Abstract
Hepatocellular carcinoma (HCC) has a poor prognosis due to high recurrence rate. Aspartate-β-hydroxylase (ASPH) is a highly conserved transmembrane protein, which is over expressed in HCC and promotes a malignant phenotype. The capability of ASPH protein-derived HLA Class I and II peptides to generate antigen specific CD4+ and CD8+ immune responses is unknown. Therefore, these studies aim to define the epitope specific components required for a peptide based candidate vaccine. Monocyte-derived dendritic cells (DCs) generated from the peripheral blood mononuclear cells (PBMCs) of HCC patients were loaded with ASPH protein. Helper CD4+ T cells and CD8+ cytotoxic T lymphocytes (CTLs) were co-incubated with the DCs; T cell activation was evaluated by flow cytometric analysis. Immunoinformatics tools were used to predict HLA class I- and class II-restricted ASPH sequences, and the corresponding peptides were synthesized. The immunogenicity of each peptide in cultures of human PBMCs was determined by IFN-γ ELISpot assay. ASPH protein-loaded DCs activated both CD4+ and CD8+ T cells contained within the PBMC population derived from HCC patients. Furthermore, the predicted HLA class I- and class II-restricted ASPH peptides were significantly immunogenic. Both HLA class I- and class II-restricted peptides derived from ASPH induce T cell activation in HCC. We observed that ASPH protein and related peptides were highly immunogenic in patients with HCC and produce the type of cellular immune responses required for generation of anti-tumor activity.
Keywords: aspartate-β-hydroxylase, epitope, hepatocellular carcinoma, immunotherapy, T cells
INTRODUCTION
Hepatocellular carcinoma, the third most common cause of cancer-related death worldwide, is characterized by a very poor prognosis and a high mortality rate [1, 2]. Available therapeutic modalities are largely inadequate. Currently, surgical resection is considered the optimal treatment approach. Only a small proportion of patients qualify for treatment, however, and a recurrence of disease is common following surgery [3, 4]. Therefore, the development of new treatment strategies is a priority.
Aspartate-β-hydroxylase (ASPH, also known as aspartyl-asparaginyl-β-hydroxylase) is a type 2, 758 amino acid, transmembrane protein that belongs to the α-ketoglutarate-dependent dioxygenase family [5]. It is a highly conserved enzyme, which catalyzes the hydroxylation of aspartyl and asparaginyl residues in epidermal growth factor-like domains of proteins including Notch and homologs [6–8]. ASPH is over expressed in HCC compared to normal liver tissue; over expression produces a malignant phenotype characterized by increased cell motility, invasion and metastasis [6, 9]. The level of ASPH protein expression in HCC correlates significantly with postoperative prognosis [10]. ASPH is not expressed in normal liver or regenerating nodules. Indeed, most if not all HCC cells within a given tumor highly express ASPH and not the surrounding uninvolved liver [11].
Previous studies demonstrated that immunization with ASPH protein-loaded dendritic cells (DCs) exerted a substantial anti-tumor effect in an experimental murine model [12, 13]. Moreover, recombinant ASPH protein stimulated a significant increase in antigen-specific CD4+ T cells in PBMC cultures derived from both healthy volunteers and HCC patients [12]. These findings suggest that ASPH may serve as a potential target for immunotherapy since it is expressed on the cell surface. The response of human, cytotoxic CD8+ T lymphocytes to ASPH protein, however, remains to be determined. In the present study, we first demonstrated that full-length ASPH protein induced significant activation of CTLs, as well as CD4+ helper T cells, obtained from individuals with HCC before and after depletion of Treg cells. Next, immunoinformatics tools were used to predict 15 HLA-DRB1-restricted immunogenic consensus sequences (ICS, each composed of multiple epitopes) contained within ASPH. These predicted ICS were synthesized as peptides and their capacities to bind multiple HLA-DRB1 alleles were determined. Thirty HLA class I-restricted ASPH epitopes were also predicted and synthesized. Subsequently, each HLA class I- and class II-restricted peptide was evaluated for immunogenicity in HCC patients.
MATERIALS AND METHODS
Recombinant ASPH and ASPH peptides
Full-length human ASPH (GenBank Accession No. 583325) was cloned into the EcoRI site of the pcDNA vector (Invitrogen, Carlsbad, CA). Recombinant protein was produced in a Baculovirus system (Invitrogen) according to the manufacturer’s instruction [12, 13].
ASPH ICS were predicted using methods previously described [14, 15]. Briefly, the entire ASPH protein was parsed into overlapping nine amino acid frames (9-mers), which constitute the length of the peptide chain that fits into the binding grooves of HLA class I and class II molecules. Each frame was then evaluated using EpiMatrix for its potential to bind a panel of eight common HLA-DRB1 alleles (DRB1*0101, DRB1*0301, DRB1*0401, DRB1*0701, DRB1*0801, DRB1*1101, DRB1*1301, and DRB1*1501), which represent >95% of the MHC diversity in the human population [16]. HLA-DRB1-restricted ICS were constructed from those frames that exhibited potential binding activity using EpiAssembler, an algorithm that maximizes epitope density by assembling immunogenic 9-mers into 18–25 amino acid stretches (ICS) [17]. Each “promiscuous” ICS that resulted was comprised of multiple epitopes capable of binding more than one HLA-DRB1 allele. Additionally, all parsed 9-mers were scored for their potential to bind a panel of six common class I “supertype” alleles; A*0101, A*0201, A*0301, A*2402, B*0702, and B*4403, which cover over 95% of the human population [18]. Based upon these predictions, the peptide sequences were synthesized using FMOC chemistry and purified >85% by HPLC (21st Century Biochemicals, Marlboro, MA); each was dissolved in 100% DMSO (100 mg/ml) and stored at –80°C. Stock peptide solutions were diluted 1:1,000 in medium just prior to culture; 0.1% DMSO in medium alone served as the negative control. The amino acid sequences of the HLA-DRB1-restricted peptides and HLA class I-restricted peptides are shown in Table I and Table II, respectively.
Table I.
Predicted, HLA-DRB1-restricted ASPH immunogenic consensus sequences
| Peptide ID# | Peptide name | AA position | AA sequence |
|---|---|---|---|
| 1 | p52 | 52–71 | TSFFTWFMVIALLGVWTSVA |
| 2 | p103 | 103–117 | AKVLLGLKERSTSEP |
| 3 | p148 | 148–166 | KEQIQSLLHEMVHAEHVEG |
| 4 | p322 | 322–337 | QKAKVKKKKPKLLNKF |
| 5 | p415 | 415–432 | PADLLKLSLKRRSDRQQF |
| 6 | p427 | 427–444 | SDRQQFLGHMRGSLLTLQ |
| 7 | p437 | 437–452 | RGSLLTLQRLVQLFPN |
| 8 | p443 | 443–458 | LQRLVQLFPNDTSLKN |
| 9 | p492 | 492–509 | VHYGFILKAQNKIAESIP |
| 10 | p557 | 557–576 | ASVWQRSLYNVNGLKAQPWW |
| 11 | p581 | 581–597 | TGYTELVKSLERNWKLI |
| 12 | p588 | 588–607 | KSLERNWKLIRDEGLAVMDK |
| 13 | p725 | 725–738 | HEVWQDASSFRLIF |
| 14 | p731 | 731–747 | ASSFRLIFIVDVWHPEL |
| 15 | p740 | 740–758 | VDVWHPELTPQQRRSLPAI |
ASPH, aspartate-β-hydroxylase; AA, amino acid
Table II.
