Abstract
Fetal nicotine exposure from smoking during pregnancy causes long-lasting cognitive impairments in offspring, yet little is known about the mechanisms that underlie this effect. Here we demonstrate that early postnatal exposure of mouse pups to nicotine via maternal milk impairs long-term, but not short-term, hippocampus-dependent memory during adolescence. At the Schaffer collateral (SC) pathway, the most widely studied synapses for a cellular correlate of hippocampus-dependent memory, the induction of N-methyl-d-aspartate receptor-dependent transient long-term potentiation (LTP) and protein synthesis-dependent long-lasting LTP are not diminished by nicotine exposure, but rather unexpectedly the threshold for LTP induction becomes lower after nicotine treatment. Using voltage sensitive dye to visualize hippocampal activity, we found that early postnatal nicotine exposure also results in enhanced CA1 depolarization and hyperpolarization after SC stimulation. Furthermore, we show that postnatal nicotine exposure induces pervasive changes to the nicotinic modulation of CA1 activity: activation of nicotinic receptors no longer increases CA1 network depolarization, acute nicotine inhibits rather than facilitates the induction of LTP at the SC pathway by recruiting an additional nicotinic receptor subtype, and acute nicotine no longer blocks LTP induction at the temporoammonic pathway. These findings reflect the pervasive impact of nicotine exposure during hippocampal development, and demonstrate an association of hippocampal memory impairments with altered nicotinic cholinergic modulation of LTP, but not impaired LTP. The implication of our results is that nicotinic cholinergic-dependent plasticity is required for long-term memory formation and that postnatal nicotine exposure disrupts this form of plasticity.
Keywords: CA1 region, Schaffer collateral pathway, temporoammonic pathway, object location memory, object recognition memory
1. Introduction
Smoking during pregnancy can have severe impacts on the mental and physical health of offspring, including long-lasting impairments in IQ and memory (Batstra et al, 2003; Bruin et al, 2010; Fried et al, 2003). Still, as of 2010 an estimated 12.3% of expectant mothers in the United States continued to smoke during pregnancy (Tong et al, 2013). Cigarette smoke has been shown to contain more than 4,000 chemicals, but among these nicotine is thought to be the primary neuroteratogen (Pauly and Slotkin, 2008). Indeed, several studies using rodent models of perinatal nicotine treatment have demonstrated that exposure to the drug during early development causes long-lasting deficits in learning and memory (Ankarberg et al, 2001; Eppolito and Smith, 2006; Sorenson et al, 1991; Vaglenova et al, 2004; Yanai et al, 1992; Portugal et al, 2012). However, the outstanding question in the field remains which cellular and molecular changes induced by nicotine underlie this cognitive impairment.
Nicotine activates nicotinic acetylcholine receptors (nAChRs), which are pentameric associations of α2–α10 and β2–β4 subunits. nAChRs are found in abundance throughout the brain, including in the hippocampus, an area critical for certain forms of learning and memory. In humans, significant hippocampal development occurs during the third trimester of pregnancy, whereas roughly equivalent development in rodents happens during the first two postnatal weeks (de Graaf-Peters and Hadders-Algra, 2006; Seress, 2007; Seress et al, 2001). This period of axon sprouting, dendritic arborization and robust synaptogenesis (Danglot et al, 2006; de Graaf-Peters and Hadders-Algra, 2006; Dwyer et al, 2009) is also, importantly, a time of rapid development of the cholinergic system, and coincides with a sharp spike in nAChR subunit upregulation (Adams et al, 2002; Shacka and Robinson, 1998; Son and Winzer-Serhan, 2006; Winzer-Serhan and Leslie, 2005). During these two weeks, nAChRs modulate the strength of newly forming excitatory synapses, and mediate the switch in the role of γ-aminobutyric acid (GABA) from being an excitatory to an inhibitory neurotransmitter (Liu et al, 2006, 2007). It is therefore not surprising that some studies have found that this two week window of hippocampal development in rodents encompasses a “critical period” during which nicotine exposure causes long-lasting molecular and cognitive effects (Eriksson et al, 2000; Miao et al, 1998). Given the many roles of nAChRs during development, prolonged nicotine exposure during this time could alter multiple aspects of hippocampal function.
In the current study, in order to identify long-lasting cellular, molecular and circuitry changes in the hippocampus that may underlie nicotine-induced cognitive impairments, we used a model of early postnatal nicotine exposure in mice from postnatal day 1–15 (P1–P15) that targeted this critical period of hippocampal development and cholinergic activity. We tested our model to determine whether it resulted in impaired memory during adolescence. Furthermore, we used electrophysiological, pharmacological and voltage-sensitive dye imaging techniques to identify nicotine-induced changes in long-term potentiation (LTP), which is thought to be the cellular substrate of learning and memory, as well as hippocampal network activity and the nicotinic modulation of hippocampal function – each possible causes of memory deficits.
2. Materials and methods
2.1. Animals and nicotine treatment
All animal procedures were conducted in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals, and with protocols approved by the Institutional Animal Care and Use Committee of the University of California at Irvine. C57BL/6 mouse litters were adjusted to five or six male and female pups, and were exposed to nicotine through maternal milk during postnatal days 1–15 by subcutaneously implanting nursing dams with Alzet osmotic minipumps (approximate nicotine output: 21 mg/kg/day). Here, we refer to these pups as maternal-nicotineexposed mice. Naïve mice and mouse pups from dams implanted with saline-containing minipumps were used as controls. Both gave similar electrophysiological and behavioral results, thus data obtained from those groups were combined for statistical analysis. Also, because electrophysiological recordings from male and female mice yielded equivalent results, their data were combined for statistical analysis. Behavioral studies were performed using male adolescent mice.
2.2. Object location and object recognition memory tasks
Training and testing for object location and object recognition memory were conducted on P45 and P46, respectively, and were carried out as previously described (Barrett et al, 2011; McQuown et al, 2011; Roozendaal et al, 2010). Briefly, before training, mice were handled 1–2 min daily for 5 days and then habituated to the experimental arena (white rectangular open field, 30 × 23 × 21.5 cm) 5 min a day for 6 days in the absence of objects. During training, mice were placed into the experimental arena with two identical objects (100 mL beakers, lightbulbs or vases) and were allowed to explore for 10 min (Stefanko et al, 2009). During the retention test (90 min later for short-term memory, or 24 h later for long-term memory), mice explored the experimental apparatus for 5 min. For the object location task, one familiar object was placed in a novel location, and another familiar object was placed in the same location as during training. For the object recognition task, a familiar and a novel object were placed in the same locations as used during training. All combinations of locations and objects were balanced across trials to eliminate bias. Training and testing trials were videotaped and analyzed by individuals blind to the treatment condition. A mouse was scored as exploring an object when its head was oriented toward the object and within a distance of 1 cm, or when its nose was touching the object. The relative exploration time was recorded and expressed by a discrimination index (DI=(tnovel−tfamiliar)/(tnovel+tfamiliar) × 100%).
2.3. Elevated-plus maze
The elevated plus-maze task was performed as previously described (Vogel-Ciernia et al, 2013). The maze consisted of two open arms and two enclosed arms extending from a central platform, raised to a height of 40 cm above the floor. The light level in the testing room was adjusted to 15 lux. Testing consisted of placing a mouse onto the central platform of the maze facing an open arm, and recording its locomotion for 5 min. The percentage of time spent in the closed and open arms was scored using EthoVision 3.1 (Noldus Information Technology). Between subjects, the maze was cleaned with 70% ethanol.
2.4. Slice preparation
Transverse hippocampal slices (300–400 µm) were prepared from mice (age 4–6 weeks) anesthetized with urethane. Slices were maintained at 30°C for at least 1 h to recover in artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl, 124; KCl, 5; NaH2PO4, 1.25; MgSO4, 2; CaCl2, 2.5; NaHCO3, 22; glucose, 10; and oxygenated with 95% O2 and 5% CO2.
2.5. Extracellular field recordings
Slices were submerged in a recording chamber and continually superfused at 2–3 ml/min with oxygenated ACSF at 30°C. A bipolar stimulating electrode was placed at the Schaffer collateral (SC) pathway, and the slice stimulated with short current pulses (200 ms duration) every 20 s. Field excitatory postsynaptic potentials (fEPSPs) were recorded from the stratum radiatum of the CA1 region using glass electrodes filled with ACSF (3–8 MΩ). At the beginning of each experiment, a stimulus response curve was established by measuring the slope of fEPSPs. The strength of the stimulus was adjusted to elicit fEPSPs that were 30–50% of the maximum response (requiring stimulus intensities of 40–80 µA). The intensity and duration of each stimulus pulse remained invariant thereafter for each experiment. Baseline responses were recorded to establish the stability of the slice. LTP was induced by theta burst stimulation (TBS; 10 theta bursts, with each burst containing 4 pulses at 100 Hz and individual bursts separated by 200 ms), weak TBS (two theta bursts of four pulses at 100 Hz), or by four trains of tetanus (100 pulses at 100 Hz, in 5 min intervals), as indicated. To evaluate the magnitude of early-LTP, the mean values of the slopes of fEPSPs from 40–50 min after TBS stimulation were calculated and expressed as a percentage of the mean baseline fEPSPs slopes. Late-LTP magnitude was evaluated by comparing baseline slopes to those from 176–180 min after tetanus. Paired-pulse facilitation was determined using the stimulus intensity required to induce a half-maximum response with interpulse intervals of 25–200 ms. Recorded signals were amplified (A-M Systems) and digitized, and analyzed using NAC 2.0 software (Theta Burst Corp.).
