Abstract
Human drug addiction is a complex disorder, in which exogenous substances are able to recruit and maintain behaviors involved in drug taking. Many drugs that are addictive in humans are able to act on natural brain systems for learning and memory, and while many memory systems may be affected by addictive drugs, work with operant tasks has shown that addictive drugs (e.g. cocaine and alcohol) are particularly effective in recruiting habit learning systems, compared to natural rewards. It is currently unknown if the ability of addictive drugs to facilitate habit learning depends on a direct action on habit learning systems in the brain, versus the rewarding properties of drug administration. To differentiate between these options, rats were trained to perform two actions (lever pressing), each of which was rewarded with a different natural reward. After acquiring the behavior, rats received three training sessions which were followed by post-training injections of saline or cocaine (5 or 10 mg/kg, i.p.). Using sensory-specific satiety, extinction tests revealed that lever pressing for actions which were paired with saline were sensitive to devaluation (typical of goal-directed behaviors) while actions which were paired with cocaine were not sensitive to devaluation (typical of habitual behaviors). Lesions of the infralimbic or dorsolateral striatum were able to block the action of post-training cocaine injections. These data indicate that, within individual rats, cocaine injections facilitate the transition of behavior to habitual control for actions that have been recently performed, without a general facilitation of habit learning, and that this action of cocaine requires brain areas that are critical for learning natural habits.
Keywords: Cocaine, memory, operant learning, dopamine, habit learning, drug abuse, infralimbic, dorsolateral striatum
Humans self-administer a wide variety of drugs for recreational purposes, and individuals who transition from recreational use to drug addiction incur substantial costs to both themselves and society. To better intervene in drug addiction, many research studies today focus on the mechanisms by which drugs are able to recruit and sustain self-administration. Several studies have shown that experience with addictive drugs can bias rats to use habitual behaviors (in which responses are driven by stimulus–response associations) over goal-directed behaviors (in which responses are guided by action-outcome associations, and the motivation to obtain an outcome, Dickinson, 1985). For instance, Dickinson and colleagues have shown that actions reinforced with alcohol (Dickinson, Wood, & Smith, 2002) or cocaine (Miles, Everitt, & Dickinson, 2003) become resistant to devaluation of the outcomes, an index of the development of habitual behaviors. In these same animals, actions which were reinforced with natural rewards (such as sucrose) remained sensitive to outcome devaluation, an indication that these actions were goal-directed. Similarly, a study by Gabriele, Setlow and Packard (2009) demonstrated that in extinction training in a straight alley maze, training that had been reinforced with oral cocaine led to more habitual behavior during extinction, compared to training that had been reinforced with a sucrose solution. As proposed by White (1996), these drug effects may be produced in several ways, either through the reinforcing properties of the drugs themselves, through the action of the drugs on memory systems in the brain which support habitual behavior, or by the incentive learning effects of the drugs. Both amphetamine sensitization (Nelson & Killcross, 2006; Nordquist et al., 2007) and exposure to cocaine (LeBlanc, Maidment, & Ostlund, 2013) has been demonstrated to bias rats to perform habitually actions which were learned subsequently (in a drug free state). Similarly, alcohol dependent humans also show accelerated development of habitual behavior (Sjoerds et al., 2013). Together, these studies show that in nonhuman animals, drugs which are addictive in humans can selectively facilitate the transition to habitual control when behaviors are reinforced with drugs, and can more globally facilitate habit learning (or suppress goal-directed learning) after drug exposure. And, correlational studies suggest that these results may be observed in human drug dependence.
Currently, it is unclear if selective facilitation of habit learning depends on the action of drugs on the habit learning system, or in the reinforcing properties of the drug. To address this question, the present study used post-training (non-contingent) injections to test the ability of cocaine to selectively facilitate habit learning for a recently trained action, without causing a global facilitation of habit learning. Post-training injection of addictive drugs (such as cocaine and amphetamine) as well as dopaminergic agonists and antagonists can facilitate learning in a wide variety of tasks, including inhibitory avoidance (Introini-Collison & McGaugh, 1989), active avoidance (Janak & Martinez, 1992; Janak, Keppel, & Martinez, 1992), Pavlovian conditioning (Leri et al., 2013; Simon & Setlow, 2006), and win-stay learning (Leri et al., 2013), presumably by impacting post-training consolidation of learning. Rather than facilitating task acquisition, our goal was to use post-training drug administration to test the ability of cocaine to shift rats from goal-directed to habitual behavior.
Experiment 1
Rats were trained to perform two actions, each reinforced with a different natural reward. Once rats had acquired each behavior, post-training injections of either saline or cocaine were given immediately after each training session for three days. After training, rats were tested in extinction after devaluing one of the rewards, using sensory-specific satiety (Berridge, 1991; Rolls, Rolls, Rowe, & Sweeney, 1981), to differentiate between goal-directed and habitual behavior. In the extinction tests, actions that were paired with cocaine injections were not sensitive to devaluation of the outcome, while actions paired with saline injections remained sensitive to devaluation, indicating that post-training cocaine injections facilitated the transition to habitual lever pressing.
Methods
Animals
Twenty male four Sprague-Dawley rats (Harlan, Indianapolis, IN, USA, mean ad lib weight = 437g, SD = 26g) were used in the experiment. Rats were placed on food-restriction approximately 1 week before training began, and maintained at approximately 80% of their ad lib weight throughout the experiment. Rats were either pair-housed (n = 4) or individually housed (n = 20) in plastic cages in a vivarium (pair housing was discontinued after the initial set of four rats, in order to conduct prefeeding in the home cage for the satiety tests described below). The vivarium was on a 12:12 light/dark schedule, and rats were tested in the light phase.
Training
Training was conducted using a set of four standard operant chambers (Med-Associates, St. Albans, VA) controlled by a computer running Med-PC IV. Each operant chamber was equipped with a magazine for food and liquid delivery, two retractable levers (one on either side of the magazine), a stimulus light over each lever, and a house light on the chamber wall opposite the magazine and levers. Each chamber was placed in a sound-attenuating cubicle equipped with a fan for ventilation. Rats were trained to perform two actions, each in a separate chamber. To make each chamber more discriminable, two of the boxes had white backgrounds and paper in the removable tray at the bottom of the chamber. The other two boxes had a black background and cage bedding in the removable tray at the bottom of the chamber.
