Abstract
Background
The biological factors associated with shoulder osteoarthritis (OA) have not been elucidated. The purpose of this study was to investigate putative osteoarthritic biomarkers of the shoulder. To our knowledge, this is the first study to analyze shoulder cartilage for OA-associated genes and examine human shoulder cartilage for a novel biomarker, connexin 43 (Cx43).
Materials and methods
Cartilage from 16 osteoarthritic and 10 non-osteoarthritic humeral heads was assessed for expression of the following genes via real-time polymerase chain reaction: types I, II, and X collagen, metalloproteinases (MMP), tissue inhibitors of MMP (TIMP), interleukins, versican, cyclooxygenase-2 (Cox-2), inducible nitric oxide synthase (iNOS), tumor necrosis factor alpha (TNFα), aggrecanase-2 (ADAMTS5), and Cx43.
Results
In osteoarthritic shoulders, gene expression of Cx43, ADAMTS5, collagen type I, Cox-2, versican, and TIMP-3 showed predominance (85-, 33-, 13-, 12-, 11.5-, and 3-fold increases, respectively) relative to non-osteoarthritic controls. Spearman correlation analysis showed significant correlations between Cx43 and collagen types I, II, and X, MMP-9, TIMP-2 and -3, versican, Cox-2, iNOS, and ADAMTS5. In osteoarthritic shoulders, Cx43, Cox-2, versican, collagen type I, ADAMTS5, MMP3, and TNFα expressions were significantly increased compared with controls. TIMP-3 and iNOS trended toward significance, with robust expression in osteoarthritic shoulders and low expression in non-osteoarthritic shoulders.
Conclusions
Certain genes are markedly up-regulated in osteoarthritic shoulders compared with non-osteoarthritic shoulders, with Cx43, Cox-2, versican, collagen type I, ADAMTS5, MMP3, and TNFα expression being significantly increased. These genes might be useful biomarkers for examining shoulder OA.
Keywords: Shoulder, osteoarthritis, biomarkers, connexin 43
Introduction
Osteoarthritis (OA) of the shoulder is both common and debilitating but is far less studied than OA of other joints. In contrast, knee OA has been widely explored regarding biomarkers, risk factors, natural history, and treatments, including total joint replacements. Extensive studies have elucidated markers of knee OA, including interleukin 1 (IL-1), tumor necrosis factor alpha (TNFα), nitric oxide, prostaglandin E2 (PGE2), matrix metalloproteinases (MMP), and aggrecanases (ADAMTS-4 and -5), factors that control the breakdown of cartilage.4,6,18,22,39,52
The shoulder joint, unlike the more widely studied knee and hip joints, is not a weight-bearing joint. Considering that shoulders experience a very different mechanical environment from that of knee joints, it is unclear whether the same catabolic biomarkers found in knee OA are ubiquitous among all joints with OA. Studies of OA biomarkers within the glenohumeral joint, or shoulder joint, are not only far sparser but are limited to the synovial fluid or subacromial bursa.53,54 These studies elucidated the biomarkers in patients with rotator cuff tears and suggested that such biomarkers might be related to shoulder OA. This indirect association was postulated because many patients with rotator cuff tears develop OA.13,32 One study40 compared those with and without OA in which the condition of the cartilage was intraoperatively determined based on a grading system.2,37,40 After the cartilage was graded, synovial fluid was analyzed but the cartilage, which plays a significant role in the development of OA, was not analyzed.40
The aim of this study was to determine the markers associated with osteoarthritic cartilage obtained directly from the cartilage of glenohumeral joints. The chondrocyte markers investigated include well-known biomarkers that have been found in studies of other joints, such as the knee and hip joints. This study also investigated a novel biomarker of joint cartilage, connexin 43 (Cx43), a gap junction protein. Gap junctions are specialized communicative cell structures present in the plasma membrane of cells. They are made up of connexin monomers that assemble to form a hemichannel. Hemichannels provide a pathway for direct intercellular communication when paired with a hemichannel on an adjacent cell. The resultant gap junction channel permits the direct exchange of second messengers, metabolites, ions, and other small molecules among coupled cells. Gap junctions aggregate into large gap junction plaques at the interface of adjacent cells, forming a functional syncytium for the coordinated function of a tissue.30 Several studies have implicated Cx43 in the etiology of OA.19,25,49,50 Synovial biopsies from patients with OA were shown to have an increase in Cx43 expression and an increase in the size and number of gap junction plaques.25 Ex vivo analysis of these cells revealed that pharmacological inhibition of Cx43 function could reduce the basal and IL-1β-stimulated production of collagenase activity.19,25 Additionally, the inflammatory cytokine IL-1β has been shown to increase the expression of Cx43 in articular chondrocytes49,50 and synovial fibroblasts.34 Recently, a study by Mayan et al30 investigated cartilage from osteoarthritic knees and femoral heads and found significantly elevated levels of Cx43 compared with non-osteoarthritic cartilage.
Cx43 can affect the responsiveness of cells to extracellular cues by modulating signal transduction and gene transcription.23,33,35,44,45 In addition to the impact of inflammation on Cx43 expression, Cx43 has long been linked to mechanical load.5 Cx43 expression is dramatically increased by mechanical loading in skeletal tissues, and Cx43 function has been implicated in the production of prostaglandins in response to mechanical perturbation in bone cells.15 Both mechanical load and prostaglandin production are also factors that influence joint destruction in OA, circumstantially providing additional implication of a role for Cx43 in OA. Further, the up-regulation of Cx43 is implicated in the production of both pro-inflammatory and catabolic factors by synovial fibroblasts.19,25 No study has investigated OA-associated catabolic factors from cartilage of the glenohumeral joint, and Cx43 has not been studied in human humeral head cartilage.
