Abstract
Both egocentric route-based learning and spatial learning, as assessed by the Cincinnati water maze (CWM) and Morris water maze (MWM), respectively, are impaired following an 80% dopamine (DA) loss in the neostriatum after 6-hydroxydopamine (6-OHDA) administration in rats. The dorsolateral striatum (DLS) and the dorsomedial striatum (DMS) are implicated in different navigational learning types, namely the DLS is implicated in egocentric learning while the DMS is implicated in spatial learning. This experiment tested whether selective DA loss through 6-OHDA lesions in the DMS or DLS would impair one or both types of navigation. Both DLS and DMS DA loss significantly impaired route-based CWM learning, without affecting spatial or cued MWM performance. DLS 6-OHDA lesions produced a 75% DA loss in this region, with no changes in other monoamine levels in the DLS or DMS. DMS 6-OHDA lesions produced a 62% DA loss in this region, without affecting other monoamine levels in the DMS or DLS. The results indicate a role for DA in DLS and DMS regions in route-based egocentric but not spatial learning and memory. Spatial learning deficits may require more pervasive monoamine reductions within each region before deficits are exhibited. This is the first study to implicate DLS and DMS DA in route-based egocentric navigation.
Keywords: dorsolateral striatum, dorsomedial striatum, dopamine, egocentric, allocentric
Introduction
Impairments in navigational ability are present in numerous human disorders where they impair the quality of life and increase dependency (Aguirre and D’Esposito, 1999, Weniger and Irle, 2006, Livingstone and Skelton, 2007, Sanders et al., 2008, Iaria et al., 2009). Successful navigation requires complex interactions among multiple distinct, but parallel cognitive processes that can be subdivided into egocentric (self-oriented path integration and route-based) and allocentric (map-based) way finding. Route-based navigation involves a representation of space connected by “nodes” or choice points representing successive decision points in a grid or pathway (Byrne, 1982, Aguirre and D’Esposito, 1999). In the allocentric process, the navigator’s spatial orientation to distal cues in the environment is fluid and represented in a common coordinate map system external to the navigator (Byrne, 1982, Garber, 2000).
Considerable behavioral, anatomical, and electrophysiological evidence suggests that the neostriatum is an important modulator in both egocentric and allocentric learning (Potegal, 1969, 1972, Whishaw and Dunnett, 1985, Whishaw et al., 1987, Cook and Kesner, 1988, McGeorge and Faull, 1989, Packard et al., 1989, McDonald and White, 1994, Taube, 1998, Devan et al., 1999, Devan and White, 1999, Jog et al., 1999, Ragozzino et al., 2001, Mizumori et al., 2004, Mizumori et al., 2009, Packard, 2009, Braun et al., 2012, Penner and Mizumori, 2012). The neostriatum is a heterogeneous structure with anatomical subregions for different functions. The dorsomedial striatum (DMS) receives primary inputs from multiple sensory and association areas, such as the hippocampus and medial prefrontal cortex, and while lesions in this area have widespread effects, they often produce impairments in allocentric learning (Whishaw et al., 1987, Colombo et al., 1989, McGeorge and Faull, 1989, Devan et al., 1999, Devan and White, 1999). For example, DMS lesions or DMS dopamine (DA) depletion result in allocentric learning and place strategy deficits in the Morris water maze (MWM) and T-maze, respectively (Devan et al., 1999, Devan and White, 1999, Lex et al., 2011). Sensory and motor cortices have major projections to the dorsolateral striatum (DLS), that are associated with egocentric or response learning and stimulus-response habit formation (McGeorge and Faull, 1989, Reading et al., 1991, Packard and McGaugh, 1996, White, 1997, Devan and White, 1999, Yin and Knowlton, 2004, Yin et al., 2004, Palencia and Ragozzino, 2005, Yin and Knowlton, 2006, Yin et al., 2006). However, this heterogeneity of function within the neostriatum may not be fully preserved in regard to egocentric learning. Excitotoxic lesions of the DMS and DLS each result in a severe learning impairment in a 14-unit T-maze procedural learning task, implicating both regions in egocentric learning (Pistell et al., 2009).
The focus of the present experiments was to elucidate the regionally-specific role of neostriatal DA in egocentric and allocentric navigation. DA in the neostriatum influences both glutamatergic afferents and striatal medium spiny neuronal efferents that modulate striatal output (Penner and Mizumori, 2012). Previously, we showed that widespread neostriatal 6-hydroxydopamine (6-OHDA)-induced DA reduction impaired learning in both the allocentric MWM and route-based Cincinnati water maze (CWM) (Braun et al., 2012). While DMS DA has been implicated in allocentric T-maze learning strategy (Lex et al., 2011), it has not been tested for involvement in either route-based or allocentric navigation. Moreover, the role of DA in DLS-mediated route-based or allocentric navigation has yet to be tested. Accordingly, we tested groups of animals given selective 6-OHDA injections in either the DMS or DLS and evaluated them in the CWM and MWM, respectively (test order was examined previously (Broening et al., 2001, Skelton et al., 2009) compared with sham-operated controls. Motivation and swimming ability were assessed to control for potential performance changes not associated with learning.
Methods
Animals
Adult male Sprague-Dawley CD IGS rats (225–250 g at the time of arrival) were purchased from Charles River Laboratories, Raleigh, NC (strain 001). Animals were pair-housed in polypropionate cages (46 × 24 × 20 cm) containing woodchip bedding for at least a 1-week acclimation period prior to surgery. Animals had free access to food and water, were housed in an environmentally controlled vivarium (21 ± 1°C), and were on a 14 h light-dark cycle (lights on at 600 h). All procedures were in compliance with the Institutional Animal Care and Use Committee and the vivarium is fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
Surgery
Rats were anesthetized with 2–4% isoflurane (IsoThesia; Butler Animal Health Supply, Dublin, OH) with continuous administration via a nose cone throughout surgery. Rats were placed in a motorized, computer-controlled stereotaxic apparatus (StereoDrive, Stoelting Co., Wood Dale, IL), and were given bilateral injections of 6-OHDA (Sigma, St. Louis, MO) using a 26 gauge 10 μl Hamilton Gastight syringe (Reno, NV). Coordinates were based on the Paxinos and Watson brain atlas (Paxinos et al., 1985). For the DLS lesions, a volume of 3 μl [4 μg/μl 6-OHDA in 0.2% ascorbic acid saline solution] was injected over 9 min (from bregma: AP: +0.2 mm; ML: ± 3.5 mm; from skull: DV: −4.8 mm), with the needle left in place for 1 min following injection. For the DMS lesions, a volume of 0.4 μl [30 μg/μl] was injected in each site over 4 min (from bregma: AP: +1.0 mm; ML: ± 1.7 mm; DV: −5.0 mm; and AP: −0.4 mm; ML: ± 2.6 mm; DV: −4.5 mm), with the needle left in place for 5 min following completion of injection. Control animals (SHAM) received an identical amount of saline in 0.2% ascorbic acid vehicle (VEH) using the same procedure for its particular group. Following surgery, animals were given 0.1 ml buprenorphine hydrochloride to minimize pain. Animals were allowed to recover for 2 weeks before the beginning of testing. The number of animals represented in each group is given in the figure legends.