Behavioral training followed the procedures described by Nelson and Killcross (2006), with the following modifications. Rats were trained to perform two actions, each of which was paired with a different reinforcer. Throughout training, rats received two sessions per day, one in each type of operant chamber (described above). Training sessions were separated by several hours, and the order of training (which chamber rats began in each day) was constant throughout training. For each type of operant chamber, rats were assigned at the start of training one action (left or right lever) and one reinforcer (a 30% sucrose solution or chocolate flavored pellets). Levers, reinforcers and contexts were counterbalanced across rats.
Training began with two days of magazine training, with one session for each reinforcer. Magazine training sessions lasted 30 minutes, during which 30 reinforcers were given on a random-time 60 second schedule (RT-60s), with the restriction that after each delivery of a reinforcer, the next random interval did not begin until the rat had made an entry into the magazine. If rats did not obtain 30 reinforcers within 30 minutes, the session continued for a maximum of 90 minutes. After the first day of training, rats typically completed magazine training in 30 minutes. After completing magazine training, instrumental training began with a single day of continuous reinforcement. For all instrumental training sessions, rats received 40 reinforcers in each session. At the start of each session, one lever (left or right) was extended and the stimulus light over that lever was illuminated at the same time that the house light was illuminated. Rats that failed to acquire the lever press in the first session were given additional sessions of continuous reinforcement.
Rats then received one day of random interval training on a 10 second schedule (RI-10s). As in magazine training, after the delivery of a reinforcer, the next random interval did not begin until rats had made an entry into the magazine. After these training sessions on the RI-10s schedule, rats received an intraperitoneal (i.p.) saline injection (1 ml/kg). Rats then completed three days of training on a RI-30s schedule. After each training session, rats received an i.p. injection of either saline or cocaine (5 or 10 mg/kg cocaine HCL, Sigma-Aldrich, dissolved in saline, injected at volumes of 1 ml/kg) and were returned to their home cages. For half of the rats, the morning session was followed by cocaine, for the other half, the afternoon session was followed by cocaine.
After finishing the final day of training on the RI-30s schedule, rats began extinction tests on the following day. Rats were pre-fed for one hour with one of the reinforcers. Then, rats received two 10 minute extinction tests (one for each action, in the appropriate operant chamber, in the same order as the training sessions) conducted back-to-back. After the first extinction test, rats were returned to the animal colony briefly while the second extinction test was prepared. The tests were conducted in the same order as was done in acquisition: the first extinction test was conducted for the lever trained in the morning, and the second extinction test was conducted for the lever trained in the afternoon. The following day, rats were retrained in both chambers, reinforced on the RI-30s schedule, trained in the morning and afternoon, as was done during acquisition. No injections were given following the retraining sessions. On the day after retraining, rats were pre-fed with the alternate reinforcer (not used in the first extinction test) and the extinction tests were repeated.
Results
Acquisition
Rats readily acquired both the lever press followed by saline injections (the Saline-paired action) and the lever press followed by cocaine injections (the Cocaine-paired action). In the final training session before extinction tests began, rats pressed the lever at similar rats for both the action paired with saline injections (mean rate for the 5 mg/kg group was 18.1 [SD = 4.3] presses per minute, mean rate for the 10 mg/kg group was 20.3 [SD = 4.3] presses per minute) and the action paired with cocaine injections (mean rate for the 5 mg/kg group was 17.7 [SD = 8.6] presses per minute, mean rate for the 10 mg/kg group was 17.3 [SD = 5.4] presses per minute). Lever pressing rates in the final VI-30s session before the first satiety test are given in Table 2. Lever pressing rates during the three days of RI-30s training were analyzed with a 3-way ANOVA with Injection (Cocaine, Saline), Value (Non-devalued, Devalued) and Day (1, 2, 3) as within-subjects factors and Dose (5, 10 mg/kg) as a between subjects factor. The only significant effect was a main effect of day (F(2,44) = 5.8, p = 0.006, ), which was due to an increase in lever pressing rates across the three days of training. Neither the main effects of Injection nor Dose nor any of the interactions were significant (all Fs < 1, ). These results indicate that, relative to saline injections, post-training injections of cocaine did not disrupt acquisition of lever pressing for natural rewards.
Table 2.
Lever pressing (presses/min) and magazine entries (entries/min) for the final VI-30s session before the first satiety test (Acquisition) and for the entire extinction session (10 minutes for Experiment 1, 5 minutes for Experiment 2). Values in parentheses indicate standard deviation.