2.6. Voltage-sensitive dye imaging
Voltage-sensitive dye (VSD) imaging and recording was performed as previously described (Nakauchi et al, 2007; Tominaga et al, 2000). Briefly, slices were submerged in a recording chamber mounted on the stage of a fluorescence microscope (BX51WI; Olympus). A 4× objective lens (0.28 NA; Olympus) focused the excitation light on the CA1 region of the hippocampus. VSD imaging was performed with a CCD camera (MiCAM02; BrainVision) which has a 6.4 × 4.4 mm2 imaging area. To avoid bleaching of the dye, an electronically controlled shutter remained closed until 100 ms before the start of each recording. In each stimulation trial, frames were recorded at 250 Hz for 1024 ms. Eight or 16 trials were averaged to improve the signal-to-noise ratio. Extracellular potential recordings were preformed simultaneously with the optical recordings to ensure that the optical response was consistent with the electrical response. The fractional change in fluorescence intensity (ΔF/F) was used to normalize the difference in the amount of VSD in each slice, and signal gain and threshold levels were adjusted to optimize the signal-to-noise ratio of the response relative to background. Activated areas were smoothed by averaging images with spatial and cubic filters. Data were analyzed and displayed using BV-Analyser (BrainVision). To quantitatively compare optical responses across different slices, the maximum optical responses to a single stimulus were sampled at 3 × 21 grid points along the stratum oriens, stratum pyramidale and stratum radiatum layers of the hippocampal CA1, anchored to the stimulation site in the stratum radiatum. The 21 points of each layer were divided into two groups (proximal and distal to the site of stimulation), and the average optical responses in each group were calculated. As the outcomes were not substantially different between the proximal and distal groups, we have reported only the distal results below. Peak depolarization and hyperpolarization amplitudes were measured and compared. Measurement of the integrated negative area under the baseline was calculated for responses to a single stimulation with a cut-off time of 500 ms, a time point selected to minimize variability.
2.7. Drugs
Nicotine, AP5, picrotoxin, methyllycaconitine (MLA), dihydro-β-erythroidine (DHβE), mecamylamine and DNQX were obtained from Sigma. All drugs were dissolved in ACSF and bath-applied for approximately 5–10 min.
2.8. Statistical analysis
Behavior datasets were analyzed using Student's t-tests or one-way analysis of variance (ANOVA) with Bonferroni post hoc tests where appropriate. Alpha levels were set at 0.05. Electrophysiological data was normalized relative to baseline, expressed as mean ± SEM, and analyzed for significance using ANOVAs and post hoc Tukey HSD tests. In all graphs, p values are depicted as follows: *p < 0.05, **p <0.01, ***p < 0.001. Optical and physiological data were plotted and analyzed using Origin 8.1 (OriginLab).
3. Results
3.1. Early postnatal nicotine exposure disrupts hippocampus-dependent memory and increases anxiety
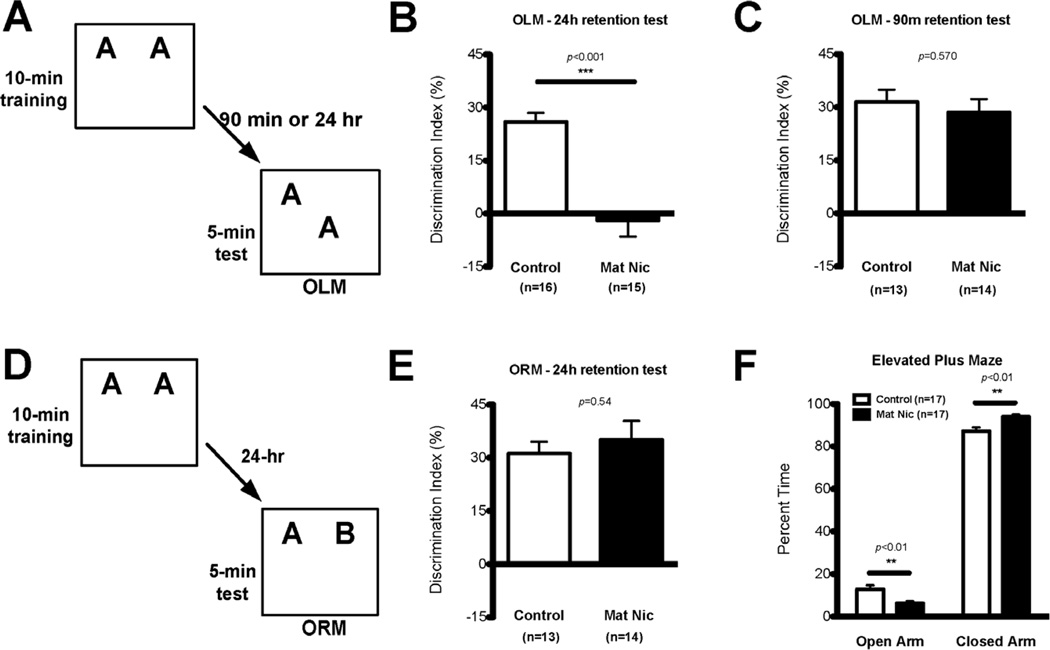
The overall aim of this study was to examine the long-lasting impact of early postnatal nicotine exposure on hippocampal CA1 function. Therefore, we first tested maternal nicotine (MN)-exposed and control mice for long- and short-term object location memory (Fig. 1A–C), a CA1-dependent task (Assini et al, 2009; Barrett et al, 2011; Haettig et al, 2013; McQuown et al, 2011). MN-exposed mice exhibited significant deficits in long-term object location memory as compared to control mice (Fig. 1B; Control, n=16, mean DI±SEM: 25.89±2.60; MN, n=15, mean DI±SEM: -1.97±4.58; Student’s t-test: t29=5.37, p<0.0001). Importantly, there were no differences between groups with regard to total exploration time during training (Control, n=16, mean DI±SEM: 30.14±2.07; MN, n=15, mean DI±SEM: 30.55±2.03; t-test: t29=0.14, p=0.88) and testing (Control, n=16, mean DI±SEM: 6.25±0.59; MN, n=15, mean DI±SEM: 5.59±0.63; t-test: t29=0.76, p=0.46). We next examined short-term object location memory in a different cohort of animals at 90 min after training. Short-term object location memory did not differ between MN mice and controls (Fig. 1C; Control, n=13, mean DI±SEM: 31.47±3.47; MN, n=14, mean DI±SEM: 28.49±3.79; Student’s t-test: t25=0.58, p=0.57). Again, there were no significant differences in total exploration time between groups (training: Control, n=13, mean DI±SEM: 17.46±1.49; MN, n=14, mean DI±SEM: 16.17±1.06; Student’s t-test: t25=0.72, p=0.48; test: Control, n=13, mean DI±SEM: 4.28±0.47; MN, n=14, mean DI±SEM: 5.01±0.88; t-test: t25=0.82, p=0.41). Together, these results indicate that maternal nicotine-exposed mice exhibit specific long-term memory impairments for object location, a hippocampus-dependent task.
Figure 1. Maternal nicotine-exposed mice have impaired long-term spatial memory and increased anxiety.
(A) For the hippocampus-dependent object location memory (OLM) task, mice were trained for 10 min with two identical objects, and tested either 90 min or 24 hrs later with one object moved to a new location. (B) MN mice (n=15) showed significantly impaired 24-hr long-term OLM compared to controls (n=16), and had a discrimination index not significantly different from zero. There were no significant differences between groups in total exploration time during training or testing. (C) In the OLM task, MN mice (n=14) did not show any difference in 90-min short-term memory from controls (n=13). There were no significant differences in total exploration time during training or testing. (D) For the hippocampus-independent object recognition memory (ORM) task, mice were trained for 10 min with two identical objects, and tested 24 hrs later after one object was replaced by a novel item. (E) In the ORM task, there was no difference in the 24-hour long-term memory demonstrated by MN mice (n=14) or controls (n=13), and no significant differences between the groups in total exploration time during training or testing. (F) MN mice (n=17) spent significantly less time in the open arm of the elevated plus maze than did control mice (n=17), demonstrating increased anxiety.
To examine whether the postnatal nicotine exposure induced more broad long-term memory impairments in MN mice, we used the hippocampus-independent object recognition memory task (Fig. 1D). MN mice showed similar long-term memory for object recognition to control mice (Fig. 1E; MN, n=14, mean DI±SEM: 36.91±5.79; Control, n=13, mean DI±SEM: 30.38±3.52; Student’s t-test: t25=0.94, p=0.35). Additionally, both groups showed similar total exploration times during training (Control, n=16, mean DI±SEM: 32.61±1.44; MN, n=15, mean DI±SEM: 31.46±1.43; t-test: t29=0.57, p=0.57) and testing (Control, n=16, mean DI±SEM: 10.82±0.91; MN, n=15, mean DI±SEM: 8.33±1.03; t-test: t29=1.82, p=0.08). Thus, MN mice exhibit normal long-term memory for object recognition, a hippocampus-independent task.
Although we observed no differences in total exploration time during the object location and object recognition experiments that would confound interpretation of the performance measures on memory, we did observe that MN mice appeared to be more anxious. Thus, we examined anxiety more directly using an elevated plus maze. MN mice (n=17) exhibited a modest yet significant increase in anxiety, spending less time in the open arms than control mice (n=17; Fig. 1F; two-way ANOVA, main effect of Arm F(1,62)=97.52, p<0.0001, no effect of Treatment F(1,62)=0.00, p=1.00, significant interaction F(1,62)=0.67, p<0.0001; Bonferroni post hoc test: Control vs MN: open arm, p<0.01; closed arm, p<0.01). It is unlikely that the increased anxiety exhibited by MN mice affected the memory experiments (Fig 1B, C, and E) because MN mice had normal total exploration times, normal short-term memory for object location, and normal long-term memory for object recognition. Together, these results suggest that MN mice have an impairment in hippocampal function that gives rise to the specific hippocampus-dependent long-term memory impairment for object location.