Behavioral Testing
Straight Channel
One day prior to CWM testing, animals were tested for swimming ability in a 244 cm long × 15 cm wide × 51 cm high water filled (38 cm deep) straight channel for 4 consecutive trials with a maximum time limit of 2 min/trial (Herring et al., 2008, Vorhees et al., 2008). Straight channel swimming served three functions: (a) to acclimate animals to swimming, (b) to teach that escape was possible by climbing on the submerged platform at the opposite end of the channel, and (c) to determine if animals had comparable swimming ability.
Cincinnati water maze
The CWM is a nine-unit multiple T water maze (21 ± 1°C) as described previously (Vorhees, 1987, Vorhees et al., 1991, Vorhees et al., 2008). Animals had to locate a submerged escape platform; the room was illuminated with infrared lighting in order to eliminate visual cues; a video camera was mounted above the maze sensitive to light in the infrared range and fed to a monitor in another room. Two trials/day (5 min limit/trial) were given. If an animal failed to find the escape within 5 min on trial-1 of each day, there was at least a 5 min intertrial interval (ITI) before trial-2. If they found the escape on trial-1 in less than 5 min, trial-2 was given immediately. Animals reaching the time limit were removed from the maze from wherever they were when the time limit was reached. Latency to escape and number of errors (defined as head and shoulder entry in a stem or arm of a T or reentry into the start channel) were recorded. To correct for animals that stopped searching, they were given an error score equal to the number of errors + 1 made by the animal that found the escape and made the most errors in < 5 min. Animals that never found the platform were removed from analysis. Data for the CWM were analyzed in 2-day (4 trials) blocks similar to the 4-trial blocks used to analyze MWM data.
Morris water maze hidden platform
To test spatial navigational learning, MWM hidden platform testing began the day following CWM completion (Morris, 1981). Animals were placed in a 244 cm diameter tank of water (21 ± 1 °C) and were required to find a submerged platform (10 cm diameter) in a stationary position with pseudo-randomized, balanced cardinal and ordinal start positions. For 6 days, rats were given 4 trials/day with a 2 min trial limit and an ITI of 15 s (on the platform). If a rat failed to find the platform within the time limit, it was placed on the platform. On the 7th day, a 30 s probe trial was given from a novel start position with the platform removed. Data were collected using video tracking software (AnyMaze, Stoelting Co., Wood Dale, IL).
Morris water maze cued
Cued MWM testing began the day following the hidden platform testing and was conducted over two days. A yellow plastic ball was attached to the top of a brass rod mounted in the center of the submerged platform (10 cm diameter) to mark its location. On each day, rats were given 4 trials with the locations of the platform and starting positions randomized (2 min trial limit with an ITI of 15 s on the platform + 15–20 s to reposition the platform). Latency was recorded (AnyMaze could not track rats under these lighting conditions).
Tissue Collection
Tissue collection took place following the completion of testing. Animals were brought to an adjacent suite and decapitated. Brains were removed and the neostriatum dissected and further segmented into the DMS and DLS. Brain regions were rapidly frozen for later monoamine assay as described (Williams et al., 2007).
Monoamine assays
Monoamines were assayed via high performance liquid chromatography with electrochemical detection (HPLC-ECD). Frozen tissues were weighed, thawed, and sonicated in appropriate volumes of 0.1 N perchloric acid (Fisher Scientific, Pittsburgh, PA). Samples were centrifuged for 14 min at 13,000 RCF at 4°C. The supernatant sample was transferred to a new vial for injection on a Supelco Supelcosil™ LC-18 column (150 × 4.6 mm, 3 μm; Sigma-Aldrich Co., St. Louis, MO). The HPLC system consisted of a Waters 717 plus autosampler (Waters Corp., Milford, MA), ESA 584 pump, and Coulochem III electrochemical detector. The potential settings were −150 mV for E1 and +250 mV for E2, with a guard cell potential set at +350 mV. MD-TM mobile phase (ESA, Inc.) was used and consisted of 75 mM sodium dihydrogen phosphate (monohydrate), 1.7 mM 1-octanesulfonic acid sodium salt, 100 μl/l triethylamine, 25 μM EDTA, and 10% acetonitrile, with a final pH of 3.0. The pump flow rate was set at 0.7 ml/min, and the samples were run at 28°C. Standards for DA, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), norepinephrine (NE), 5-HT, and 5-hydroxyindoleacetic acid (5-HIAA) (all obtained from Sigma-Aldrich Co., St. Louis, MO) were prepared in 0.1 N perchloric acid. All neurotransmitters were run on a single chromatogram.
Immunohistochemistry
Using the same surgical procedures as in behavioral testing, a different set of rats were given a unilateral injection of 6-OHDA in the DLS (N = 3) or DMS (N = 3) and a VEH injection on the contralateral side using the coordinates described. Two weeks after surgery the animals were brought into an adjacent suite, perfused transcardially with 4% paraformaldehyde, and the brains dissected, postfixed, and sunk in sucrose overnight. Brains were sectioned (at 30-μm thickness) on a microtome, and the free-floating sections processed for tyrosine hydroxylase (TH) immunohistochemistry as previously described (Hemmerle et al., 2014), using mouse monoclonal anti-TH primary antibody (MAB318, diluted 1:8000; EMD Millipore, Telecuma, CA), biotinylated horse anti-mouse IgG secondary antibody (BA-2000, diluted 1:200; Vector Laboratories, Burlingame, CA), and ABC Elite Kit reagents (Vector Laboratories) with diaminobenzidine as chromagen. Neostriatal immunostaining for TH was analyzed for the regional specificity of 6-OHDA injections as indicated by TH depletion in the DMS or DLS. Sections were viewed and scanned at 20X on an Aperio AT2 slide scanner and uploaded to Aperio eSlide Manager (Leica Biosystems, Buffalo Grove, IL).
Statistical Analysis
DLS and DMS groups were tested separately, therefore data from each experiment were analyzed independently. Data were analyzed using mixed linear ANOVA models (SAS Proc Mixed, SAS Institute 9.2, Cary, NC). The covariance matrix for each dataset was checked using best fit statistics. In most cases, the best fit was to the autoregressive-1 covariance structure. Kenward-Rodger adjusted degrees of freedom were used. Measures taken repetitively on the same animal, such as day or block, were within-subject factors. Significant interactions were analyzed using simple-effect slice ANOVAs at each level of the repeated measure factor. Biochemical data were analyzed using two-tailed t-tests. Significance was set at p ≤ 0.05. Data are presented as least square (LS) mean ± LS SEM.
Results
Immunohistochemistry
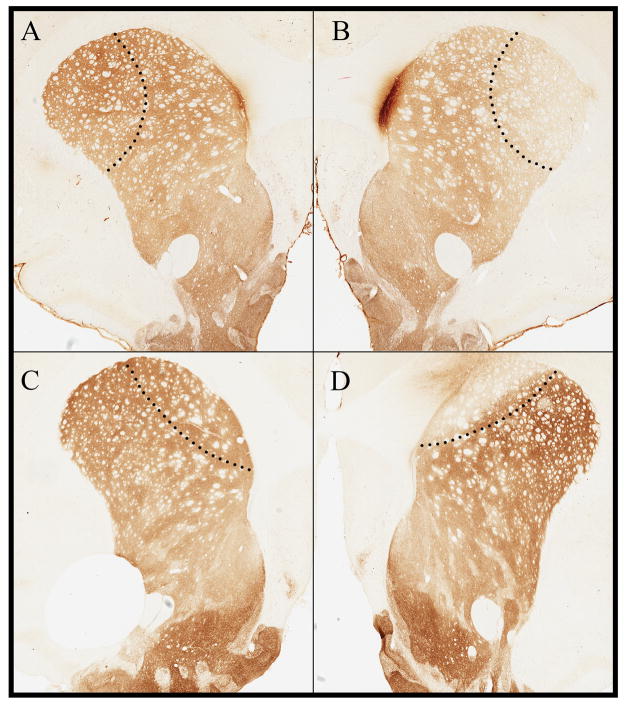
Representative sections from one animal that received a DLS or DMS lesion are shown (Fig 1). Unilateral 6-OHDA injection in the DLS resulted in DLS-specific loss of TH immunostaining (Fig 1B). No reduction of striatal TH immunoreactivity was observed in the contralateral DLS with VEH injection (Fig 1A).