| Lever-pressing | |||||||
|---|---|---|---|---|---|---|---|
| Saline-paired Lever | Cocaine-paired Lever | ||||||
| Acquisition | Extinction | Acquisition | Extinction | ||||
| Non- devalued |
Devalued | Non- devalued |
Devalued | ||||
| Experiment 1 | 5 mg/kg (n=12) |
18.1 (4.3) | 4.3 (2.3) | 1.8(1.9) | 17.7 (8.6) | 3.6 (3.4) | 2.4 (1.9) |
| 10 mg/kg (n=12) |
20.3 (4.3) | 5.6 (3.5) | 2.7 (1.7) | 17.3 (5.4) | 3.6 (2.1) | 2.7 (2.1) | |
| Experiment 2 | Sham (n=12) |
15.7 (4.6) | 6.6 (3.7) | 2.8 (1.8) | 12.5 (5.6) | 3.3 (2.8) | 3.5 (2.8) |
| IL (n=10) | 15.1 (4.6) | 6.1 (3.8) | 3.6 (1.9) | 12.1 (5.6) | 5.7 (4.8) | 4.2 (3.1) | |
| DLS (n=9) |
18.9 (5.4) | 6.5 (5.0) | 4.1 (2.3) | 20.1 (5.3) | 6.5 (2.7) | 2.9 (2.6) | |
| Magazine entries | |||||||
| Saline-paired Lever | Cocaine-paired Lever | ||||||
| Acquisition | Extinction | Acquisition | Extinction | ||||
| Non- devalued |
Devalued | Non- devalued |
Devalued | ||||
| Experiment 1 | 5 mg/kg (n=12) |
8.9 (3.8) | 2.4(2.0) | 0.7 (0.9) | 8.3 (2.8) | 1.7 (1.8) | 0.8 (0.6) |
| 10 mg/kg (n=12) |
11.9 (4.1) | 2.6 (1.7) | 1.2 (0.7) | 9.9 (2.8) | 1.6 (0.8) | 1.3 (1.0) | |
| Experiment 2 | Sham (n=12) |
10.4 (3.6) | 3.5 (2.2) | 1.5 (1.2) | 8.2 (3.1) | 2.5 (2.1) | 1.3 (0.9) |
| IL (n=10) | 10.2 (3.1) | 3.0 (1.8) | 1.6 (1.7) | 9.1 (3.1) | 1.9 (1.4) | 1.4 (1.2) | |
| DLS (n=9) |
11.3 (4.2) | 2.6 (1.2) | 1.9 (2.0) | 9.3 (4.0) | 2.5 (1.7) | 1.4 (0.8) | |
Magazine entries during acquisition were also not affected by post-training cocaine injections. In the final training session before extinction tests began, rats made similar numbers of magazine entries for the reinforcer paired with saline injections (mean rate for the 5 mg/kg group was 8.9 [SD = 3.8] entries per minute, mean rate for the 10 mg/kg group was 10.8 [SD = 3.6] entries per minute) and the reinforcer paired with cocaine injections (mean rate for the 5 mg/kg group was 8.3 [SD = 3.1] entries per minute, mean rate for the 10 mg/kg group was 9.9 [SD = 2.4] entries per minute). Magazine entry rates in the final VI-30s session before the first satiety test are given in Table 2. Magazine entry rates during the three days of RI-30s training were analyzed with a 3-way ANOVA with Injection (Cocaine, Saline), Value (Non-devalued, Devalued) and Day (1, 2, 3) as within-subjects factors and Dose (5, 10 mg/kg) as a between subjects factor. The only significant effect was a main effect of Day (F(2,44) = 7.6, p = 0.001, ), which was due to a decrease in magazine entries on the second day of the RI-30s schedule, and then a return to levels of magazine entries on the third day that were intermediate between the first and second days. The main effect of dose approached significance (F(1,22) = 3.9, p = 0.061, ), but none of the interactions of dose with session and injection were significant (all Fs < 1.5, ps > 0.24, ), indicating that any difference in magazine entries was present before cocaine injections began. The Injection × Day interaction was also not significant (F(1,22) < 1, n.s., ). This indicates that, relative to saline injections, posttraining injections of cocaine did not disrupt magazine entries for natural rewards.
Extinction tests
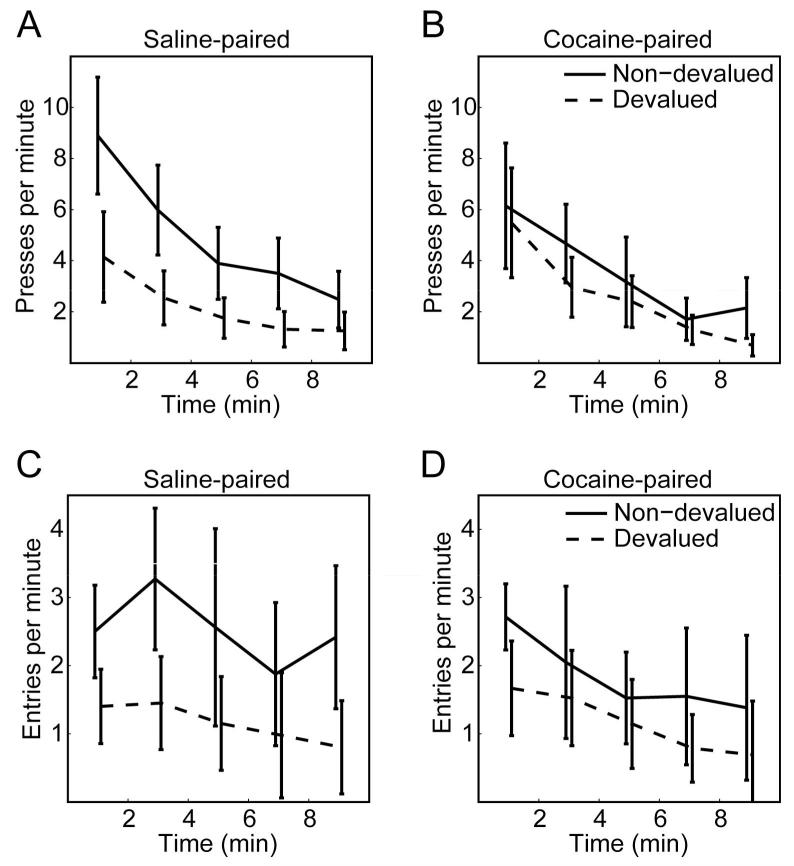
Lever pressing rates during the extinction tests are shown in Figure 1A-B, with the ten minute extinction tests divided into five 2-minute bins. Lever pressing rates across the entire 10 minute extinction test are given in Table 2. For both the Saline- and Cocaine-paired actions, lever pressing rates decreased across the ten minute extinction session, and on average lever pressing rates were lower when rewards were devalued compared to when they were not devalued. However, the size of devaluation effect was much larger for Saline-paired actions (see Figure 1A) than for Cocaine-paired actions (see Figure 1B). Lever pressing rates during the extinction tests were analyzed using a 4-way ANOVA with Injection (Cocaine, Saline), Value (Non-devalued, Devalued) and Block (the ten minute extinction session was divided into five 2-minute blocks) as within-subject factors and Dose (5, 10 mg/kg) as a between-subjects factor. There was no main effect of Dose (F(1, 22) < 1, n.s., ), nor was there any significant interaction of Dose with any other factor (all Fs < 1.1, ps > 0.3, ), indicating that the 5 and 10 mg/kg doses were equally effective. There was a significant main effect of Value (F(1,22) = 46.5, p < 0.001, ), Block (F(4,88) = 25.8, p < 0.001, ), and a significant Value × Block interaction (F(4,22) = 2.6, p = 0.043, ) and most importantly there was a significant 3-way interaction of Injection × Value × Block (F(4,88) = 2.7, p = 0.035, ). No other main effect or interaction was significant (Injection × Value: F(1,22) = 2.8, p = 0.11, , and all other ps > 0.26, ). Post-hoc tests (Tukey HSD, = 0.05) revealed that for the Saline-paired actions, rats pressed the lever significantly more during the first two blocks (the first four minutes of the test) when the reward was not devalued compared to when it was devalued. However, for the Cocaine-paired action, lever pressing rates did not significantly differ for any of the blocks depending on the value of the reward.
Figure 1.