Predicted, HLA class I-restricted ASPH peptide sequences
| Peptide ID# | Peptide name | AA position | AA sequence | Restricting HLA allele |
|---|---|---|---|---|
| 1 | ASPH48 | 48–56 | GLSGTSFFT | A*0201 |
| 2 | ASPH53 | 53–61 | SFFTWFMVI | A*2402 |
| 3 | ASPH58 | 58–66 | FMVIALLGV | A*0201 |
| 4 | ASPH62 | 62–70 | ALLGVWTSV | A*0201 |
| 5 | ASPH72 | 72–80 | VVWFDLVDY | A*0101, A*0301, B*0702 |
| 6 | ASPH79 | 79–87 | DYEEVLGKL | A*2402 |
| 7 | ASPH81 | 81–89 | EEVLGKLGI | B*4403 |
| 8 | ASPH252 | 252–260 | TDDVTYQVY | A*0101, B*4403 |
| 9 | ASPH258 | 258–266 | QVYEEQAVY | A*0101, A0301 |
| 10 | ASPH261 | 261–269 | EEQAVYEPL | B*4403 |
| 11 | ASPH371 | 371–379 | YPQSPRARY | A*0101, B*0702 |
| 12 | ASPH374 | 374–382 | SPRARYGKA | B*0702 |
| 13 | ASPH406 | 406–414 | QEVASLPDV | B*4403 |
| 14 | ASPH411 | 411–419 | LPDVPADLL | B*0702 |
| 15 | ASPH475 | 475–483 | KVYEEVLSV | A*0201 |
| 16 | ASPH478 | 478–486 | EEVLSVTPN | B*4403 |
| 17 | ASPH484 | 484–492 | TPNDGFAKV | B*0702 |
| 18 | ASPH488 | 488–496 | GFAKVHYGF | A*2402 |
| 19 | ASPH491 | 491–499 | KVHYGFILK | A*0301 |
| 20 | ASPH503 | 503–511 | KIAESIPYL | A*0201 |
| 21 | ASPH521 | 521–529 | GTDDGRFYF | A*0101 |
| 22 | ASPH537 | 537–545 | RVGNKEAYK | A*0301 |
| 23 | ASPH557 | 557–565 | ASVWQRSLY | A*0101, A*0301, B*4403 |
| 24 | ASPH563 | 563–571 | SLYNVNGLK | A*0301 |
| 25 | ASPH582 | 582–590 | GYTELVKSL | A*2402 |
| 26 | ASPH611 | 611–619 | LFLPEDENL | A*2402 |
| 27 | ASPH681 | 681–689 | GPTNCRLRM | B*0702 |
| 28 | ASPH693 | 693–701 | LVIPKEGCK | A*0301 |
| 29 | ASPH701 | 701–709 | KIRCANETR | A*0301 |
| 30 | ASPH711 | 711–719 | WEEGKVLIF | B*4403 |
ASPH, aspartate-β-hydroxylase; AA, amino acid
Healthy blood donors and HCC patients
Blood samples were obtained from 12 HCC patients (HCC #1–12) and 5 healthy blood donors (HD #1–5). The peripheral blood mononuclear cells (PBMCs) were purified from whole blood of the HCC patients as previously described [12]. Hartford Hospital Transplantation Research Laboratory (Hartford, CT) performed HLA typing on DNA extracted from each sample.
De-identified whole-blood leukocyte reduction filters (blood filters; Sepacell RZ-2000, Baxter Healthcare Corporation, Irvine CA), obtained after use from the Rhode Island Blood Center (Providence, RI), served as the source of PBMCs derived from blood donated with informed consent by healthy volunteers. The PBMCs were recovered by back-flushing the filters according to the methods of Meyer et al. and purified by centrifugation on Ficoll-Paque Plus (1.077; Pharmacia, Uppsala, Sweden) gradient as we described previous [19, 20]. The Rhode Island Hospital Institutional Review Board approved this study.
Epitope-specific T cell induction
Epitope-specific T cells were induced according to methods we described previously [21]. Briefly, 2.5 × 105 PBMCs/200 µl X-VIVO 15 medium supplemented with 1 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, and 50 U/ml recombinant human IL-2 (R&D Systems) in round-bottom 96-well plates were cultured for 2 weeks with 10 µg/ml individual peptide.
Alternatively, ASPH-specific T cells were generated by co-culturing purified T lymphocytes with protein-pulsed DCs in accordance with methods we also reported previously [12]. Briefly, monocytes were isolated from PBMCs using anti-CD14 microbeads (Miltenyi Biotec, Auburn, CA) and cultured for 5 days in X-VIVO 15 medium (Lonza, Walkerville, MD) supplemented with human GM-CSF (R&D Systems, Minneapolis, MN) and IL-4 (R&D Systems). ASPH protein (1 µg/ml) was added on day 5; TNF-α (R&D Systems) was added on the following day to stimulate DC maturation, and the cells were incubated for another 48 hours. DCs incubated with α-fetoprotein (AFP; Zynaxis Cell Science, Malvern, PA) or alone served as the control. Mature, epitope-expressing DCs were collected at the end of the incubation period.
T cells were isolated from PBMCs by negative selection using the Pan T Cell Isolation Kit II (Miltenyi Biotec). Regulatory T(reg) cells were removed by the addition of anti-CD25 microbeads (Miltenyi Biotec) where indicated. Treg cell-depleted or non-depleted T lymphocytes (2.4 × 106) were co-cultured for 8 days with 4 × 104 mature DCs loaded with relevant antigen in 24-well plates [12].
Enzyme-linked immunospot (ELISpot) assay
Human IFN-γ ELISpot assays were performed as we described previously using a kit purchased from eBioscience (San Diego, CA) to determine T cell immune-reactivity [21]. Cells (5 × 104/well) collected after induction were added to ELISpot plates (Millipore, Bedford, MA) pre-coated with anti-IFN-γ capture antibody and incubated with peptides (10 µg/ml) for 20 hours. Subsequently, the plates were washed and incubated sequentially with biotinylated IFN-γ detection antibody then avidin-HRP. The plates were developed by adding substrate, 3-amino-9-ethyl carbazole, and the number of spots/well was quantified using a CTL-immunospot S5 UV Analyzer (Cellular Technology Limited, Shaker Heights, OH).
Blocking of T cell response
To demonstrate the contribution of HLA molecules to ASPH peptide-dependent T cell activation, the cells were incubated with antibodies specific for HLA class I (clone W6/32; BioLegend, San Diego, CA) or HLA-DR (clone L432; BioLegend) (15 µg/ml) for 1 hour at 37°C prior to analyses.
Flow cytometric analysis
Flow cytometric analysis was conducted as previously described [12]. Intracellular cytokine staining was performed to evaluate T cell activation. Conjugated mouse monoclonal antibodies specific for the following determinants were used: CD4 (clone OKT4; BioLegend, San Diego, CA), CD8a (clone RPA-T8; BioLegend), CD137 (clone 4B4-1; BD Biosciences, San Diego, CA), CD154 (clone TRAP1; BD Biosciences), and IFN-γ (clone B27; BD Biosciences). Appropriate isotype controls were included in each analysis.
Enzyme-linked immunosorbent assay
ELISAs were performed to quantify IFN-γ in cell culture supernatants using a human IFN-γ ELISA kit (eBioscience) as previously described [12].
Statistical analysis
Data analyses were performed using StatView (version 5.0; SAS Institute Inc., Cary, NC). Differences were assessed using the Mann-Whitney U test for unpaired samples and the Wilcoxon signed rank tests for paired samples. A p value < 0.05 was considered statistically significant.
RESULTS
Immunogenic response to ASPH
Experiments were undertaken to determine the capacity of ASPH to activate CD8+ CTLs, as well as helper CD4+ T cells, contained in the PBMC populations derived from HCC patients. The experimental procedure for evaluating ASPH-specific T cell activation was optimized in a previous investigation using PBMCs derived from a healthy donor [12]. In that study, pan T cells were co-cultured with autologous, ASPH protein-pulsed DCs; T cells co-incubated with AFP-pulsed or non-pulsed DCs served as controls. After 8 days culture, the T cells were collected and re-incubated with freshly prepared DCs pre-loaded with the same antigen; antigen-specific T cell activation was evaluated by flow cytometric analysis. In addition to IFN-γ, the expression of CD154 and CD137 was used as surrogate markers for antigen-specific activation of CD4+ T cells and CD8+ CTLs, respectively [22, 23]. These experiments revealed significant increases in the activation of both CD4+ T cells and CTLs in response to ASPH protein compared to AFP or no antigen (Supplementary Figure S1). Moreover, they support the concept that immunization with ASPH protein may activate specific CD4+ and CD8+ T cells in HCC patients.