Figure 1. Immunohistochemistry.
VEH injections in the DLS (A) compared with contralateral DLS 6-OHDA injections (B). DLS 6-OHDA selectively destroyed TH neurons in the DLS. TH loss was limited to the DMS following unilateral 6-OHDA injections in the DMS (D) compared with contralateral DMS VEH injections (C).
The striatal regional specificity of the DMS injections of 6-OHDA was also confirmed by TH immunostaining. Unilateral 6-OHDA injections in the DMS resulted in DMS-specific loss of TH immunostaining (Fig 1D) compared with the contralateral DMS injected with VEH (Fig 1C).
Dorsolateral Striatal 6-OHDA Lesions
Straight Channel
No difference in time to swim the straight channel was observed across trials between DLS 6-OHDA-treated and SHAM animals (LS mean ± LS SEM across trials: 6-OHDA: 13.8 ± 1.1 s; SHAM: 12.3 ± 1.2 s).
Cincinnati water maze
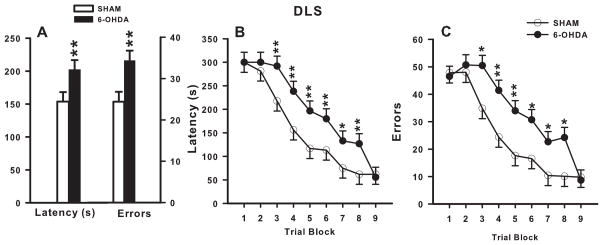
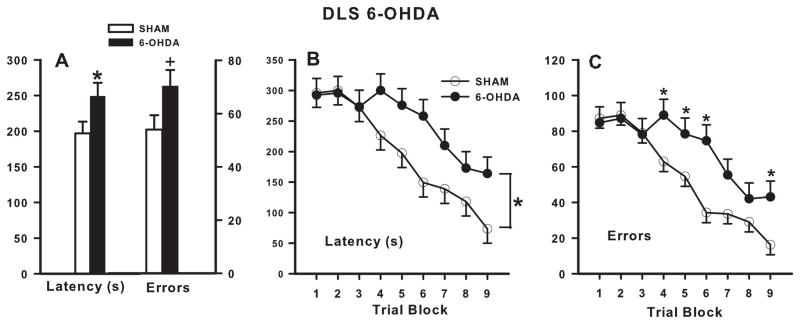
6-OHDA-treated animals had significantly increased latencies to find the platform compared with SHAM animals (F (1, 37.7) = 5.75, p ≤ 0.05; Fig 2A) with significantly longer latencies observed from block-3 through block-8 (treatment x block: F(8, 177) = 2.18, p ≤ 0.05; Fig 2B). DLS 6-OHDA-treated animals committed significantly more errors compared with SHAM animals (F(1,39) = 6.56, p ≤ 0.05; Fig 2A) with significantly more errors observed during block-3 through block-6 and block-8 (treatment x block: F(8,177) = 2.24, p ≤ 0.05; Fig 2C).
Figure 2. DLS Cincinnati water maze latency and errors.
There was a main effect of lesion: DLS lesioned animals had a longer latency and made more errors than SHAMs (A). Across days, DLS lesioned animals had significantly longer latencies during blocks 3–8 (B) and made significantly more errors during blocks 3–6 and block 8, compared with SHAMs. N = 12/group. *p ≤ 0.05, **p ≤ 0.01.
Morris water maze
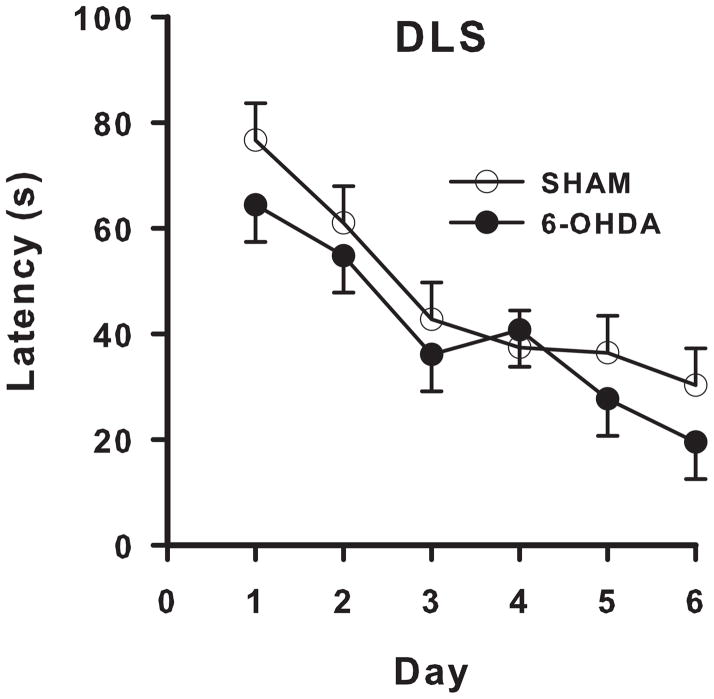
6-OHDA-treated animals showed no difference in MWM performance compared with SHAM animals. No significant difference was found in latency to find the platform (Fig 3), path length, or cumulative distance to the platform. Swim speed did not differ between groups (6-OHDA: 0.41 ± 0.07 m/s; SHAM: 0.47 ± 0.09 m/s). Initial heading error and average heading error were not significantly different between 6-OHDA-treated animals and SHAM controls. During the probe trial, 6-OHDA-treated animals were not affected on the number of platform crossovers (6-OHDA: 0.66 ± 0.28; SHAM: 0.5 ± 0.26) or average distance from the platform site compared with SHAM animals (6-OHDA: 0.83 ± 0.06 m; SHAM: 0.85 ± 0.06 m). For cued platform trials, there was no significant latency difference between 6-OHDA-treated animals and SHAM controls (averaged across days and trials: 6-OHDA: 28.76 ± 3.31 s; SHAM: 23.48 ± 3.54 s).
Figure 3. DLS Morris water maze latency.
6-OHDA lesions in the DLS did not have a significant effect on latency (main effect or day x lesion interaction) in the MWM to find the hidden platform compared with SHAMs.