Behavior during the 10 minute extinction tests in Experiment 1. Data from the 5 and 10 mg/kg cocaine doses have been combined. A-B: Lever pressing. A: Levers which were paired with saline injections. Prefeeding rats with the reward that was normally delivered during training strongly decreased the amount that rats responded during the extinction test, indicating that this behavior was goal-directed. B: Levers which were paired with cocaine injections. Prefeeding rats with the reward that was normally delivered during training had no significant effect on the rate that rats pressed the lever during the 10 minute extinction test, indicating that this behavior had become habitual. C-D: Magazine entries. C: Reinforcers which were paired with saline injections. D: Reinforcers which were paired with cocaine injections. Magazine entry rates were significantly reduced by prefeeding the reinforcer before the extinction test, and did not differ significantly by injection type.
Magazine entry rates during the extinction tests are shown in Figure 1C-D, with the ten minute extinction tests divided into five 2-minute bins. Magazine entry rates across the entire 10 minute extinction test are given in Table 2. For both the Saline- and Cocaine-paired reinforcers, lever pressing rates decreased across the ten minute extinction session, and on average lever pressing rates were lower when rewards were devalued compared to when they were not devalued, for both the Saline-paired and Cocaine-paired reinforcers. Magazine entry rates were analyzed using a 4-way ANOVA with Injection (Cocaine, Saline), Value (Non-devalued, Devalued) and Block (the ten minute extinction session was divided into five 2-minute blocks) as within-subject factors and Dose (5, 10 mg/kg) as a between-subjects factor. There was no main effect of Dose (F(1,22) < 1, n.s., ), nor was there any significant interaction of Dose with any other factor (all ps > 0.12, ), indicating that the 5 and 10 mg/kg doses were equally effective. There was a significant main effect of Value (F(1,22) = 23.8, p < 0.001, ) and Block (F(4,88) = 5.0, p = 0.001, ). There was a non-significant trend for magazine entry rates to be higher for the Saline-paired reinforcer compared to the Cocainepaired reinforcer (main effect of Injection: F(1,22) = 3.2, p = 0.087, ). No other interaction was significant (Injection × Value: F(1,22) = 2.8, p = 0.11, , all other ps > 0.35, ). These data indicate that devaluation of a reinforcer by sensory specific satiety was effective in decreasing magazine entries for both the Saline-paired and Cocaine-paired reinforcer.
Discussion
These results reported here demonstrate that cocaine injections given immediately after operant training can facilitate the development of habitual behavior, without affecting the acquisition of other goal-directed behaviors. The facilitation of habit learning was very specific: the same rats which showed habitual responding for the lever paired with cocaine showed goal-directed behavior for the lever paired with saline. These results are consistent with reports that actions which are reinforced with drugs such as cocaine and alcohol become insensitive to outcome devaluation, while in the same animals actions reinforced with natural rewards remain sensitive to the value of the outcome (Dickinson et al., 2002; Miles et al., 2003). Our results extend this work by showing that the facilitation of habit learning by cocaine can be demonstrated when cocaine is given non-contingently, during post-training memory consolidation. Also, these results are consistent with reports that amphetamine sensitization (Nelson & Killcross, 2006; Nordquist et al., 2007) and cocaine exposure (LeBlanc et al., 2013) conducted before training could bias rats to respond habitually for actions trained later, in a drug free state. These studies suggest that, under some conditions, experience with amphetamine and cocaine may produce a general facilitation of habit learning (or a general suppression of goal-directed learning). Such an effect is distinct from the very specific action of cocaine on a single action that we have reported here, and that reported by Miles and colleagues have reported (2003), in which cocaine administration is able to facilitate habitual control of one behavior, without an effect on other behaviors which are not paired with cocaine.
Experiment 2
The ability of cocaine to facilitate habit learning suggests that cocaine is able to act during memory consolidation on brain systems involved in habitual behavior, or goal-directed behavior, or both. Goal-directed and habitual behaviors involve distinct brain systems, involving prefrontal and basal ganglia structures in rats. Goal-directed behaviors require the prelimbic division of the medial prefrontal cortex (Killcross & Coutureau, 2003) and the dorsomedial striatum (Yin, Ostlund, Knowlton, & Balleine, 2005), while habitual behavior requires the infralimbic cortex (Killcross & Coutureau, 2003) and dorsolateral striatum (Yin, Knowlton, & Balleine, 2004). Presumably, cocaine is able to facilitate habit learning by acting on either the goal-directed system (to suppress acquisition of a goal-directed behavior) or on the habitual system (to promote the transition to a habitual behavior) during memory consolidation. Whether this action is to suppress activity in brain areas critical for goal-directed behavior (the prelimbic cortex and dorsomedial striatum) or to enhance activity in brain areas critical for habitual behavior (the infralimbic cortex and dorsolateral striatum), or to act in both systems, is an important question for future study. Interesting, the psychostimulant methamphetamine has been demonstrated to induce synaptic plasticity in the dorsolateral, but not the dorsomedial, striatum, consistent with a recruitment of brain areas involved in habit learning (Jedynak, Uslaner, Esteban, & Robinson, 2007). And, Corbit, Nie and Janak (2012) have demonstrated that habitual responding for ethanol depends on activity in the dorsolateral striatum. Other work has demonstrated that exposure to cocaine before training, which promotes the development of habitual behavior, found changes in glutamate transmission in the dorsomedial, but not the dorsolateral striatum (Corbit, Chieng, & Balleine, 2014).
The results of Experiment 1 demonstrated that systemic post-training cocaine administration facilitates habit learning in rats. Presumably, if the facilitation of habit learning by cocaine involved suppressing activity in the goal directed system during consolidation, then disruption of the habit learning system (combined with cocaine administration) could produce deficits in acquisition of a behavior or in disruption of goal directed behavior on an extinction test. If, however, the facilitation of habit learning by cocaine involved promoting activity in brain areas required for habit learning, then disruption of the habit system could block the facilitation of habit learning by cocaine, while leaving goal-directed behavior intact. To determine the effect of damage to the habit learning system on the facilitation of habit learning, rats received pre-training lesions of the infralimbic cortex, dorsolateral striatum, or sham surgery. Rats then completed the same training protocol as in Experiment 1, with slight modifications. Based on the result that lever pressing rates decreased significantly across the 10 minute extinction test, we reduced the duration of the extinction tests to 5 minutes.
Methods
Animals
Forty Sprague-Dawley rats (36 male, Harlan, Indianapolis, IN, USA, mean weight at surgery = 389g, SD = 91g) were used in the experiment. One infralimbic lesion rat died during post-operative recovery, and did not complete behavioral testing. Rats were placed on food-restriction and moved to individual housing after surgery and maintained at approximately 80% of their ad lib weight throughout the experiment. The vivarium was on a 12:12 light/dark schedule, and rats were tested in the light phase.
