Abstract
Although imidazoline I2 receptor ligands have been used as discriminative stimuli, the role of efficacy of I2 receptor ligands as a critical determinant in drug discrimination has not been explored. This study characterized the discriminative stimulus effects of selective imidazoline I2 receptor ligands BU224 (a low-efficacy I2 receptor ligand) and phenyzoline (a higher efficacy I2 receptor ligand) in rats. Two groups of male Sprague-Dawley rats were trained to discriminate 5.6 mg/kg BU224 or 32 mg/kg phenyzoline (i.p.) from their vehicle in a two-lever food-reinforced drug discrimination procedure, respectively. All rats acquired the discriminations after an average of 18 (BU224) and 56 (phenyzoline) training sessions, respectively. BU224 and phenyzoline completely substituted for one another symmetrically. Several I2 receptor ligands (tracizoline, CR4056, RS45041, and idazoxan) all occasioned > 80% drug-associated lever responding in both discriminations. The I2 receptor ligand 2-BFI and a monoamine oxidase inhibitor harmane occasioned > 80% drug-associated lever responding in rats discriminating BU224. Other drugs that occasioned partial or less substitution to BU224 cue included clonidine, methamphetamine, ketamine, morphine, methadone and agmatine. Clonidine, methamphetamine and morphine also only produced partial substitution to phenyzoline cue. Naltrexone, dopamine D2 receptor antagonist haloperidol and serotonin (5-HT) 2A receptor antagonist MDL100907 failed to alter the discriminative stimulus effects of BU224 or phenyzoline. Combined, these results are the first to demonstrate that BU224 and phenyzoline can serve as discriminative stimuli and that the low-efficacy I2 receptor ligand BU224 shares similar discriminative stimulus effects with higher-efficacy I2 receptor ligands such as phenyzoline and 2-BFI.
Keywords: BU224, phenyzoline, imidazoline I2 receptors, drug discrimination, rats
1. Introduction
Imidazoline receptors are a group of heterogeneous binding sites which are further classified as I1, I2 and I3 subtypes. Emerging evidence suggests that these subtypes are involved in differential physiological and/or pharmacological functions (Eglen et al., 1998; Li and Zhang, 2011; Parini et al., 1996). Recently, there is a revival in the functional study of I2 receptors and the discovery of several highly selective ligands facilitates this process. For example, it has been shown that selective I2 receptor ligands dose-dependently reduce body temperature (hypothermia) in rats and the effect is mediated through I2 receptors (Thorn et al., 2012). More importantly, increasing data indicate that I2 receptor ligands have antinociceptive effects in several rodent models of acute and chronic pain, suggesting that drugs acting on this receptor may represent a new class of analgesics (Ferrari et al., 2011; Lanza et al., 2014; Li et al., 2014; Li and Zhang, 2011; Li et al., 2011; Meregalli et al., 2012; Thorn et al., 2011).
Studies also suggest that certain I2 receptor ligands can serve as discriminative stimuli in rats. For example, 2-BFI (2-(2-Benzofuranyl)-2-imidazoline hydrochloride), a widely used selective I2 receptor ligand, exerts robust discriminative stimulus effects in rats, which is probably partially mediated through reversible inhibition of monoamine oxidase (MAO) A (Jordan et al., 1996; MacInnes and Handley, 2002, 2003). We recently reported that a new selective I2 receptor ligand CR4056 also can serve as a discriminative stimulus in rats (Qiu et al., 2014a). Importantly, we find that other reported I2 receptor ligands produced varied magnitude of substitution for CR4056 (from full substitution to no substitution), suggesting that although the currently available I2 receptor ligands are invaluable research tools for the study of I2 receptors, they have important differences and require further comparative pharmacological characterizations.
BU224 (2-(4, 5-Dihydroimidazol-2-yl) quinoline hydrochloride) is a widely used selective I2 receptor ligand in research (Thorn et al., 2012). In some studies, BU224 behaves like 2-BFI. In rats discriminating 2-BFI, BU224 fully substituted for 2-BFI (MacInnes and Handley, 2002). Both 2-BFI and BU224 increase rotational behavior in rats with unilateral 6-hydroxydopamine lesion of the nigrostriatal pathway (Macinnes and Duty, 2004) and produce antinociception in a rat writhing test (Li et al., 2011). However, notable differences exist between these two compounds. For example, although both 2-BFI and BU224 produce hypothermia, the effect of BU224 is less effective and reaches a plateau at a moderate dose (Thorn et al., 2012). In acute nociception tests, although 2-BFI enhances the antinociceptive effect of morphine, BU224 has no effect but blocks 2-BFI-induced enhancement (Sanchez-Blazquez et al., 2000; Thorn et al., 2011). Indeed, BU224 is sometimes used as an I2 receptor antagonist (Chen et al., 2014; Yang et al., 2013). These findings suggest that BU224 may be a low efficacy I2 receptor agonist, although other mechanisms cannot be ruled out under certain conditions (Min et al., 2013). Phenyzoline, 4,5-dihidro-2-(2-phenylethyl)-1H-imidazole, is a recently described selective I2 receptor ligand, which shows remarkable receptor binding selectivity for I2 receptors over I1 and α2 adrenoceptors (Gentili et al., 2006). Our receptor binding screening results confirmed its pharmacological selectivity (Table 1). Functionally, phenyzoline increases morphine-induced antinociception and produces hypothermia, and both of the effects are mediated through I2 receptors (Gentili et al., 2006; Thorn et al., 2012). These results suggest that phenyzoline may have higher efficacy than BU224 on I2 receptors.
Table 1.
Binding selectivity screening for phenyzoline.