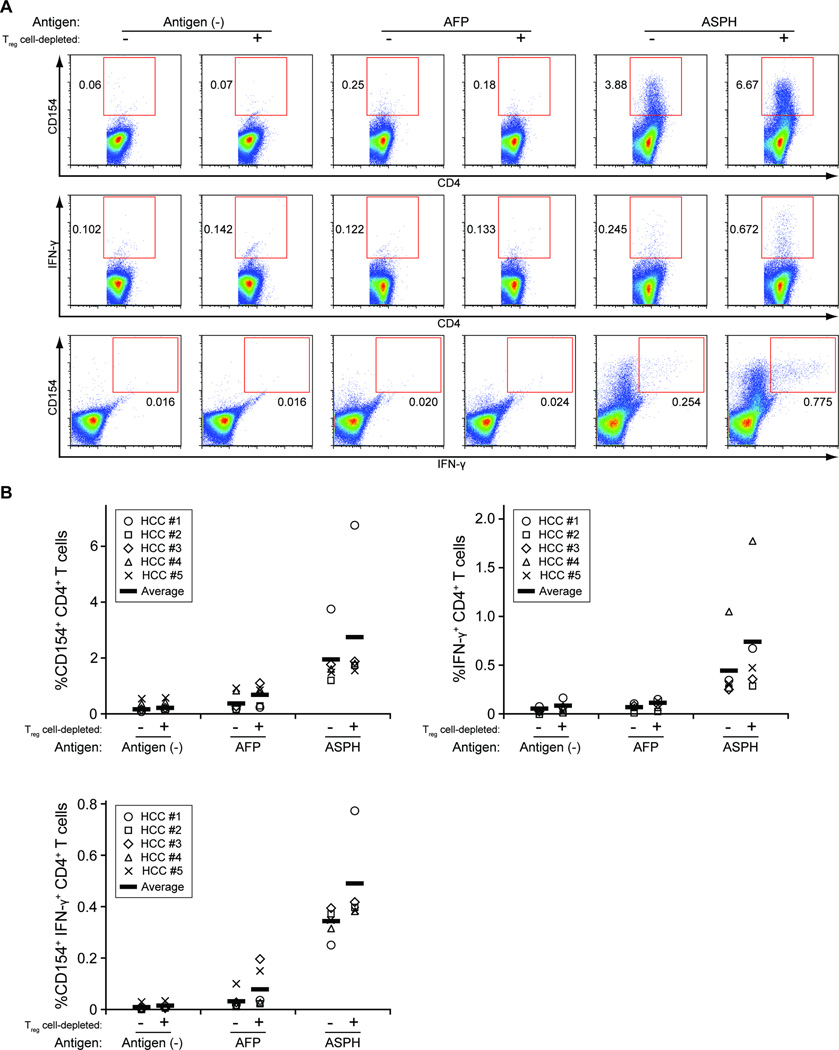
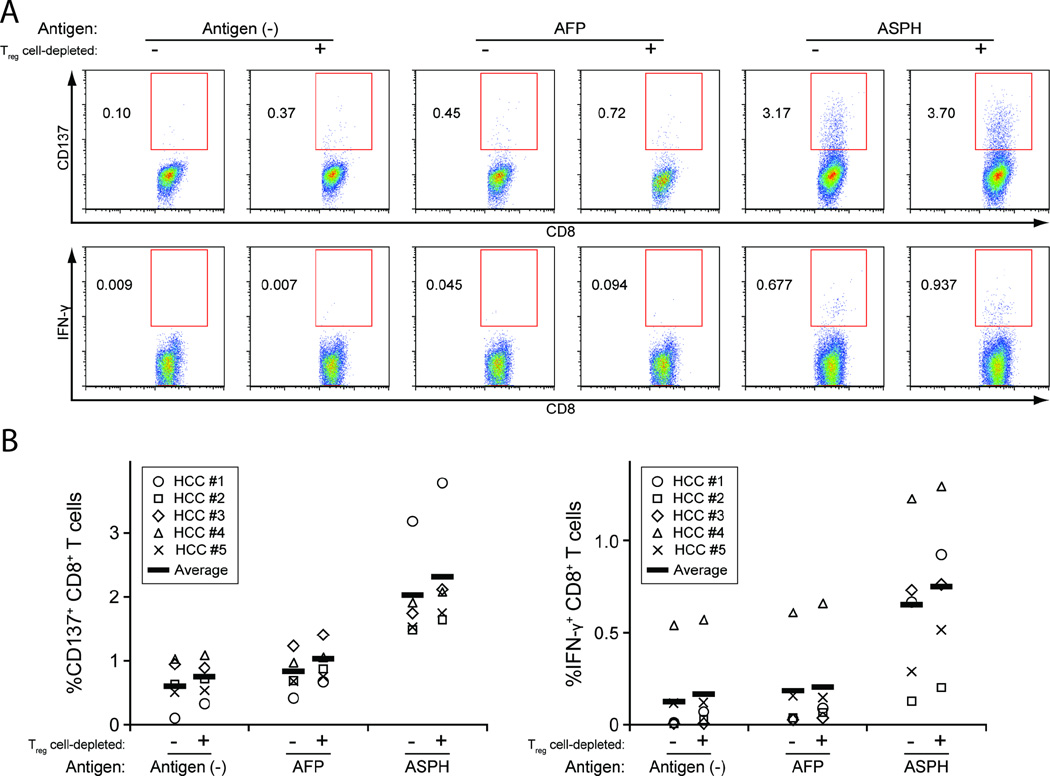
Using the same experimental approach, ASPH-specific T cell activation was evaluated in PBMCs derived from five HCC patients (HCC #1–5) characterized in Supplementary Table S1. As shown in Figures 1A and B, incubation with ASPH protein-pulsed DCs resulted in significant increases in CD4+CD154+ and CD4+IFN-γ+ T cells compared to incubation with AFP-pulsed or non-pulsed DCs. CD4+ T cells expressing both IFN-γ and CD154 were also expanded significantly. In addition, the percentage of CD8+ CTLs expressing CD137 and IFN-γ was significantly higher in co-cultures that contained ASPH protein-pulsed DCs compared to co-cultures that contained AFP-pulsed or non-pulsed DCs (Figures 2A and B). Moreover, in accordance with the published results of other investigators, Treg cell depletion using anti-CD25 microbeads prior to co-culture enhanced ASPH protein-specific T cell activation [24]. These results demonstrate the ASPH-specific generation of CD4+ and CD8+ T cells among PBMCs derived from HCC patients.
Figure 1. ASPH protein-specific CD4+ T cell response in HCC patients.
CD25-depleted or non-depleted, pan T cells purified from the PBMCs of HCC patients (HCC #1–5) were co-cultured with monocyte-derived DCs loaded with recombinant ASPH protein, AFP or no antigen (control). After 8 days incubation, the T cells were collected and re-stimulated by co-culture with DCs loaded with the same antigen. The response of CD4+ T cells was evaluated by quantifying the percentage of CD154+, IFN-γ+, and IFN-γ+CD154+ cells (A: representative flow cytometric analyses; B: summary of the analyses of 5 HCC patients). The percentages of responsive cells in the ASPH sample are significantly greater than the percentages found in the AFP sample or antigen control; the Treg cell-depleted and non-depleted ASPH samples are significantly different from each other, p < 0.05.
Figure 2. ASPH protein-specific CD8+ CTL response in HCC patients.
The response of CD8+ CTLs present in the same DC-purified T cell co-cultures described in Figure 1 was evaluated by quantifying the percentage of CD137+ and IFN-γ+ cells (A: representative flow cytometric analyses, B: summary of the analyses in 5 HCC patients). The percentages of responsive cells in the ASPH sample are significantly greater than the percentages found in the AFP sample or antigen control; the Treg cell-depleted and non-depleted ASPH samples are significantly different from each other, p < 0.05.