Surgery
Rats received stereotaxic surgery targeting either the infralimbic cortex (IL: +3mm AP, ±0.7mm ML, −5.4mm DV) or the dorsolateral striatum (DLS: +0.7mm AP, ±3.4mm ML, −5mm DV). IL rats (n = 15) received bilateral infusions of NMDA, ibotenic acid. DLS rats (n = 12) received bilateral infusions of NMDA. Sham rats (n = 13) received vehicle infusions (PBS) in the IL or DLS. After surgery, rats were allowed to recover for a minimum of 5 days before experimental training began.
Training
Training was conducted as in Experiment 1, with the following modifications. For the liquid reinforcer, rats were trained with either 30% or 20% (w/v) sucrose, and for the pellet reinforcer, rats were trained with either chocolate or fruit punch flavored pellets (Research Diets). Also, the duration of each extinction test was reduced from 10 minutes to 5 minutes, and all cocaine infusions were made using a dose of 10 mg/kg.
Histology
After the completion of experiments, rats were given an overdose of sodium pentobarbital and perfused with 0.9% saline followed by 10% formalin. Brains were stored for at least two days in 10% formalin at 4°C before being transferred to a 30% (w/v) sucrose formalin solution for at least one week. Brains were sliced frozen (50-70 m) and stained with cresyl violet. Lesion extent was assessed under a light microscope by a rater who was blind to the experimental group of each rat.
Results
Histology
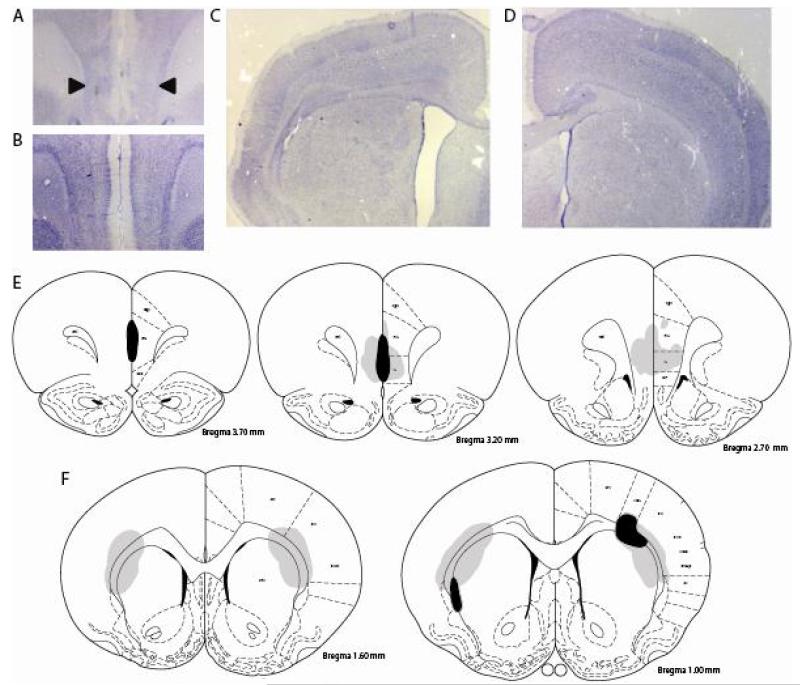
Examples of infralimbic and dorsolateral striatal lesions are shown in Figure 2A-B. Of the lesioned rats, nine rats with lesions targeting the infralimbic cortex were judged to involve primarily the infralimbic cortex bilaterally or unilaterally. A total of eight rats with lesions targeting the dorsolateral striatum were judged to have damage involving the dorsolateral striatum. In the infralimbic lesion group, several rats had damage that extended dorsally into the ventral prelimbic cortex (Figure 2D), and in the dorsolateral striatal group, the white matter and cortex overlying the striatum showed evidence of damage in many cases (see Figure 2C and the example in 2B). The remaining rats were excluded from the analyses, and were generally found in infralimbic rats to have damage in more anterior regions involving primarily the prelimbic or medial orbital cortices, or were not able to be judged based on the histological specimens. One additional rat in the sham group was judged to be an outlier based on performance during the extinction tests (z = −2.85 for the Saline-paired lever, +2.81 for the Cocaine-paired lever) and was removed from the behavioral analyses. The final sample of 29 rats (12 sham [11 male], 9 infralimbic [7 male] and 8 dorsolateral striatum [8 male]) were analyzed below.
Figure 2.
Experiment 2 histology. A-D: Examples of infralimbic (A) and dorsolateral striatal (C) lesions and comparisons from shams (B, D). A: Arrows show the location of infralimbic lesion, visible in the disrupted cell layers and compression of the medial wall of the cortex. B: Damage to the dorsolateral striatum is visible, and noticeable enlargement of the lateral ventricle is observed. The lesion also involves the overlying white matter and cortex. E-F: Summary of the infralimbic lesions (E) and dorsolateral striatal (F) lesions. Gray shaded area indicates the overlap across lesioned rats, and black shaded area indicates the area of the smallest lesion included in each group. Outlines are modified from Paxinos and Watson (1998). Abbreviations: IL: infralimbic cortex, PrL: prelimbic cortex, DP: dorsal peduncular cortex, fmi: forceps minor corpus callosum, Cg1: cingulate cortex, area 1, M1: primary motor cortex, CPu: striatum (caudate putamen), S1: primary somatosensory cortex, GI: granular insular cortex.
Acquisition
As in Experiment 1, rats were able to acquire both the Saline-paired and Cocaine-paired action, and acquisition was not affected by IL or DLS lesions. Lever pressing rates in the final VI-30s session before the first satiety test are given in Table 2. Lever pressing rates during the three days of drug injections were analyzed for each group using a 3-way ANOVA with Day (1, 2, 3) and Injection (Saline, Cocaine) as within-subjects factors and Group (Sham, IL, DLS) as a between-subjects factor. There was main effect Group was not significant (F(2,28) = 2.9, p = 0.074, ), but approached significance due to a non-significant trend for DLS lesioned rats to press at higher rates (18.2 [SE = 1.3]) than Sham (15.1 [1.1]) and IL lesioned (14.3 [1.2]) rats. No other main effect or interactions were significant (all ps > 0.27, all ).