| Target | Radioligand | % inhibition (10nM) | pKi |
|---|---|---|---|
| 5-HT1A | [3H]8-OH-DPAT | - | <5.0 |
| 5-HT1B | [3H]GR125743 | −2.6 | |
| 5-HT1D | [3H]GR125743 | 5.0 | |
| 5-HT1E | [3H]5HT | −4.2 | |
| 5-HT2A | [3H]Ketanserin | 38.4 | |
| 5-HT2C | [3H]Mesulergine | 14.3 | |
| 5-HT3 | [3H]LY278584 | −13.3 | |
| 5-HT5A | [3H]LSD | 4.6 | |
| 5-HT7 | [3H]LSD | 23.7 | |
| alpha 1A | [3H]Prazosin | 17.3 | |
| alpha 2B | [3H]Prazosin | 6.3 | |
| alpha 1D | [3H]Prazosin | −0.6 | |
| alpha 2A | [3H]-Rauwolscine | - | 6.07±0.09 |
| alpha 2B | [3H]-Rauwolscine | 42.7 | |
| alpha 2C | [3H]-Rauwolscine | 29.6 | |
| β 1 | [125I]Pindolol | −10.1 | |
| β 2 | [3H]CGP12177 | 22.9 | |
| β 3 | [3H]CGP12177 | −5.5 | |
| D1 | [3H]SCH23390 | 0.9 | |
| D2 | [3H]N-Methylspiperone | −7.3 | |
| D3 | [3H]N-Methylspiperone | −4.1 | |
| D4 | [3H]N-Methylspiperone | 9.1 | |
| D5 | [3H]SCH23390 | −11.6 | |
| DAT | [3H]WIN35428 | 43.6 | |
| δ opioid receptor | [3H]DADLE | −2.7 | |
| GABAa | [3H]Muscimol | −1.7 | |
| H1 | [3H]Pyrilamine | −10.9 | |
| H3 | [3H]Alpha-methylhistamine | 10.4 | |
| H4 | [3H]Histamine | 15.2 | |
| κ opioid receptor | [3H]U69593 | 7.1 | |
| M1 | [3H]QNB | −14.1 | |
| M2 | [3H]QNB | 11.1 | |
| M3 | [3H]QNB | −2.0 | |
| M4 | [3H]QNB | −0.5 | |
| M5 | [3H]QNB | 25.9 | |
| μ opioid receptor | [3H]DAMGO | 5.4 | |
| NET | [3H]Nisoxetine | 6.8 | |
| peripheral BZP receptor | [3H]PK11195 | −0.7 | |
| SERT | [3H]Citalopram | 21.9 | |
| Sigma 2 | [3H]DTG | 12.6 |
Note: Data represent mean % inhibition (N = 4 determinations) for compound tested at receptor subtypes. Significant inhibition is considered > 50%. In cases where negative inhibition (−) is seen, this represents a stimulation of binding. The default concentration for primary binding experiments is 10uM.
Drug discrimination is a widely used and powerful behavioral pharmacological approach to discern the receptor mechanisms mediating the subjective effects of centrally-active compounds (Colpaert, 1999). Training doses, efficacy and selectivity of the training drugs are some crucial determinants of the discriminative stimulus properties of drugs (Colpaert, 1988; Comer et al., 1991; Lelas et al., 2000; Porter and Prus, 2009). Regarding the discriminative stimulus effects of imidazoline I2 receptor ligands, little is known of the roles these parameters play in determining their stimulus properties. In order to better understand the discriminative stimulus effects and the in vivo pharmacology of imidazoline I2 receptor ligands, the present study attempted to train rats to discriminate BU224, a low efficacy I2 receptor ligand, or phenyzoline, an I2 receptor ligand with higher efficacy, from its vehicle. Because reliable discriminative control was established with BU224 and phenyzoline, we then characterized their respective discriminative stimuli in rats by conducting substitution and antagonism studies. Given the low efficacy of BU224 at I2 receptors, it is predicted that an asymmetrical substitution should be observed such that the higher efficacy I2 receptor ligand phenyzoline may fully substitute for BU224 in rats discriminating BU224 and the opposite should not be the case.
2. Methods
2.1 Subjects
Two groups of eight adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) each were housed individually on a 12/12-h cycle (discrimination sessions occurred during the light period) with free access to water and restricted access to food and used in BU224 discrimination and phenyzoline discrimination, respectively. The body weights of the rats were maintained at 85% of their free-feeding body weights by adjusting the amount of standard rodent chow that was provided in the home cages after daily sessions. One rat in the BU224 discrimination group was euthanized due to study-unrelated health issue. Animals were maintained and experiments conducted in accordance with the Institutional Animal Care and Use Committee, University at Buffalo, and the 2011 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences).
2.2 Apparatus
Drug discrimination experiments were conducted in commercially available operant chambers within sound-attenuating, ventilated enclosures (Coulbourn Instruments Inc., Allentown, PA, USA) (Qiu et al., 2014a; Qiu et al., 2014b). Each chamber includes an open space (30.5 cm by 24.5 cm by 21.0 cm), a grid floor, and a pellet dispenser with a pellet receptacle that was centered between two response levers, above which were stimulus lights. A 28 V house light was mounted on the rear aluminum wall of the chamber. All experimental events and data recording were controlled by a computer running Graphic State 3.03 software and an interface (Coulbourn Instruments Inc.).
2.3 Drug Discrimination Procedure
Rats were trained to discriminate the training drug (5.6 mg/kg BU224 or 32 mg/kg phenyzoline) intraperitoneally (i.p.) from vehicle while responding under a fixed ratio (FR) 10 schedule of food presentation according to published protocol (Li et al., 2013; Qiu et al., 2014a). The dose of 5.6 mg/kg BU224 was chosen because this dose produces significant antinociceptive effects in several rat models of pain without significantly altering other behavioral indices such as spontaneous locomotor activity and food-maintained operant responding (An et al., 2012; Li et al., 2014; Li et al., 2011; Thorn et al., 2012). The dose of 32 mg/kg phenyzoline was chosen because this dose is a behaviorally active dose that produces significant hypothermic effects without significantly altering spontaneous activity in rats (Thorn et al., 2012).
Briefly, daily 20-min sessions included a 10-min timeout period, during which the chamber was dark and lever presses had no programmed consequence, and a 10-min response period, during which stimulus lights above both levers were illuminated and an FR 10 schedule of food presentation was active. Either saline or training drug was injected 10 min before the initiation of daily sessions. After saline injection, only responding on the saline-associated (left) lever resulted in food delivery (45 mg; BioServ Inc., Frenchtown, New Jersey, USA), and after training drug injection only responding on the other (drug-associated) lever resulted in food delivery. During each session, the rats can earn a maximum of 10 food reinforcers. The session ended either after the animals earned all the reinforcers or after the 10-min response period passed, whichever occurred first. Sessions were conducted 7 days per week according to a double alternation schedule (e.g., saline, saline, drug, drug).
The following criteria were used to indicate that the rats successfully acquired the discrimination: in five consecutive or six of seven sessions, rats pressed at least 90% of the total responses on the correct lever and fewer than ten responses on the incorrect lever before the first reinforcer was earned. Thereafter, tests were conducted every 3rd day provided that the criteria were satisfied during two (one drug and one vehicle) intervening training sessions. If rats failed to satisfy these criteria in any session, discrimination training continued until the criteria were met again for two consecutive sessions.
Test sessions were identical to training sessions except that ten consecutive responses on either lever resulted in the delivery of food and different doses of training drugs or other drugs were administered. In combination studies that involved two drug injections, the pretreatment drug was always administered 10 min before the second drug injection. In general, drugs were studied up to doses that produced at least 80% responding on the drug-associated lever or to doses that significantly decreased the rate of responding.