Prediction and validation of HLA-DRB1-restricted ASPH peptide sequences
The demonstrated immunogenicity of ASPH affords the potential development of an ASPH epitope-based immunotherapeutic approach. Both MHC class II-restricted (CD4+) and MHC class I-restricted (CD8+) T cells are absolutely required for sustained anti-tumor immunity [25, 26]. Initially, HLA-DRB1-restricted ICS composed of multiple epitopes and covering full-length human ASPH were predicted and constructed using bioinformatics tools [14, 15]. Fifteen promiscuous ISC, each capable of binding multiple HLA-DRB1 alleles representing >95% of the human population, were synthesized (Table I) [16].
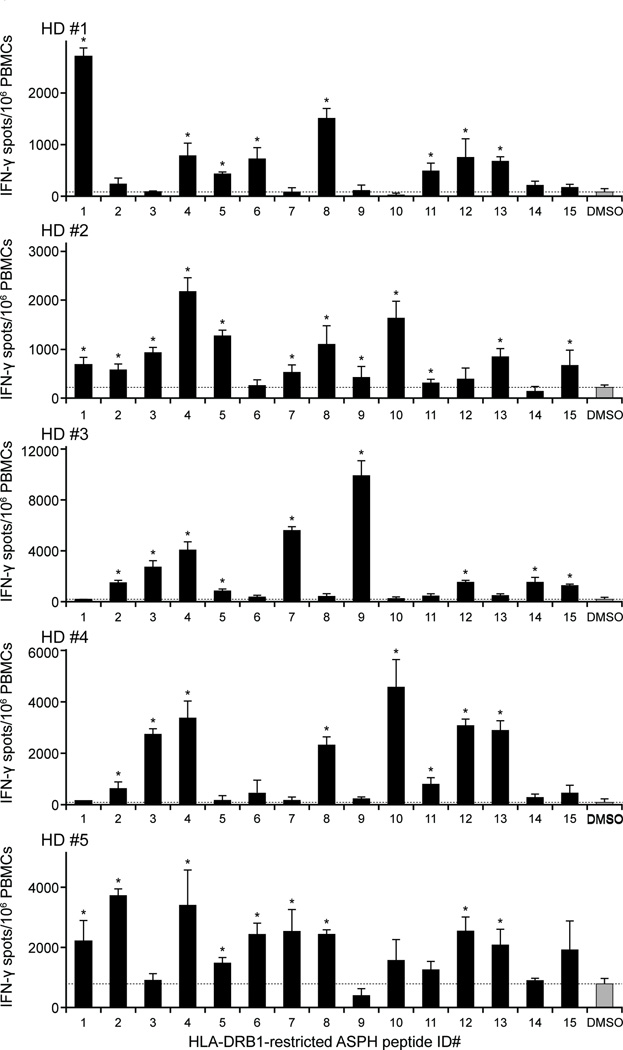
The validity of these predicted ASPH epitopes (ICS) was documented by demonstrating their ability to induce the response of naïve T cells contained within PBMC populations derived from 5 healthy blood donors (HD #1–5). The PBMCs obtained from each donor were transferred to triplicate wells in a 96-well round bottom plate and cultured with a single, ASPH peptide sequence. After 2 weeks, the capacity each sequence to induce a peptide-specific response was evaluated by IFN-γ ELISpot assay. As shown in Figure 3, each peptide sequence induced a statistically significant increase in IFN-γ producing cells, albeit, the response to any single sequence varied among donors. While these ICS are promiscuous and bind multiple HLA-DRB1 alleles, it is likely that differences in the donors’ MHC backgrounds contribute to this varied response. Notably, however, all the ASPH peptides exhibited immunogenicity in at least one healthy blood donor, and p322 (ID #4, Table I) was immunogenic for all 5 donors.
Figure 3. The response of healthy donors to predicted ASPH immunogenic consensus sequences.
PBMCs isolated from healthy donors (HD #1–5) were cultured with the single HLA-DRB1-restricted ASPH peptide (10 µg/ml) enumerated according to the list in Table I. The cells were collected after 2 weeks and the peptide-specific response was quantified by IFN-γ ELISpot assay. Values are expressed as the means ± SD of triplicate determination. The dotted lines represent the level of control (0.1% DMSO). *Significantly greater than the control, p < 0.05.
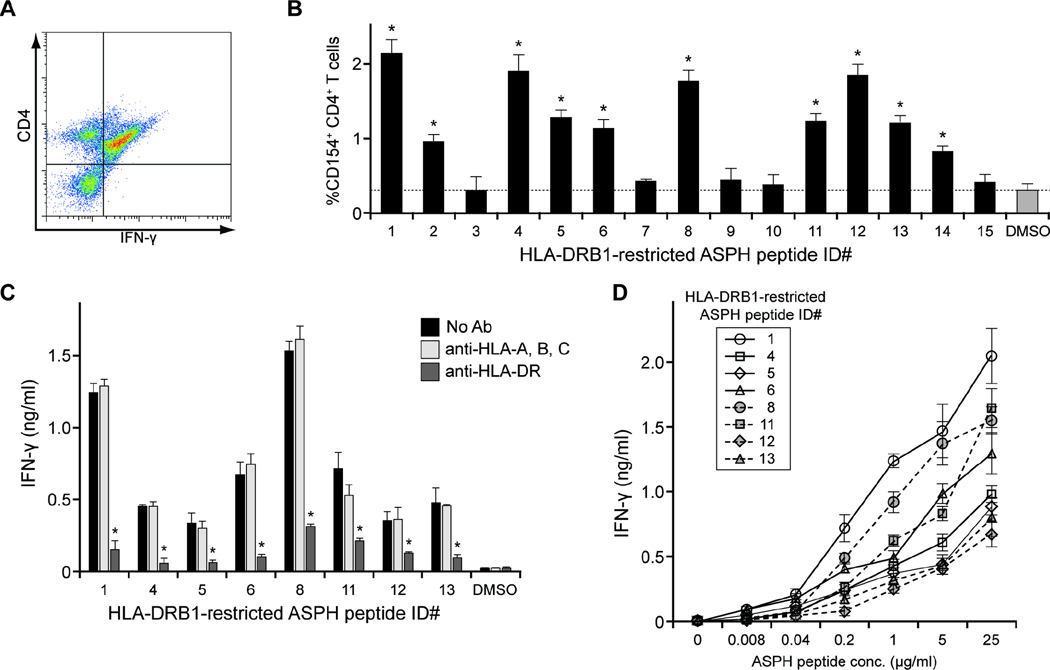
To quantify and characterize the IFN-γ-producing cells, the PBMCs derived from HD #1 following induction by p52 (ID #1, Table I) were stained and evaluated by flow cytometric analysis. As predicted, essentially all HLA-DRB1-restricted, p52-reactive, IFN-γ-producing cells expressed CD4 (Figure 4A). In addition, CD154 expression was assessed as a measure of CD4+ T cells activation following incubation with each ASPH peptide (Figure 4B). The pattern of CD154 expression was consistent with the IFN-γ ELISpot data presented above. HLA blocking experiments confirmed the dependence of ASPH peptide-induced IFN-γ production on HLA-DRB1 expression. IFN-γ production by the ASPH peptide-responsive cells was significantly abrogated by the presence of anti-HLA-DR, but not anti-HLA-class I, antibody (Figure 4C). Titration experiments revealed dose-dependent increases in IFN-γ production following ASPH peptide stimulation (Figure 4D). Together, these results substantiate the HLA-DR-restricted, dose-dependent CD4+ T cell responses induced by the ASPH peptides. IL-4 production was not observed following incubation with any of the ASPH peptide sequences indicating that these sequences induced Th1-like CD4+ T cell responses (data not shown).
Figure 4. Characterization of ASPH peptide-specific T cell responses.
