Abstract
We have recently reported that transactivation of cytochrome P450 (CYP) 2D6 promoter by hepatocyte nuclear factor (HNF) 4α is enhanced during pregnancy, and this is triggered in part by altered expression of small heterodimer partner (SHP) and Krüppel-like factor 9 (KLF9). The objective of this study is to determine whether this is conserved for mouse endogenous Cyp2d gene(s). Among the eight Cyp2d homologs of mouse we examined, only Cyp2d40 expression was found induced (by 6-fold) at term pregnancy as compared to pre-pregnancy level. In mice where hepatic Hnf4α was knocked-down, the pregnancy-mediated increase in Cyp2d40 expression was abrogated. Results from transient transfection, promoter reporter assays, and electrophoretic mobility shift assays indicated that HNF4α transactivates Cyp2d40 promoter via direct binding to −117/−105 of the gene. Chromatin immunoprecipitation assay showed a 2.3-fold increase in HNF4α recruitment to Cyp2d40 promoter during pregnancy. Results from mice treated with an SHP inducer (i.e., GW4064) and HepG2 cells co-transfected with KLF9 suggest that neither SHP nor KLF9 is involved in the increased HNF4α transactivation of Cyp2d40 promoter during pregnancy. Together, our results indicate that while the underlying molecular mechanism is different from that for CYP2D6, Cyp2d40 is induced during pregnancy through enhanced transactivation by HNF4α.
Keywords: Cyp2d40, pregnancy, regulation, HNF4α
1. Introduction
Cytochrome P450 (CYP) 2D6 is a major drug-metabolizing enzyme and expressed in the liver, intestine, kidney, and brain [1, 2]. CYP2D6 mediates hepatic metabolism of approximately 25% of marketed drugs including antidepressants and antipsychotics [3, 4]. Also, decreased CYP2D6 activity levels are associated with increased susceptibility to Parkinson’s disease [5, 6] or higher blood perfusion levels in brain regions linked to alertness in humans [7]. The detailed roles of CYP2D6 in altered biological functions in extrahepatic tissues (such as brain) remains unclear, in part due to a lack of animal models to study CYP2D6 function in vivo. Transgenic mice such as CYP2D6-humanized (Tg-CYP2D6) mice that carry human CYP2D6 gene in the mouse genome exhibit robust CYP2D6 expression in liver and intestine [1, 8, 9]. However, CYP2D6 expression is absent in the brain of the transgenic mice [1, 9]. Identification and characterization of mouse Cyp2d homologs whose expression or activities are co-regulated as CYP2D6, if any, may potentially enable establishing study models to explore the biological functions of CYP2D6 in in vivo systems.
CYP2D6-mediated drug metabolism is increased during human pregnancy [10–12]. For example, metoprolol clearance increases 2–13 fold during pregnancy as compared to that after delivery [11]. We have recently shown that CYP2D6 expression is enhanced by 4-fold at term pregnancy (as compared to the pre-pregnancy or postpartum period) in Tg-CYP2D6 mice, and this was accompanied by increased transactivation of CYP2D6 promoter by hepatocyte nuclear factor 4α (HNF4α, NR2A1) [13]. HNF4α is a nuclear receptor known to play a critical role in regulating expression of liver-specific genes, including drug-metabolizing enzymes [14–17]. Our studies also demonstrated that the increased HNF4α transactivation of CYP2D6 promoter is in part attributed to two transcription factors, namely small heterodimer partner (SHP, NR0B2) and Krüppel-like Factor 9 (KLF9), whose hepatic expression is differentially regulated during pregnancy [13, 18]. SHP, a member of nuclear receptor superfamily, lacks the DNA-binding domain [19] and interacts with HNF4α to function as a transcriptional repressor [20, 21]. During pregnancy, hepatic SHP expression decreases, and this leads to de-repression of CYP2D6 promoter [13]. On the other hand, KLF9 transactivates CYP2D6 promoter in synergy with HNF4α [18]. During pregnancy, hepatic KLF9 expression increases, further potentiating HNF4α transactivation of CYP2D6 promoter [18]. Importantly, these results provide a potential platform to identify and characterize mouse Cyp2d homologs that are regulated similarly as CYP2D6.