3.2. Early postnatal nicotine exposure lowers the LTP induction threshold
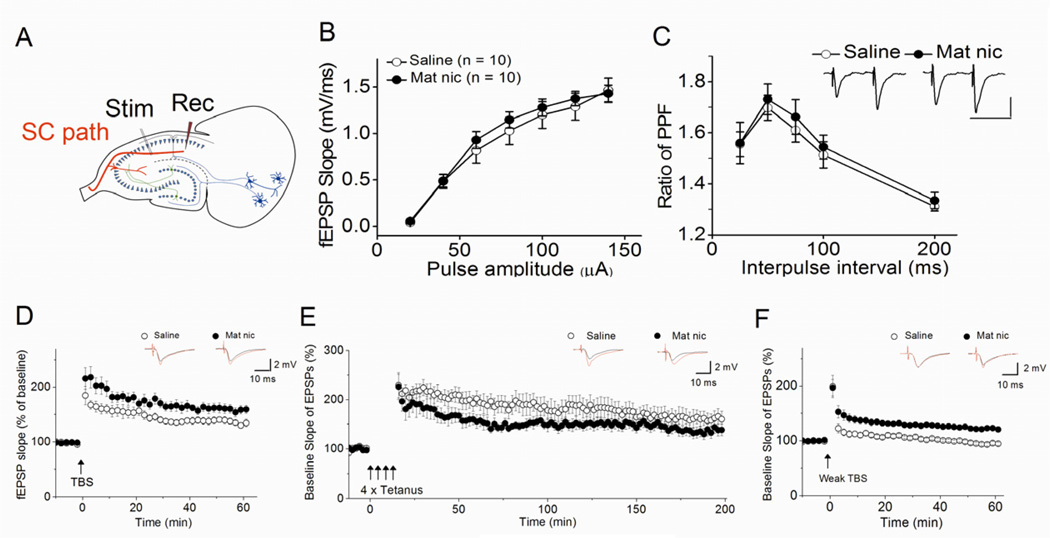
To gain insight into the mechanism underlying the observed deficit in hippocampal memory, we first looked for MN-induced changes in synaptic transmission at the SC pathway by recording fEPSPs in hippocampal slices (Fig. 2A). We found no significant differences between slices from control or MN mice in either the stimulus-response relationships (Fig. 2B; F(1, 134)=1.07, p=0.30) or in paired-pulse facilitation (Fig. 2C; F(1, 69)=0.69, p=0.41), suggesting that early postnatal nicotine exposure significantly alters neither the basal synaptic transmission nor the probability of transmitter release.
Figure 2. Early postnatal nicotine exposure facilitates the induction of LTP in the SC pathway of adolescent mice.
(A) The placement of the stimulating and recording electrodes used to measure fEPSPs in the CA1. There was no significant difference between slices from control and MN mice (B) in the stimulus-response relationship (control: n=10, MN: n=10) or (C) in paired-pulse facilitation (control: n=6, MN: n=8), as shown by the ratio of the second fEPSP slope to the first fEPSP slope at different interpulse intervals (insert: representative traces for the 50 ms interpulse interval; horizontal calibration bar: 50 ms; vertical calibration bar: 1 mV). (D) MN slices (n=8) showed a trend for a small increase in the magnitude of early-LTP induced by TBS. (E) There was no difference between MN (n=6) and control (n=7) hippocampal slices in the magnitude of late-LTP induced by four bursts of high frequency stimulation. However, (F) weak TBS, which does not induce LTP in control hippocampal slices (n=8), induced LTP in MN slices (n=6). (D–F) Traces above each graph are representative waveforms recorded before and 40–50 min (D, F) or 176–180 min (E) after LTP-inducing stimulation.
Because LTP at the SC pathway has been strongly implicated as one of the cellular mechanisms of hippocampal learning and memory, we next examined whether MN impaired LTP at SC synapses. Theta burst stimulation (TBS) was used to induce early-LTP in hippocampal slices from nicotine-treated and control mice. Contrary to our expectations, postnatal nicotine exposure resulted in a strong, but not statistically significant, trend for a small increase in LTP magnitude (Fig. 2D; saline, 139 ± 6%, n=6, vs. MN, 161 ± 8%, n= 8, F(1,13)=4.19, p=0.06). We also used four bursts of tetanus stimulation to induce long-lasting, protein-synthesis and dopamine-dependent late-LTP. Hippocampal slices from MN mice showed no difference in late-LTP from control slices (Fig. 2E; control, 160 ± 14%, n=7, vs. MN, 138 ± 11%, n= 6, F(1,12)=1.39, p=0.23).
Because these results suggested that, if anything, postnatal nicotine exposure enhances LTP at the SC pathway, we also explored whether it altered the threshold for LTP induction. We found that weak TBS, which is sub-threshold for LTP induction in hippocampal slices from control animals, is able to induce LTP in MN slices (Fig. 2F; saline, 96 ± 3%, n=8, vs. MN, 122 ± 3%, n= 6, F(1,13)=44.91, p<0.001). Thus early postnatal nicotine exposure, which causes CA1-dependent memory impairments, unexpectedly facilitates LTP at SC synapses. The observation of increased TBS-induced LTP following maternal nicotine has been previously reported in the dentate gyrus of rats (Mahar et al, 2012).
3.3. Early postnatal nicotine enhances depolarizing and hyperpolarizing neuronal activity in the CA1 region
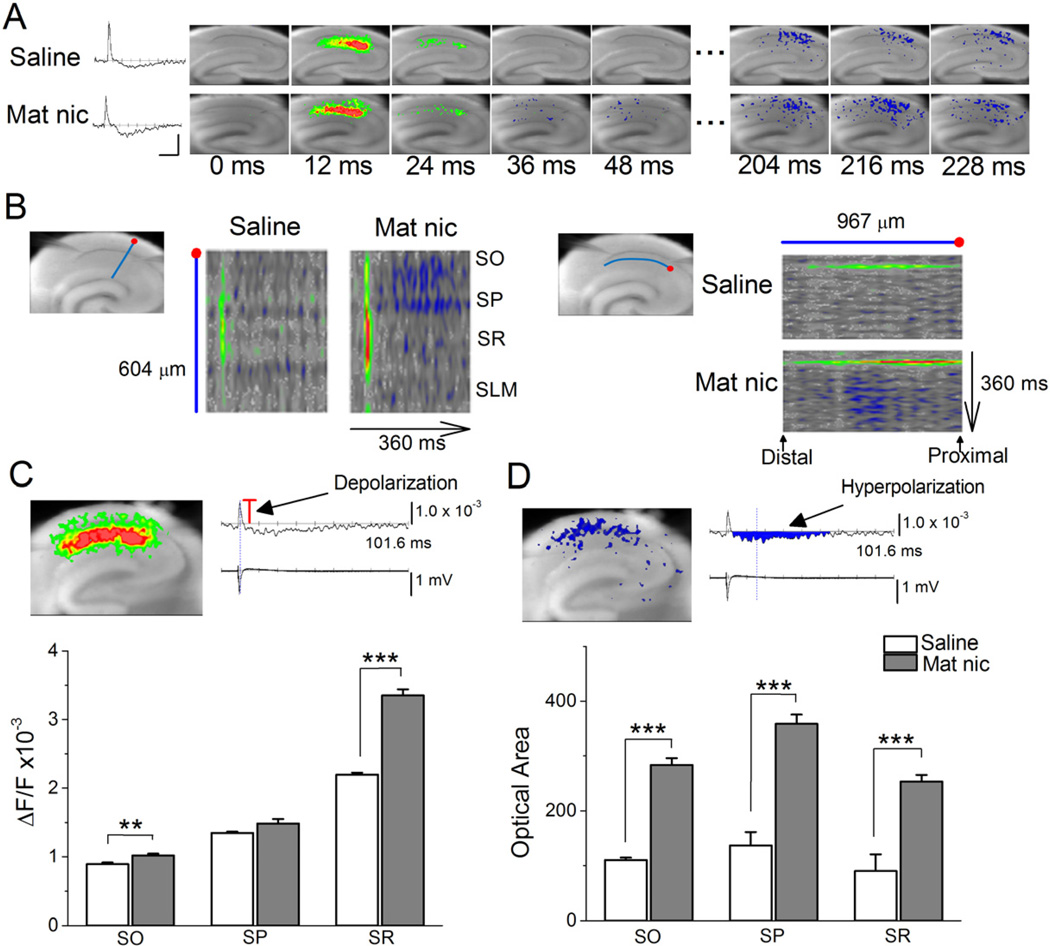
In the absence of clear impairments in SC-LTP that might underlie the observed deficit in hippocampal memory, we next used voltage-sensitive dye imaging as a more sensitive measure of network activity, because it allows for the visualization of changes in neuronal membrane potential, not just synaptic activity. This approach has the further benefit of quantifying neural activity over a much wider region of the CA1 than is possible with fEPSP recordings. Electrical stimulation of the SC pathway, the intensity of which was adjusted to evoke similar amplitudes of fEPSPs between different slices, caused the spread of optical signal in all anatomical layers of the CA1. Such signals can be presented as traces or as pseudocolor images of the fractional change in fluorescence intensity (ΔF/F; Fig. 3A). Depolarizing responses originating from the site of stimulation peaked at 12 ms in slices from both control and MN mice, but the peak signals in the stratum radiatum and stratum oriens were significantly stronger in the MN slices (Fig. 3A–C). Furthermore, in MN slices, we observed significantly stronger hyperpolarizing responses in all anatomical layers of the CA1 region at ~210 ms (Fig. 3A, B, D). The MN-induced changes are clearly visible in pseudocolor representations of line scans across the anatomical layers of the CA1 (Fig. 3B, left), and along the stratum radiatum (Fig. 3B, right) over time. Depolarizing activity was blocked with the addition of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor and N-methyl-d-aspartate receptor antagonists DNQX and AP5; hyperpolarizing activity was partially blocked with the GABAA antagonist picrotoxin, and completely blocked with the further addition of the GABAB antagonist CGP55845 (data not shown). These results indicate that MN treatment significantly increases both excitatory and inhibitory neuronal activity throughout the hippocampal CA1 after SC stimulation.