Several reported I2 receptor ligands with varied receptor selectivity including 2-BFI, tracizoline (2-styryl-4, 5-dihydro-lH-imidazole), RS45041((4-chloro-2-(imidazolin-2-yl) isoindoline), CR4056 (2-phenyl-6-(1H-imidazol-1-yl) quinazoline) and phenyzoline (4, 5-dihidro-2-(2-phenylethyl)-1H-imidazole) (Eglen et al., 1998; Thorn et al., 2012) were studied in substitution tests to examine the generalization of the pharmacological selectivity of the discriminative stimulus effects of BU224 and phenyzoline. Idazoxan was studied as a potential I2 receptor antagonist to confirm the I2 receptor mechanism of the discriminations. Harmane was studied because it is a MAO inhibitor and produces 2-BFI-like but not CR4056-like discriminative stimulus effect (MacInnes and Handley, 2002; Qiu et al., 2014a). Clonidine was studied to test the pharmacologic selectivity of BU224 discrimination because it has high affinity at imidazoline I1 receptors/α2 adrenoceptors (Eglen et al., 1998). The opioids morphine and methadone were studied because there is evidence suggesting a cross-talk interaction between I2 receptors and opioid system (Chang et al., 2010; Ruiz-Durantez et al., 2003). Agmatine was studied because it is a proposed endogenous imidazoline receptor ligand (Li et al., 1994). Since I2 receptor ligands including BU224 modulate dopaminergic activity (Hudson et al., 1999) which may partially attribute to the discriminative stimulus effects of I2 receptor ligands, the dopamine releaser methamphetamine was studied. Ketamine was studied as a negative control. For antagonism studies, naltrexone was tested to assess the potential role of opioid receptors in BU224 and phenyzoline discrimination because opioids partially substituted for BU224 and phenyzoline. The nonselective dopamine receptor antagonist haloperidol and the selective serotonin 5-HT2A receptor antagonist MDL100907 ((R)-(2, 3-dimethoxyphenyl)-[1-[2-(4-fluorophenyl) ethyl]-4-piperidyl] methanol) were tested because BU224 enhances both dopamine and serotonin activity by inhibiting MAO (Hudson et al., 1999) which may contribute to its discriminative stimulus effects. For the antagonists, a 10-min pretreatment was used because our own experience and other studies indicate that this pretreatment time is adequate for the drug to show significant receptor blockade (Li et al., 2009; unpublished observations)
2.4 Data Analyses
The mean percentage of responses on training drug-associated lever (± 1 S.E.M.) was plotted as a function of dose. When an animal responded at a rate less than 20% of the vehicle control rate, discrimination data from that test were not included in the average. The discrimination data were not plotted if the operant response was substantially suppressed such that less than half of the animals responded. Dose-effect curves that attained at least 80% phenyzoline-appropriate responding were analyzed by linear regression to estimate the dose required to generate 50% responding on the training drug-appropriate lever (ED50), along with 95% confidence limits (CLs). Full substitution for the discriminative stimulus was defined as ≥ 80% training drug-appropriate responding, while partial substitution was defined as training drug-appropriate responding ≥ 60% and < 80% (Donahue et al., 2014). In drug combination studies that determined complete dose-effect curves, dose ratios (ED50 values of the training drugs after drug treatment divided by those before drug treatment) were calculated to estimate the magnitude of shift in the training drug dose-effect curves produced by other drugs. When the 95% CLs of the mean dose ratio did not encompass 1, the phenyzoline dose-effect curve was considered to be shifted significantly. Response rates were expressed as the average (± 1 S.E.M.) number of responses per second on both levers and analyzed using one way repeated measures analysis of variance followed by post hoc Bonferroni’s test (GraphPad Prism 6.0, GraphPad Software Inc., La Jolla, CA). P < 0.05 was considered statistically significant.
2.5 Drugs
BU224 hydrochloride, 2-BFI hydrochloride, tracizoline oxalate, phenyzoline oxalate and CR4056 were synthesized according to standard procedures at the Research Triangle Institute and fully characterized by NMR and elemental analysis. RS45041 ((4-chloro-2-(imidazolin-2-yl) isoindoline) was kindly provided by National Institute of Mental Health’s Chemical Synthesis and Drug Supply program (Bethesda, MD, USA). Idazoxan hydrochloride, agmatine hydrochloride, clonidine hydrochloride, naltrexone hydrochloride, harmane hydrochloride, haloperidol, and MDL100907 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ketamine hydrochloride was purchased from Patterson Veterinary (Devens, MA, USA). Morphine sulfate, methadone hydrochloride and methamphetamine hydrochloride were provided by Research Technology Branch, National Institute on Drug Abuse, National Institutes of Health (Rockville, MD, USA). All drugs were dissolved in 0.9% physiological saline except otherwise noted. CR4056 was dissolved in 20% dimethyl sulfoxide (DMSO) with saline and a drop of hydrochloric acid. Haloperidol was dissolved in 0.9% physiological saline with a drop of acetic acid. MDL100907 was dissolved in 20% DMSO with saline.
3. Results
All rats acquired the discriminations after varying training sessions. At the training doses used (5.6 mg/kg for BU224 and 32 mg/kg for phenyzoline), rats acquired BU224 discrimination [18 (range = 10–23) training sessions] quicker than phenyzoline discrimination [56 (range = 27–69) training sessions].
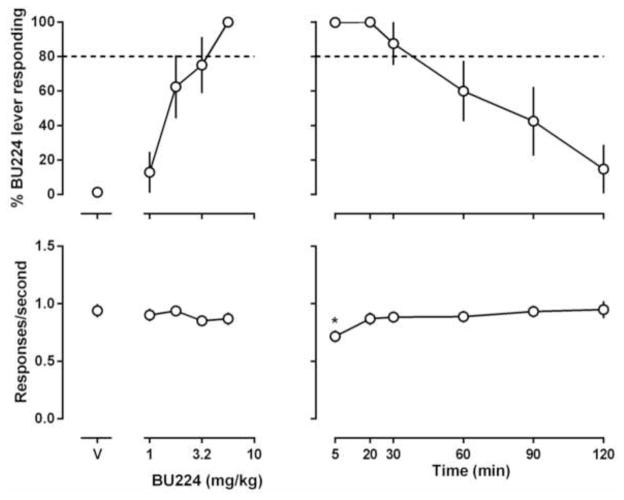
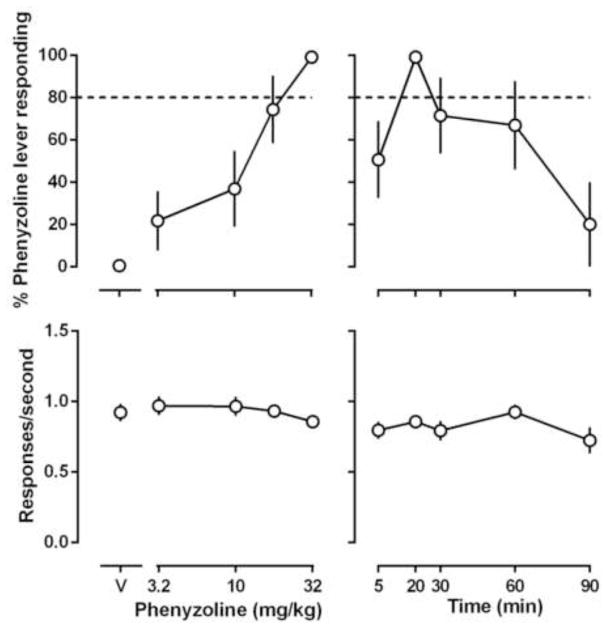
Both BU224 and phenyzoline dose-dependently increased training drug-associated lever responding without significantly altering response rate (left panels, Figs. 1 and 2). The training doses of BU224 and phenyzoline induced the discriminative stimulus effects in a time-dependent manner with 5.6 mg/kg BU224 lasting 120 min and 32 mg/kg phenyzoline lasting 90 min (right panels, Figs. 1 and 2). The ED50 (95% CLs) values of BU224 and phenyzoline were 1.9 (1.3, 2.9) mg/kg and 13.7 (6.6, 19.0) mg/kg, respectively (Table 2). In addition, phenyzoline (Fig. 3) increased BU224-associated lever responding to near 100% in rats discriminating BU224 [ED50: 31.3 (18.1, 54.1) mg/kg] and BU224 (Fig. 4) increased phenyzoline-associated lever responding to near 100% in rats discriminating phenyzoline [ED50: 2.9 (1.8, 3.7) mg/kg], demonstrating a clear symmetrical substitution.