PBMCs obtained from patient HD #1 were cultured with the ASPH peptides indicated. After 2 weeks incubation, the cells were collected and re-cultured with the corresponding peptides for 20 hours, followed by analyses. p52-reactive PBMCs were stained and the percentage of IFN-γ+CD4+ T cells was determined by flow cytometric analysis (A). The percentage of CD154-expressing CD4+ T cells assessed by flow cytometric analysis was determined as a measure of the response of cells to the ASPH peptide listed in accordance with Table I (B). The results are the means ± SD of triplicate determinations; dotted lines represent the control (0.1% DMSO) values. *Significantly greater than control, p < 0.05. PBMCs cultured 2 weeks with the peptides listed were not treated or pretreated with anti-HLA-A, B, C or anti-HLA-DR for 1 hour; the same corresponding peptide (10 µg/ml) was then added and IFN-γ in the culture supernates collected after 5 days was quantified by ELISA (C). Results are the means ± SD of triplicate determinations. *Significantly less than non-antibody or anti-HLA-A, B, C-treated; p < 0.05 Peptide titration assay for the immune response of ASPH peptides-specific cells (D). PBMCs, cultured 2 weeks with the ASPH peptides listed, were collected and reincubated with increasing concentrations of the same peptide. IFN-γ in the culture supernates collected after 5 days was quantified by ELISA.
ASPH peptide-specific T cell activation in HCC patients
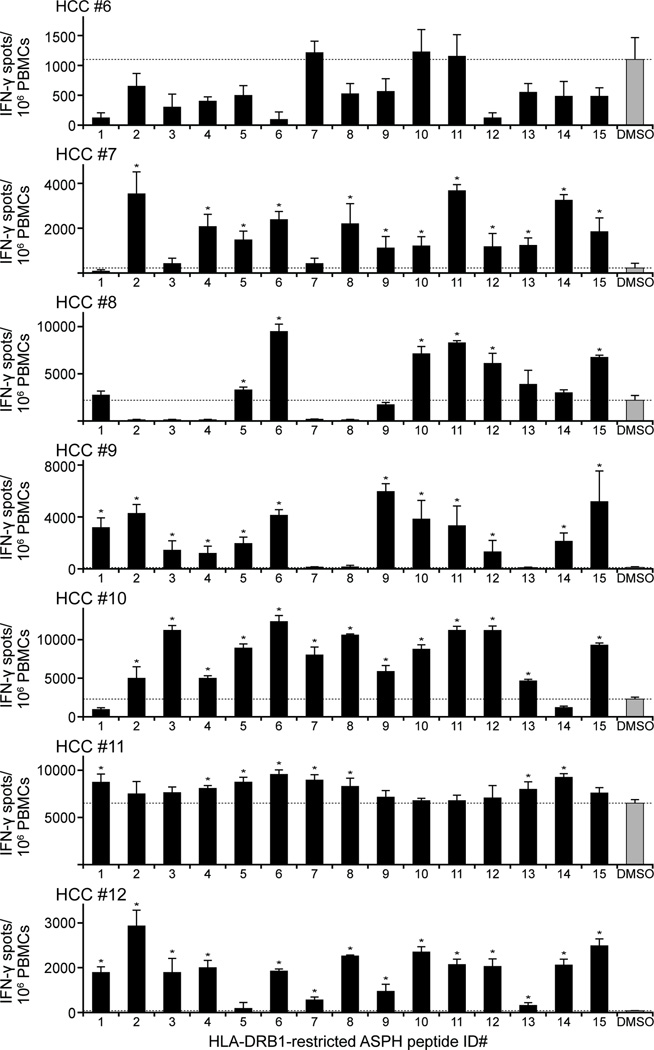
Based upon the results demonstrating the HLA-DRB1-restricted, ASPH ICS-specific T cell response of healthy blood donors, the response of CD4+ T cells derived from seven HCC patients (HCC #6–12) to the same peptides was evaluated. Each HLA class II-restricted peptide sequence induced a statistically significant increase in IFN-γ producing cells, though the response to individual peptides varied among patients (Figure 5). In this regard, none of the peptides elicited a significant response in HCC patient #6. All the predicted ASPH peptides exhibited immunogenicity in at least one HCC patients, however, a pattern similar to that found in the healthy blood donors shown above.
Figure 5. HCC patients respond to HLA-DRB1-restricted ASPH peptides.
PBMCs obtained from 7 HCC patients (HCC #6–12) were cultured for 2 weeks with HLA-DRB1-restricted ASPH peptide enumerated in accordance with Table I. Peptide-specific T cell recognition was evaluated by IFN-γ ELISpot assay. Values are the means ± SD of triplicate determination. The dotted lines represent the level of control (0.1% DMSO). *Significantly greater than the control, p < 0.05.
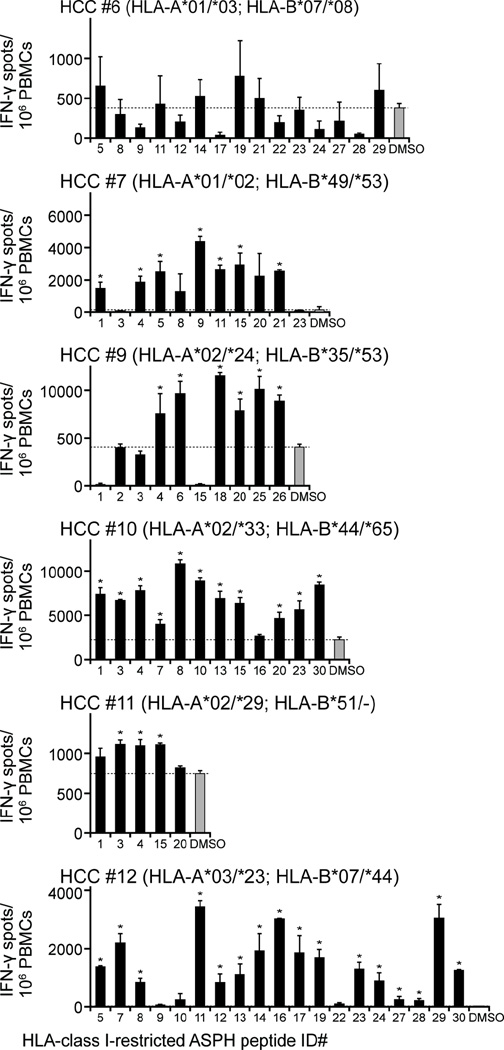
CD8+, as well as CD4+, T cells are required for anti-tumor immunity. As such, HLA class I-restricted ASPH protein-derived epitopes were also predicted and 30 HLA-A- and HLA-B-restricted peptides were synthesized (Table II). The response of CD8+ CTLs derived from the HCC patients to the peptides compatible with their HLA class I alleles was subsequently evaluated. All the HLA class I-restricted peptides induced significant increases in IFN-γ producing cells albeit, as in the case of those restricted by HLA-DRB1, the response to individual peptides varied substantially among patients (Figure 6). HCC patient #6, for example, failed to exhibit a significant response to any peptide among a panel of 11 HLA-A*01/*03- and 5 HLA-B*07-restricted peptides. HCC patient #12, on the other hand, responded to 85% of the peptides compatible with his HLA background.
Figure 6. HCC patients respond to HLA-class I-restricted ASPH peptides.
PBMCs obtained from 7 HCC patients (HCC #6–12) were cultured for 2 weeks with HLA-class I-restricted ASPH peptides enumerated in accordance with Table II and compatible with the patients’ HLA background (indicated in parentheses). Peptide-specific T cell recognition was evaluated by IFN-γ ELISpot assay. Values are the means ± SD of triplicate determination. The dotted lines represent the level of control (0.1% DMSO). *Significantly greater than the control, p < 0.05.