Monoamine Assessment
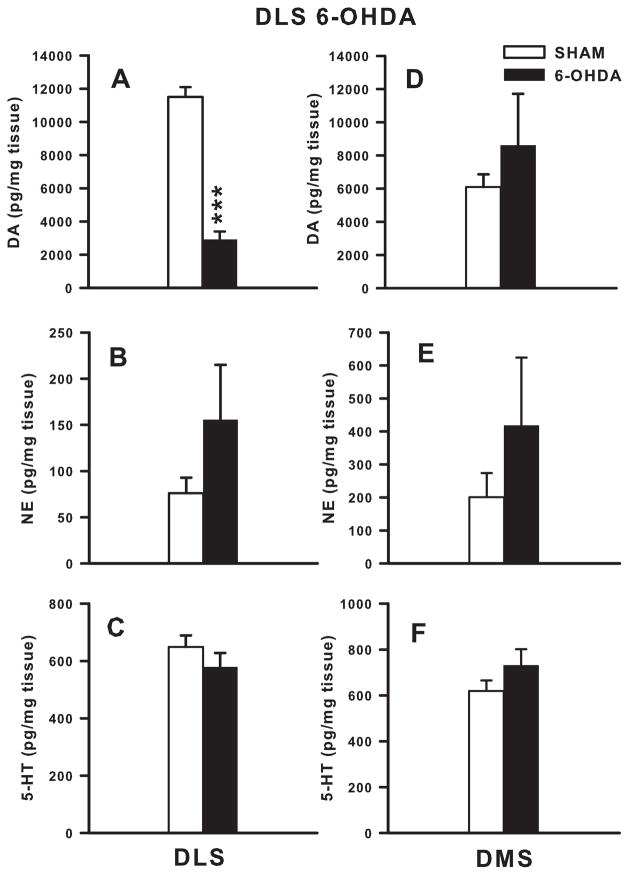
6-OHDA injection caused a 75% decrease in DLS DA compared with SHAM animals (t(22) = 11.2, p ≤ 0.001; Fig 4A) with significant decreases in DA metabolites (DOPAC: t(22) = 6.36, p ≤ 0.001; HVA = t(20) = 6.06, p ≤ 0.001) and utilization ratios (DOPAC/DA = t(22) = 5.38, p ≤ 0.001; overall turnover ratio: t(20) = 3.10, p ≤ 0.001) (Table 1). NE and 5-HT levels in the DLS were not altered in 6-OHDA-treated animals compared with SHAM controls (Fig 4B and C, respectively). To determine if DLS 6-OHDA injections affected the DMS this region was also analyzed. DA concentrations (Fig 4D), metabolites and turnover in the DMS after 6-OHDA DLS injection were not significantly different compared with SHAM animals. NE (Fig 4E) and 5-HT (Fig 4F) levels were also not different.
Figure 4. DLS Monoamine levels.
6-OHDA lesions in the DLS significantly decreased DA levels in the DLS by 75% (A), with no change in NE (B), or 5-HT (C) DLS levels, compared with SHAMS. DMS DA (D), NE (E), and 5-HT (F) levels were not altered following DLS DA depletion. ***p ≤ 0.001.
Table 1.
Dorsolateral striatal (DLS) dopamine metabolite levels (3, 4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA)) and turnover rate after DLS 6-OHDA injection
| DLS Lesion | DOPAC (pg/mg) | HVA (pg/mg) | DOPAC/DA | HVA/DA | (DOPAC + HVA)/DA |
|---|---|---|---|---|---|
| 6-OHDA | 499.9 ± 92.4*** | 232.4 ± 49.7*** | 0.18 ± 0.01*** | 0.08 ± 0.01 | 0.25 ± 0.02** |
| SHAM | 1308.2 ± 85.9 | 721.7 ± 60.8 | 0.11 ± 0.004 | 0.06 ± 0.005 | 0.17 ± 0.008 |
p ≤ 0.01;
p ≤ 0.001
Dorsomedial Striatal 6-OHDA Lesions
Straight Channel
No difference in time to swim the straight channel was observed between 6-OHDA-treated animals and SHAM controls (LS mean ± LS SEM across trials: 6-OHDA: 18.53 ± 2.52 s; SHAM: 17.33 ± 2.22 s).
Cincinnati water maze
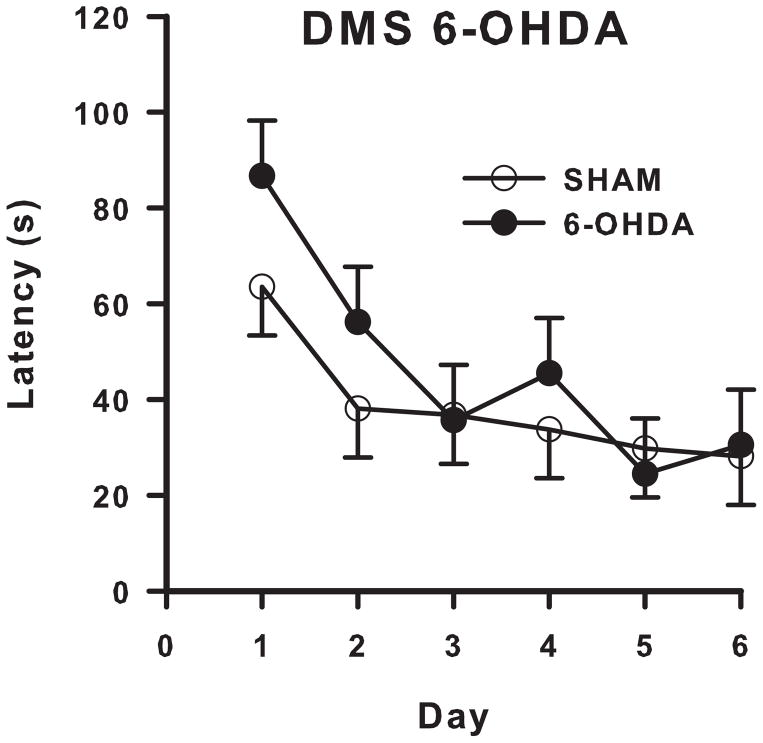
6-OHDA-treated rats had increased latency to find the platform compared with SHAM animals (F(1,19.4) = 4.36, p ≤ 0.05; Fig 5A), but the treatment x block interaction was not significant (Fig 5B). A trend towards significantly more errors overall in 6-OHDA-treated animals was also seen (F(1, 19.8) = 4.04, p ≤ 0.10; Fig 5A), however on blocks 4–6 and block 9 lesioned animals made significantly more errors (treatment x block: F(8, 109) = 2.59, p ≤ 0.05; Fig 5C) compared with SHAM control animals.
Figure 5. DMS Cincinnati water maze latency and errors.
(A) 6-OHDA treated animals in the DMS had significantly longer latencies to find the platform, and a trend toward significantly making more errors compared with controls over the duration of testing. DMS lesioned animals had significantly longer latencies than SHAMs that were unaffected by time (B), but made significantly more errors than SHAM controls on blocks 4–6 and block 9 (C). N = 7/6-OHDA; 9/SHAM. +p ≤ 0.1, *p ≤ 0.05
Morris water maze
6-OHDA-treated animals showed no difference in MWM performance compared with SHAM animals. No significant differences were found in latency to reach the platform (Fig 6), path length, or cumulative distance to the platform. Swim speed was not significantly different between the groups (6-OHDA: 0.29 ± 0.01 m/s; SHAM: 0.28 ± 0.01 m/s). Initial and average heading errors were not significantly altered. During the probe trial, 6-OHDA-treated rats showed no significant effect on the number of platform crossings (6-OHDA: 1.00 ± 0.58; SHAM: 1.00 ± 0.38), or on average distance from the platform site (6-OHDA: 0.93 ± 0.06 m; SHAM: 0.73 ± 0.07 m; t(13) = 2.07, p ≤ 0.10). For cued platform trials, there was no significant latency difference between 6-OHDA-treated animals and SHAM animals (averaged across days and trials: 6-OHDA: 27.66 ± 9.30 s; SHAM: 36.27 ± 8.21 s).
