] [PubMed] [Google Scholar]
- 38.Barr RG, Mesia-Vela S, Austin JH, et al. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the EMphysema and Cancer Action Project (EMCAP) Study. Am J Respir Crit Care Med. 2007;176:1200–1207. doi: 10.1164/rccm.200707-980OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hogg JC, Walker BA. Polymorphonuclear leucocyte traffic in lung inflammation. Thorax. 1995;50:819–820. doi: 10.1136/thx.50.8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soler Artigas M, Loth DW, Wain LV, et al. Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet. 2011;43:1082–1090. doi: 10.1038/ng.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manichaikul A, Hoffman EA, Smolonska J, et al. Genome-wide study of percent emphysema on computed tomography in the general population: the Multi-Ethnic Study of Atherosclerosis Lung/SNP Health Association REsource Study. Am J Respir Crit Care Med. 2014;189:408–418. doi: 10.1164/rccm.201306-1061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Repapi E, Sayers I, Wain LV, et al. Genome-wide association study identifies five loci associated with lung function. Nat Genet. 2010;42:36–44. doi: 10.1038/ng.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong X, Cho MH, Anderson W, et al. Genome-wide association study identifies bicd1 as a susceptibility gene for emphysema. Am J Respir Crit Care Med. 2011;183:43–49. doi: 10.1164/rccm.201004-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eschen O, Christensen JH, Dethlefsen C, Schmidt EB. Cellular Adhesion Molecules in Healthy Subjects: Short Term Variations and Relations to Flow Mediated Dilation. Biomark Insights. 2008;3:57–62. [PMC free article] [PubMed] [Google Scholar]
- 45.Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burkart KM, Enright P, Ahmed F, et al. Dyspnea and measures of subclinical cardiopulmonary disease in a multi-ethnic cohort: the MESA Lung Study. 2010;A2853 [Google Scholar]
- 47.Tager IB. Outcomes in cohort studies. Epidemiol Rev. 1998;20:15–28. doi: 10.1093/oxfordjournals.epirev.a017969. [DOI] [PubMed] [Google Scholar]
- 48.Ahmed FS, Jiang XC, Schwartz JE, et al. Plasma sphingomyelin and longitudinal change in percent emphysema on CT: the MESA Lung Study. Biomarkers. 2014;19:207–13. doi: 10.3109/1354750X.2014.896414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim DK, Cho MH, Hersh CP, et al. Genome-wide association analysis of blood biomarkers in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:1238–1247. doi: 10.1164/rccm.201206-1013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Witkowska AM, Borawska MH. Soluble intercellular adhesion molecule-1 (sICAM-1): an overview. Eur Cytokine Net. 2004;15:91–9851. [PubMed] [Google Scholar]
- 51.Newell JD, Jr., Hogg JC, Snider GL. Report of a workshop: quantitative computed tomography scanning in longitudinal studies of emphysema. Eur Respir J. 2004;23:769–775. doi: 10.1183/09031936.04.00026504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.