Figure 1.
Effects of BU224 in rats trained to discriminate between 5.6 mg/kg BU224 (i.p) and vehicle using a two-lever food-reinforced procedure. The mean (± 1 S.E.M.) percentage of responses on the BU224-appropriate lever (top panels) and the mean (± 1 S.E.M.) rate of responding (bottom panels) are plotted as a function of dose in the left panels and as a function of time after i.p. administration of 5.6 mg/kg BU224 in the right panels. Points above “V” indicate vehicle. Asterisks indicate mean rate of responding that were significantly different from control.
Figure 2.
Effects of phenyzoline in rats trained to discriminate between 32 mg/kg phenyzoline (i.p) and vehicle using a two-lever food-reinforced procedure. The mean (± 1 S.E.M.) percentage of responses on the phenyzoline-appropriate lever (top panels) and the mean (± 1 S.E.M.) rate of responding (bottom panels) are plotted as a function of dose in the left panels and as a function of time after i.p. administration of 32 mg/kg phenyzoline in the right panels. Points above “V” indicate vehicle.
Table 2.
ED50 values (95% CL) of the test drugs in substitution tests and BU224 in combination studies.
| Test drugs | ED50 (95% CL) (mg/kg) | |
|---|---|---|
| BU224 discrimination | Phenyzoline discrimination | |
| Substitution studies | ||
| BU224 | 1.9 (1.3, 2.9) | 2.9 (1.8, 3.7) |
| 2-BFI | 3.0 (2.0, 4.6) | 2.3 (0.9, 3.3) |
| Tracizoline | 34.9 (22.1, 55.3) | 24.2 (7.7, 31.9) |
| RS45041 | 9.8 (5.3, 18.2) | 3.9 (2.8, 4.7) |
| CR4056 | 5.0 (2.9, 8.6) | 4.8 (2.2, 6.2) |
| Idazoxan | 6.4 (3.8, 11.0) | 4.1 (0.6, 5.1) |
| Phenyzoline | 31.3 (18.1, 54.1) | 13.7 (6.6, 19.1) |
|
| ||
| Combination studies | ||
|
| ||
| 0.32 mg/kg naltrexone | 3.1 (2.9, 3.2) | 13.9 (8.1, 18.3) |
| 1.0 mg/kg naltrexone | 3.0 (2.9, 3.1) | - |
| 0.1 mg/kg haloperidol | 2.4 (2.3, 2.5) | 13.0 (7.2, 17.2) |
| 0.1 mg/kg MDL100907 | 2.3 (2.2, 2.5) | 12.0 (7.0, 16.4) |
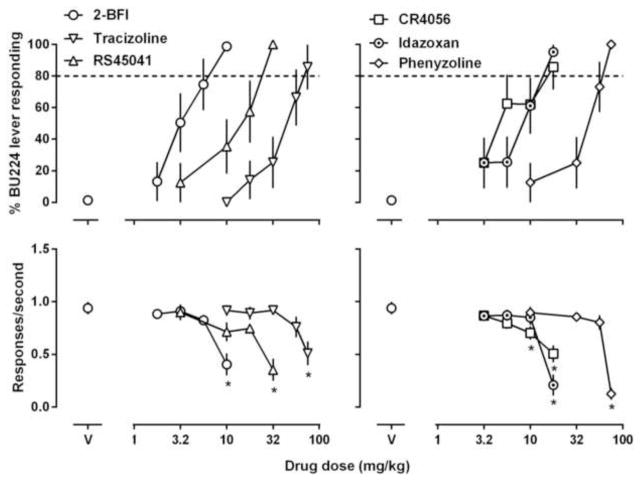
Figure 3.
Effects of 2-BFI, tracizoline, phenyzoline, CR4056, RS45041 and idazoxan in rats discriminating 5.6 mg/kg BU224 (i.p). See Figures 1 and 2 for other details.
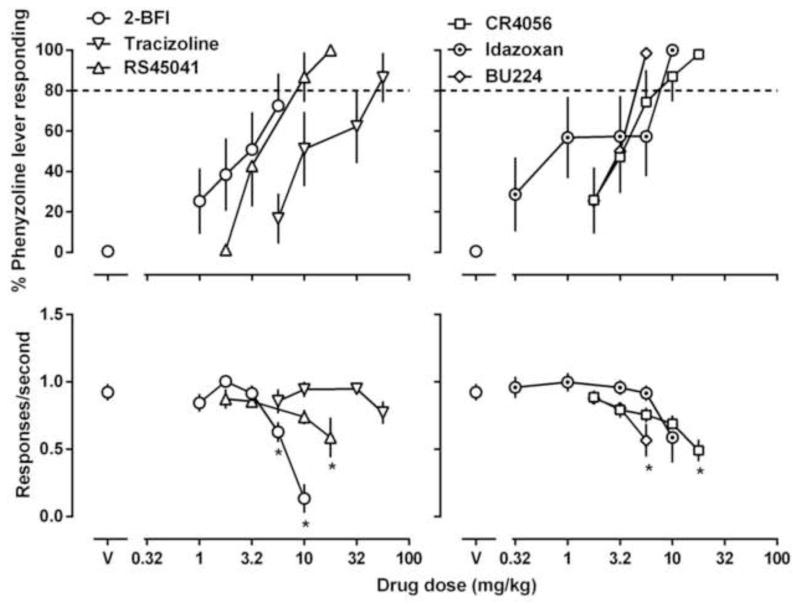
Figure 4.
Effects of 2-BFI, tracizoline, BU224, CR4056, RS45041 and idazoxan in rats discriminating 32 mg/kg phenyzoline (i.p). See Figures 1 and 2 for other details.