Humans express only one functional CYP2D (i.e., CYP2D6), but mouse genome harbors the following nine Cyp2d homologs: Cyp2d9, 2d10, 2d11, 2d12, 2d13, 2d22, 2d26, 2d34, and 2d40. How the expression of mouse Cyp2d genes is regulated remains largely unknown. Also unknown is whether the regulatory mechanisms leading to CYP2D6 induction during pregnancy is conserved for endogenous mouse Cyp2d genes.
In this study, we identified Cyp2d40 as an endogenous mouse Cyp2d gene whose expression is induced during pregnancy. We further evaluated the role of HNF4α and its modulators (i.e., SHP and KLF9) in the regulation of Cyp2d40 expression during pregnancy, to explore conserved physiological/functional significance of CYP2D induction during pregnancy.
Materials and Methods
1.1. Animals
Tg-CYP2D6 and Hnf4α/AlbCre transgenic mice were previously described [8, 22]. Adult female (8 weeks old) mice were mated with male mice of similar age. Mating between adult mice was confirmed by the presence of vaginal plugs (day 0). Male mice were separated from female mice immediately after a vaginal plug was found. Virgin mice were group-housed so that their estrous cycles were suppressed. All procedures were approved by the Institutional Animal Care and Use Committee in the University of Illinois at Chicago.
1.2. GW4064 treatment
Male C57BL/6J mice were injected with GW4064 (Sigma-Aldrich, St. Louis, MO) at 15 mg/kg/day or vehicle (olive oil) for 5 days. Livers were collected 6 hours after the last injection for examination of gene expression.
1.3. Plasmids
The upstream region of Cyp2d40 (GenBank accession no. NC_000081.6) was PCR-amplified using genomic DNA of C57BL/6J mouse and the primer set listed in Table 1. The PCR product was digested by KpnI and SacI restriction enzymes (New England Biolabs, Ipswich, MA) and cloned into promoterless pGL3-basic vector (Promega, Madison, WI) digested by the same enzymes, yielding pGL3-Cyp2d40 (−2000/+6). For cloning of 5′-deletion constructs, different regions of Cyp2d40 promoter were PCR-amplified using pGL3-Cyp2d40 (−2000/+6) as template and respective forward primers listed in Table 1. The PCR product was digested by KpnI and SacI restriction enzymes and cloned into pGL3-basic vector digested by the same enzymes. To construct mutant vectors of Cyp2d40 promoter at HNF4α binding site, pGL3-CYP2d40 (−171/+6) was PCR-amplified by using Phusion High-Fidelity DNA Polymerase (New England Biolabs, Ipswich, MA) and 5′-phosphorylated primers harboring mutation or deletion of the HNF4α binding site listed in Table 1. The PCR products were then ligated by using T4 ligase (New England Biolabs, Ipswich, MA).
Table 1.
Primers used in the study
| Cloning primers | ||
| pGL3-Cyp2d40 (−2000/+6) | Forward | 5′-ATCGGGTACCCACAGGACTGCAAAGCACTC -3′ |
| Reverse | 5′-ATCGGAGCTCCCACTGCTTCCCAGGCTTCA -3′ | |
| pGL3-Cyp2d40 (−1451/+6) | Forward | 5′-ATCGGGTACCAGCATCCTGAGACTGTCA -3′ |
| pGL3-Cyp2d40 (−981/+6) | Forward | 5′-ATCGGGTACCGGATCCAGCAAGGACATG -3′ |
| pGL3-Cyp2d40 (−471/+6) | Forward | 5′-ATCGGGTACCCTCTTCCAATGCCAAGAG -3′ |
| pGL3-Cyp2d40 (−171/+6) | Forward | 5′-ATCGGGTACCCTGGCCTGTCTCTACACT -3′ |
| pGL3-Cyp2d40 (−86/+6) | Forward | 5′-ATCGGGTACCAAGGTGGTAGGATCCAAG -3′ |
| pGL3-Cyp2d40 (−171/+6) HNF4α site mutation |
Forward | [Phos]5′-AATTCACACCTGGACACTCCTTTATAAGG -3′ |
| Reverse | [Phos]5′-GTCAATCTGCCCCCAACCCCAATCCT -3′ | |