Figure 3. Early postnatal nicotine exposure increases depolarization and hyperpolarization in the CA1 region after SC stimulation (A–D).
Voltage-sensitive dye imaging, which detects changes in neuronal activity not restricted to synapses, showed that MN exposure increased depolarization and hyperpolarization in the CA1 after SC stimulation. (A) Left, sample trace of optical response (ΔF/F) over time for a point in control and MN slices. Horizontal calibration scale: 82 ms, vertical scale: 1.0×10−3. Right, sample pseudocolor representations of VSD signal after SC stimulation in control and MN slices. Red: depolarization, blue: hyperpolarization. (B) Left, pseudocolor representation of line scanning across CA1 layers (along the blue line beginning at the red dot, as shown in the slice image) over time in control and MN hippocampi. Scanning began 100 ms before stimulation and peaked at 8 ms after stimulation. Length of line is 604 um. Right, pseudocolor representation of line scanning along the stratum radiatum (along the blue line, starting from the red dot) over time in control and MN-treated hippocampi. Length of line is 967 um. (C) Top, sample pseudocolor image of maximum depolarizing responses (left), and simultaneous optical (ΔF/F) and fEPSP recordings (right). Bottom, averages of maximum optical responses (shown by the red bar in the sample optical trace) within CA1 layers in control (n=6) and MN (n=10) slices show that nicotine treatment enhanced depolarizing responses in the stratum oriens and radiatum. SO: Control; 0.89 ± 0.03, n=6 vs. MN; 1.02 ± 0.03, n=10; F(1,15)=10.22, p<0.01. SP: Control; 1.35 ± 0.02, n=6 vs. MN; 1.49 ± 0.07, n=10; F(1,15)=2.60, p=0.13. SR: Control; 2.20 ± 0.03, n=6 vs. MN; 3.35 ± 0.09, n=10; F(1,15)=92.16, p<0.001 (D) Top, sample pseudocolor image of inhibitory optical response (left), alongside simultaneous optical (ΔF/F) and fEPSP recordings (right). Bottom, averages of the areas of inhibitory optical responses (as shown by blue region in sample optical trace) within CA1 layers in control (n=6) and MN (n=10) slices show that nicotine treatment enhanced hyperpolarizing responses in the stratum oriens, pyrimidale and radiatum. SO: Control, 110.43 ± 4.28, n=6 vs. MN, 283.95 ± 11.92, n=10, F(1,15)=118.59, p<0.001. SP: Control, 137.14 ± 24.01, n=6 vs. MN, 359.05 ± 16.11, n=10, F(1,15)=63.44, p<0.001. SR: Control, 90.17 ± 30.64, n=6 vs. MN, 253.54 ± 11.97, n=10, F(1,15)=34.13, p<0.001. SO: stratum oriens; SP: stratum pyrimidale; SR: stratum radiatum. **p< 0.01, *** p< 0.001
3.4. Early postnatal nicotine exposure alters nicotinic modulation of depolarizing neuronal activity and LTP in the CA1
nAChRs are key modulators of neuronal activity, and prolonged exposure to nicotine is known to alter the number and function of certain nAChR subtypes. In order to understand possible mechanisms for the MN-induced enhancement of depolarization and hyperpolarization in the CA1, we therefore investigated the possibility that early postnatal nicotine treatment alters nicotinic modulation of neuronal activity.
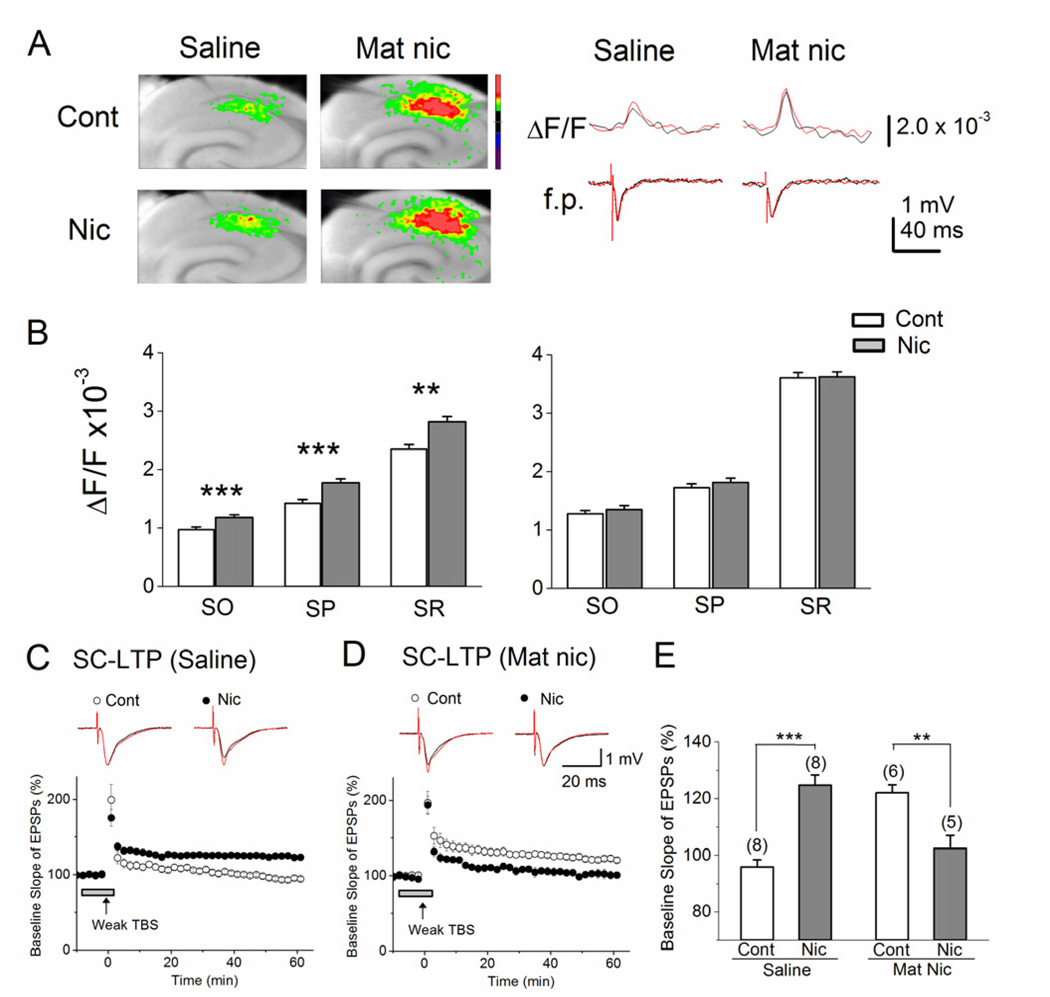
Using hippocampal slices from control and MN mice, we simultaneously recorded fEPSPs and VSD optical signal, both in the presence and absence of bath application of nicotine (1 µM; Fig 4A). In hippocampi from saline-treated controls, acute nicotine significantly increased depolarization in all anatomical regions measured, as determined by maximum optical signal (Fig. 4B left graph). However, bath application of nicotine did not cause detectable changes in the amplitude of fEPSPs (Fig. 4A right, bottom traces), suggesting that in control slices, acute nicotine acts indirectly on pyramidal cells, as in wild-type mice (Nakauchi et al, 2007). By contrast, in slices from MN mice – which, in the absence of acute nicotine, show stronger optical signals than slices from saline-treated mice (Fig. 4A) – bath application of nicotine did not increase either fEPSP amplitude (Fig. 4A, right, bottom traces) or excitatory optical signal (Fig. 4B, right graph) in any of the anatomical regions recorded in the CA1. These results indicate that early postnatal nicotine exposure causes long-lasting disruptions to the nicotinic modulation of excitatory activity in the CA1 region.
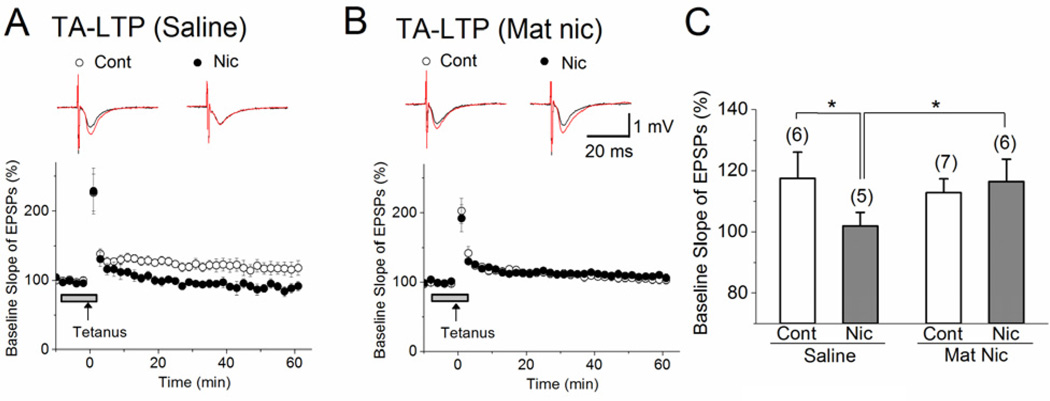
Figure 4. Early postnatal nicotine exposure alters acute nicotinic modulation of depolarizing neuronal activity and LTP at SC pathway.