Four reported imidazoline I2 receptor ligands tracizoline, CR4056, RS45041 and idazoxan produced greater than 80% drug-associated lever responding in both groups of rats and they often significantly reduced response rate at the largest doses studied (Figs. 3 and 4). The ED50 (95% CLs) values of these drugs for substituting both BU224 and phenyzoline are presented in Table 2. It is important to note that despite the differences of the absolute ED50 values in the two discrimination assays, the rank orders of these I2 receptor ligands for fully substituting BU224 and phenyzoline are similar: BU224 ≥ CR4056 ≥ idazoxan ≥ RS45041 > phenyzoline ≥ tracizoline (Table 2). In contrast, the I2 receptor ligand 2-BFI fully substituted for BU224 (Fig. 3) but only partially substituted for phenyzoline (Fig. 4).
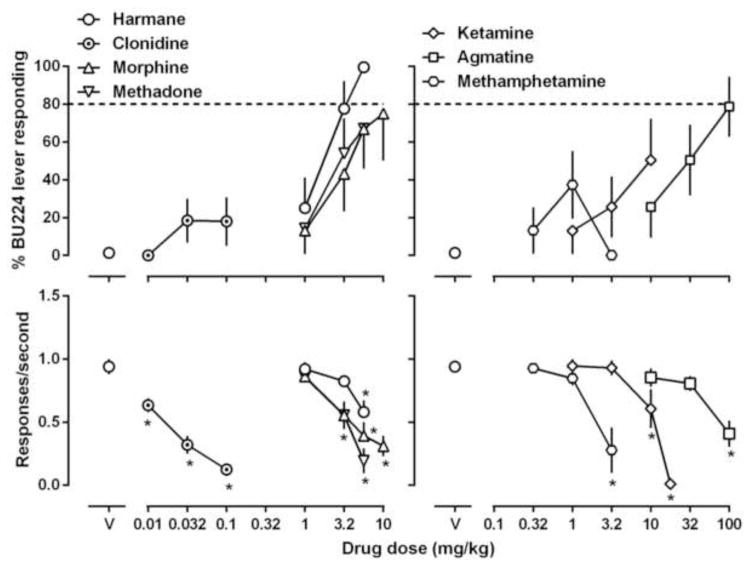
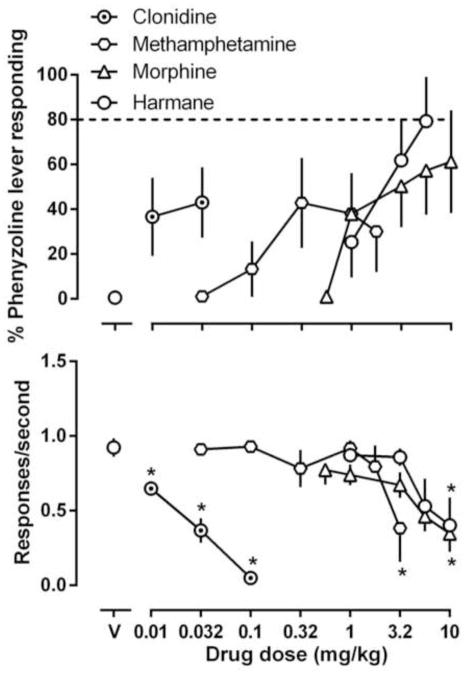
We also tested drugs that exert their pharmacological effects primarily through non-I2 receptors to test the pharmacological specificity of the discriminations. In general, these compounds only produced partial or less training drug-like discriminative stimulus effects. The opioid morphine increased BU224-appropriate responding to 75% and phenyzoline-appropriate responding to 61% (upper panel, Figs. 5 and 6). The monoamine releaser methamphetamine produced a maximum of 40% BU224-appropriate responding and 42% phenyzoline-appropriate responding up to a dose (3.2 mg/kg) that markedly suppressed operant responding (hexagon, Figs. 5 and 6). A β-carboline harmane produced near maximal BU224-appropriate responding and 79% phenyzoline-appropriate responding up to doses that significantly decreased response rate (open squares, Figs. 5 and 6). Clonidine produced a maximum of 18% BU224-appropriate responding and 43% phenyzoline-appropriate responding up to a dose that eliminated the operant responding in all animals (circled dot, Figs. 5 and 6). In rats discriminating BU224, three other compounds were also studied for substitution test, and each of the three compounds [methadone (67%), ketamine (50%) and agmatine (78%)] produced partial or no substitution up to doses that significantly suppressed response rate (Fig. 5).
Figure 5.
Effects of harmane, clonidine, morphine, methadone, ketamine, agmatine and methamphetamine in rats discriminating 5.6 mg/kg BU224. See Figures 1 and 2 for other details.
Figure 6.
Effects of harmane, clonidine, morphine and methamphetamine in rats discriminating 32 mg/kg phenyzoline (i.p). See Figures 1 and 2 for other details.
The opioid receptor antagonist naltrexone, the dopamine D2 receptor antagonist haloperidol and the selective 5-HT2A receptor antagonist MDL100907 failed to antagonize the discriminative stimulus effects of BU224 and phenyzoline (Table 2). In each case, the dose ratios (95% CLs) of BU224 and phenyzoline in the presence and absence of the individual antagonists are not significantly different from 1 because the 95% CLs of the dose ratios included 1 (Table 2).
4. Discussion
The primary findings of the current study were that both BU224 and phenyzoline could serve as discriminative stimuli in a time- and dose-dependent manner in rats and that both compounds produced symmetrical substitution to each other. It was also found that compounds with reported imidazoline I2 receptor binding affinities produced similar substitution profiles with similar rank orders in both discrimination assays. Drugs from different pharmacological classes such as morphine, methamphetamine and clonidine did not produce prominent substitution in both assays, suggesting that the discriminative stimulus effects of BU224 and phenyzoline are pharmacologically selective. Antagonism studies suggest that opioid receptors, dopamine D2, and 5-HT2A receptors do not play major roles in the discriminative stimulus effects of either BU224 or phenyzoline. Together, these results demonstrate that the previous prediction that the low efficacy I2 ligand BU224 and the higher efficacy I2 ligand phenyzoline would produce asymmetrical substitution was incorrect, and that although differences exist, the discriminative stimulus effects of BU224 and phenyzoline are similar.
Imidazoline receptors refer to the non-adrenergic binding sites that drugs such as clonidine and p-aminoclonidine bind to (Ernsberger et al., 1987). Although the molecular detail of I2 receptors remains elusive, it is known that the receptor is heterogeneous and includes several different proteins including one that has recently been identified as brain creatine kinase (Kimura et al., 2009). Nonetheless, medicinal chemistry efforts have led to a number of highly selective I2 receptor ligands, including those tested in this study (Thorn et al., 2012). For example, RS45041 is a highly selective I2 receptor ligand with high affinity for I2 receptors and very low affinity (> 1000-fold) for adrenoceptors, dopamine, 5-HT, muscarinic receptors and dihydropyridine binding sites (Brown et al., 1995). Tracizoline is a cirazoline analog with high affinity and selectivity for I2 receptors over α2 adrenoceptors (7431-fold) and less selective for I1 receptors (10-fold) (Pigini et al., 1997). CR4056 is a highly selective moderate affinity I2 receptor ligand (IC50=596 nM) which shows negligible binding affinities for over 35 other receptors, enzymes and ion channels (Ferrari et al., 2011). All three of these compounds produced greater than 80% BU224-like discriminative stimulus effects, strongly supporting the notion that the discriminative stimulus effects of BU224 is primarily mediated through I2 receptors. In addition, phenyzoline shows a remarkable selectivity for I2 receptors over a battery of 40 other receptors (Table 2). The finding that phenyzoline fully substituted for CR4056 (Qiu et al., 2014a) and BU224 (current study) further supports the I2 receptor-mediated mechanism.