Materials and methods
Participant selection
The study was conducted prospectively at a single institution. Patients with and without OA of the shoulder who were undergoing either arthroscopic or open shoulder surgery were recruited. Twenty-six patients were selected from the clinical practice of one orthopaedic surgeon who specializes in shoulder and elbow surgery. All patients provided consent according to Institutional Review Board protocol at the surgeon’s institution. Patients were defined as having OA whether they developed OA from a degenerative wear-and-tear process or from a secondary process, such as rotator cuff arthropathy or instability, in accordance with a study by Ratcliffe et al.40 Exclusion criteria included inability to provide consent and presence of fracture. All patients undergoing either arthroscopic or open surgery had their cartilage intraoperatively graded by a single orthopaedic surgeon. The cartilage was graded according to a previously described grading system for OA:2,37,40 grade I (normal), the articular cartilage is smooth and shiny with an intact surface that is firm when probed; grade II, the articular surface has localized softening of the surface and fibrillation; grade III, the articular cartilage has extensive softening, pervasive fibrillation and/or fissuring, and clefts; and grade IV, eburnated bone and osteophyte formation are present, and the articular cartilage shows pitting, with tufts and fronds.40
Ten patients with grade I cartilage comprised the non-osteoarthritic (control) group. The average age of this group of four men and six women was 59 years (age range, 29-72 years). This group underwent arthroscopic surgery for rotator cuff repairs (nine patients) and instability (one patient). Sixteen patients with grade IV cartilage comprised the osteoarthritic group. The average age of this group of five men and 11 women was 66 years (45-82 years). This group underwent total or reverse shoulder arthroplasty for OA (10 patients), avascular necrosis (three patients), cuff tear arthropathy (two patients), and rheumatoid arthritis (one patient). No patients recruited were found to have grade II or grade III cartilage intraoperatively. Patients who underwent arthroscopic surgery required surgical suture anchors for tissue repair. A small piece of cartilage at the edge of the suture anchors was obtained for specimen. All patients had cartilage removed from their humeral heads, except the patient with instability who had cartilage obtained from the glenoid. For patients undergoing total shoulder arthroplasty, the corresponding area on the humeral head from which cartilage was procured in the non-OA group might have been devoid of cartilage. The surgeon therefore obtained a sample of the most severely degraded area of cartilage on the humeral head. In patients with avascular necrosis, the area from which cartilage was obtained did not include the area of avascular necrosis.
Analysis
Cartilage biopsies were placed immediately into ribonucleic acid (RNA), later solution (Life Technologies Corporation, Carlsbad, CA, USA) at the time of procurement. Each cartilage specimen had RNA extracted with TRIzol reagent (Life Technologies) according to the manufacturer’s directions. The RNA was reverse-transcribed, and quantitative real-time polymerase chain reaction was performed using the SYBR Green method (Life Technologies) on an Applied Biosystems 7300 sequence detection system (Applied Biosystems, Foster City, CA, USA), as described previously.34
Catabolic factors, such as MMP-1, -3, -9, and -13, and ADAMTS5 destroy extracellular matrix, leading to a loss of articular cartilage.4 Anti-catabolic factors include the tissue inhibitors of MMP (TIMP). To study the status of the articular cartilage, we looked at four extracellular matrix proteins: collagen types I, II, and X and versican. Finally, we studied Cx43, a novel putative mediator of shoulder OA. We and others have shown that Cx43 gets up-regulated in cells of the joint during inflammation and OA.19,25,30,34,49,50 The 19 genes tested in this study were collagen (types I, II, and X), MMP-1, -3, -9, and -13, TIMP-1, -2, and -3, IL-1β, -6, and -17, versican, cyclooxygenase 2 (Cox-2), inducible nitric oxide synthase (iNOS), TNFα, aggrecanase-2 (ADAMTS5), and Cx43 (Table I). Gene expression was normalized to the expression of 18S ribosomal RNA.
Table I.
Nineteen genes evaluated
| Gene Tested | Gene Name | Primer 1 | Primer 2 |
|---|---|---|---|
| Collagen type I | COL1A1 | 5′-CCT GCG TGT ACC CCA CTC A-3′ | 5′-ACC AGA CAT GCC TCT TGT CCT T-3′ |
| Collagen type II | COL2A1 | 5′-TTG CCT ATC TGG ACG AAG CA-3′ | 5′-TCA TTG GAG CCC TGG ATG A-3′ |
| Collagen type X | COLlOAl | 5′-CCA AAG CTT ACC CAG CAA TAG G-3′ | 5′-TGC TGT TGC CTG TTA TAC AAA ATT T-3′ |
| Metalloproteinase 1 | MMP1 | 5′-TTT GAT GGA CCT GGA GGA AAT C-3′ | 5′-TGA GCA TCC CCT CCA ATA CC-3′ |
| Metalloproteinase 3 | MMP3 | 5′-CCT GGT ACC CAC GGA ACC T-3′ | 5′-GGA CAA AGC AGG ATC ACA GTT G-3′ |
| Metalloproteinase 9 | MMP9 | 5′-GAT CCA AAA CTA CTC GGA AGA CTT G-3′ | 5′-GAA GGC GCG GGC AAA-3′ |
| Metalloproteinase 13 | MMP13 | 5′-ATT AAG GAG CAT GGC GAC TTC T-3′ | 5′-CCC AGG AGG AAA AGC ATG AG-3′ |
| TIMP1 | TIMP1 | 5′-CAC CCA CAG ACG GCC TTC T-3′ | 5′-TGG TGT CCC CAC GAA CTT G-3′ |
| TIMP2 | TIMP2 | 5′-CAC CCA GAA GAA GAG CCT GAA-3′ | 5′-GGC AGC GCG TGA TCT TG-3′ |
| TIMP3 | TIMP3 | 5′-CCA GGA CGC CTT CTG CAA-3′ | 5′-AGC TTC TTC CCC ACC ACC TT-3′ |
| Interleukin 1β | IL1B | 5′-CGA ATC TCC GAC CAC CAC TAC-3′ | 5′-TCC ATG GCC ACA ACA ACT GA-3′ |
| Interleukin 6 | IL6 | 5′-TGT AGC CGC CCC ACA CA-3′ | 5′-GGA TGT ACC GAA TTT GTT TGT CAA-3′ |
| Interleukin 17 | IL17 | 5′-TGA TGG GAA CGT GGA CTA CCA-3′ | 5′-TGC GCA GGA CCA GGA TCT-3′ |
| Versican | VCAN | 5′-GCC TAC TTT GCC ACC CAG TTA C-3′ | 5′-TTT TGT CCA CTT CAA TCT TAG ACC AT-3′ |
| Cyclooxygenase-2 | PTGS2 | 5′-CAG CAC TTC ACG CAT CAG TTT T-3′ | 5′-CCA GCC CGT TGG TGA AAG-3′ |
| iNOS | NOS2 | 5′-TGG ATG CAA CCC CAT TGT C-3′ | 5′-CGC TGC CCC AGT TTT TGA T-3′ |
| TNFα | TNF | 5′-CTC GAA CCC CGA GTG ACA A-3′ | 5′-AGC TGC CCC TCA GCT TGA-3′ |
| ADAMTS5 | ADAMTS5 | 5′-AAT AAC CCT GCT CCC AGA AAC A-3′ | 5′-GCG GTA GAT GGC CCT CTT C-3′ |
| Connexin 43 | GJA1 | 5′-AAC AAT TCT TCT TGC CGC AAT TAC-3′ | 5′-CAT TCG ATT TTG TTC TGC ACT GTA-3′ |
iNOS, inducible nitric oxide synthase; TIMP, tissue inhibitors of metalloproteinase; ADAMTS5, aggrecanase-2; TNFα, tumor necrosis factor alpha.