DISCUSSION
The scientific rationale for immunotherapy resides in the fact that tumors are replete with potential antigens. With respect to HCC, TAAs fall into the following three categories [27–29]: (1) proteins with increased expression in tumor cells, such as α-fetoprotein, human telomerase reverse transcriptase (hTERT), glypican-3, and other cancer-associated antigens including melanoma antigen gene (MAGE) family and NY-ESO-1; (2) proteins whose gene is mutated in HCC cells, such as p53; (3) non-cellular proteins encoded by hepatitis viral genomes, such as HBx, HCV core, and HCV NS5. In this context, naturally occurring cellular immunity against α-fetoprotein, hTERT, MAGE, and NY-ESO-1 has been demonstrated in patients with HCC [30–33]. Although these findings suggest that these antigens are immunogenic, the immune responses to these antigens fail to control tumor growth. Inducible cellular immunity against α-fetoprotein, glypican-3, mutant p53, and proteins derived from hepatitis viruses have been demonstrated in human subjects as well as in mice studies [34–39], but there are few reports regarding clinical trials of vaccines that employ these antigens. The only antigen that has been used in a clinical HCC treatment paradigm is α-fetoprotein. However, these investigations revealed that immunization with α-fetoprotein had only minimal impact on patients’ outcome [34, 40]. On the other hand, several studies successfully showed prolonged overall survival when patients were immunized with tumor or formalin-fixed HCC tissue lysates [41–43]. These studies indicate that unidentified antigen(s) may be present in HCC cells that induce antitumor immunity and therefore there is an opportunity to discover and employ novel TAAs for immunotherapy.
The goal of cancer immunotherapy is to induce an effective immune response that specifically targets tumor cells [44]. Success is predicated upon the existence of a tumor-associated antigen (TAA), which is over expressed in tumor tissue and not healthy organs, and elicits a specific immune response in the tumor-bearing host. As examples, several HCC-related TAAs have been identified: AFP, glypican-3, and NY-ESO-1 [45–48]. While T cell responses specific for these TAAs are often observed, however, they are not sufficiently robust to affect clinical outcomes. Although the factors that contribute to these outcomes are poorly understood and require further exploration, one condition in common was a diminished CD4+ helper T cell response. It is well established that both activated CD4+ T cells and CD8+ CTLs are absolutely required to maintain a sustained anti-tumor response [25, 26]. Here, we demonstrated that ASPH protein elicits antigen-specific activation of CD4+ and CD8+ T cells among human PBMCs indicating that ASPH possesses both HLA class I- and class II-restricted epitopes which makes it an attractive candidate for HCC immunotherapy.
The responses observed to total protein raised the possibility of an ASPH epitope-based immunotherapeutic approach for HCC consisting of a combination of peptides (epitopes) capable of eliciting robust anti-tumor responses generated by CD4+ and CD8+ T cells. Toward this end, immunoinformatics tools were used to predict 30 ASPH protein-derived HLA class I-restricted and 15 class II-restricted epitopes. These epitopes induced both CD8+ CTL and CD4+ T cell responses in six out of seven HCC patients suggesting that such identified ASPH peptides may be useful for HCC immunotherapy. One patient (HCC #6), however, failed to respond to any of the epitopes compatible with the HLA background suggesting that the individual would not be a good candidate for this vaccine formulation.
In addition to selecting the ideal TAA to target, strategies to optimize anti-tumor immunity may also require additional approaches to prevent the escape of tumor cells from immune recognition. A myriad of reports address the mechanisms that underlie cancer cell escape, e.g., cell surface inhibitory molecules and immunoregulatory cells including Treg and myeloid-derived suppressor cells [49–52]. Indeed, our findings demonstrated an enhanced T cell response to ASPH protein following CD25+ Treg cell depletion. Moreover, we previously reported that residual tumor cells still expressed cell surface ASPH in a murine model following immunization with ASPH protein-loaded DCs implying the existence of tumor cells that escape immunotherapy [13]. Thus, ASPH-based immunotherapy in combination with other approaches (e.g., antagonizing Treg cell function) may be required to circumvent immune escape and achieve a successful clinical outcome [53]. Low doses of cyclophosphamide, for example, reportedly decrease the number and inhibitory activity of Treg cells by suppressing the expression of important functional markers: forkhead box P3 and glucocorticoid-induced TNF-receptor-related protein [54]. In a tumor-bearing mouse model, cyclophosphamide treatment suppressed Treg cell function and augmented the immune response and tumor eradication [55]. Similarly, sorafenib (a multi-targeted anti-angiogenic tyrosine kinase inhibitor used to treat patients with advanced HCC) reduced Treg cell function and enhanced anti-tumor immunity in mice bearing established orthotropic HCC [56].
Other options such as transarterial embolization or local ablation therapy could also be used to augment ASPH-based HCC immunotherapy. Transarterial embolization, for example, not only induced tumor necrosis directly, but also expanded the T cell-mediated immune response [57]. Additionally, Nobuoka and co-workers reported that radiofrequency HCC ablation induced peptide-specific CTLs [58]. Such studies provide a rationale for combining ASPH-based immunotherapy with additional approaches to optimize treatment of HCC.
Our results suggest that Treg cells comprise a component of the T cell population activated by the entire ASPH protein sequence. Additional studies are required to explore this effect further and to identify the epitope(s) that induces Treg cell function. This observation, however, demonstrates a major advantage of peptide-based, versus whole protein-based, immunotherapy, i.e., Treg cell epitopes can readily be identified and omitted.
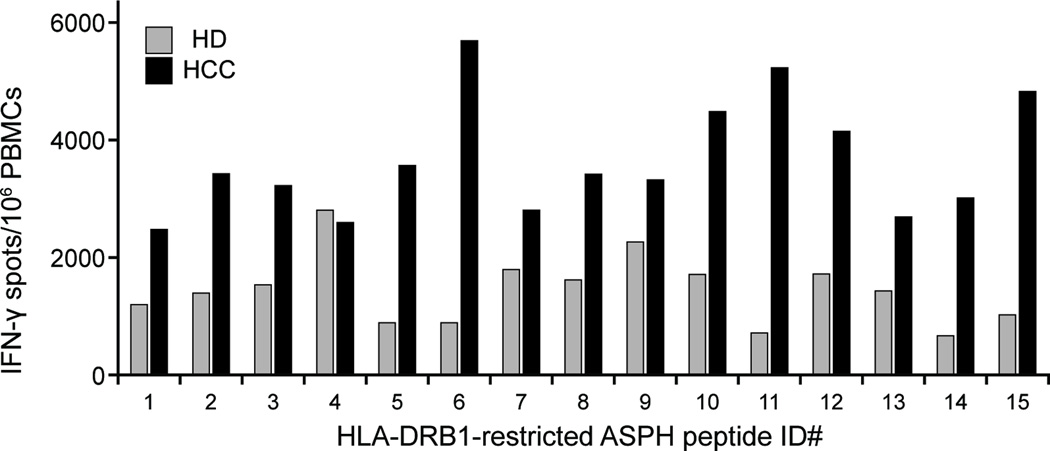
In summary, we demonstrated that ASPH protein elicited antigen-specific CD4+ T cell and CD8+ CTL activation, and that ASPH-derived HLA class I- and class II-restricted peptides can activate CD4+ T cells and CD8+ CTLs derived from HCC patients. The average responses of the healthy donors and the HCC patients to the HLA-DRB1-restricted ASPH peptides (shown in Figures 3 and 5, respectively) are summarized and compared in Figure 7. In the case of normal healthy volunteers, it is presumed that naïve T cells represent the vast majority of induced, antigen-specific T cells. The composition of the antigen-specific T cell population induced in cultures derived from HCC patients is much less clear, however, it is likely comprised of both naïve cells, as well as cells previously sensitized. This contention is supported by the relative increased response of HCC patients to 14 of the 15 peptides tested. Notably, the sample sizes are small and the responses of individuals comprising either population to the same peptide/epitope are extremely variable although all the peptides promoted a response in more than one individual. Undoubtedly, a number of factors contribute to the varied responses observed. While the 15 ICS are promiscuous in their ability to bind multiple DRB1 alleles, for example, it is not known whether each allele capable of binding an ICS and distinguishing individuals within a group is equally capable in inducing a response. Taken together, these findings provide a rationale for development of prophylactic (to prevent or reduce micrometastases following surgical removal of HCC) or therapeutic peptide based approach that may require augmentation with other agents to generate optimal anti-tumor responses.
Figure 7. Summary and comparison of the responses of the healthy donors and HCC patients to HLA-DRB1-restricted ASPH peptides.