Drugs with different pharmacological mechanisms such as methamphetamine and clonidine did not produce prominent BU224-, CR4056-, or phenyzoline-like discriminative stimulus effects, suggesting that I2 receptor ligands induced discriminative stimulus effects are pharmacologically specific (Qiu et al., 2014a). Although the opiate morphine partially substituted for I2 receptor ligands including CR4056, BU224 and phenyzoline (Qiu et al., 2014a, current study), antagonism studies suggested that opioid receptors do not play a major role. However, some evidence suggests that there may exist a crosstalk between I2 receptors and opioid systems. Acute 2-BFI treatment attenuates morphine-induced inhibition of locus coeruleus neuron activity in vivo (Ruiz-Durantez et al., 2003). The purported endogenous imidazoline receptor ligand agmatine stimulates beta-endorphin secretion in rat adrenal gland via I2 receptor-mediated mechanism (Chang et al., 2010). Moreover, I2 receptor ligands including phenyzoline enhance the antinociceptive effects of opioids such as morphine and tramadol (Gentili et al., 2006; Sanchez-Blazquez et al., 2000; Thorn et al., 2011). It seems likely that activation of μ opioid receptors partially mimic the stimulus effects of phenyzoline via indirect mechanism.
Early studies indicate that the imidazoline I2 receptors overlap with MAO and some I2 receptor ligands inhibit MAO activity, which led to the hypothesis that I2 receptors may represent an allosteric binding site on MAO that is different from the catalytic site (Eglen et al., 1998). However, later studies suggest that this view is too simplistic and I2 receptors may be heterogeneous and dynamic in nature and include other proteins such as brain creatine kinase (Kimura et al., 2009). In rats discriminating 2-BFI, MAO-A inhibitors such as harmane and harmaline, which also have high affinity for I2 receptors, fully substitute for 2-BFI (MacInnes and Handley, 2002). However, although harmane also fully substitutes for BU224, it only partially substitutes for phenyzoline and does not substitute for CR4056 (Qiu et al., 2014a). This inconsistency is remarkable because CR4056 inhibits MAO activity (Ferrari et al., 2011). Thus, the substitution of harmane for 2-BFI and BU224 cannot simply attribute to its MAO inhibition property. This seems to be consistent with antagonist studies. If MAO inhibition plays a critical role in the discriminative stimulus effects of I2 receptor ligands, then it should be expected that pharmacological blockade of downstream monoamine receptors can counteract the effects of MAO inhibition. This is not the case. This and other studies consistently show that blockade of specific adrenergic, dopaminergic or serotonergic receptors does not attenuate the discriminative stimulus effects of I2 receptor ligands (Qiu et al., 2014a, current study).
One caveat of the current study is that we could not find a compound to block the discriminative stimulus effects of I2 receptor ligands. Idazoxan is the prototypic compound that was used to define I2 receptors (Eglen et al., 1998). It is often used to block some behavioral effects of other I2 receptor ligands (Ferrari et al., 2011; Gentili et al., 2006; Lanza et al., 2014; Li et al., 2014; Li et al., 2011; Meregalli et al., 2012; Sanchez-Blazquez et al., 2000; Thorn et al., 2012; Tonello et al., 2012). However, idazoxan fully substitutes for 2-BFI in rats discriminating 2-BFI (Jordan et al., 1996; MacInnes and Handley, 2002). In the present study, idazoxan also fully substituted for BU224 and phenyzoline. This is intriguing and we speculate that the discriminative stimulus effects and other behavioral effects (e.g., antinociceptive effects) of I2 receptor ligands might be mediated through different components of I2 receptors. Thus, idazoxan may be an antagonist on one component (which mediates I2 receptor ligand-induced antinociception) but an agonist on another component (which mediates I2 receptor ligand-induced discriminative stimulus effects) (Qiu et al., 2014a). Although this possibility exists given the heterogeneous nature of I2 receptors (Olmos et al., 1999), it is too early to associate individual I2 receptor components with specific functional effects. More extensive studies that simultaneously examine multiple I2 receptor ligands in multiple functional assays should be able to eventually address this issue.
The pharmacological selectivity of drug discrimination procedures can be greatly influenced by the training dose (Colpaert et al., 1980). In general, decreasing the training dose decreases pharmacologic selectivity and the efficacy requirements of the assay. In the current study and previous studies using 2-BFI as the training drug, the training doses of 2-BFI and BU224 do not affect the rate of operant responding, although they are behaviorally active (i.e., antinociception) (Li et al., 2014; Li et al., 2011). Substantially larger doses of 2-BFI (10 mg/kg) and BU224 (17.8 mg/kg) can significantly suppress food-maintained operant responding under the same schedule of reinforcement (An et al., 2012). Throughout the course of the study, no systematic deterioration of the stimulus control was observed (e.g., the number of training sessions between tests did not significantly decrease over time). However, it is possible that the training dose of BU224 (5.6 mg/kg) used in the study is relatively low and as such the pharmacologic specificity of the discriminative stimulus might be low. Increasing the training dose of BU224 might increase the pharmacological selectivity, which in turn may decrease the substitution magnitudes of the test drugs, in particular those that produced intermediate substitutions in the present study. In addition, although 2-BFI, phenyzoline and BU224 produce symmetrical substitution in the present study and previous studies (MacInnes and Handley, 2002; Qiu et al., 2014a), given the clear evidence that BU224 has lower efficacy than 2-BFI at I2 receptors (Thorn et al., 2011), it is possible that BU224 might only partially substitute or does not substitute for 2-BFI in rats discriminating a larger dose of 2-BFI. The finding that BU224 and phenyzoline shows symmetrical substitution suggests that the efficacy demand of drug discrimination may be quite low such that a limited efficacy is sufficient to induce discriminative stimulus effects. If so, then drug discrimination may be insufficient to differentiate I2 receptor ligands with varying efficacies.
A preliminary analysis of the substitution profiles of the available data using different I2 receptor ligands as training drugs is presented in Table 3. It is clear that although all the I2 receptor ligands are highly selective for I2 receptors than for other receptors (Ferrari et al., 2011; Thorn et al., 2012; Table 1), these compounds have important differences. It remains unclear what contributes to the differences. As discussed before (Qiu et al., 2014a), one possibility is that these compounds bind to the different components of I2 receptors differently, which cannot be differentiated by using [3H] idazoxan or [3H] 2-BFI as the radioligands as essentially all the existing literature does. These data underscore the importance of utilizing multiple existing I2 receptor ligands in future studies to examine the generality rather than specificity of pharmacological effects thought to attribute to I2 receptor modulation.