Similar results were obtained for magazine entries made during training. Magazine entry rates in the final VI-30s session before the first satiety test are given in Table 2. Magazine entry rates during the three days of drug injections were analyzed using a 3-way ANOVA with Day and Injection (Saline, Cocaine) as within-subjects factors and Group (Sham, IL, DLS) as a between-subjects factor. There was no main effect Group, nor were there any significant interactions of Group with any other factor (all Fs < 1, n.s. , ). The main effect of Day approached significance (F(2,56) = 2.92, p = 0.062, ), which was due to a non-significant trend for rats to make more magazine entries on Day 1 (10.1 [SE = 0.52]) compared to Day 2 (9.1 [0.43]), with Day 3 rates falling in-between (9.7 [0.44]). No other main effect or interactions were significant (all ps ≥ 0.14, ).
Extinction
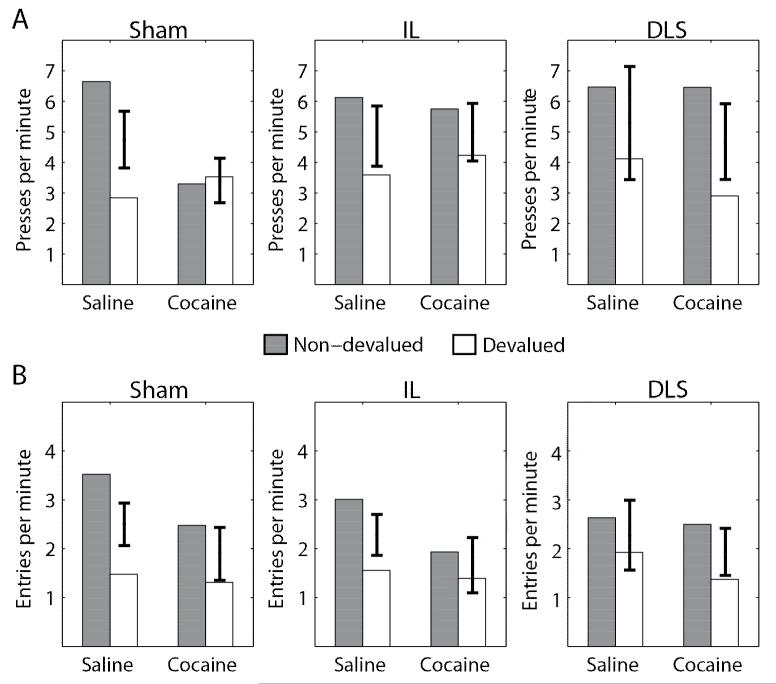
Lever pressing rates during the extinction tests are shown in Figure 3A and Table 2. An initial 3-way ANOVA was conducted, with Value (Non-devalued, Devalued) and Injection (Saline, Cocaine) as within-subjects factors and Group (Sham, IL, DLS) as a between-subjects factor. The interaction of Group × Value × Injection was not significant (F(2,28) = 2.5, p = 0.097, ). However, when data from the two lesion groups (IL and DLS) were combined, a second ANOVA found a significant interaction of Group (Sham, Lesion) × Value × Injection (F(1,29) = 4.3, p = 0.048, ).
Figure 3.
Behavior during the extinction tests in Experiment 2. A: Lever-pressing during extinction testing in Experiment 2. Sham rats replicated the results of Experiment 1. For lesioned rats, the effect of satiety on lever pressing did not differ for the saline- and cocaine-paired levers, indicating that both behaviors were goal-directed. B: Magazine entries during extinction testing in Experiment 2. Across all groups, the effect of satiety on magine entries did not differ for the saline- and cocaine-paired levers, replicating the results of Experiment 1. Error bars represent ±SEM for the difference between Non-devalued and Devalued scores, centered at the average (across levels of satiety).
Lever pressing during extinction was then examined separately for the sham and the combined lesion group using 2-way ANOVAs with Value (Non-devalued, Devalued) and Injection (Saline, Cocaine) as within-subjects factors. As in Experiment 1, in the Sham group there was a significant interaction between Value × Injection (F(1,11) = 15.5, p = 0.002, ), as well as a significant main effect of Value (F(1,11) = 7.4, p = 0.020, ) while the main effect of Injection was not significant (F(1,11) = 1.7, p = 0.22, ). Post-hoc tests (Tukey HSD, = 0.05) indicated that Sham animals showed a reduction in lever pressing for the Saline-paired action after devaluation, and no change in lever pressing for the Cocaine-paired action after devaluation.
In the combined lesion group, the interaction between Value × Injection was not significant (F(1,18) < 1, n.s., ), while there was a significant main effect of Value (F(1,9) = 20.8, p < 0.001, ). The main effect of injection was not significant (IL: F(1,9) < 1, n.s., ). These results demonstrate that lesions of the IL or DLS block the effects of post-training cocaine administration on habit learning: for both lesion groups, lever-pressing rates were sensitive to devaluation, for both the Saline-paired and Cocaine-paired levers.
When the outlier Sham rat (described above) was included in these analyses, the 3-way interaction of Group (Lesion, Sham) × Value × Injection was not significant (F(1,30) < 1, n.s, ) and the 2-way interaction of Value × Injection for the Sham group was also not significant (F(1,12) < 1, n.s., ). However, paired-tests (testing the change in lever pressing following devaluation) for the Sham group showed that there was a nonsignificant trend towards a decrease in lever pressing for the Saline-paired lever (t(12) = 2.0, p = 0.073, 95% CI for the difference: [−5.7 0.3]) while there was no significant change in lever pressing for the Cocaine-paired lever (t(12) = 0.6, p = 0.54, 95% CI: [−3.3 1.8]), which follows the pattern we observed in Experiment 1.
Magazine entry rates during the extinction tests are shown in Figure 3B and Table 2. As in Experiment 1, devaluation was effective in reducing magazine entry rates for both the Salineand Cocaine-paired action, and this effect was not affected by lesions of IL or DLS. Magazine entries were somewhat higher for the Saline-paired lever than the Cocaine-paired lever (though this difference failed to reach significance, similar to the pattern observed in Experiment 1). An initial 3-way ANOVA was conducted, with Value (Non-devalued, Devalued) and Injection (Saline, Cocaine) as within-subjects factors and Group (Sham, IL, DLS) as a between-subjects factor. The interaction of Group × Value × Injection was not significant (F(2,28) < 1, n.s., ). There was a significant main effect of Value (F(1,28) = 34.9, p < 0.001, ) and the main effect of Injection approached significance (F(1,28) = 3.6, p = 0.069, ). The main effect of group and all other interactions failed to reach significance (all ps > 0.29, ).