(A) Left, sample pseudocolor representations of maximum optical signal from voltage sensitive dye after SC stimulation, recorded first in the absence and then in the presence of nicotine, for control and MN slices. Right, sample simultaneous optical (ΔF/F) and fEPSP (f.p.) traces comparing responses to SC stimulation under baseline (black) and bath nicotine (red) conditions for saline and MN slices. ΔF/F increased in the presence of nicotine in saline but not MN slices, while fEPSP amplitude showed no changes. (B) Averages of maximum optical responses within CA1 layers in saline (left graph, n=9) and MN (right graph, n=13) slices show that, although MN hippocampi show higher baseline activity than saline hippocampi, they show no change in response to bath application of nicotine, unlike control slices. Saline - SO: Control, 0.97 ± 0.03, n=9 vs. Nicotine, 1.18 ± 0.03, n=9, F(1,17)=23.3, p<0.001. SP: Control, 1.42 ± 0.05, n=9 vs. Nicotine, 1.77 ± 0.05, n=9, F(1,17)=25.22, p<0.001. SR: Control, 2.35 ± 0.10, n=9 vs. Nicotine, 2.93 ± 0.11, n=9, F(1,17)=15.37, p<0.01. MN - SO: Control, 1.33 ± 0.07, n=13 vs. Nicotine, 1.35 ± 0.07, n=13, F(1,25)=0.04, p=0.83. SP: Control, 1.72 ± 0.09, n=13 vs. Nicotine, 1.82 ± 0.08, n=13, F(1,25)=0.63, p=0.44. SR: Control, 3.61 ± 0.15, n=13 vs. Nicotine, 3.62 ± 0.14, n=13, F(1,25)=0.005, p=0.94. (C) Weak TBS induces LTP in slices from saline-treated control mice in the presence (n=8), but not in the absence (n=8), of acute nicotine. However, (D) in MN slices, weak TBS induces LTP in the absence of nicotine (n=6), but this LTP induction is blocked in the presence of nicotine (n=5). (E) MN treatment reverses the effect of nicotine on SC-LTP, as shown by the percent change in the slope of fEPSPs with and without bath nicotine, measured 50–55 min after weak TBS stimulation. (C, D) LTP-inducing stimulation was delivered at the time indicated by the arrow, and nicotine administration is indicated by the horizontal bar. Traces above each graph are representative waveforms recorded before (black) and 50 min after (red) LTP-inducing stimulation in control and bath-nicotine-treated slices. Cont: control; Nic: nicotine. **p< 0.01, *** p< 0.001.
Similarly, we found alterations in the nicotinic cholinergic modulation of LTP after MN treatment. We have previously shown that weak TBS, which alone is sub-threshold for LTP induction, induces LTP at SC synapses of naïve mice with the addition of 1 µM nicotine (Nakauchi et al, 2007; Nakauchi and Sumikawa, 2012). Here, we confirmed this nicotinic modulation in saline-treated control mice (Fig. 4C, E; Control, 96 ± 3%, n=8, vs. Acute nicotine, 125 ± 3%, n= 8, F(1,15)=41.00, p<0.001). However, in hippocampal slices from MN mice, when weak TBS – which alone induces LTP – was administered in the presence of nicotine, the induction of LTP was unexpectedly blocked (Fig. 4D, E; Control, 122 ± 3%, n= 6, vs. Acute nicotine, 102 ± 5%, n=5, F(1,10)=14.12, p<0.01).
We also observed a similar effect of MN exposure on nicotinic modulation when stimulating another excitatory input onto CA1 pyramidal neurons, the temporoammonic (TA) pathway. In hippocampal slices from saline-exposed mice, the induction of LTP by tetanic stimulation of the TA pathway (Fig. 5A, C; 118 ± 9% of basal levels) is blocked by the addition of 1 µM nicotine (Fig. 5A, C; Control, 118 ± 9%, n=6, vs. Acute nicotine, 90 ± 5%, n= 5, F(1,10)=6.48, p<0.05), as with naïve mice (Nakauchi et al, 2007). However, in slices from MN mice, although tetanic stimulation induced similar LTP as in controls (Fig. 5A–C; Saline, 118 ± 9%, n=6, vs. MN, 113 ± 5%, n=7, F(1,12)=0.27, p=0.61), nicotine failed to block LTP induction (Fig. 5B, C; Control, 113 ± 5%, n=7, vs. Acute nicotine, 116 ± 9%, n= 6, F(1,12)=0.12, p=0.73). Combined, these results suggest that early postnatal nicotine exposure cripples the ability of nAChRs to modulate hippocampal CA1 activity.
Figure 5. Early postnatal nicotine exposure alters acute nicotinic modulation of LTP at TA pathway.
(A) At TA synapses in hippocampal slices from saline-treated control mice (n=6), tetanus stimulation (100 pulses at 100 Hz) induced LTP; LTP induction was blocked in the presence of bath application of 1 µM nicotine (n=5). (B) In hippocampal slices from MN mice, bath application of nicotine failed to block the TA-LTP (n=6) that was induced by tetanus stimulation in the absence of nicotine (n=7). (C) MN treatment blocks the effect of nicotine on TA-LTP, as shown by the percent change in the slope of fEPSPs with and without bath nicotine, measured 50–55 min after tetanus stimulation. (A, B) LTP-inducing stimulation was delivered at the time indicated by the arrow, and nicotine administration is indicated by the horizontal bar. Traces above each graph are representative waveforms recorded before (black) and 50 min after (red) LTP-inducing stimulation in control and bath-nicotine-treated slices. Cont: control; Nic: nicotine. *p< 0.05.
3.5. Nicotinic modulation of LTP in MN mice is driven by a newly recruited nAChR subtype
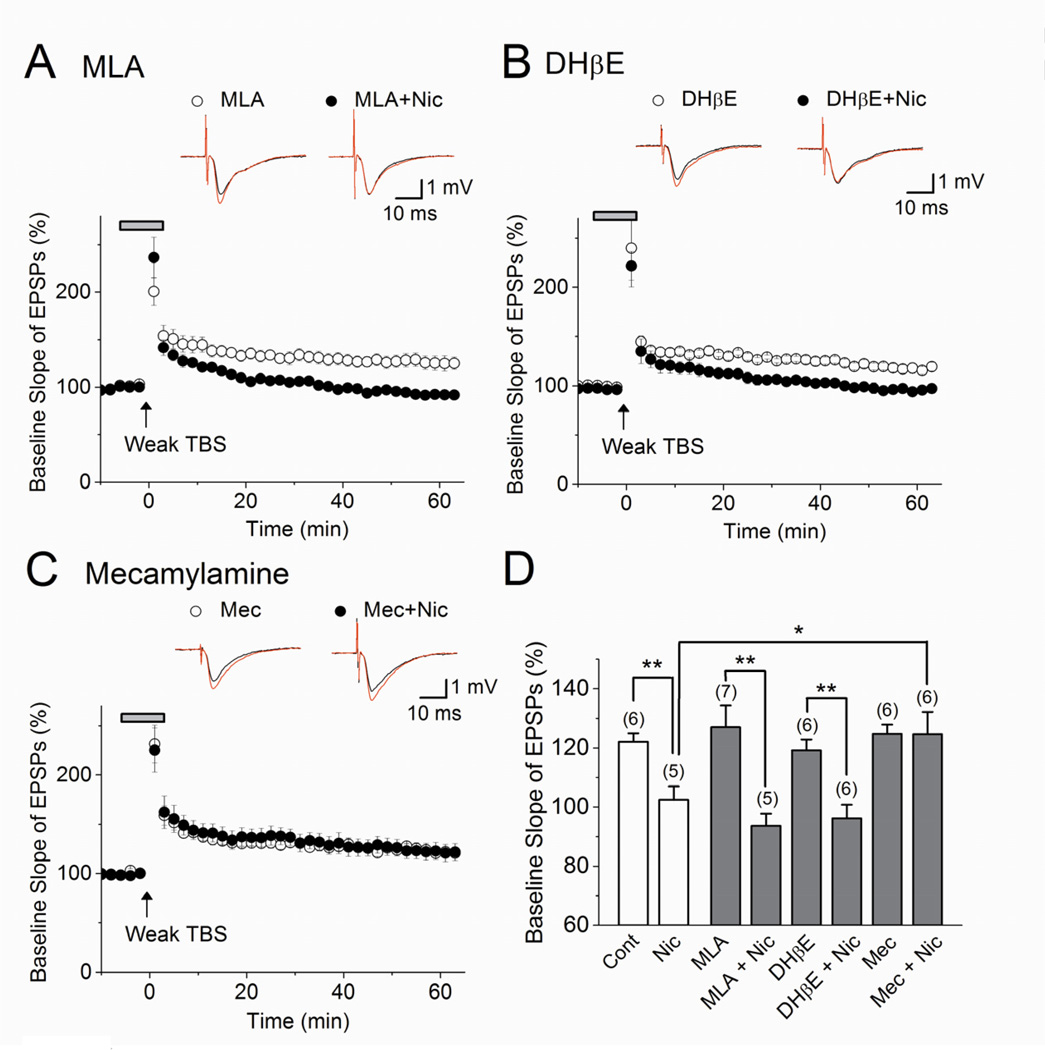
In order to begin identifying the alterations in molecular and cellular mechanisms driving these changes in network activity and synaptic plasticity, we next investigated which nAChRs mediated LTP induction at SC synapses in MN mice. To start, we bathed MN slices in MLA (20 nM), an antagonist selective for α7 nAChRs, DhβE (500 nM), an antagonist of β2-containing nAChRs (e.g., α2β2, α4β2), or the non-selective nAChR antagonist mecamylamine (3 µM), and administered weak TBS. MN-facilitated LTP induction persisted with each of these antagonists (Fig. 6A–D; Control, 122 ± 3%, n=6, vs. MLA, 127 ± 7%, n=7, F(1,12)=0.39, p=0.54; Control, 122 ± 3%, n=6, vs. DHβE, 119 ± 3%, n=6, F(1,11)=0.39, p=0.55; Control, 122 ± 3%, n=6, vs. Mecamylamine, 125 ± 3%, n=6, F(1,11)=0.46, p=0.51). This suggests that activation of nAChRs by TBS-induced ACh release is not driving the facilitation of LTP that results from early prenatal nicotine treatment. However, when these antagonists were applied in the presence of nicotine (1 µM), we found that the suppressive effect of acute nicotine on LTP induction in MN slices was blocked by mecamylamine, but not MLA or DHβE (Fig. 6A–D; Nicotine, 102 ± 5%, n=5, vs. Nicotine + Mecamylamine, 125 ± 8%, n=6, F(1,10)=5.65, p<0.05; Mecamylamine, 125 ± 3%, n=6, vs. Nicotine + Mecamylamine, 125 ± 8%, n=6, F(1,11)=4.57, p=0.98; Nicotine, 102 ± 5%, n=5, vs. Nicotine + MLA, 94 ± 4%, n=5, F(1,9)=2.12, p=0.18; MLA, 127 ± 7%, n=7, vs. Nicotine + MLA, 94 ± 4%, n=5, F(1,11)=12.87, p<0.01; Nicotine, 102 ± 5%, n=5, vs. Nicotine + DHβE, 96 ± 5%, n=6, F(1,10)=0.95, p=0.36; DHβE, 119 ± 3%, n=6, vs. Nicotine + DHβE, 96 ± 5%, n=6, F(1,11)=17.06, p<0.01). We have previously shown that in naive mice, DHβE inhibits the facilitation of LTP induced by acute nicotine (Nakauchi and Sumikawa, 2012). Early postnatal nicotine exposure, however, appears to recruit a different nAChR subtype, containing neither β2 nor α7 subunits, for the nicotinic modulation of LTP. However, the identity and location of the nAChR subtype involved in this nicotinic suppression of LTP in MN mice remains to be determined.