Figure 6. DMS Morris water maze latency.
6-OHDA lesions in the DMS did not have a significant effect on latency (main effect or day x lesion interaction) to find the hidden platform in the MWM compared with SHAMs.
Monoamine Assessment
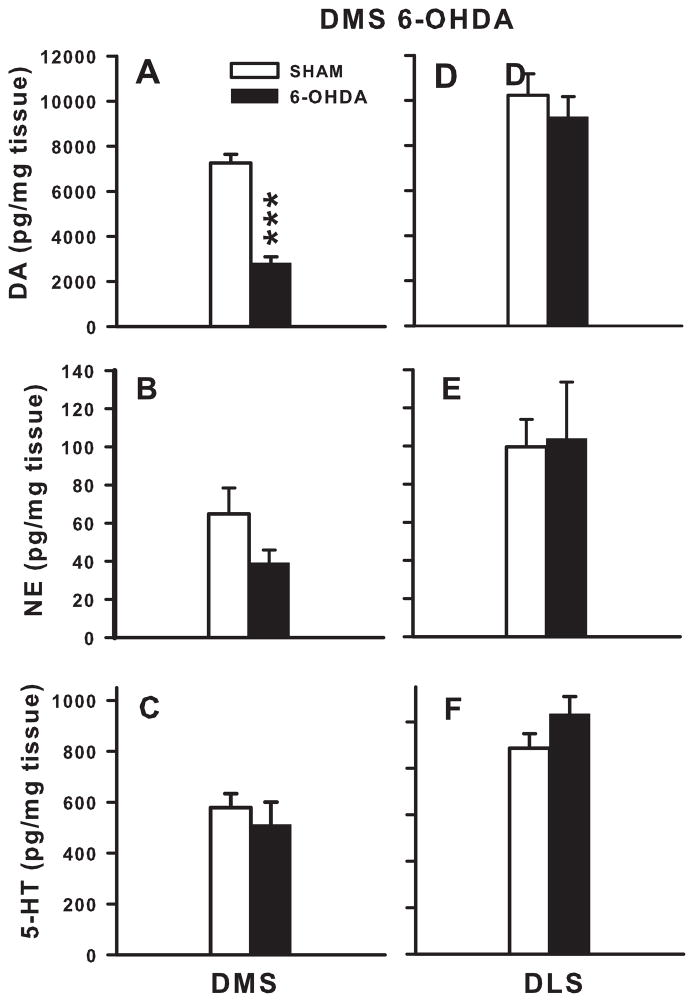
Following 6-OHDA injection in the DMS, DA concentrations were decreased 62% compared with SHAM controls (t(14) = 8.87, p ≤ 0.001; Fig 7A). 6-OHDA injection in the DMS also decreased DA metabolites (DOPAC: t(14) = 3.86, p ≤ 0.01; HVA: t(14) = 2.74, p ≤ 0.05) and increased turnover (DOPAC/DA: t(14) = 2.42, p ≤0.01; HVA/DA: t(14) = 5.29, p ≤ 0.001; overall turnover: t(14) = 3.47, p ≤ 0.01) compared with SHAM controls (Table 2). DMS NE (Fig 7B) and 5-HT (Fig 7C) were not altered following DMS 6-OHDA injection compared with SHAM injections.
Figure 7. DMS Monoamine levels.
6-OHDA injection in the DMS significantly decreased DA levels in the DMS by 62% (A), with no change in NE (B), or 5-HT (C) DMS levels compared with SHAMS. DLS DA (D), NE (E), and 5-HT (F) levels were not altered following DMS 6-OHDA lesions. ***p ≤ 0.001.
Table 2.
Dorsomedial striatal (DMS) dopamine metabolite levels (3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA)) and turnover rate after DMS 6-OHDA injection
| DMS Lesion | DOPAC (pg/mg) | HVA (pg/mg) | DOPAC/DA | HVA/DA | (DOPAC + HVA)/DA |
|---|---|---|---|---|---|
| 6-OHDA | 695.4 ± 111.8 ** | 346.9 ± 29.9 * | 0.25 ± 0.03 * | 0.12 ± 0.01 *** | 0.38 ± 0.04 ** |
| SHAM | 1324.8 ± 114.2 | 482.2 ± 36.7 | 0.18 ± 0.01 | 0.06 ± 0.004 | 0.24 ± 0.01 |
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001
DA concentrations following 6-OHDA DMS injection were not significantly different in the DLS compared with SHAM controls (Fig 7D). DA metabolites and turnover and NE (Fig 7E) and 5-HT (Fig 7F) were not significantly altered in the DLS following 6-OHDA DMS injection compared with SHAM controls.
Discussion
6-OHDA injections in the DLS reduced DA levels by 75% and resulted in CWM route-based navigation deficits, but had no effect on hidden platform allocentric learning in the MWM. DMS DA depletion of 62% also resulted in route-based CWM navigational deficits, without altering MWM-based allocentric learning. These deficits were independent of motivational or motoric impairments (no differences in straight channel, cued platform MWM, or swim speed in the MWM). The DMS has been implicated in other behaviors, such as modulation of expected reward value, initiation behavior, and goal-directed behavior (White, 1997, Calaminus and Hauber, 2009, Mizumori et al., 2009, Penner and Mizumori, 2012, Fouquet et al., 2013, Kim et al., 2013). These other processes are likely not contributing to the egocentric impairment seen in the present study as the other tested behaviors were unaltered. For each of the striatal subregions, the 6-OHDA injection damage was limited to the targeted area, precluding potential effects from the other region to explain the route-based navigational deficits. NE and 5-HT were not altered, regardless of which striatal subregion was lesioned, leaving DA loss to account for the observed learning impairments. It is unlikely that brain regions outside of the neostriatum were involved, as whole neostriatal DA loss has not been shown to affect monoamine levels in other brain regions associated with learning (Braun et al., 2012). The observed changes in DA metabolites and turnover are consistent with what others have found for these regions (Henze et al., 2005, Chen et al., 2007, Aguiar et al., 2008, Tadaiesky et al., 2008, Braun et al., 2012).