Discussion
The results of Experiment 2 demonstrate that the infralimbic cortex and dorsolateral striatum are required for habitual behavior facilitated by post-training cocaine injections. Pretraining lesions of the infralimbic cortex or the dorsolateral striatum blocked the ability of post-training cocaine injections to facilitate habit learning, while sham surgery had no effect, and replicated the findings of Experiment 1 on the ability of post-training cocaine administration to facilitate habit learning. These results indicate that when the habit system is impaired, through lesions of the infralimbic cortext and dorsolateral striatum, facilitation of habit learning is blocked (as would be expected), but goal-directed learning is not disrupted, as might be expected if the mechanism for habit facilitation relied on disrupting activity in the goal directed system.
General Discussion
Together, the data from these experiments indicate that administration of cocaine during memory consolidation can facilitate the transition from goal-directed to habitual behavior. And, that habits facilitated by cocaine administration depend on the integrity of the dorsolateral striatum and infralimbic cortex, which are also necessary for natural habits. While these data implicate the infralimbic cortex and dorsolateral striatum in the actions of cocaine on habit learning, it is unclear if the facilitation of habit learning by cocaine depends on activity in these brain regions during memory consolidation. Studies which test the effects of post-training infusion of cocaine directly into the infralimbic cortex or dorsolateral striatum, rather than systemically, could determine if local administration of cocaine into either region alone can facilitate habit learning to the same degree as systemic injections.
Rather than producing a global impairment in goal-directed behavior, cocaine caused a selective facilitation of habit learning: a within-subjects design demonstrated that the saline-paired action remained goal-directed, while the cocaine-paired action became habitual. These data extend the literature on the effects of addictive drugs on goal-directed and habitual memory systems, which has demonstrated that many drugs of abuse are effective at promoting habitual behaviors (Corbit et al., 2012; Dickinson et al., 2002; Miles et al., 2003) or are effective at producing relatively permanent deficits in goal-directed learning (LeBlanc et al., 2013; Nelson & Killcross, 2006; Nordquist et al., 2007), or short-term suppressions of goal-directed behaviors (Ostlund, Maidment, & Balleine, 2010). Drug abuse and addiction are complicated phenomena, and cannot be reduced to simply a promotion of habitual behavior, however deficits in goal-directed behavior and aggressive recruitment of habitual memory systems may be important in maintaining drug use late in the process of addiction.
Our demonstration of the ability of post-training injections of cocaine to facilitate habit learning are also consistent with a broad literature in learning and memory which have demonstrated that manipulations of dopaminergic systems during memory consolidation to facilitate memory in a variety of tasks such as inhibitory avoidance (Introini-Collison & McGaugh, 1989), active avoidance (Janak & Martinez, 1992; Janak et al., 1992), Pavlovian conditioning (Simon & Setlow, 2006). However, post-training injections of cocaine in the present study had no impact on task acquisition or retention. Rather, our data indicate that post-training injections of cocaine can facilitate a transfer of control from goal-directed to habitual behavior. Rats acquired the instrumental response before cocaine injections began, and presumably lever pressing at this point was a goal-directed behavior, and sensitive to outcome devaluation. By administering cocaine after training, one of these behaviors (the action paired with cocaine) was transferred to habitual control, while the second behavior (the action paired with saline) remained goal-directed.
To account for the present findings, we have proposed that cocaine is selectively acting during memory consolidation to promote the use of a habitual behavior (or to suppress the use of a goal-directed behavior), and as such is facilitating the transition from goal-directed to habitual control of a behavior. One alternative explanation for the effect cocaine in the present study is that rather than selectively enhancing habit learning, contextual cues (present in the Cocaine-paired context) have become associated with cocaine exposure, and these cues may be selectively suppressing the expression of a goal-directed behavior during the extinction test. Ostlund, Maidment and Balleine (2010) have demonstrated that after pairing alcohol exposure with a distinct context, presentation of that context during an extinction test causes a suppression of goal-directed responding, and that rats act habitually, similar to our findings in Experiment 1. In the Ostlund study, rats experienced alcohol while in the context, while in our experiments all cocaine exposure was done after rats were removed from the operant chambers, and as rats were returned to the home cage. However, it is possible that rats developed an expectation for cocaine while in the cocaine-paired context.
These data suggest that that the use of cocaine, and perhaps other dopamine agonists, can facilitate the development of rigid, habitual control of behaviors which are unrelated to drug use (i.e. noncontingent) but happen to be performed immediately before drug use. Whether such cocaine-facilitated habits can be identified in humans abusing cocaine and other addictive drugs, and if such habits are involved in maintaining drug use, is an important question for future research. We might expect that similar administration of cocaine, or other addictive drugs such as alcohol, nicotine, etc., may show similar facilitation of habitual behavior in rats and other animals including humans. If so, then these results would have important implications for humans, where learning that occurred before the use of alcohol or nicotine (drugs which are commonly used) may become habitual at a faster than natural rate. To our knowledge, such an effect has not been demonstrated to date.
Drugs of abuse continue to pose challenges for our society and are and active area of research. While drug abuse and addiction are unlikely to be simply a case of habits gone awry, a better understanding of how drugs interact with goal-directed and habitual behavior systems may have implications for the treatment of drug addiction, and for improving our understanding of how goal-directed and habitual memory systems interact for normal learning.
Highlights.
We tested the effects of post-training cocaine administration on habit learning in rats in a lever pressing task.
Cocaine given after lever training facilitated the development of habits in rats.
In the same animals, saline injections had no effect on habit learning for a second behavior.
Damage to the infralimbic cortex or dorsolateral striatum blocked the effect of cocaine on habit facilitation.
Table 1.