Figure 6. Nicotinic modulation of LTP in maternal nicotine-exposed mice is not driven by α7 or α4β2 nAChRs.
In MN hippocampal slices, (A, D) the α7 antagonist MLA (20 nM) alone has no effect on LTP induced by weak TBS (n=7) and did not block the suppressive effect of 1 µM nicotine (n=5). Likewise, (B, D) DhβE (500 nM), an antagonist of certain heteromeric nAChRs including α2β2 and α4β2, did not affect LTP in MN slices (n=6) and did not block the suppressive effect of 1 µM nicotine (n=6). (C, D) Mecamylamine (3 µM) also does not affect the induction of LTP by weak TBS in MN slices (n=6), but does block the effect of 1 µM nicotine (n=6). (A–C) Traces above each graph are representative waveforms recorded before (black) and 50–55 min after (red) LTP-inducing stimulation. Weak TBS stimulation was delivered at the time indicated by the arrow. Administration of drugs is indicated by the horizontal bar. (D) Histograms show the percent change in the slope of fEPSPs, measured 50–55 min after weak TBS. Nic = nicotine; Mec = mecamylamine. *p< 0.05, **p< 0.01
4. Discussion
Several studies have attempted to model the cognitive impact to offspring of smoking during pregnancy by characterizing the memory impairments in rodents exposed to perinatal nicotine. Using different protocols of nicotine administration and testing, some found clear impairments in learning and memory (Sorenson et al, 1991; Vaglenova et al, 2004; Yanai et al, 1992; Portugal et al, 2012), whereas others reported no nicotine effects (Cutler et al, 1996; Huang et al, 2007), only subtle impairments (Levin et al, 1993), or only dose- or sex-specific impairments (Ankarberg et al, 2001; Eppolito and Smith, 2006). Combined, this body of work demonstrates that the effects of nicotine exposure during development are sensitive to a combination of factors including sex, dose and timing of exposure. It is therefore particularly important, if aiming to identify long-term physiological changes that might underlie nicotine-induced cognitive deficits, to validate that the model of nicotine treatment being studied affects behavior. To our knowledge, this is the first study that uses a nicotine model with demonstrated learning and memory impairments to identify specific functional changes that may be the cause of nicotine-induced cognitive impairments.
Our behavioral experiments were conducted in adolescent mice one month after the end of nicotine exposure from P1 to P15 via maternal milk. We found that this early postnatal nicotine treatment resulted in a long-lasting impairment in long-term memory for the hippocampus-dependent object location task, but no impairment in either short-term object location memory or in long-term object recognition memory, a task that is thought to be dependent on the perirhinal cortex (Moses et al, 2005; Norman and Eacott, 2004). This indicates that the cognitive impairments induced by early postnatal nicotine exposure are not global, but rather that there are certain learning and memory processes that are particularly sensitive to nicotine effects. Nearly all previous investigations of the effect of perinatal nicotine exposure on memory in rodents have studied hippocampus-dependent tasks, with the exception of one study showing that prenatal nicotine exposure also impairs active avoidance (Vaglenova et al, 2004), a limbic-system- and prefrontal-cortex-dependent task (McNew and Thompson, 1966; Moscarello and LeDoux, 2013). However, studies of the effect of chronic nicotine exposure in adult rodents do suggest that the hippocampus is particularly sensitive to nicotine (Kenney and Gould, 2008). Similarly, human studies of the impact of smoking during pregnancy have found that it causes impairments in some aspects of learning and memory, but not others (Fried et al, 2003). We also observed increased anxiety in MN mice, which others have shown using different models of perinatal nicotine exposure (Huang et al, 2007; Vaglenova et al, 2004). Interestingly, the ventral hippocampus is required for anxiety-like behavior (Bannerman et al, 2004; Kjelstrup et al, 2002), again suggesting the hippocampus's sensitivity to the effects of nicotine.
This study identified several significant changes in electrophysiological and network activity in the hippocampus that could underlie the learning and memory impairments we observed after early postnatal nicotine exposure. Because LTP is a leading candidate for many forms of memory, we had expected that we would see MN-induced impairments in LTP magnitude or induction. However, early nicotine exposure resulted in facilitated LTP induction and a trend for a small increase in the magnitude of LTP. This raises the possibility that facilitated LTP can induce behavioral impairments by strengthening synapses that compete with those required for object location memory, or that, the observed memory impairments are driven by a different mechanism or form of synaptic plasticity.
Among the changes we observed in MN mice during adolescence was increased depolarization and hyperpolarization in the CA1 region of the hippocampus after stimulation of the Schaffer collateral. One possibility is that this reflects a pervasive restructuring of CA1 connectivity, brought about by the prolonged, nicotine-induced activation of nAChRs during a period critical for hippocampal development. In rodents, nAChRs are present and functional in the brain during late gestation (Naeff et al, 1992; Tribollet et al, 2004; Zoli et al, 1995), and the expression of nAChRs transiently increases during the first two postnatal weeks (Shacka and Robinson, 1998), with particular increases in α2, α5 and α7 subunits (Adams et al, 2002; Son and Winzer-Serhan, 2006; Winzer-Serhan and Leslie, 2005). During this period, nicotinic receptors are important modulators of the strength of newly formed excitatory synapses (Maggi et al, 2003, 2004). Additionally, in this two-week period, nicotinic receptors containing α7 subunits drive the transition in GABAergic signaling from being excitatory to inhibitory (Liu et al, 2006, 2007), which further shapes hippocampal network development. Therefore, the presence of nicotine during this critical window could have caused long-term changes in hippocampal circuitry or synaptic connectivity. Indeed, voltage-sensitive dye imaging revealed significant changes in the network activity of hippocampal slices from MN mice. A similar increase in CA1 excitatory activity has also been observed in rats exposed to one week of postnatal nicotine (Damborsky et al, 2012). Interestingly, we did not observe any corresponding change in fEPSP stimulus response-curves or in paired pulse facilitation, suggesting that these changes in hippocampal physiology are not occurring solely at the SC synapse.