| pGL3-Cyp2d40 (−171/+6) HNF4α site deletion |
Forward | [Phos]5′-ACCTGGACACTCCTTTATAAGG -3′ |
| Reverse | [Phos]5′-CTGCCCCCAACCCCAATCCT -3′ | |
| qRT-PCR primers | ||
| Shp | Forward | 5′-GGCCTCTACCCTCAAGAACAT -3′ |
| Reverse | 5′-TGTCAACGTCTCCCATGATAGG -3′ | |
| Gapdh | Forward | 5′-AGGTCGGTGTGAACGGATTTG -3′ |
| Reverse | 5′-TGTAGACCATGTAGTTGAGGTCA -3′ | |
| ChIP PCR primers | ||
| Cyp2d40 | Forward | 5′-TCTCTGGCCTGTCTCTACAC -3′ |
| Reverse | 5′-GCTTGGATCCTACCACCTTA -3′ | |
1.4. Cell culture and luciferase reporter assay
HEK293T cells were cultured in RPMI-1640 media supplied with 10 mM Hepes buffer, 100 μM non-essential amino acids, 5,000 U penicillin/streptomycin (Life Technologies, Carlsbad, CA) and 10% fetal bovine serum (Gemini Bio-products, West Sacramento, CA). For assays, HEK-293T cells were seeded in 24-well plates at a density of 1×105 cells per well. On the next day, the cells were transfected with 0.3 μg of luciferase construct, 0.1 μg of expression plasmid (or empty vector as a control), and 0.002 μg of Renilla expression vector (Promega, Madison, WI) per well, using Fugene HD transfection reagent (Promega, Madison, WI) according to the manufacturer’s protocol. After 48 hours, the transfected cells were harvested for determination of luciferase activities using Dual-Luciferase® Reporter Assay System (Promega, Madison, WI).
1.5. RNA isolation and quantitative real time-PCR (qRT-PCR)
Total RNAs were isolated from mouse liver tissues using Trizol (Life Technologies, Carlsbad, CA) and used as template for cDNA synthesis using High-Capacity cDNA Reverse Transcription Kit (Life Technologies, Foster City, CA). Using the cDNA as template, qRT-PCR was performed using StepOnePlus™ Real-Time PCR System and the following TaqMan® Gene expression assays (Integrated DNA technologies, Coralville, IA) for Cyp2ds and mouse Actb. Shp and Gapdh mRNA levels were determined using SYBR® Green Real Time PCR Master Mix (Life technologies, Foster City, CA) and primer sets listed in Table 1. The relative expression in mRNA levels was determined after normalizing the gene expression levels by those of mouse Gapdh or Actb (2−ΔΔCt method) [23].
1.6. Chromatin Immunoprecipitation (ChIP) assay
ChIP assays with mouse liver were performed as previously described [13]. Brie y, livers were nely minced and incubated in PBS containing 1% formaldehyde at room temperature for 15 min, and glycine was added to stop the crosslinking reaction. Cell pellets were resuspended in hypotonic buffer (15 mM HEPES, 60 mM KCl, 2 mM EDTA, 0.5% BSA, 0.15 mM spermine, 0.5 mM spermidine, 0.32 M sucrose, pH 7.9) and lysed by homogenization. Nuclei were pelleted and resuspended in nuclei lysis buffer (50 mM Tris-HCl, 2 mM EDTA, 1% SDS, pH 8.0). The samples were sonicated to shear DNA at the length from 100 to 500 bp. After centrifuge, the chromatin samples were immunoprecipitated using magnetic beads coated with 2 μg antibody (HNF4α, sc-6556x, Santa Cruz Biotechnology, Dallas, TX) or immunoglobulin G (IgG, sc-2028, Santa Cruz, Dallas, TX) at 4°C overnight. The immune complex on the magnetic beads was collected and extensively washed, and the bound chromatin was eluted. Genomic DNA was puri ed by phenol chloroform extraction followed by Wizard® SV Gel and PCR Clean-Up System (Promega, Madison, WI). qRT-PCR was performed using the genomic DNA and the primer set listed in Table 1.