Table 3.
Cross-substitution profiles of selected I2 receptor ligands.
| Substitution drugs | Training drugs | |||
|---|---|---|---|---|
| 2-BFIa | BU224 | CR4056a | Phenyzoline | |
| 2-BFI | Yes | Yes | No | No |
| BU224 | Yes | Yes | No | Yes |
| CR4056 | ND | Yes | Yes | Yes |
| Phenyzoline | ND | Yes | Yes | Yes |
| Harmane | Yes | Yes | No | No |
Note: Yes=full substitution; No = no full substitution; ND=not determined.
Data about 2-BFI and CR4056 discrimination were adapted from (MacInnes and Handley, 2002; Qiu et al., 2014a).
In summary, this study successfully trained rats to discriminate and compared the discriminative stimulus effects of a low efficacy I2 receptor ligand BU224 and a higher efficacy I2 receptor ligand phenyzoline. BU224 and phenyzoline demonstrated symmetrical substitution and the discriminative stimulus effects of both compounds were largely similar, showing pharmacological selectivity related to I2 receptors. A further analysis of currently available discrimination data of I2 receptor ligands identified important differences among some I2 receptor ligands, which caution careful interpretation of data in future studies that use one I2 receptor ligand. This study also demonstrates the power of drug discrimination as an in vivo research tool to understand I2 receptor pharmacology and also encourages the application of multiple I2 receptor ligands for detailed pharmacological analysis of I2 receptor-mediated effects across different assays.
Acknowledgments
Receptor binding profiles were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract # HHSN-271-2008-00025-C (NIMH PDSP). The NIMH PDSP is directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD, USA. This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (Awards no. R01DA034806 and R21DA033426).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- An XF, Zhang Y, Winter JC, Li JX. Effects of imidazoline I(2) receptor agonists and morphine on schedule-controlled responding in rats. Pharmacol Biochem Behav. 2012;101:354–359. doi: 10.1016/j.pbb.2012.01.024. [DOI] [PubMed] [Google Scholar]
- Brown CM, MacKinnon AC, Redfern WS, Williams A, Linton C, Stewart M, Clague RU, Clark R, Spedding M. RS-45041-190: a selective, high-affinity ligand for I2 imidazoline receptors. Br J Pharmacol. 1995;116:1737–1744. doi: 10.1111/j.1476-5381.1995.tb16656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Wu HT, Cheng KC, Lin HJ, Cheng JT. Increase of beta-endorphin secretion by agmatine is induced by activation of imidazoline I(2A) receptors in adrenal gland of rats. Neurosci Lett. 2010;468:297–299. doi: 10.1016/j.neulet.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Chen MF, Tsai JT, Chen LJ, Wu TP, Yang JJ, Yin LT, Yang YL, Chiang TA, Lu HL, Wu MC. Characterization of imidazoline receptors in blood vessels for the development of antihypertensive agents. BioMed Res Intl. 2014;2014:182846. doi: 10.1155/2014/182846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpaert FC. Intrinsic activity and discriminative effects of drugs. Psychopharmacology Ser. 1988;4:154–160. doi: 10.1007/978-3-642-73223-2_12. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. Drug discrimination in neurobiology. Pharmacol Biochem Behav. 1999;64:337–345. doi: 10.1016/s0091-3057(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Colpaert FC, Niemegeers CJ, Janssen PA. Factors regulating drug cue sensitivity: the effect of training dose in fentanyl-saline discrimination. Neuropharmacology. 1980;19:705–713. doi: 10.1016/0028-3908(80)90061-1. [DOI] [PubMed] [Google Scholar]
- Comer SD, France CP, Woods JH. Training dose: influences in opioid drug discrimination. NIDA Res Monogr. 1991:145–161. doi: 10.1037/e496182006-010. [DOI] [PubMed] [Google Scholar]
- Donahue TJ, Hillhouse TM, Webster KA, Young R, De Oliveira EO, Porter JH. (S)-amisulpride as a discriminative stimulus in C57BL/6 mice and its comparison to the stimulus effects of typical and atypical antipsychotics. Eur J Pharmacol. 2014;734:15–22. doi: 10.1016/j.ejphar.2014.03.047. [DOI] [PubMed] [Google Scholar]
- Eglen RM, Hudson AL, Kendall DA, Nutt DJ, Morgan NG, Wilson VG, Dillon MP. ‘Seeing through a glass darkly’: casting light on imidazoline ‘I’ sites. Trends Pharmacol Sci. 1998;19:381–390. doi: 10.1016/s0165-6147(98)01244-9. [DOI] [PubMed] [Google Scholar]
- Ernsberger P, Meeley MP, Mann JJ, Reis DJ. Clonidine binds to imidazole binding sites as well as alpha 2-adrenoceptors in the ventrolateral medulla. Eur J Pharmacol. 1987;134:1–13. doi: 10.1016/0014-2999(87)90125-7. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Fiorentino S, Mennuni L, Garofalo P, Letari O, Mandelli S, Giordani A, Lanza M, Caselli G. Analgesic efficacy of CR4056, a novel imidazoline-2 receptor ligand, in rat models of inflammatory and neuropathic pain. J Pain Res. 2011;4:111–125. doi: 10.2147/JPR.S18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentili F, Cardinaletti C, Carrieri A, Ghelfi F, Mattioli L, Perfumi M, Vesprini C, Pigini M. Involvement of I2-imidazoline binding sites in positive and negative morphine analgesia modulatory effects. Eur J Pharmacol. 2006;553:73–81. doi: 10.1016/j.ejphar.2006.09.031. [DOI] [PubMed] [Google Scholar]
- Hudson AL, Gough R, Tyacke R, Lione L, Lalies M, Lewis J, Husbands S, Knight P, Murray F, Hutson P, Nutt DJ. Novel selective compounds for the investigation of imidazoline receptors. Ann N Y Acad Sci. 1999;881:81–91. doi: 10.1111/j.1749-6632.1999.tb09344.x. [DOI] [PubMed] [Google Scholar]
- Jordan S, Jackson HC, Nutt DJ, Handley SL. Discriminative stimulus produced by the imidazoline I2 site ligand, 2 -BFI. J Psychopharmacol. 1996;10:273–278. doi: 10.1177/026988119601000403. [DOI] [PubMed] [Google Scholar]
- Kimura A, Tyacke RJ, Robinson JJ, Husbands SM, Minchin MC, Nutt DJ, Hudson AL. Identification of an imidazoline binding protein: creatine kinase and an imidazoline-2 binding site. Brain Res. 2009;1279:21–28. doi: 10.1016/j.brainres.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza M, Ferrari F, Menghetti I, Tremolada D, Caselli G. Modulation of imidazoline I2 binding sites by CR4056 relieves postoperative hyperalgesia in male and female rats. Br J Pharmacol. 2014;171:3693–3701. doi: 10.1111/bph.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelas S, Spealman RD, Rowlett JK. Using behavior to elucidate receptor mechanisms: a review of the discriminative stimulus effects of benzodiazepines. Exp Clin Psychopharmacol. 2000;8:294–311. doi: 10.1037//1064-1297.8.3.294. [DOI] [PubMed] [Google Scholar]
- Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Agmatine: an endogenous clonidine-displacing substance in the brain. Science. 1994;263:966–969. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- Li JX, Thorn DA, Jin C. The GPR88 receptor agonist 2-PCCA does not alter the behavioral effects of methamphetamine in rats. Eur J Pharmacol. 2013;698:272–277. doi: 10.1016/j.ejphar.2012.10.037. [DOI] [PubMed] [Google Scholar]
- Li JX, Thorn DA, Qiu Y, Peng BW, Zhang Y. Antihyperalgesic effects of imidazoline I(2) receptor ligands in rat models of inflammatory and neuropathic pain. Br J Pharmacol. 2014;171:1580–1590. doi: 10.1111/bph.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Zhang Y. Imidazoline I2 receptors: target for new analgesics? Eur J Pharmacol. 2011;658:49–56. doi: 10.1016/j.ejphar.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Li JX, Zhang Y, Winter JC. Morphine-induced antinociception in the rat: supra-additive interactions with imidazoline I(2) receptor ligands. Eur J Pharmacol. 2011;669:59–65. doi: 10.1016/j.ejphar.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Macinnes N, Duty S. Locomotor effects of imidazoline I2-site-specific ligands and monoamine oxidase inhibitors in rats with a unilateral 6-hydroxydopamine lesion of the nigrostriatal pathway. Br J Pharmacol. 2004;143:952–959. doi: 10.1038/sj.bjp.0706019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnes N, Handley SL. Characterization of the discriminable stimulus produced by 2-BFI: effects of imidazoline I(2)-site ligands, MAOIs, beta-carbolines, agmatine and ibogaine. Br J Pharmacol. 2002;135:1227–1234. doi: 10.1038/sj.bjp.0704579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacInnes N, Handley SL. Potential serotonergic and noradrenergic involvement in the discriminative stimulus effects of the selective imidazoline I2-site ligand 2-BFI. Pharmacol Biochem Behav. 2003;75:427–433. doi: 10.1016/s0091-3057(03)00136-9. [DOI] [PubMed] [Google Scholar]
- Meregalli C, Ceresa C, Canta A, Carozzi VA, Chiorazzi A, Sala B, Oggioni N, Lanza M, Letari O, Ferrari F, Avezza F, Marmiroli P, Caselli G, Cavaletti G. CR4056, a new analgesic I2 ligand, is highly effective against bortezomib-induced painful neuropathy in rats. J Pain Res. 2012;5:151–167. doi: 10.2147/JPR.S32122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min JW, Peng BW, He X, Zhang Y, Li JX. Gender difference in epileptogenic effects of 2-BFI and BU224 in mice. Eur J Pharmacol. 2013;718:81–86. doi: 10.1016/j.ejphar.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos G, Alemany R, Boronat MA, Garcia-Sevilla JA. Pharmacologic and molecular discrimination of I2-imidazoline receptor subtypes. Ann N Y Acad Sci. 1999;881:144–160. doi: 10.1111/j.1749-6632.1999.tb09354.x. [DOI] [PubMed] [Google Scholar]
- Parini A, Moudanos CG, Pizzinat N, Lanier SM. The elusive family of imidazoline binding sites. Trends Pharmacol Sci. 1996;17:13–16. doi: 10.1016/0165-6147(96)81564-1. [DOI] [PubMed] [Google Scholar]
- Pigini M, Bousquet P, Carotti A, Dontenwill M, Giannella M, Moriconi R, Piergentili A, Quaglia W, Tayebati SK, Brasili L. Imidazoline receptors: qualitative structure-activity relationships and discovery of tracizoline and benazoline. Two ligands with high affinity and unprecedented selectivity. Bioorg Med Chem. 1997;5:833–841. doi: 10.1016/s0968-0896(97)00009-6. [DOI] [PubMed] [Google Scholar]
- Porter JH, Prus AJ. Discriminative stimulus properties of atypical and typical antipsychotic drugs: a review of preclinical studies. Psychopharmacology. 2009;203:279–294. doi: 10.1007/s00213-008-1308-3. [DOI] [PubMed] [Google Scholar]
- Qiu Y, He XH, Zhang Y, Li JX. Discriminative stimulus effects of the novel imidazoline I2 receptor ligand CR4056 in rats. Sci Rep. 2014a;4:6605. doi: 10.1038/srep06605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Thorn DA, Zhang Y, He X, Li JX. Behavioral effects of the imidazoline I(2) receptor ligand BU99006 in rats. Behav Pharmacol. 2014b;25:130–136. doi: 10.1097/FBP.0000000000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Durantez E, Torrecilla M, Pineda J, Ugedo L. Attenuation of acute and chronic effects of morphine by the imidazoline receptor ligand 2-(2-benzofuranyl)-2-imidazoline in rat locus coeruleus neurons. Br J Pharmacol. 2003;138:494–500. doi: 10.1038/sj.bjp.0705052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Blazquez P, Boronat MA, Olmos G, Garcia-Sevilla JA, Garzon J. Activation of I(2)-imidazoline receptors enhances supraspinal morphine analgesia in mice: a model to detect agonist and antagonist activities at these receptors. Br J Pharmacol. 2000;130:146–152. doi: 10.1038/sj.bjp.0703294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, An XF, Zhang Y, Pigini M, Li JX. Characterization of the hypothermic effects of imidazoline I(2) receptor agonists in rats. Br J Pharmacol. 2012;166:1936–1945. doi: 10.1111/j.1476-5381.2012.01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Zhang Y, Peng BW, Winter JC, Li JX. Effects of imidazoline I(2) receptor ligands on morphine- and tramadol-induced antinociception in rats. Eur J Pharmacol. 2011;670:435–440. doi: 10.1016/j.ejphar.2011.09.173. [DOI] [PubMed] [Google Scholar]
- Tonello R, Villarinho JG, da Silva Sant’Anna G, Tamiozzo L, Machado P, Trevisan G, Pinto Martins MA, Ferreira J, Rubin MA. The potential antidepressant-like effect of imidazoline I2 ligand 2-BFI in mice. Prog Neuropsychopharmacol Bio Psychiatry. 2012;37:15–21. doi: 10.1016/j.pnpbp.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Yang TT, Ku PM, Hsu CT, Chung HH, Lee WJ, Cheng JT. Mediation of AMP kinase in the increase of glucose uptake in L6 cells induced by activation of imidazoline I-2 receptors. Horm Metab Res. 2013;45:359–363. doi: 10.1055/s-0032-1331184. [DOI] [PubMed] [Google Scholar]