Statistics
Differences between the gene expression in the non-osteoarthritic and osteoarthritic groups were analyzed by conducting a Mann-Whitney test for non-parametric samples using Graph Pad Prism software. An x-fold difference between these two groups, for each biomarker, was also calculated. Analysis was conducted to examine the correlation of Cx43 messenger RNA (mRNA) expression to the mRNA expression of other OA-associated genes. Statistical significance was calculated by a two-sided non-parametric Spearman’s correlation using Graph Pad Prism software. A P value < .05 was considered statistically significant.
Results
Comparisons of gene expression between osteoarthritic and non-osteoarthritic specimens
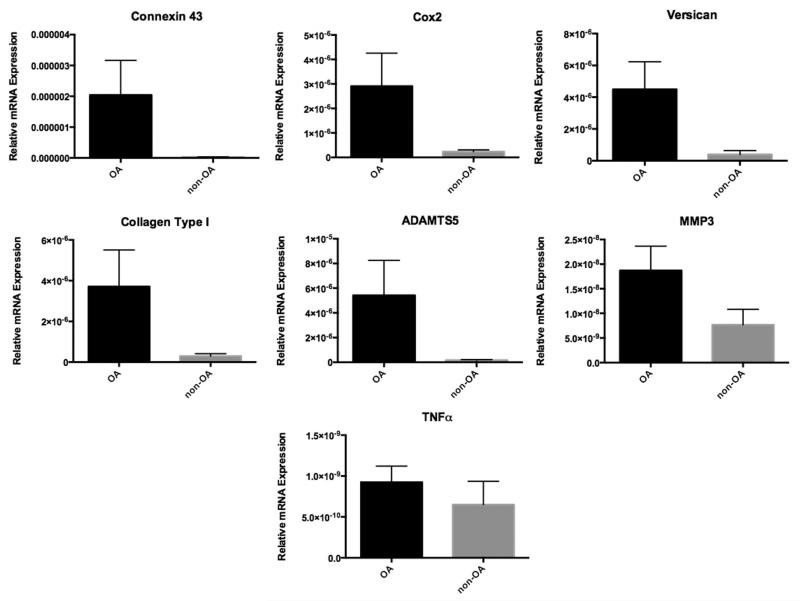
Of the 19 genes analyzed as putative markers of OA, only the expressions of Cx43, Cox-2, versican, collagen type I, ADAMTS5, MMP3 and TNFα were statistically increased (Fig. 1) when comparing RNA from biopsies of patients with grades I (non-OA) and IV (OA) cartilage. Their respective P values were .03, .04, .002, .007, .04, .05, and .05. TIMP-3 and iNOS were also elevated but did not reach statistical significance (P = .23 and .08, respectively).
Figure 1.
Bar graph shows average relative expression of biomarkers that were significantly elevated in osteoarthritic (black bar) versus non-osteoarthritic (gray bar) shoulders (P < .05).
When x-fold differences were calculated, the expression of Cx43, ADAMTS5, collagen type I, Cox-2, and versican showed the greatest abundance in osteoarthritic shoulders, increasing 85-, 33-, 13-, 12-, and 11.5-fold, respectively, in osteoarthritic humeral head cartilage compared with non-osteoarthritic humeral head cartilage. The expression of the other tested genes showed a less than 3-fold increase.
Correlation of Cx43 expression to the expression of OA-associated genes
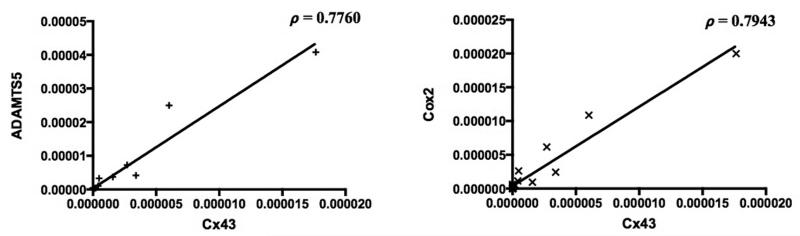
When Cx43 was correlated with the other biomarkers studied, Spearman correlation analysis showed significant positive correlations between Cx43 and collagen types I, II, and X, MMP-9, TIMP-2 and -3, versican, Cox-2, iNOS, and ADAMTS5 (Table II) (P < .05). Of note, significant positive correlation was shown between Cx43 and four biomarkers that were significantly elevated in osteoarthritic shoulders: collagen type I, versican, Cox-2, and ADAMTS5 (P < .05) (Fig. 2).