1.7. Electrophoretic mobility shift assay (EMSA)
EMSA was performed as previously described [24]. Briefly, recombinant HNF4α protein (with C-terminal MYC/DDK tag; 0.2 μg; TP317863, OriGene Technologies. Rockville, MD) or nuclear protein extracts (5 μg) from HEK293T cells transfected with HNF4α expression vector (or empty vector) were preincubated with binding buffer (4% glycerol, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, 50 mM NaCl, 10 mM Tris-HCl, pH 7.5) at room temperature in the presence or absence of unlabeled DNA probes or HNF4α antibody (2 μg, sc-6556x, Santa Cruz). After 10 min, the binding reaction was initiated by adding 0.035 pmol of 5′-end 32P-labeled probes harboring Cyp2d40 putative HNF4α-binding sequence or CYP2D6 HNF4α-binding sequence. The reaction mixture was incubated at room temperature for 20 min. Protein-bound probes were separated from free probes on 4% (w/v) non-denaturing polyacrylamide gel. The gel was dried, and radioactivity visualized using PhosphorImager (GE Healthcare Bio-Sciences, Pittsburgh, PA).
1.8. Statistical analysis
All data were presented as means ± SD. Statistical analyses were performed using one-way ANOVA followed by Dunnett’s post-hoc test. A value of p < 0.05 was considered statistically significant.
2. Results
2.1. Cyp2d40 expression is enhanced during pregnancy
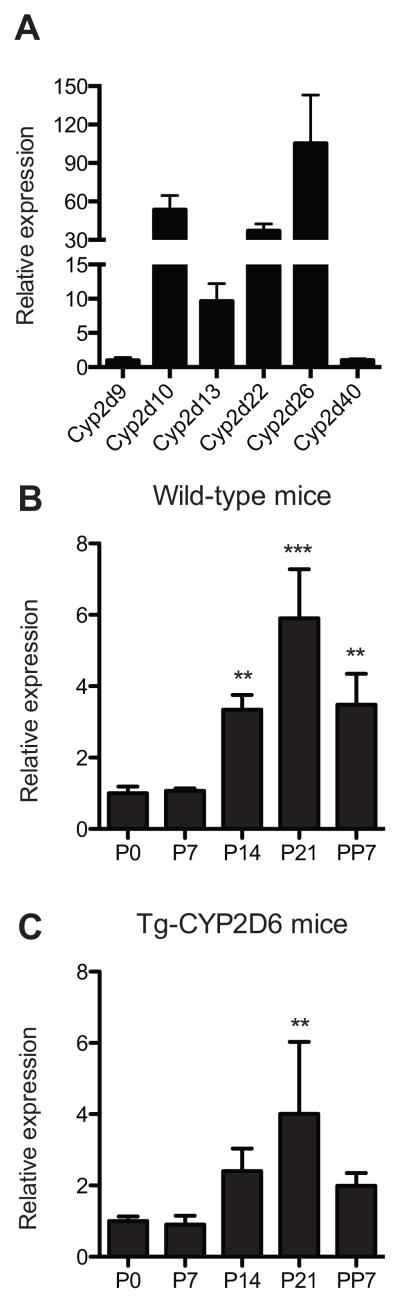
To identify the mouse Cyp2d genes that exhibit similar upregulation as CYP2D6 does during pregnancy, we examined the mRNA expression levels of the following Cyp2d homologs in livers of Tg-CYP2D6 or wild type mice at different gestational time points: Cyp2d9, 2d10, 2d12, 2d13, 2d22, 2d26, 2d34, and 2d40. Cyp2d11 was not examined in this study because its expression is male-specific [25]. Cyp2d12 and Cyp2d34 expression was not detectable in the mouse liver tissues (data not shown). In the livers of nonpregnant mice, Cyp2d26 expression was most abundant, followed by Cyp2d10, Cyp2d22, Cyp2d13, Cyp2d9 and Cyp2d40 (Fig. 1A). Among the six Cyp2d isoforms, Cyp2d40 expression increased significantly at term as compared to pre-pregnancy level in both Tg-CYP2D6 and wild-type mice (by 6- and 4-fold, respectively) (Fig. 1B and 1C), suggesting that the presence of transgene (i.e., CYP2D6) does not impact Cyp2d40 regulation during pregnancy. On the other hand, mRNA expression of the remaining five Cyp2d isoforms slightly decreased during pregnancy (data not shown).