The neostriatum has long been associated with egocentric learning (Potegal, 1969, 1972, Cook and Kesner, 1988, Packard et al., 1989, Packard and McGaugh, 1996, White, 1997, Taube, 1998, Devan et al., 1999, Devan and White, 1999, Packard and Knowlton, 2002, White and McDonald, 2002, Mizumori et al., 2004, Yin and Knowlton, 2004, Yin et al., 2004, Mizumori et al., 2005, Palencia and Ragozzino, 2005, Yin and Knowlton, 2006, Yin et al., 2006, Packard, 2009, Braun et al., 2012, Penner and Mizumori, 2012). While a separation of function between the DMS and DLS in regard to allocentric learning tasks has been observed, both regions have independently been implicated in egocentric learning. Glutamate in the DLS has a modulatory role in egocentric response learning in a T-maze and post-training silencing of the DLS inhibits egocentric response (Packard and McGaugh, 1996, Palencia and Ragozzino, 2005). Recently, Etienne et al. showed that pharmacological inhibition of the DMS reduced route-based (direction-based) learning, but not allocentric or cued learning in rhesus macaques (Etienne et al., 2012). Excitotoxic lesions of either the DMS or DLS impaired procedural learning in a 14-unit T-maze (Pistell et al., 2009). While both Pistell et al. and this study implicate the DMS and DLS in complex egocentric learning, the 14-unit T-maze and the CWM have several differences that distinguish the CWM as a test of route-based egocentric navigation rather than a task of procedural memory as is the 14 unit T-maze. For example, in the CWM there are no spatial cues available, it uses water as the motivator, and animals are not forced into making a left-right response choice during navigation. The 14 unit T-maze has spatial cues available, uses shock for the motivator, guillotine doors close off previously visited arms, and there is a forced left-right response to navigate correctly. Testing in the CWM lasts for 18 days (2 trials/day) allowing animals to demonstrate long-term learning and memory ability, whereas testing in the 14 unit T-maze lasts for 1 day with 15 trials.
How DA signaling in the DMS and DLS modulates egocentric learning is currently unknown. The DMS and DLS possess both allocentric place cells and neurons that fire only to specific egocentric response movements such as turns, forward movement, and head direction (Wiener, 1993, Lavoie and Mizumori, 1994, Ragozzino et al., 2001). The egocentric response cells in the DLS and/or DMS could be influenced by DA projections and compromised following DA loss. Striatal DA could also be influencing egocentric learning through its direct regulation of glutamatergic input to medium spiny neurons, inputs that are necessary for initial egocentric learning in the DLS (Sesack et al., 2003, Palencia and Ragozzino, 2005).
The finding that allocentric learning was spared following DLS DA loss is consistent with the literature that shows this area is not necessary for this type of learning, regardless of lesion type (Devan et al., 1999, Yin and Knowlton, 2004, Yin et al., 2006, Mizumori et al., 2009, Packard, 2009). Electrolytic and excitotoxic lesions of the DMS cause deficits in hidden platform MWM learning (Devan et al., 1999, Devan and White, 1999). DA in the posterior DMS has been implicated in place learning, however not specifically in MWM-based allocentric learning (Lex et al., 2011). In the latter study, when given a choice between solving a T-maze using an egocentric response strategy or an allocentric place strategy subsequent to posterior DMS DA depletion, a significantly higher proportion of animals utilized an egocentric response (83%) compared with SHAM controls (50%) early in testing. During later phases of learning no differences were observed between groups. As it is more common for animals to utilize a place strategy during early training and transition into the response strategy following continued training, the inference was that DA-depleted animals exhibited a deficit in allocentric performance during the phase of acquisition when place learning normally dominates. No overall learning deficit was observed in that both groups learned the task; only the strategy used initially differed.
Differences between mazes may explain the lack of effect in the current study for place learning compared with the Lex et al. (2011) study. The T-maze is more rudimentary than the CWM, making it easier to solve. While the T-maze gives a choice between two strategies, the CWM and MWM are configured such that only one strategy or the other is effective. The CWM is tested under infrared light eliminating spatial cues, and animals do not develop an egocentric learning strategy in the MWM using the testing protocol herein (Morris, 1981). While animals in the Lex et al. (2011) study resorted to a response strategy over a place learning strategy in the T-maze, animals in the present study learned at the same rate as controls when given only the option of allocentric learning in the MWM, but had deficits when given only the option of egocentric learning in the CWM. Because the CWM is a more complex egocentric learning task, it was able to uncover the involvement of DA in the DMS for this type of learning.
It is unlikely that greater DA loss would have resulted in a MWM allocentric learning impairment. Allocentric learning deficits in the MWM require a threshold of about 60% neostriatal DA depletion (Whishaw and Dunnett, 1985, Lindner et al., 1999, Miyoshi et al., 2002, Da Cunha et al., 2003, Mura and Feldon, 2003, De Leonibus et al., 2007, Braun et al., 2012), a level of reduction exceeded in the present experiments. Since DA loss in the DLS and DMS lesioned groups surpassed this level of reduction, it suggests that more widespread neostriatum DA loss is necessary before allocentric learning deficits are observed rather than greater subregional loss. In agreement with this, genetically DA-deficient mice unable to show allocentric MWM learning exhibit a restoration of learning following DA supplementation to either the DMS or DLS; this also indicates that allocentric learning does not depend on DA signaling in a single striatal subregion (Darvas and Palmiter, 2010). Taken together with previous studies, it appears that allocentric learning deficits following DMS lesions require a lesion of more than DA alone.
Limitations to the present study include: attempts to deplete DA further without causing 6-OHDA damage to other regions were unsuccessful; that we tested only CWM egocentric learning and it is possible that other tasks might show different effects; that navigation is undoubtedly the product of complex interactions among different neurotransmitters and receptors in different regions, such that isolating only the role of DA in two neostriatal subregions is necessarily artificial; and test order may have contributed to the findings since there may have been positive transfer from the CWM to the MWM that, if it occurred, would have benefited MWM performance and reduced apparent effects on allocentric navigation. This is unlikely though, as mice lacking neostriatal DA are impaired in strategy-switching, and excitotoxic lesions of the DMS increase perseverative behavior in both rats and marmoset monkeys (Rogers et al., 2001, Clarke et al., 2008, Castane et al., 2010, Darvas and Palmiter, 2010). Future studies are needed, however, to further clarify each of these points.
While neostriatal DA has been shown to be involved in egocentric navigation (Anguiano-Rodriguez et al., 2007, Braun et al., 2012), this is the first experiment to implicate both DLS and DMS DA as modulatory factors in egocentric route-based navigation. This study is also the first to directly implicate the DMS in route-based learning. Taken together with our previous data where DA was depleted throughout the neostriatum (Braun et al., 2012), the findings support the view that neostriatal DA involvement in allocentric learning requires contributions from both the DLS and DMS. Conversely, the DLS and DMS can each influence egocentric learning independently.
Highlights.
Egocentric and allocentic learning investigated after dorsolateral or dorsomedial striatal 6-OHDA lesions.
Egocentric learning in the Cincinnati water maze was compromised regardless of region lesioned.
Spatial learning in the Morris water maze was unaffected.
The results suggest that dopamine modulates egocentric learning in discrete regions of the striatum.