Table II.
Spearman’s rank correlation coefficient (ρ) between Connexin 43 and other putative biomarkers
| TIMP-3 | Cox-2 | ADAMTS5 | Versican | iNOS | Col. Type I | TIMP-2 | Col. Type X | MMP-9 | Col. Type II | |
|---|---|---|---|---|---|---|---|---|---|---|
| ρ | 0.88 | 0.8 | 0.78 | 0.75 | 0.73 | 0.66 | 0.51 | 0.5 | 0.47 | 0.44 |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0003 | <0.0078 | <0.0086 | <0.0154 | <0.0242 |
TIMP, tissue inhibitors of metalloproteinases; Cox-2, cyclooxygenase-2; ADAMTS5, aggrecanase-2; iNOS, inducible nitric oxide synthase; Col., collagen; MMP, metalloproteinases.
Figure 2.
Spearman correlation between Cx43 and ADAMTS5 and between Cx43 and Cox-2 in osteoarthritic shoulders (ρ = rho = Spearman correlation coefficient).
Discussion
This study aimed to explore the expression of 19 genes and their association with OA in the shoulder joint. We looked at cytokines, catabolic factors, inhibitors of catabolic factors, extracellular matrix proteins, and the gap junction Cx43. These molecules were evaluated in osteoarthritic and non-osteoarthritic shoulder joints. Gene expression for Cx43, versican, Cox-2, collagen type I, ADAMTS5, MMP3, and TNFα were all found to be significantly elevated in osteoarthritic shoulders (P < .05). Although TIMP-3 and iNOS were also elevated, they did not reach statistical significance (P = .13 and .1, respectively).
When the x-fold increases for chondrocyte markers were calculated for the osteoarthritic group compared with the non-osteoarthritic group, Cx43 had the largest fold increase in the osteoarthritic groups: an 85-fold increase. This increase is nearly three times more than that of ADAMTS5, which is the biomarker with the second largest increase (33-fold).
Considering that Cx43 was found to be significantly increased in osteoarthritic shoulders compared with non-osteoarthritic shoulders and considering that this protein had an 85-fold increase in osteoarthritic shoulders, we correlated the Cx43 to the other 18 putative biomarkers. Of these, Cx43 was found to be significantly correlated to 10 of these biomarkers: collagen types I, II, and X, versican, MMP-9, TIMP-2 and -3, ADAMTS5, Cox-2, and iNOS. Of these 10 molecules, four were shown to be significantly increased by Mann-Whitney statistical analysis: collagen type I, versican, Cox-2, and ADAMTS5. Two markers, MMP3 and TNFα, were found to be significantly increased in shoulders with OA (by Mann-Whitney test) but were not shown to be significantly correlated to Cx43. These two markers showed less than a 3-fold increase in abundance compared with the 85-fold increase observed with Cx43, which could account for the lack of significant correlation.
Our findings regarding Cx43 are not surprising based on recent literature. Cx43 was approximately 50% greater in the synovial lining cells of patients with OA compared with those without OA.25 More recently, this gap junction protein has been investigated in human knee and femoral head cartilage and found to be significantly elevated in osteoarthritic cartilage versus non-osteoarthritic cartilage (P < .05 and P < .01, depending on depths of cartilage compared; Kruskal-Wallis test with Dunn’s multiple comparison test).30 To date, the role of Cx43 in shoulder OA has not been investigated. This study provides strong evidence that Cx43 is significantly increased and has a high predominance in osteoarthritic shoulders compared with non-osteoarthritic shoulders. Additionally, the abundance of Cx43 in osteoarthritic shoulders is shown to be significantly correlated to other known biomarkers of OA in the human body.
Versican was also significantly increased in osteoarthritic shoulders. Versican is a structural component of cartilage, providing resistance to compression but increasing in OA when the tissue is attempting to repair itself. Similar up-regulation of versican in the osteoarthritic joints of lower limbs was reported previously and, although the difference did not reach statistical significance, this might indicate subtle differences between joints.27 Nishida et al36 showed increased expression of versican in osteoarthritic hip joints, whereas no expression was detected in non-osteoarthritic knee cartilage. Another study demonstrated that both the versican mRNA level and protein content were significantly higher in osteoarthritic knees compared with age-matched normal samples.8 The findings in our study, which show that versican was significantly increased in the OA group, had an 11.5-fold increase compared with the non-OA group, and was significantly correlated to Cx43, strongly suggest that versican might be a biomarker of shoulder OA.
Likewise, we found that Cox-2 was significantly increased in the shoulders of our osteoarthritic group, increased 12-fold compared with the non-OA group, and significantly correlated to Cx43. This is not surprising considering that elevated Cox-2 levels have been identified in other osteoarthritic joints and inflamed synovial tissue.3,14 Furthermore, Cox-2 inhibitors are known pharmacological agents in the treatment of OA.9,28
Collagen type I was significantly elevated in osteoarthritic shoulders compared with non-osteoarthritic shoulders and has been implicated in the pathogenesis of OA. Investigating the expression of collagen type I in consecutive stages of human OA revealed increasing amounts of collagen type I mRNA with the progression of the disease.31
Biomarker ADAMTS5 was found to have the second highest predominance in the OA group: a 33-fold increase, compared with Cx43 at an 85-fold increase. Additionally, ADAMTS5 was significantly correlated to Cx43. Considering this, and considering that ADAMTS5 has a known role in OA, it is not surprising that it was significantly elevated in shoulders with OA. Matrilin-3 expression is increased in OA and has been found to induce the expression of MMP, Cox-2, iNOS, and ADAMTS5 in chondrocytes from human knees. Furthermore, ADAMTS5 has been shown to cleave matrilin-3, which is thought to have a positive feedback effect in leading to further induction of proinflammatory cytokines and proteases.17
Matrix metalloproteinases have been implicated in the progression of OA. A study by Masuhara et al29 found serum and plasma levels of MMP-3 to be significantly elevated in patients with rapidly destructive hip OA. A more recent study showed that MMP-3 rises in the early and middle grades of knee OA.1 In our study, MMP3 was shown to be significantly elevated in the OA group.