The responses (IFN-γ spots/106 PBMCs) of 5 healthy donors (#1–5) and 6 HCC patients (#7–12) to the 15 individual HLA-DRB1-restricted peptides shown in Figures 3 and 5, respectively, were combined and averaged; the background, DMSO control was subtracted.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jan A. Clark (Department of Medicine, Division of Gastroenterology, Rhode Island Hospital) for coordinating the human studies. We are also grateful to Joe Desrosiers (Cell-mediated Immunity Core Laboratory, University of Rhode Island, Providence, RI) for his help in conducting ELISpot assays.
FINANCIAL SUPPORT: National Institutes of Health Research Grants CA-123544 and U19 AI082642 supported this study.
ABBREVIATIONS USED
- HCC
hepatocellular carcinoma
- ASPH
aspartate-β-hydroxylase
- DCs
dendritic cells
- PBMCs
peripheral blood mononuclear cells
- CTLs
cytotoxic T lymphocytes
- ICS
immunogenic consensus sequences
- Treg cell
regulatory T cell
- AFP
α-fetoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTERESTS: William Martin and Anne S. De Groot are senior officers and shareholders at EpiVax, Inc. None of the remaining co-authors has any financial conflict of interest related to the manuscript to disclose.
REFERENCES
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Schutte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma--epidemiological trends and risk factors. Digestive diseases. 2009;27:80–92. doi: 10.1159/000218339. [DOI] [PubMed] [Google Scholar]
- 3.Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Annals of surgery. 1999;229:790–799. doi: 10.1097/00000658-199906000-00005. discussion 9-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu AX. Systemic therapy of advanced hepatocellular carcinoma: how hopeful should we be? The oncologist. 2006;11:790–800. doi: 10.1634/theoncologist.11-7-790. [DOI] [PubMed] [Google Scholar]
- 5.Jia S, VanDusen WJ, Diehl RE, Kohl NE, Dixon RA, Elliston KO, et al. cDNA cloning and expression of bovine aspartyl (asparaginyl) beta-hydroxylase. The Journal of biological chemistry. 1992;267:14322–14327. [PubMed] [Google Scholar]
- 6.Lavaissiere L, Jia S, Nishiyama M, de la Monte S, Stern AM, Wands JR, et al. Overexpression of human aspartyl(asparaginyl)beta-hydroxylase in hepatocellular carcinoma and cholangiocarcinoma. The Journal of clinical investigation. 1996;98:1313–1323. doi: 10.1172/JCI118918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gronke RS, VanDusen WJ, Garsky VM, Jacobs JW, Sardana MK, Stern AM, et al. Aspartyl beta-hydroxylase: in vitro hydroxylation of a synthetic peptide based on the structure of the first growth factor-like domain of human factor IX. Proc Natl Acad Sci U S A. 1989;86:3609–3613. doi: 10.1073/pnas.86.10.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gronke RS, Welsch DJ, VanDusen WJ, Garsky VM, Sardana MK, Stern AM, et al. Partial purification and characterization of bovine liver aspartyl beta-hydroxylase. The Journal of biological chemistry. 1990;265:8558–8565. [PubMed] [Google Scholar]
- 9.de la Monte SM, Tamaki S, Cantarini MC, Ince N, Wiedmann M, Carter JJ, et al. Aspartyl- (asparaginyl)-beta-hydroxylase regulates hepatocellular carcinoma invasiveness. Journal of hepatology. 2006;44:971–983. doi: 10.1016/j.jhep.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 10.Wang K, Liu J, Yan ZL, Li J, Shi LH, Cong WM, et al. Overexpression of aspartyl- (asparaginyl)-beta-hydroxylase in hepatocellular carcinoma is associated with worse surgical outcome. Hepatology. 2010;52:164–173. doi: 10.1002/hep.23650. [DOI] [PubMed] [Google Scholar]
- 11.Aihara A, Huang CK, Olsen MJ, Lin Q, Chung W, Tang Q, et al. A cell-surface betahydroxylase is a biomarker and therapeutic target for hepatocellular carcinoma. Hepatology. 2014;60:1302–1313. doi: 10.1002/hep.27275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimoda M, Tomimaru Y, Charpentier KP, Safran H, Carlson RI, Wands J. Tumor progression-related transmembrane protein aspartate-beta-hydroxylase is a target for immunotherapy of hepatocellular carcinoma. Journal of hepatology. 2012;56:1129–1135. doi: 10.1016/j.jhep.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noda T, Shimoda M, Ortiz V, Sirica AE, Wands JR. Immunization with aspartate-betahydroxylase- loaded dendritic cells produces antitumor effects in a rat model of intrahepatic cholangiocarcinoma. Hepatology. 2012;55:86–97. doi: 10.1002/hep.24629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Groot AS, Jesdale BM, Szu E, Schafer JR, Chicz RM, Deocampo G. An interactive Web site providing major histocompatibility ligand predictions: application to HIV research. AIDS research and human retroviruses. 1997;13:529–531. doi: 10.1089/aid.1997.13.529. [DOI] [PubMed] [Google Scholar]
- 15.Schafer JR, Jesdale BM, George JA, Kouttab NM, De Groot AS. Prediction of wellconserved HIV-1 ligands using a matrix-based algorithm, EpiMatrix. Vaccine. 1998;16:1880–1884. doi: 10.1016/s0264-410x(98)00173-x. [DOI] [PubMed] [Google Scholar]
- 16.Southwood S, Sidney J, Kondo A, del Guercio MF, Appella E, Hoffman S, et al. Several common HLA-DR types share largely overlapping peptide binding repertoires. Journal of immunology. 1998;160:3363–3373. [PubMed] [Google Scholar]
- 17.De Groot AS, Bishop EA, Khan B, Lally M, Marcon L, Franco J, et al. Engineering immunogenic consensus T helper epitopes for a cross-clade HIV vaccine. Methods. 2004;34:476–487. doi: 10.1016/j.ymeth.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Sette A, Sidney J. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics. 1999;50:201–212. doi: 10.1007/s002510050594. [DOI] [PubMed] [Google Scholar]
- 19.Meyer TP, Zehnter I, Hofmann B, Zaisserer J, Burkhart J, Rapp S, et al. Filter Buffy Coats (FBC): a source of peripheral blood leukocytes recovered from leukocyte depletion filters. Journal of immunological methods. 2005;307:150–166. doi: 10.1016/j.jim.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. The Journal of clinical investigation. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mishra S, Losikoff PT, Self AA, Terry F, Ardito MT, Tassone R, et al. Peptide-pulsed dendritic cells induce the hepatitis C viral epitope-specific responses of naïve human T cells. Vaccine. doi: 10.1016/j.vaccine.2014.03.083. in press. [DOI] [PubMed] [Google Scholar]
- 22.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nature medicine. 2005;11:1118–1124. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 23.Wolfl M, Kuball J, Ho WY, Nguyen H, Manley TJ, Bleakley M, et al. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood. 2007;110:201–210. doi: 10.1182/blood-2006-11-056168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moser JM, Sassano ER, Leistritz del C, Eatrides JM, Phogat S, Koff W, et al. Optimization of a dendritic cell-based assay for the in vitro priming of naive human CD4+ T cells. Journal of immunological methods. 2010;353:8–19. doi: 10.1016/j.jim.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunological reviews. 2008;222:129–144. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 26.Marzo AL, Kinnear BF, Lake RA, Frelinger JJ, Collins EJ, Robinson BW, et al. Tumorspecific CD4+ T cells have a major "post-licensing" role in CTL mediated anti-tumor immunity. Journal of immunology. 2000;165:6047–6055. doi: 10.4049/jimmunol.165.11.6047. [DOI] [PubMed] [Google Scholar]
- 27.Behboudi S, Boswell S, Williams R. Cell-mediated immune responses to alpha-fetoprotein and other antigens in hepatocellular carcinoma. Liver Int. 2009 doi: 10.1111/j.1478-3231.2009.02194.x. [DOI] [PubMed] [Google Scholar]