Acknowledgments
This work was supported by NIH project grant ES015689 and training grant ES07051 and the Gardner Family Center for Parkinson’s Disease and Movement Disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aguiar LM, Macedo DS, Vasconcelos SM, Oliveira AA, de Sousa FC, Viana GS. CSC, an adenosine A(2A) receptor antagonist and MAO B inhibitor, reverses behavior, monoamine neurotransmission, and amino acid alterations in the 6-OHDA-lesioned rats. Brain Res. 2008;1191:192–199. doi: 10.1016/j.brainres.2007.11.051. [DOI] [PubMed] [Google Scholar]
- Aguirre GK, D’Esposito M. Topographical disorientation: a synthesis and taxonomy. Brain. 1999;122 (Pt 9):1613–1628. doi: 10.1093/brain/122.9.1613. [DOI] [PubMed] [Google Scholar]
- Anguiano-Rodriguez PB, Gaytan-Tocaven L, Olvera-Cortes ME. Striatal serotonin depletion facilitates rat egocentric learning via dopamine modulation. Eur J Pharmacol. 2007;556:91–98. doi: 10.1016/j.ejphar.2006.10.042. [DOI] [PubMed] [Google Scholar]
- Braun AA, Graham DL, Schaefer TL, Vorhees CV, Williams MT. Dorsal striatal dopamine depletion impairs both allocentric and egocentric navigation in rats. Neurobiol Learn Mem. 2012;97:402–408. doi: 10.1016/j.nlm.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broening HW, Morford LL, Inman-Wood SL, Fukumura M, Vorhees CV. 3,4-methylenedioxymethamphetamine (ecstasy)-induced learning and memory impairments depend on the age of exposure during early development. J Neurosci. 2001;21:3228–3235. doi: 10.1523/JNEUROSCI.21-09-03228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne RW. Normality and pathology in cognitive functions. London: Academic; 1982. Geographical knowledge and orientation; pp. 239–264. [Google Scholar]
- Calaminus C, Hauber W. Modulation of behavior by expected reward magnitude depends on dopamine in the dorsomedial striatum. Neurotox Res. 2009;15:97–110. doi: 10.1007/s12640-009-9009-1. [DOI] [PubMed] [Google Scholar]
- Castane A, Theobald DE, Robbins TW. Selective lesions of the dorsomedial striatum impair serial spatial reversal learning in rats. Behav Brain Res. 2010;210:74–83. doi: 10.1016/j.bbr.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Jing FC, Li CL, Tu PF, Zheng QS, Wang ZH. Echinacoside prevents the striatal extracellular levels of monoamine neurotransmitters from diminution in 6-hydroxydopamine lesion rats. J Ethnopharmacol. 2007;114:285–289. doi: 10.1016/j.jep.2007.07.035. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Robbins TW, Roberts AC. Lesions of the medial striatum in monkeys produce perseverative impairments during reversal learning similar to those produced by lesions of the orbitofrontal cortex. J Neurosci. 2008;28:10972–10982. doi: 10.1523/JNEUROSCI.1521-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo PJ, Davis HP, Volpe BT. Allocentric spatial and tactile memory impairments in rats with dorsal caudate lesions are affected by preoperative behavioral training. Behav Neurosci. 1989;103:1242–1250. doi: 10.1037//0735-7044.103.6.1242. [DOI] [PubMed] [Google Scholar]
- Cook D, Kesner RP. Caudate nucleus and memory for egocentric localization. Behav Neural Biol. 1988;49:332–343. doi: 10.1016/s0163-1047(88)90338-x. [DOI] [PubMed] [Google Scholar]
- Da Cunha C, Wietzikoski S, Wietzikoski EC, Miyoshi E, Ferro MM, Anselmo-Franci JA, Canteras NS. Evidence for the substantia nigra pars compacta as an essential component of a memory system independent of the hippocampal memory system. Neurobiol Learn Mem. 2003;79:236–242. doi: 10.1016/s1074-7427(03)00008-x. [DOI] [PubMed] [Google Scholar]
- Darvas M, Palmiter RD. Restricting dopaminergic signaling to either dorsolateral or medial striatum facilitates cognition. J Neurosci. 2010;30:1158–1165. doi: 10.1523/JNEUROSCI.4576-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leonibus E, Pascucci T, Lopez S, Oliverio A, Amalric M, Mele A. Spatial deficits in a mouse model of Parkinson disease. Psychopharmacology (Berl) 2007;194:517–525. doi: 10.1007/s00213-007-0862-4. [DOI] [PubMed] [Google Scholar]
- Devan BD, McDonald RJ, White NM. Effects of medial and lateral caudate-putamen lesions on place- and cue-guided behaviors in the water maze: relation to thigmotaxis. Behav Brain Res. 1999;100:5–14. doi: 10.1016/s0166-4328(98)00107-7. [DOI] [PubMed] [Google Scholar]
- Devan BD, White NM. Parallel information processing in the dorsal striatum: relation to hippocampal function. J Neurosci. 1999;19:2789–2798. doi: 10.1523/JNEUROSCI.19-07-02789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne S, Guthrie M, Gross C, Boraud T. Dissociation of spatial navigation strategies in primates by inhibiting various territories of the striatum. Society for Neuroscience; 2012. p. Abstracts 38. [Google Scholar]
- Fouquet C, Babayan BM, Watilliaux A, Bontempi B, Tobin C, Rondi-Reig L. Complementary Roles of the Hippocampus and the Dorsomedial Striatum during Spatial and Sequence-Based Navigation Behavior. PloS one. 2013;8:e67232. doi: 10.1371/journal.pone.0067232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber PA. Evidence for the use of spatial, temporal, and social information by some primate foragers. In: Boinski SB, Garber PA, editors. On the move: how and why animals travel in groups. Chicago: University of Chicago Press; 2000. pp. 261–298. [Google Scholar]
- Hemmerle AM, Dickerson JW, Herman JP, Seroogy KB. Stress exacerbates experimental Parkinson’s disease. Molecular psychiatry. 2014;19:638–640. doi: 10.1038/mp.2013.108. [DOI] [PubMed] [Google Scholar]
- Henze C, Earl C, Sautter J, Schmidt N, Themann C, Hartmann A, Oertel WH. Reactive oxidative and nitrogen species in the nigrostriatal system following striatal 6-hydroxydopamine lesion in rats. Brain Res. 2005;1052:97–104. doi: 10.1016/j.brainres.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. Effect of +-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology (Berl) 2008;199:637–650. doi: 10.1007/s00213-008-1183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G, Palermo L, Committeri G, Barton JJ. Age differences in the formation and use of cognitive maps. Behav Brain Res. 2009;196:187–191. doi: 10.1016/j.bbr.2008.08.040. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Kim H, Lee D, Jung MW. Signals for previous goal choice persist in the dorsomedial, but not dorsolateral striatum of rats. J Neurosci. 2013;33:52–63. doi: 10.1523/JNEUROSCI.2422-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie AM, Mizumori SJ. Spatial, movement- and reward-sensitive discharge by medial ventral striatum neurons of rats. Brain Res. 1994;638:157–168. doi: 10.1016/0006-8993(94)90645-9. [DOI] [PubMed] [Google Scholar]
- Lex B, Sommer S, Hauber W. The role of dopamine in the dorsomedial striatum in place and response learning. Neuroscience. 2011;172:212–218. doi: 10.1016/j.neuroscience.2010.10.081. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Cain CK, Plone MA, Frydel BR, Blaney TJ, Emerich DF, Hoane MR. Incomplete nigrostriatal dopaminergic cell loss and partial reductions in striatal dopamine produce a kinesia, rigidity, tremor and cognitive deficits in middle-aged rats. Behav Brain Res. 1999;102:1–16. doi: 10.1016/s0166-4328(98)00160-0. [DOI] [PubMed] [Google Scholar]