TNFα is a well-known inflammatory factor involved in both cartilage degradation and nociceptive stimulation.21 Stannus et al46 found that TNFα was positively associated with change in total knee pain and change in pain while standing. In another study, Stannus et al47 found that TNFα serum levels were associated with knee cartilage loss in older people. In our study, TNFα was found to be significantly elevated in the cartilage of those with OA of the shoulder.
Although Mann-Whitney analysis did not show statistical significance for TIMP-3 or iNOS, these cytokines were elevated in the OA group compared with the non-OA group and the elevation approached statistical significance. Additionally, these cytokines were significantly correlated to Cx43, suggesting that Cx43 might be a novel marker of shoulder OA. The role of iNOS has also been established in osteoarthritic human cartilage. When normal and osteoarthritic human cartilage samples from distal femoral condyles were compared, iNOS was found nearly twice as much in the osteoarthritic samples.41 Furthermore, diacerein, a drug shown to relieve symptoms of OA and provide chondroprotective effects, has been found to inhibit nitric oxide release via inhibition of IL-1β-induced iNOS expression.10
Results on the other MMPs and TIMPs studied were not particularly revealing considering the wide variances among the control and osteoarthritic groups. These wide variances could be explained in that MMPs and TIMPs are antagonists of each other and the abundance of one is, to a large part, contingent on the abundance of another and the stages of the inflammatory process.16,26 Nonetheless, TIMP-3 levels were elevated in the osteoarthritic group in our study. This finding was supported by a study by Su et al,48 who reported increased TIMP-3 expression in arthritic joints and suggested that its expression was associated with pathological remodeling.
Although some genes, such as those of inflammatory cytokines, did not show statistically significant differences, this might be because the non-osteoarthritic group, although devoid of degraded cartilage, had other pathological abnormalities (such as rotator cuff tears and instability) that could have affected the inflammatory state in the shoulder. Additionally, it is possible that a larger sample size would be necessary to identify differences in biomarker abundance between the two groups. Wide variances among the gene expression of some of the molecules seen in the OA groups, specifically the MMP and TIMP, could be explained by these factors being antagonists to each other and the abundance of one being, in large part, contingent on the abundance of another and the stage of the inflammatory process.16,24,26 A ratio between the two might reveal more meaningful information.11,16,38
To minimize confounding factors, the orthopaedic surgeon attempted to procure cartilage from the same relative area on the humeral head for each patient, but this was not always possible. In patients undergoing arthroscopic rotator cuff repairs with otherwise normal cartilage, we were limited to the area of the anchor placement for cartilage procurement. When the same corresponding area in an osteoarthritic shoulder was completely devoid of cartilage, another area of the humeral head was used to obtain cartilage.
This study provides insight into Cx43, a protein known to be a mediator of inflammation in certain parts of the body but not previously studied in human shoulder joint cartilage, as a potential indicator of shoulder OA. Intercellular communication through gap junctions has been shown to be critical to the ability of synovial lining cells, from osteoarthritic knees, to secrete MMP.25 Cx43 was also shown to be elevated in the actual cartilage of osteoarthritic knees and femoral heads.30 This study is novel, indicating that Cx43 might also be a biomarker in the cartilage of osteoarthritic shoulder joints. Alterations in gap junctions have been linked to diseases of chronic inflammation, and disruption of gap junction function has been suggested as a putative therapeutic target for inflammatory diseases.12 As such, Cx43 might be a target in which pharmacological agents can block the gap protein and slow or halt the progression of OA in the shoulder. A study by Tsuchida et al51 showed in a rat model of rheumatoid arthritis that inhibition of Cx43 protected against the development of inflammation and joint destruction. Increased Cx43 in osteoarthritic joints has biological plausibility considering that increased mechanical load can lead to both the degradation of joints and an increase in Cx43.15,42 This might support treatment protocols aimed at reducing stress at the glenohumeral joint.
Limitations of this study include the inherent differences among patients that might systemically affect their cartilage and the differences in activity level, which might locally affect their glenohumeral joints. We did not adjust for patient’s nutritional status, family history of OA, smoking status, or activity level. Additionally, although a correlation was found between osteoarthritic specimens and expression of biomarkers, we did not correlate these findings to functional level and, therefore, cannot comment on the clinical relevance of certain biomarker prevalence. At present, we evaluated only mRNA levels, which might not correlate to protein levels. Also, we examined cytokine expression at only one time point and, therefore, do not know whether cytokine elevation would be an early predictor of OA, which is a goal in considering treatment options. Furthermore, we tried to procure cartilage from the same area on the humeral head for each patient; however, this was not always possible because of the variation in cartilage degradation.
Future studies might also evaluate the differences or similarities in biomarker abundance between the shoulder joint and other joints. Such data could then be used to develop inhibitors of these catabolic mediators to medically manage OA. Animal and human studies have already demonstrated the ability of inhibitors to attenuate the levels of biomarkers involved in OA and decrease human cartilage degradation ex vivo.7,43 The investigation of an IL-1 inhibitor as a means to prevent posttraumatic arthritis in humans is currently ongoing.20 Additional studies could include correlating the biomarkers in the cartilage with those in the synovial fluid of an osteoarthritic joint. If a correlation is shown, it might allow synovial fluid to be tested in patients who have or are suspected of having a diagnosis of OA. If individuals can be identified early as having OA, before severe debilitation, they could potentially be treated early with medication or activity modification so that disability and invasive surgery can be delayed or prevented.