It is also possible that long-lasting compensatory changes in nAChR number or function – rather than altered network connectivity established during postnatal development – underlies the altered hippocampal activity that results from postnatal nicotine exposure. nAChRs continue to play an important role in modulating hippocampal activity throughout life, and anomalous nAChR expression is associated with cognitive impairment and memory disorders including Alzheimer's disease (reviewed in Posadas et al, 2013). Early life nicotine exposure in rodents, particularly during the second postnatal week, has been shown to cause persistent changes in nAChRs, upregulating expression of high-affinity nAChRs such as α4β2 heteromers (Huang and Winzer-Serhan, 2006; Miao et al, 1998; Narayanan et al, 2002), while drastically decreasing low affinity nAChRs such as homomeric α7-containing receptors (Eriksson et al, 2000). Furthermore, prenatal nicotine exposure in rats has been shown to stifle the increase in nAChR-mediated current that normally occurs during adolescence (Britton et al, 2007). Likewise, our results suggest long-term, MN-induced alterations in the normal nicotinic modulation of CA1 activity. We showed that early postnatal nicotine exposure blocked the effect of acute nicotine on CA1 network activity and on TA-LTP, and reversed the facilitating effect of acute nicotine on LTP induced at the SC pathway. It is therefore possible that MN-induced memory impairments are caused by a lack of nicotinic modulation crucial for the synaptic plasticity mechanisms of learning and memory.
This study has identified several significant changes in hippocampal cellular and network activity following early postnatal nicotine exposure, but it still remains to be determined what underlies these changes. We have previously shown that in naïve mice, acute nicotine enhances hippocampal network activity and LTP by driving the inhibition of feedforward inhibition in the SC pathway (Nakauchi and Sumikawa, 2012; Yamazaki et al, 2005). Our results here demonstrate that early postnatal nicotine exposure enhances hippocampal network activity and LTP, and impairs the ability of acute nicotine to enhance hippocampal network activity and LTP. Together, one possible mechanism of enhanced excitatory optical signal and LTP observed in MN mice is the persistent inhibition of feedforward inhibition, occluding the effect of acute nicotine.
In wild-type mice, the facilitation of LTP by acute nicotine is absent in α2 and β2 knockout mice and interrupted by DHβE, an antagonist of β2-containing nAChRs (Nakauchi and Sumikawa, 2012). However, in MN-treated mice, the suppressive effect of acute nicotine on LTP was blocked by the non-specific nAChR antagonist mecamylamine, but not by DHβE or by MLA, an antagonist of α7-containing nAChRs. This suggests that early postnatal nicotine exposure alters nAChR or circuit function to such a degree that a different nAChR subtype now plays the dominant role in LTP modulation, with completely opposite effect. Interestingly, α2* nAChRs, which are located on stratum oriens/ alveus (O/A) interneurons (Ishii et al, 2005; Wada et al, 1989), drive the inhibition of feedforward inhibition via circuitry-dependent mechanism, and hippocampal slices from α2 knockout mice have impaired responses to acute nicotine at both SC and TA pathways (Nakauchi et al, 2007) that are very similar to what we observed in MN slices. Thus, it is possible that the lack of acute nicotine’s effect in MN mice is due to altered function of α2* nAChR-expressing O/A interneuron. The expression of α2 mRNA in O/A interneurons is upregulated during early postnatal period (Son and Winzer-Serhan, 2006). It is possible that early postnatal nicotine exposure continuously excites these interneurons via activation of α2* nAChRs to cause a long-lasting disturbance of GABAergic inhibition and its nicotinic control, affecting nicotinic modulation of LTP and hippocampal-dependent memory in adolescent mice.
This study shows that early postnatal nicotine exposure results in long-lasting and pervasive changes to the CA1 region of the mouse hippocampus, including impairments in long-term spatial memory, and significant changes in CA1 network activity and nicotinic control of synaptic plasticity. Thus, these findings demonstrate the significance of nAChR activity during early brain development, and indicate the critical role of timing-dependent cholinergic induction of synaptic plasticity (Gu et al, 2012; Gu and Yakel, 2011; Ji et al, 2001) and other nicotinic cholinergic-dependent mechanisms of synaptic plasticity (Halff et al, 2014; Ishibashi et al, 2014; Nakauchi and Sumikawa, 2012; Yamazaki et al, 2006) in spatial memory.
Highlights.
Early postnatal nicotine exposure disrupts hippocampus-dependent memory
Early postnatal nicotine exposure lowers the LTP induction threshold
Early postnatal nicotine exposure alters nicotinic modulation of LTP
Acknowledgements
This work was supported by NIDA Grants DA025269, DA025676, and DA026458 to K.S. and NIMH and NIDA Grants MH101491, DA036984, and DA031989 to M.A.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams CE, Broide RS, Chen Y, Winzer-Serhan UH, Henderson TA, Leslie FM, et al. Development of the alpha7 nicotinic cholinergic receptor in rat hippocampal formation. Brain research Developmental brain research. 2002;139(2):175–187. doi: 10.1016/s0165-3806(02)00547-3. [DOI] [PubMed] [Google Scholar]
- Ankarberg E, Fredriksson A, Eriksson P. Neurobehavioural defects in adult mice neonatally exposed to nicotine: changes in nicotine-induced behaviour and maze learning performance. Behavioural brain research. 2001;123(2):185–192. doi: 10.1016/s0166-4328(01)00207-8. [DOI] [PubMed] [Google Scholar]
- Assini FL, Duzzioni M, Takahashi RN. Object location memory in mice: pharmacological validation and further evidence of hippocampal CA1 participation. Behavioural brain research. 2009;204(1):206–211. doi: 10.1016/j.bbr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus--memory and anxiety. Neuroscience and biobehavioral reviews. 2004;28(3):273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Barrett RM, Malvaez M, Kramar E, Matheos DP, Arrizon A, Cabrera SM, et al. Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory. Neuropsychopharmacology. 2011;36(8):1545–1556. doi: 10.1038/npp.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batstra L, Hadders-Algra M, Neeleman J. Effect of antenatal exposure to maternal smoking on behavioural problems and academic achievement in childhood: prospective evidence from a Dutch birth cohort. Early human development. 2003;75(1–2):21–33. doi: 10.1016/j.earlhumdev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Britton AF, Vann RE, Robinson SE. Perinatal nicotine exposure eliminates peak in nicotinic acetylcholine receptor response in adolescent rats. The Journal of pharmacology and experimental therapeutics. 2007;320(2):871–876. doi: 10.1124/jpet.106.112730. [DOI] [PubMed] [Google Scholar]
- Bruin JE, Gerstein HC, Holloway AC. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicological sciences. 2010;116(2):364–374. doi: 10.1093/toxsci/kfq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler AR, Wilkerson AE, Gingras JL, Levin ED. Prenatal cocaine and/or nicotine exposure in rats: preliminary findings on long-term cognitive outcome and genital development at birth. Neurotoxicology and teratology. 1996;18(6):635–643. doi: 10.1016/s0892-0362(96)00125-0. [DOI] [PubMed] [Google Scholar]
- Damborsky JC, Griffith WH, Winzer-Serhan UH. Chronic neonatal nicotine exposure increases excitation in the young adult rat hippocampus in a sex-dependent manner. Brain research. 2012;1430:8–17. doi: 10.1016/j.brainres.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danglot L, Triller A, Marty S. The development of hippocampal interneurons in rodents. Hippocampus. 2006;16(12):1032–1060. doi: 10.1002/hipo.20225. [DOI] [PubMed] [Google Scholar]
- de Graaf-Peters VB, Hadders-Algra M. Ontogeny of the human central nervous system: what is happening when? Early human development. 2006;82(4):257–266. doi: 10.1016/j.earlhumdev.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacology & therapeutics. 2009;122(2):125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppolito AK, Smith RF. Long-term behavioral and developmental consequences of pre- and perinatal nicotine. Pharmacology, biochemistry, and behavior. 2006;85(4):835–841. doi: 10.1016/j.pbb.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Ankarberg E, Fredriksson A. Exposure to nicotine during a defined period in neonatal life induces permanent changes in brain nicotinic receptors and in behaviour of adult mice. Brain research. 2000;853(1):41–48. doi: 10.1016/s0006-8993(99)02231-3. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Differential effects on cognitive functioning in 13- to 16-year-olds prenatally exposed to cigarettes and marihuana. Neurotoxicology and teratology. 2003;25(4):427–436. doi: 10.1016/s0892-0362(03)00029-1. [DOI] [PubMed] [Google Scholar]
- Gu Z, Lamb PW, Yakel JL. Cholinergic coordination of presynaptic and postsynaptic activity induces timing-dependent hippocampal synaptic plasticity. The Journal of neuroscience. 2012;32(36):12337–12348. doi: 10.1523/JNEUROSCI.2129-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z, Yakel JL. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron. 2011;71(1):155–165. doi: 10.1016/j.neuron.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haettig J, Sun Y, Wood MA, Xu X. Cell-type specific inactivation of hippocampal CA1 disrupts location-dependent object recognition in the mouse. Learning & memory. 2013;20(3):139–146. doi: 10.1101/lm.027847.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halff AW, Gomez-Varela D, John D, Berg DK. A novel mechanism for nicotinic potentiation of glutamatergic synapses. The Journal of neuroscience. 2014;34(6):2051–2064. doi: 10.1523/JNEUROSCI.2795-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LZ, Liu X, Griffith WH, Winzer-Serhan UH. Chronic neonatal nicotine increases anxiety but does not impair cognition in adult rats. Behavioral neuroscience. 2007;121(6):1342–1352. doi: 10.1037/0735-7044.121.6.1342. [DOI] [PubMed] [Google Scholar]