- Livingstone SA, Skelton RW. Virtual environment navigation tasks and the assessment of cognitive deficits in individuals with brain injury. Behav Brain Res. 2007;185:21–31. doi: 10.1016/j.bbr.2007.07.015. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 1994;61:260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Miyoshi E, Wietzikoski S, Camplessei M, Silveira R, Takahashi RN, Da CC. Impaired learning in a spatial working memory version and in a cued version of the water maze in rats with MPTP-induced mesencephalic dopaminergic lesions. Brain Res Bull. 2002;58:41–47. doi: 10.1016/s0361-9230(02)00754-2. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Canfield JG, Yeshenko O. Parallel and interrelated neural systems underlying adaptive navigation. Integr Comp Biol. 2005;45:547–554. doi: 10.1093/icb/45.3.547. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Puryear CB, Martig AK. Basal ganglia contributions to adaptive navigation. Behav Brain Res. 2009;199:32–42. doi: 10.1016/j.bbr.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Yeshenko O, Gill KM, Davis DM. Parallel processing across neural systems: implications for a multiple memory system hypothesis. Neurobiol Learn Mem. 2004;82:278–298. doi: 10.1016/j.nlm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Morris RG. Spatial Localization Does Not Require the Presence of Local Cues. Learning and Motivation. 1981;12:239–260. [Google Scholar]
- Mura A, Feldon J. Spatial learning in rats is impaired after degeneration of the nigrostriatal dopaminergic system. Mov Disord. 2003;18:860–871. doi: 10.1002/mds.10472. [DOI] [PubMed] [Google Scholar]
- Packard MG. Exhumed from thought: basal ganglia and response learning in the plus-maze. Behav Brain Res. 2009;199:24–31. doi: 10.1016/j.bbr.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Packard MG, Hirsh R, White NM. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: evidence for multiple memory systems. J Neurosci. 1989;9:1465–1472. doi: 10.1523/JNEUROSCI.09-05-01465.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Knowlton BJ. Learning and memory functions of the Basal Ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem. 1996;65:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The contribution of NMDA receptors in the dorsolateral striatum to egocentric response learning. Behav Neurosci. 2005;119:953–960. doi: 10.1037/0735-7044.119.4.953. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- Penner MR, Mizumori SJ. Neural systems analysis of decision making during goal-directed navigation. Prog Neurobiol. 2012;96:96–135. doi: 10.1016/j.pneurobio.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Nelson CM, Miller MG, Spangler EL, Ingram DK, Devan BD. Striatal lesions interfere with acquisition of a complex maze task in rats. Behav Brain Res. 2009;197:138–143. doi: 10.1016/j.bbr.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potegal M. Role of the caudate nucleus in spatial orientation of rats. J Comp Physiol Psychol. 1969;69:756–764. doi: 10.1037/h0028168. [DOI] [PubMed] [Google Scholar]
- Potegal M. The caudate nucleus egocentric localization system. Acta Neurobiol Exp(Wars) 1972;32:479–494. [PubMed] [Google Scholar]
- Ragozzino KE, Leutgeb S, Mizumori SJ. Dorsal striatal head direction and hippocampal place representations during spatial navigation. Exp Brain Res. 2001;139:372–376. doi: 10.1007/s002210100795. [DOI] [PubMed] [Google Scholar]
- Reading PJ, Dunnett SB, Robbins TW. Dissociable roles of the ventral, medial and lateral striatum on the acquisition and performance of a complex visual stimulus-response habit. Behav Brain Res. 1991;45:147–161. doi: 10.1016/s0166-4328(05)80080-4. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Baunez C, Everitt BJ, Robbins TW. Lesions of the medial and lateral striatum in the rat produce differential deficits in attentional performance. Behav Neurosci. 2001;115:799–811. doi: 10.1037//0735-7044.115.4.799. [DOI] [PubMed] [Google Scholar]
- Sanders AE, Holtzer R, Lipton RB, Hall C, Verghese J. Egocentric and exocentric navigation skills in older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1356–1363. doi: 10.1093/gerona/63.12.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann NY Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Skelton MR, Schaefer TL, Herring NR, Grace CE, Vorhees CV, Williams MT. Comparison of the developmental effects of 5-methoxy-N,N-diisopropyltryptamine (Foxy) to (+/−)-3,4-methylenedioxymethamphetamine (ecstasy) in rats. Psychopharmacology (Berl) 2009;204:287–297. doi: 10.1007/s00213-009-1459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadaiesky MT, Dombrowski PA, Figueiredo CP, Cargnin-Ferreira E, Da CC, Takahashi RN. Emotional, cognitive and neurochemical alterations in a premotor stage model of Parkinson’s disease. Neuroscience. 2008;156:830–840. doi: 10.1016/j.neuroscience.2008.08.035. [DOI] [PubMed] [Google Scholar]
- Taube JS. Head direction cells and the neurophysiological basis for a sense of direction. Prog Neurobiol. 1998;55:225–256. doi: 10.1016/s0301-0082(98)00004-5. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Maze learning in rats: a comparison of performance in two water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol Teratol. 1987;9:235–241. doi: 10.1016/0892-0362(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Herring NR, Schaefer TL, Grace CE, Skelton MR, Johnson HL, Williams MT. Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int J Dev Neurosci. 2008;26:599–610. doi: 10.1016/j.ijdevneu.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Weisenburger WP, Acuff-Smith KD, Minck DR. An analysis of factors influencing complex water maze learning in rats: effects of task complexity, path order and escape assistance on performance following prenatal exposure to phenytoin. Neurotoxicol Teratol. 1991;13:213–222. doi: 10.1016/0892-0362(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Weniger G, Irle E. Posterior parahippocampal gyrus lesions in the human impair egocentric learning in a virtual environment. Eur J Neurosci. 2006;24:2406–2414. doi: 10.1111/j.1460-9568.2006.05108.x. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Dunnett SB. Dopamine depletion, stimulation or blockade in the rat disrupts spatial navigation and locomotion dependent upon beacon or distal cues. Behav Brain Res. 1985;18:11–29. doi: 10.1016/0166-4328(85)90165-2. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Mittleman G, Bunch ST, Dunnett SB. Impairments in the acquisition, retention and selection of spatial navigation strategies after medial caudate-putamen lesions in rats. Behav Brain Res. 1987;24:125–138. doi: 10.1016/0166-4328(87)90250-6. [DOI] [PubMed] [Google Scholar]
- White NM. Mnemonic functions of the basal ganglia. Curr Opin Neurobiol. 1997;7:164–169. doi: 10.1016/s0959-4388(97)80004-9. [DOI] [PubMed] [Google Scholar]
- White NM, McDonald RJ. Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem. 2002;77:125–184. doi: 10.1006/nlme.2001.4008. [DOI] [PubMed] [Google Scholar]
- Wiener SI. Spatial and behavioral correlates of striatal neurons in rats performing a self-initiated navigation task. J Neurosci. 1993;13:3802–3817. doi: 10.1523/JNEUROSCI.13-09-03802.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Herring NR, Schaefer TL, Skelton MR, Campbell NG, Lipton JW, McCrea AE, Vorhees CV. Alterations in body temperature, corticosterone, and behavior following the administration of 5-methoxy-diisopropyltryptamine (‘foxy’) to adult rats: a new drug of abuse. Neuropsychopharmacology. 2007;32:1404–1420. doi: 10.1038/sj.npp.1301232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. Contributions of striatal subregions to place and response learning. Learn Mem. 2004;11:459–463. doi: 10.1101/lm.81004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behav Brain Res. 2006;166:189–196. doi: 10.1016/j.bbr.2005.07.012. [DOI] [PubMed] [Google Scholar]