Conclusion
We found an abundance of Cx43, ADAMTS5, collagen type I, Cox-2, versican, TIMP-3, and iNOS in osteoarthritic shoulders. Amounts of Cx43, ADAMTS5, collagen type I, Cox-2, versican, MMP3, and TNFα were statistically significant. For Cx43, an 85-fold increase was found in osteoarthritic shoulders compared with non-osteoarthritic shoulders, and its abundance was significantly correlated to the expression of other known biomarkers.
Clinical relevance.
Identification of osteoarthritic biomarkers can help us better understand shoulder OA and build the foundation for future research on disease progression and treatments.
Acknowledgment
The authors thank Senior Editor and Writer Dori Kelly, MA, for professional assistance with the manuscript and figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: None.
IRB approval: The Institutional Review Board at the University of Maryland approved this study. The assigned study number is H-30537.
References
- 1.Bassiouni HM, El-Deeb M, Kenawy N, Abdul-Azim E, Khairy M. Phonoarthrography, musculoskeletal ultrasonography, and biochemical biomarkers for the evaluation of knee cartilage in osteoarthritis. Mod Rheumatol. 2011;21:500–8. doi: 10.1007/s10165-011-0441-8. doi: 10.1007/s10165-011-0441-8. [DOI] [PubMed] [Google Scholar]
- 2.Bauer M, Jackson RW. Chondral lesions of the femoral condyles: a system of arthroscopic classification. Arthroscopy. 1988;4:97–102. doi: 10.1016/s0749-8063(88)80074-4. [DOI] [PubMed] [Google Scholar]
- 3.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–7. doi: 10.1136/ard.2004.025270. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caterson B, Flannery CR, Hughes CE, Little CB. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000;19:333–44. doi: 10.1016/s0945-053x(00)00078-0. [DOI] [PubMed] [Google Scholar]
- 5.Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, Jiang JX. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16:3100–6. doi: 10.1091/mbc.E04-10-0912. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chevalier X. Upregulation of enzymatic activity by interleukin-1 in osteoarthritis. Biomed Pharmacother. 1997;51:58–62. doi: 10.1016/s0753-3322(97)87727-x. [DOI] [PubMed] [Google Scholar]
- 7.Chockalingam PS, Sun W, Rivera-Bermudez MA, Zeng W, Dufield DR, Larsson S, et al. Elevated aggrecanase activity in a rat model of joint injury is attenuated by an aggrecanase specific inhibitor. Osteoarthritis Cartilage. 2011;19:315–23. doi: 10.1016/j.joca.2010.12.004. doi: 10.1016/j.joca.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Cs-Szabò G, Melching LI, Roughley PJ, Glant TT. Changes in messenger RNA and protein levels of proteoglycans and link protein in human osteoarthritic cartilage samples. Arthritis Rheum. 1997;40:1037–45. doi: 10.1002/art.1780400607. [DOI] [PubMed] [Google Scholar]
- 9.de Boer TN, Huisman AM, Polak AA, Niehoff AG, van Rinsum AC, Saris D, Bijlsma JW, Lafeber FJ, Mastbergen SC. The chondroprotective effect of selective Cox-2 inhibition in osteoarthritis: ex vivo evaluation of human cartilage tissue after in vivo treatment. Osteoarthritis Cartilage. 2009;17:482–8. doi: 10.1016/j.joca.2008.09.002. doi: 10.1016./j.joca.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 10.de Isla NG, Mainard D, Muller S, Stoltz JF. In vitro effects of diacerein on NO production by chondrocytes in response to proinflammatory mediators. Biomed Mater Eng. 2008;18(Suppl 1):S99–104. doi: not available. [PubMed] [Google Scholar]
- 11.Fotopoulos VC, Tzinia A, Tzurbakis M, Kalfakakou V, Levidiotou-Stefanou S, Georgoulis A. Expression levels of matrix metalloproteinase (MMP)-9 and its specific inhibitor TIMP-1, in septic and aseptic arthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20:1159–67. doi: 10.1007/s00167-011-1676-9. doi: 10.1007/s00167-011-1676-9. [DOI] [PubMed] [Google Scholar]
- 12.Green CR, Nicholson LF. Interrupting the inflammatory cycle in chronic diseases: do gap junctions provide the answer? Cell Biol Int. 2008;32:1578–83. doi: 10.1016/j.cellbi.2008.09.006. doi: 10.1016/j.cellbi.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Hamada K, Fukuda H, Mikasa M, Kobayashi Y. Roentgenographic findings in massive rotator cuff tears: a long-term observation. Clin Orthop Relat Res. 1990;254:92–6. [PubMed] [Google Scholar]
- 14.Hiraiwa H, Sakai T, Mitsuyama H, Hamada T, Yamamoto R, Omachi T, Ohno Y, Nakashima M, Ishiguro N. Inflammatory effect of advanced glycation end products on human meniscal cells from osteoarthritic knees. Inflamm Res. 2011;60:1039–48. doi: 10.1007/s00011-011-0365-y. doi: 10.1007/s00011-011-0365-y. [DOI] [PubMed] [Google Scholar]
- 15.Jiang JX, Siller-Jackson AJ, Burra S. Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Front Biosci. 2007;12:1450–62. doi: 10.2741/2159. doi: 10.2741/2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jingushi S, Lohmander LS, Shinmei M, Hoerrner LA, Lark MW, Sugioka Y, Iwamoto Y. Markers of joint tissue turnover in joint fluids from hips with osteonecrosis of the femoral head. J Orthop Res. 2000;18:728–33. doi: 10.1002/jor.1100180508. [DOI] [PubMed] [Google Scholar]