- 28.Butterfield LH. Immunotherapeutic strategies for hepatocellular carcinoma. Gastroenterology. 2004;127:S232–S241. doi: 10.1053/j.gastro.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 29.Greten TF, Blum HE, Manns MP, Geissler M. [Treatment of hepatocellular carcinoma] Z Gastroenterol. 2006;44:43–49. doi: 10.1055/s-2005-858932. [DOI] [PubMed] [Google Scholar]
- 30.Alisa A, Ives A, Pathan AA, Navarrete CV, Williams R, Bertoletti A, et al. Analysis of CD4+ T-Cell responses to a novel alpha-fetoprotein-derived epitope in hepatocellular carcinoma patients. Clin Cancer Res. 2005;11:6686–6694. doi: 10.1158/1078-0432.CCR-05-0382. [DOI] [PubMed] [Google Scholar]
- 31.Bricard G, Bouzourene H, Martinet O, Rimoldi D, Halkic N, Gillet M, et al. Naturally acquired MAGE-A10- and SSX-2-specific CD8+ T cell responses in patients with hepatocellular carcinoma. J Immunol. 2005;174:1709–1716. doi: 10.4049/jimmunol.174.3.1709. [DOI] [PubMed] [Google Scholar]
- 32.Mizukoshi E, Nakamoto Y, Marukawa Y, Arai K, Yamashita T, Tsuji H, et al. Cytotoxic T cell responses to human telomerase reverse transcriptase in patients with hepatocellular carcinoma. Hepatology. 2006;43:1284–1294. doi: 10.1002/hep.21203. [DOI] [PubMed] [Google Scholar]
- 33.Shang XY, Chen HS, Zhang HG, Pang XW, Qiao H, Peng JR, et al. The spontaneous CD8+ T-cell response to HLA-A2-restricted NY-ESO-1b peptide in hepatocellular carcinoma patients. Clin Cancer Res. 2004;10:6946–6955. doi: 10.1158/1078-0432.CCR-04-0502. [DOI] [PubMed] [Google Scholar]
- 34.Butterfield LH, Ribas A, Meng WS, Dissette VB, Amarnani S, Vu HT, et al. T-cell responses to HLA-A*0201 immunodominant peptides derived from alpha-fetoprotein in patients with hepatocellular cancer. Clin Cancer Res. 2003;9:5902–5908. [PubMed] [Google Scholar]
- 35.Ding FX, Wang F, Lu YM, Li K, Wang KH, He XW, et al. Multiepitope peptide-loaded virus-like particles as a vaccine against hepatitis B virus-related hepatocellular carcinoma. Hepatology. 2009;49:1492–1502. doi: 10.1002/hep.22816. [DOI] [PubMed] [Google Scholar]
- 36.Komori H, Nakatsura T, Senju S, Yoshitake Y, Motomura Y, Ikuta Y, et al. Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma. Clin Cancer Res. 2006;12:2689–2697. doi: 10.1158/1078-0432.CCR-05-2267. [DOI] [PubMed] [Google Scholar]
- 37.Motomura Y, Senju S, Nakatsura T, Matsuyoshi H, Hirata S, Monji M, et al. Embryonic stem cell-derived dendritic cells expressing glypican-3, a recently identified oncofetal antigen, induce protective immunity against highly metastatic mouse melanoma, B16-F10. Cancer Res. 2006;66:2414–2422. doi: 10.1158/0008-5472.CAN-05-2090. [DOI] [PubMed] [Google Scholar]
- 38.Ren J, Jia J, Zhang H, Zhang L, Ma B, Jiang H, et al. Dendritic cells pulsed with alphafetoprotein and mutant P53 fused gene induce bi-targeted cytotoxic T lymphocyte response against hepatic carcinoma. Cancer Sci. 2008;99:1420–1426. doi: 10.1111/j.1349-7006.2008.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yutani S, Komatsu N, Shichijo S, Yoshida K, Takedatsu H, Itou M, et al. Phase I clinical study of a peptide vaccination for hepatitis C virus-infected patients with different human leukocyte antigen-class I-A alleles. Cancer Sci. 2009;100:1935–1942. doi: 10.1111/j.1349-7006.2009.01256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butterfield LH, Ribas A, Dissette VB, Lee Y, Yang JQ, De la Rocha P, et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res. 2006;12:2817–2825. doi: 10.1158/1078-0432.CCR-05-2856. [DOI] [PubMed] [Google Scholar]
- 41.Kuang M, Peng BG, Lu MD, Liang LJ, Huang JF, He Q, et al. Phase II randomized trial of autologous formalin-fixed tumor vaccine for postsurgical recurrence of hepatocellular carcinoma. Clin Cancer Res. 2004;10:1574–1579. doi: 10.1158/1078-0432.ccr-03-0071. [DOI] [PubMed] [Google Scholar]
- 42.Lee WC, Wang HC, Hung CF, Huang PF, Lia CR, Chen MF. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunother. 2005;28:496–504. doi: 10.1097/01.cji.0000171291.72039.e2. [DOI] [PubMed] [Google Scholar]
- 43.Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, et al. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49:124–132. doi: 10.1002/hep.22626. [DOI] [PubMed] [Google Scholar]
- 44.Breous E, Thimme R. Potential of immunotherapy for hepatocellular carcinoma. Journal of hepatology. 2011;54:830–834. doi: 10.1016/j.jhep.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 45.Komori H, Nakatsura T, Senju S, Yoshitake Y, Motomura Y, Ikuta Y, et al. Identification of HLA-A2- or HLA-A24-restricted CTL epitopes possibly useful for glypican-3-specific immunotherapy of hepatocellular carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:2689–2697. doi: 10.1158/1078-0432.CCR-05-2267. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Daley S, Evdokimova VN, Zdobinski DD, Potter DM, Butterfield LH. Hierarchy of alpha fetoprotein (AFP)-specific T cell responses in subjects with AFP-positive hepatocellular cancer. Journal of immunology. 2006;177:712–721. doi: 10.4049/jimmunol.177.1.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shang XY, Chen HS, Zhang HG, Pang XW, Qiao H, Peng JR, et al. The spontaneous CD8+ T-cell response to HLA-A2-restricted NY-ESO-1b peptide in hepatocellular carcinoma patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10:6946–6955. doi: 10.1158/1078-0432.CCR-04-0502. [DOI] [PubMed] [Google Scholar]
- 48.Thimme R, Neagu M, Boettler T, Neumann-Haefelin C, Kersting N, Geissler M, et al. Comprehensive analysis of the alpha-fetoprotein-specific CD8+ T cell responses in patients with hepatocellular carcinoma. Hepatology. 2008;48:1821–1833. doi: 10.1002/hep.22535. [DOI] [PubMed] [Google Scholar]
- 49.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nature reviews Immunology. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 50.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annual review of immunology. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 51.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 52.Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang JY, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. International journal of cancer Journal international du cancer. 2011;128:887–896. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 53.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nature reviews Immunology. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 54.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 55.Schiavoni G, Mattei F, Di Pucchio T, Santini SM, Bracci L, Belardelli F, et al. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood. 2000;95:2024–2030. [PubMed] [Google Scholar]
- 56.Chen ML, Yan BS, Lu WC, Chen MH, Yu SL, Yang PC, et al. Sorafenib relieves cellintrinsic and cell-extrinsic inhibitions of effector T cells in tumor microenvironment to augment antitumor immunity. International journal of cancer Journal international du cancer. 2014;134:319–331. doi: 10.1002/ijc.28362. [DOI] [PubMed] [Google Scholar]
- 57.Ayaru L, Pereira SP, Alisa A, Pathan AA, Williams R, Davidson B, et al. Unmasking of alpha-fetoprotein-specific CD4(+) T cell responses in hepatocellular carcinoma patients undergoing embolization. Journal of immunology. 2007;178:1914–1922. doi: 10.4049/jimmunol.178.3.1914. [DOI] [PubMed] [Google Scholar]
- 58.Nobuoka D, Motomura Y, Shirakawa H, Yoshikawa T, Kuronuma T, Takahashi M, et al. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. International journal of oncology. 2012;40:63–70. doi: 10.3892/ijo.2011.1202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.