Experimental design for Experiment 1 and 2. O1/2 refer to reinforcers, LP1/2 to levers, S1/2 to contexts, and Inj1/2 to injection type. Refer to text for details.
| Magazine | Acquisition | Satiety Test | Retrain | Extinction | ||||
|---|---|---|---|---|---|---|---|---|
| 2 × RT60 | 1 × CRF | 1 × VI10 | 3 × VI30 | Prefeed | 1 × VI30 | Prefeed | ||
| S1: O1 | S1: LP1→ O1 | S1: LP1→ O1 Saline |
S1: LP1 →O1 Inj1 |
O1 or O2 | S1: LP1→ X | S1: LP1→ O1 | O1 or O2 | S1: LP1→ X |
| S2: O2 | S2: LP2→ O2 | S2: LP2→ O2 Saline |
S2: LP2→ O2 Inj2 |
S2: LP2→ X | S2: LP2→ O2 | S2: LP2→ X | ||
Acknowledgements
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under award R15DA029546 and was also supported by Wabash College. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank Mike Nowak, Nathan Powell, Jordon Blackwell, Xumin Sun, Drew Casey, Jacob Owens for their assistance in conducting these experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Berridge KC. Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Appetite. 1991;16(2):103–120. doi: 10.1016/0195-6663(91)90036-r. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Chieng BC, Balleine BW. Effects of repeated cocaine exposure on habit learning and reversal by N-acetylcysteine. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2014;39(8):1893–1901. doi: 10.1038/npp.2014.37. doi:10.1038/npp.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual alcohol seeking: Time course and the contribution of subregions of the dorsal striatum. Biological Psychiatry. 2012;72(5):389–395. doi: 10.1016/j.biopsych.2012.02.024. doi:10.1016/j.biopsych.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A. Actions and habits: The development of behavioural autonomy. Philosophical Transactions of the Royal Society of London, Series B. Biological Sciences. 1985;308(1135):67–78. [Google Scholar]
- Dickinson A, Wood N, Smith JW. Alcohol seeking by rats: Action or habit? The Quarterly Journal of Experimental Psychology.B, Comparative and Physiological Psychology. 2002;55(4):331–348. doi: 10.1080/0272499024400016. doi:10.1080/0272499024400016. [DOI] [PubMed] [Google Scholar]
- Gabriele A, Setlow B, Packard MG. Cocaine self-administration alters the relative effectiveness of multiple memory systems during extinction. Learning & Memory (Cold Spring Harbor, N.Y.) 2009;16(5):296–299. doi: 10.1101/lm.1253409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Introini-Collison IB, McGaugh JL. Cocaine enhances memory storage in mice. Psychopharmacology. 1989;99(4):537–541. doi: 10.1007/BF00589905. [DOI] [PubMed] [Google Scholar]
- Janak PH, Keppel G, Martinez JL. Cocaine enhances retention of avoidance conditioning in rats. Psychopharmacology. 1992;106(3):383–387. doi: 10.1007/BF02245422. [DOI] [PubMed] [Google Scholar]
- Janak PH, Martinez JL. Cocaine and amphetamine facilitate retention of jump-up responding in rats. Pharmacology Biochemistry and Behavior. 1992;41(4):837–840. doi: 10.1016/0091-3057(92)90235-8. doi:DOI: 10.1016/0091-3057(92)90235-8. [DOI] [PubMed] [Google Scholar]
- Jedynak JP, Uslaner JM, Esteban JA, Robinson TE. Methamphetamine-induced structural plasticity in the dorsal striatum. European Journal of Neuroscience. 2007;25(3):847–853. doi: 10.1111/j.1460-9568.2007.05316.x. doi:10.1111/j.1460-9568.2007.05316.x. [DOI] [PubMed] [Google Scholar]
- Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cerebral Cortex. 2003;13(4):400–408. doi: 10.1093/cercor/13.4.400. doi:10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- LeBlanc KH, Maidment NT, Ostlund SB. Repeated cocaine exposure facilitates the expression of incentive motivation and induces habitual control in rats. PLoS ONE. 2013;8(4):1–10. doi: 10.1371/journal.pone.0061355. doi:10.1371/journal.pone.0061355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Nahas E, Henderson K, Limebeer CL, Parker LA, White NM. Effects of post-training heroin and d-amphetamine on consolidation of win-stay learning and fear conditioning. Journal of Psychopharmacology (Oxford, England) 2013;27(3):292–301. doi: 10.1177/0269881112472566. doi:10.1177/0269881112472566. [DOI] [PubMed] [Google Scholar]
- Miles FJ, Everitt BJ, Dickinson A. Oral cocaine seeking by rats: Action or habit? Behavioral Neuroscience. 2003;117(5):927–938. doi: 10.1037/0735-7044.117.5.927. doi:10.1037/0735-7044.117.5.927. [DOI] [PubMed] [Google Scholar]
- Nelson A, Killcross S. Amphetamine exposure enhances habit formation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26(14):3805–3812. doi: 10.1523/JNEUROSCI.4305-05.2006. doi:10.1523/JNEUROSCI.4305-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordquist RE, Voorn P, de Mooij-van Malsen JG, Joosten RNJMA, Pennartz CMA, Vanderschuren LJMJ. Augmented reinforcer value and accelerated habit formation after repeated amphetamine treatment. European Neuropsychopharmacology. 2007;17(8):532–540. doi: 10.1016/j.euroneuro.2006.12.005. doi:10.1016/j.euroneuro.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Maidment NT, Balleine BW. Alcohol-paired contextual cues produce an immediate and selective loss of goal-directed action in rats. Frontiers in Integrative Neuroscience. 2010;4 doi: 10.3389/fnint.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in sterotaxic coordinates. 4th ed. Academic Press; New York: 1998. [Google Scholar]
- Rolls BJ, Rolls ET, Rowe EA, Sweeney K. Sensory specific satiety in man. Physiology & Behavior. 1981;27(1):137–142. doi: 10.1016/0031-9384(81)90310-3. [DOI] [PubMed] [Google Scholar]
- Simon NW, Setlow B. Post-training amphetamine administration enhances memory consolidation in appetitive pavlovian conditioning: Implications for drug addiction. Neurobiology of Learning and Memory. 2006;86(3):305–310. doi: 10.1016/j.nlm.2006.04.005. doi:DOI: 10.1016/j.nlm.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Sjoerds Z, de Wit S, van d. B., Robbins TW, Beekman ATF, Penninx BWJH, Veltman DJ. Behavioral and neuroimaging evidence for overreliance on habit learning in alcohol-dependent patients. Translational Psychiatry. 2013;3:e337–e337. doi: 10.1038/tp.2013.107. doi:10.1038/tp.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM. Addictive drugs as reinforcers: Multiple partial actions on memory systems. Addiction. 1996;91(7):921–949. doi:10.1080/09652149639826. [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. European Journal of Neuroscience. 2004;19(1):181–189. doi: 10.1111/j.1460-9568.2004.03095.x. doi:10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. European Journal of Neuroscience. 2005;22(2):513–523. doi: 10.1111/j.1460-9568.2005.04218.x. doi:10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]