- 17.Klatt AR, Klinger G, Paul-Klausch B, Kuhn G, Renno JH, Wagener R, et al. Matrilin-3 Activates the expression of osteoarthritis-associated genes in primary human chondrocytes. FEBS Lett. 2009;583:3611–7. doi: 10.1016/j.febslet.2009.10.035. doi: 10.1016/j.febslet.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, et al. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52:128–35. doi: 10.1002/art.20776. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 19.Kolomytkin OV, Marino AA, Waddell DD, Mathis JM, Wolf RE, Sadasivan KK, et al. IL-1beta-induced production of metalloproteinases by synovial cells depends on gap junction conductance. Am J Physiol Cell Physiol. 2002;282:C1254–60. doi: 10.1152/ajpcell.01166.2000. doi: 10.1152/ajpcell.01166.2000. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence JT, Birmingham J, Toth AP. Emerging ideas: prevention of posttraumatic arthritis through interleukin-1 and tumor necrosis factor-alpha inhibition. Clin Orthop Relat Res. 2011;469:3522–6. doi: 10.1007/s11999-010-1699-4. doi: 10.1007/s11999-010-1699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee AS, Ellman MB, Yan D, Kroin JS, Cole BJ, Van Wijnen AJ, Im HJ. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527:440–7. doi: 10.1016/j.gene.2013.05.069. doi: 10.1016/j.gene.2013.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeGrand A, Fermor B, Fink C, Pisetsky DS, Weinberg JB, Vail TP, et al. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum. 2001;44:2078–83. doi: 10.1002/1529-0131(200109)44:9<2078::AID-ART358>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 23.Lima F, Niger C, Hebert C, Stains JP. Connexin43 potentiates osteoblast responsiveness to fibroblast growth factor 2 via a protein kinase C-delta/Runx2-dependent mechanism. Mol Biol Cell. 2009;20:2697–708. doi: 10.1091/mbc.E08-10-1079. doi: 10.1091/mbc.E08-10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo IK, Marchuk LL, Hollinshead R, Hart DA, Frank CB. Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase mRNA levels are specifically altered in torn rotator cuff tendons. Am J Sports Med. 2004;32:1223–9. doi: 10.1177/0363546503262200. doi: 10.1177/0363546503262200. [DOI] [PubMed] [Google Scholar]
- 25.Marino AA, Waddell DD, Kolomytkin OV, Meek WD, Wolf R, Sadasivan KK, et al. Increased intercellular communication through gap junctions may contribute to progression of osteoarthritis. Clin Orthop Relat Res. 2004;422:224–32. doi: 10.1097/01.blo.0000129346.29945.3b. doi: 10.1097/01.blo.0000129346.29945.3b. [DOI] [PubMed] [Google Scholar]
- 26.Martel-Pelletier J, McCollum R, Fujimoto N, Obata K, Cloutier JM, Pelletier JP. Excess of metalloproteases over tissue inhibitor of metalloprotease may contribute to cartilage degradation in osteoarthritis and rheumatoid arthritis. Lab Invest. 1994;70:807–15. [PubMed] [Google Scholar]
- 27.Martin I, Jakob M, Schäfer D, Dick W, Spagnoli G, Heberer M. Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthritis Cartilage. 2001;9:112–8. doi: 10.1053/joca.2000.0366. [DOI] [PubMed] [Google Scholar]
- 28.Mastbergen SC, Bijlsma JW, Lafeber FP. Selective Cox-2 inhibition is favorable to human early and late-stage osteoarthritis cartilage: a human in vitro study. Osteoarthritis Cartilage. 2005;13:519–26. doi: 10.1016/j.joca.2005.02.004. doi: 10.1016/j.joca.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Masuhara K, Nakai T, Yamaguchi K, Yamasaki S, Sasaguri Y. Significant increases in serum and plasma concentrations of matrix metalloproteinases 3 and 9 in patients with rapidly destructive osteoarthritis of the hip. Arthritis Rheum. 2002;46:2625–31. doi: 10.1002/art.10547. doi: 10.1002/art.10547. [DOI] [PubMed] [Google Scholar]
- 30.Mayan MD, Carpintero-Fernandez P, Gago-Fuentes R, Martinez-de-Ilarduya O, Wang HZ, Valiunas V, Brink P, Blanco FJ. Human articular chondrocytes express multiple gap junction proteins: differential expression of connexins in normal and osteoarthritic cartilage. Am J Pathol. 2013;182:1337–46. doi: 10.1016/j.ajpath.2012.12.018. doi: 10.1016/j.ajpath.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miosge N, Hartmann M, Maelicke C, Herken R. Expression of collagen type I and type II in consecutive stages of human osteoarthritis. Histochem Cell Biol. 2004;122:229–36. doi: 10.1007/s00418-004-0697-6. doi: 10.1007/s00418-004-0697-6. [DOI] [PubMed] [Google Scholar]
- 32.Neer CS, II, Craig EV, Fukuda H. Cuff-tear arthropathy. J Bone Joint Surg Am. 1983;65:1232–44. [PubMed] [Google Scholar]
- 33.Niger C, Buo AM, Hebert C, Duggan BT, Williams MS, Stains JP. ERK acts in parallel to PKCσ to mediate the connexin43-dependent potentiation of Runx2 activity by FGF2 in MC3T3 osteoblasts. Am J Physiol Cell Physiol. 2012;302:C1035–44. doi: 10.1152/ajpcell.00262.2011. doi: 10.1152/ajpcell.00262.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niger C, Howell FD, Stains JP. Interleukin-1beta increases gap junctional communication among synovial fibroblasts via the extracellular-signal-regulated kinase pathway. Biol Cell. 2009;102:37–49. doi: 10.1042/BC20090056. doi: 10.1042/BC20090056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niger C, Luciotti MA, Buo AM, Hebert C, Ma V, Stains JP. The regulation of runt-related transcription factor 2 by fibroblast growth factor-2 and connexin43 requires the inositol polyphosphate/protein kinase Cσ cascade. J Bone Miner Res. 2013;28:1468–77. doi: 10.1002/jbmr.1867. doi: 10.1002/jbmr.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishida Y, Shinomura T, Iwata H, Miura T, Kimata K. Abnormal occurrence of a large chondroitin sulfate proteoglycan, PG-M/versican in osteoarthritic cartilage. Osteoarthritis Cartilage. 1994;2:43–9. doi: 10.1016/s1063-4584(05)80005-6. [DOI] [PubMed] [Google Scholar]