- Huang LZ, Winzer-Serhan UH. Chronic neonatal nicotine upregulates heteromeric nicotinic acetylcholine receptor binding without change in subunit mRNA expression. Brain research. 2006;1113(1):94–109. doi: 10.1016/j.brainres.2006.06.084. [DOI] [PubMed] [Google Scholar]
- Ishibashi M, Yamazaki Y, Miledi R, Sumikawa K. Nicotinic and muscarinic agonists and acetylcholinesterase inhibitors stimulate a common pathway to enhance GluN2B-NMDAR responses. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(34):12538–12543. doi: 10.1073/pnas.1408805111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Wong JK, Sumikawa K. Comparison of alpha2 nicotinic acetylcholine receptor subunit mRNA expression in the central nervous system of rats and mice. The Journal of comparative neurology. 2005;493(2):241–260. doi: 10.1002/cne.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31(1):131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Molecular neurobiology. 2008;38(1):101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach HA, Murison R, Moser EI, Moser MB. Reduced fear expression after lesions of the ventral hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(16):10825–10830. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Briggs SJ, Christopher NC, Rose JE. Chronic nicotinic stimulation and blockade effects on working memory. Behavioural pharmacology. 1993;4(2):179–182. [PubMed] [Google Scholar]
- Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314(5805):1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang J, Berg DK. Role of endogenous nicotinic signaling in guiding neuronal development. Biochemical pharmacology. 2007;74(8):1112–1119. doi: 10.1016/j.bcp.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Le Magueresse C, Changeux JP, Cherubini E. Nicotine activates immature "silent" connections in the developing hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):2059–2064. doi: 10.1073/pnas.0437947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Sola E, Minneci F, Le Magueresse C, Changeux JP, Cherubini E. Persistent decrease in synaptic efficacy induced by nicotine at Schaffer collateral-CA1 synapses in the immature rat hippocampus. The Journal of physiology. 2004;559(Pt 3):863–874. doi: 10.1113/jphysiol.2004.067041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahar I, Bagot RC, Davoli MA, Miksys S, Tyndale RF, Walker CD, Maheu M, Huang SH, Wong TP, Mechawar N. Developmental hippocampal neuroplasticity in a model of nicotine replacement therapy during pregnancy and breastfeeding. PLos One. 2012;7:e37219. doi: 10.1371/journal.pone.0037219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNew JJ, Thompson R. Role of the limbic system in active and passive avoidance conditioning in the rat. Journal of comparative and physiological psychology. 1966;61(2):173–180. doi: 10.1037/h0023127. [DOI] [PubMed] [Google Scholar]
- McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, et al. HDAC3 is a critical negative regulator of long-term memory formation. The Journal of neuroscience. 2011;31(2):764–774. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Liu C, Bishop K, Gong ZH, Nordberg A, Zhang X. Nicotine exposure during a critical period of development leads to persistent changes in nicotinic acetylcholine receptors of adult rat brain. Journal of neurochemistry. 1998;70(2):752–762. doi: 10.1046/j.1471-4159.1998.70020752.x. [DOI] [PubMed] [Google Scholar]
- Moscarello JM, LeDoux JE. Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions. The Journal of neuroscience. 2013;33(9):3815–3823. doi: 10.1523/JNEUROSCI.2596-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses SN, Cole C, Driscoll I, Ryan JD. Differential contributions of hippocampus, amygdala and perirhinal cortex to recognition of novel objects, contextual stimuli and stimulus relationships. Brain research bulletin. 2005;67(1–2):62–76. doi: 10.1016/j.brainresbull.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Naeff B, Schlumpf M, Lichtensteiger W. Pre- and postnatal development of high-affinity [3H]nicotine binding sites in rat brain regions: an autoradiographic study. Brain research Developmental brain research. 1992;68(2):163–174. doi: 10.1016/0165-3806(92)90058-5. [DOI] [PubMed] [Google Scholar]
- Nakauchi S, Brennan RJ, Boulter J, Sumikawa K. Nicotine gates long-term potentiation in the hippocampal CA1 region via the activation of alpha2* nicotinic ACh receptors. The European journal of neuroscience. 2007;25(9):2666–2681. doi: 10.1111/j.1460-9568.2007.05513.x. [DOI] [PubMed] [Google Scholar]
- Nakauchi S, Sumikawa K. Endogenously released ACh and exogenous nicotine differentially facilitate long-term potentiation induction in the hippocampal CA1 region of mice. The European journal of neuroscience. 2012;35(9):1381–1395. doi: 10.1111/j.1460-9568.2012.08056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan U, Birru S, Vaglenova J, Breese CR. Nicotinic receptor expression following nicotine exposure via maternal milk. Neuroreport. 2002;13(7):961–963. doi: 10.1097/00001756-200205240-00012. [DOI] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Impaired object recognition with increasing levels of feature ambiguity in rats with perirhinal cortex lesions. Behavioural brain research. 2004;148(1–2):79–91. doi: 10.1016/s0166-4328(03)00176-1. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Slotkin TA. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta paediatrica. 2008;97(10):1331–1337. doi: 10.1111/j.1651-2227.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- Posadas I, Lopez-Hernandez B, Cena V. Nicotinic receptors in neurodegeneration. Current neuropharmacology. 2013;11(3):298–314. doi: 10.2174/1570159X11311030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Turner JR, Blendy JA, Gould TJ. Developmental effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Neurobiol Learn Mem. 2012;97(4):482–494. doi: 10.1016/j.nlm.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, et al. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. The Journal of neuroscience. 2010;30(14):5037–5046. doi: 10.1523/JNEUROSCI.5717-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seress L. Comparative anatomy of the hippocampal dentate gyrus in adult and developing rodents, non-human primates and humans. Progress in brain research. 2007;163:23–41. doi: 10.1016/S0079-6123(07)63002-7. [DOI] [PubMed] [Google Scholar]
- Seress L, Abraham H, Tornoczky T, Kosztolanyi G. Cell formation in the human hippocampal formation from mid-gestation to the late postnatal period. Neuroscience. 2001;105(4):831–843. doi: 10.1016/s0306-4522(01)00156-7. [DOI] [PubMed] [Google Scholar]
- Shacka JJ, Robinson SE. Exposure to prenatal nicotine transiently increases neuronal nicotinic receptor subunit alpha7, alpha4 and beta2 messenger RNAs in the postnatal rat brain. Neuroscience. 1998;84(4):1151–1161. doi: 10.1016/s0306-4522(97)00564-2. [DOI] [PubMed] [Google Scholar]
- Son JH, Winzer-Serhan UH. Postnatal expression of alpha2 nicotinic acetylcholine receptor subunit mRNA in developing cortex and hippocampus. Journal of chemical neuroanatomy. 2006;32(2–4):179–190. doi: 10.1016/j.jchemneu.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson CA, Raskin LA, Suh Y. The effects of prenatal nicotine on radial-arm maze performance in rats. Pharmacology, biochemistry, and behavior. 1991;40(4):991–993. doi: 10.1016/0091-3057(91)90117-k. [DOI] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for object recognition via HDAC inhibition. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(23):9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T, Tominaga Y, Yamada H, Matsumoto G, Ichikawa M. Quantification of optical signals with electrophysiological signals in neural activities of Di-4-ANEPPS stained rat hippocampal slices. Journal of neuroscience methods. 2000;102(1):11–23. doi: 10.1016/s0165-0270(00)00270-3. [DOI] [PubMed] [Google Scholar]
- Tong VT, Dietz PM, Morrow B, D'Angelo DV, Farr SL, Rockhill KM, et al. Trends in smoking before, during, and after pregnancy--Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000–2010. Morbidity and mortality weekly report Surveillance summaries. 2013;62(6):1–19. [PubMed] [Google Scholar]
- Tribollet E, Bertrand D, Marguerat A, Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain. Neuroscience. 2004;124(2):405–420. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vaglenova J, Birru S, Pandiella NM, Breese CR. An assessment of the long-term developmental and behavioral teratogenicity of prenatal nicotine exposure. Behavioural brain research. 2004;150(1–2):159–170. doi: 10.1016/j.bbr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Vogel-Ciernia A, Matheos DP, Barrett RM, Kramar EA, Azzawi S, Chen Y, et al. The neuron-specific chromatin regulatory subunit BAF53b is necessary for synaptic plasticity and memory. Nature neuroscience. 2013;16(5):552–561. doi: 10.1038/nn.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, et al. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. The Journal of comparative neurology. 1989;284(2):314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Leslie FM. Expression of alpha5 nicotinic acetylcholine receptor subunit mRNA during hippocampal and cortical development. The Journal of comparative neurology. 2005;481(1):19–30. doi: 10.1002/cne.20357. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Jia Y, Hamaue N, Sumikawa K. Nicotine-induced switch in the nicotinic cholinergic mechanisms of facilitation of long-term potentiation induction. The European journal of neuroscience. 2005;22(4):845–860. doi: 10.1111/j.1460-9568.2005.04259.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Jia Y, Niu R, Sumikawa K. Nicotine exposure in vivo induces long-lasting enhancement of NMDA receptor-mediated currents in the hippocampus. The European journal of neuroscience. 2006;23(7):1819–1828. doi: 10.1111/j.1460-9568.2006.04714.x. [DOI] [PubMed] [Google Scholar]
- Yanai J, Pick CG, Rogel-Fuchs Y, Zahalka EA. Alterations in hippocampal cholinergic receptors and hippocampal behaviors after early exposure to nicotine. Brain research bulletin. 1992;29(3–4):363–368. doi: 10.1016/0361-9230(92)90069-a. [DOI] [PubMed] [Google Scholar]
- Zoli M, Le Novere N, Hill JA, Jr, Changeux JP. Developmental regulation of nicotinic ACh receptor subunit mRNAs in the rat central and peripheral nervous systems. The Journal of neuroscience. 1995;15(3 Pt 1):1912–1939. doi: 10.1523/JNEUROSCI.15-03-01912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]