- 37.Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med. 1989;17:505–13. doi: 10.1177/036354658901700410. [DOI] [PubMed] [Google Scholar]
- 38.Omura K, Takahashi M, Omura T, Miyamoto S, Kushida K, Sano Y, Miura M, Nagano A. Changes in the concentration of plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases-1 (TIMP-1) after total joint replacement in patients with arthritis. Clin Rheumatol. 2002;21:448–92. doi: 10.1007/s100670200120. doi: not available. [DOI] [PubMed] [Google Scholar]
- 39.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–47. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 40.Ratcliffe A, Flatow EL, Roth N, Saed-Nejad F, Bigliani LU. Biochemical markers in synovial fluid identity early osteoarthritis of the glenohumeral joint. Clin Orthop Relat Res. 1996;330:45–53. doi: 10.1097/00003086-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Rosa SC, Judas F, Lopes MC, Mendes AF. Nitric oxide synthase isoforms and NF-kappaB activity in normal and osteoarthritic human chondrocytes: regulation by inducible nitric oxide. Nitric Oxide. 2008;19:276–83. doi: 10.1016/j.niox.2008.07.005. doi: 10.1016/j.niox.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez C, Pesesse L, Gabay O, Delcour JP, Msika P, Baudouin C, et al. Regulation of subchondral bone osteoblast metabolism by cyclic compression. Arthritis Rheum. 2012;64:1193–203. doi: 10.1002/art.33445. doi: 10.1002/art.33445. [DOI] [PubMed] [Google Scholar]
- 43.Settle S, Vickery L, Nemirovskiy O, Vidmar T, Bendele A, Messing D, et al. Cartilage degradation biomarkers predict efficacy of a novel, highly selective matrix metalloproteinase 13 inhibitor in a dog model of osteoarthritis: confirmation by multivariate analysis that modulation of type II collagen and aggrecan degradation peptides parallels pathologic changes. Arthritis Rheum. 2010;62:3006–15. doi: 10.1002/art.27596. doi: 10.1002/art.27596. [DOI] [PubMed] [Google Scholar]
- 44.Stains JP, Civitelli R. Gap junctions regulate extracellular signal-regulated kinase signaling to affect gene transcription. Mol Biol Cell. 2005;16:64–72. doi: 10.1091/mbc.E04-04-0339. doi: 10.1091/mbc.E04-04-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stains JP, Lecanda F, Screen J, Towler DA, Civitelli R. Gap junctional communication modulates gene transcription by altering the recruitment of Sp1 and Sp3 to connexin-response elements in osteoblast promoters. J Biol Chem. 2003;278:24377–87. doi: 10.1074/jbc.M212554200. doi: 10.1074/jbc.M212554200. [DOI] [PubMed] [Google Scholar]
- 46.Stannus OP, Jones G, Blizzard L, Cicuttini FM, Ding C. Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis. 2013;72:535–40. doi: 10.1136/annrheumdis-2011-201047. doi: 10.1136/annrheumdis-2011-201047. [DOI] [PubMed] [Google Scholar]
- 47.Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J, Ding C. Circulating levels of IL-6 and TNFα are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthritis Cartilage. 2010;18:1441–7. doi: 10.1016/j.joca.2010.08.016. doi: 10.1016/j.joca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 48.Su S, Grover J, Roughley PJ, DiBattista JA, Martel-Pelletier J, Pelletier JP, Zafarullah M. Expression of the tissue inhibitor of metalloproteinases (TIMP) gene family in normal and osteoarthritic joints. Rheumatol Int. 1999;18:183–91. doi: 10.1007/s002960050083. [DOI] [PubMed] [Google Scholar]
- 49.Tonon R, D’Andrea P. The functional expression of connexin 43 in articular chondrocytes is increased by interleukin 1beta: evidence for a Ca2+-dependent mechanism. Biorheology. 2002;39:153–60. doi: not available. [PubMed] [Google Scholar]
- 50.Tonon R, D’Andrea P. Interleukin-1beta increases the functional expression of connexin 43 in articular chondrocytes: evidence for a Ca2+-dependent mechanism. J Bone Miner Res. 2000;15:1669–77. doi: 10.1359/jbmr.2000.15.9.1669. [DOI] [PubMed] [Google Scholar]
- 51.Tsuchida S, Arai Y, Kishida T, Takahashi KA, Honjo K, Terauchi R, Inoue H, Oda R, Mazda O, Kubo T. Silencing the expression of connexin 43 decreases inflammation and joint destruction in experimental arthritis. J Orthop Res. 2013;31:525–30. doi: 10.1002/jor.22263. doi:10.1002/jor.22263. [DOI] [PubMed] [Google Scholar]
- 52.van den Berg WB. The role of cytokines and growth factors in cartilage destruction in osteoarthritis and rheumatoid arthritis. Z Rheumatol. 1999;58:136–41. doi: 10.1007/s003930050163. [DOI] [PubMed] [Google Scholar]
- 53.Voloshin I, Gelinas J, Maloney MD, O’Keefe RJ, Bigliani LU, Blaine TA. Proinflammatory cytokines and metalloproteases are expressed in the subacromial bursa in patients with rotator cuff disease. Arthroscopy. 2005;21:1076.e1–9. doi: 10.1016/j.arthro.2005.05.017. doi: 10.1016/j.arthro.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 54.Yoshihara Y, Hamada K, Nakajima T, Fujikawa K, Fukuda H. Biochemical markers in the synovial fluid of glenohumeral joints from patients with rotator cuff tear. J Orthop Res. 2001;19:573–9. doi: 10.1016/S0736-0266(00)00063-2. [DOI] [PubMed] [Google Scholar]