Figure 1. Hepatic Cyp2d40 is induced in both wild-type and Tg-CYP2D6 mice during pregnancy.
(A) Liver tissues of wild-type nonpregnant female mice were collected (n=4). mRNA expression levels of Cyp2ds were determined by qRT-PCR and normalized by Cyp2d40 expression. (B) and (C) Liver tissues of wild-type (B) and Tg-CYP2D6 (C) mice were collected at different gestational time points: pre-pregnancy (P0), 7, 14, or 21 days of pregnancy (P7, P14, P21, respectively), 7 days postpartum (PP7). mRNA levels of Cyp2d40 were determined by qRT-PCR and normalized by that in the pre-pregnancy group (n=4, mean ± S.D.; **, p<0.01, ***, p<0.001 versus P0 group).
2.2. HNF4α is critical for Cyp2d40 induction during pregnancy
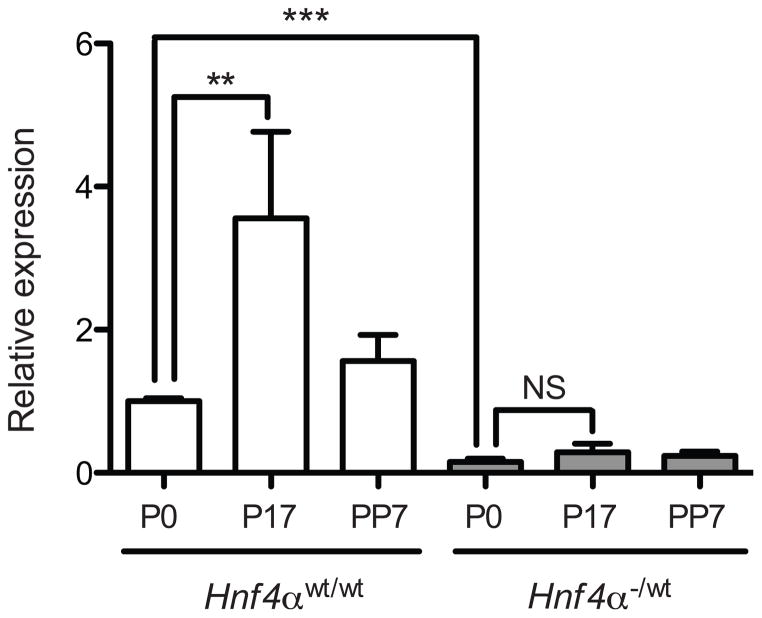
We have previously shown that CYP2D6 induction during pregnancy is attributable to enhanced transactivation of CYP2D6 promoter by HNF4α [13]. To define the role of HNF4α in Cyp2d40 induction during pregnancy, we examined whether reduced hepatic HNF4α expression alters the extent of Cyp2d40 up-regulation seen at term pregnancy. To this end, the pregnancy-mediated changes in Cyp2d40 expression were compared between mice carrying two vs. one copy of Hnf4α allele in the liver (noted as Hnf4α(wt/wt) and Hnf4α(−/wt) mice, respectively). Hepatic HNF4α protein expression level in Hnf4α(− /wt) mice was previously shown to be about half of that in Hnf4α(wt/wt) mice [13]. In Hnf4α(−/wt) mice, the basal mRNA expression of Cyp2d40 decreased dramatically as compared to that in Hnf4α(wt/wt) mice (Fig. 2), indicating that HNF4α is a major regulator of basal Cyp2d40 expression. More importantly, Cyp2d40 induction during pregnancy was abrogated in Hnf4α(−/wt) mice, suggesting a key role of HNF4α in Cyp2d40 induction during pregnancy.
Figure 2. HNF4α is critical for Cyp2d40 basal expression and induction during pregnancy.
Liver tissues were collected from Hnf4α(wt/wt) and Hnf4α(−/wt) mice at pre-pregnancy (P0), 17 days of pregnancy (P17), and 7 days post-partum (PP7). mRNA levels of Cyp2d40 were determined by qRT-PCR and normalized by that of Hnf4α(wt/wt) mice at P0 (n=4, mean ± S.D.; **, p<0.01; ***, p<0.001; NS, not significant).
2.3. HNF4α transactivates Cyp2d40 promoter
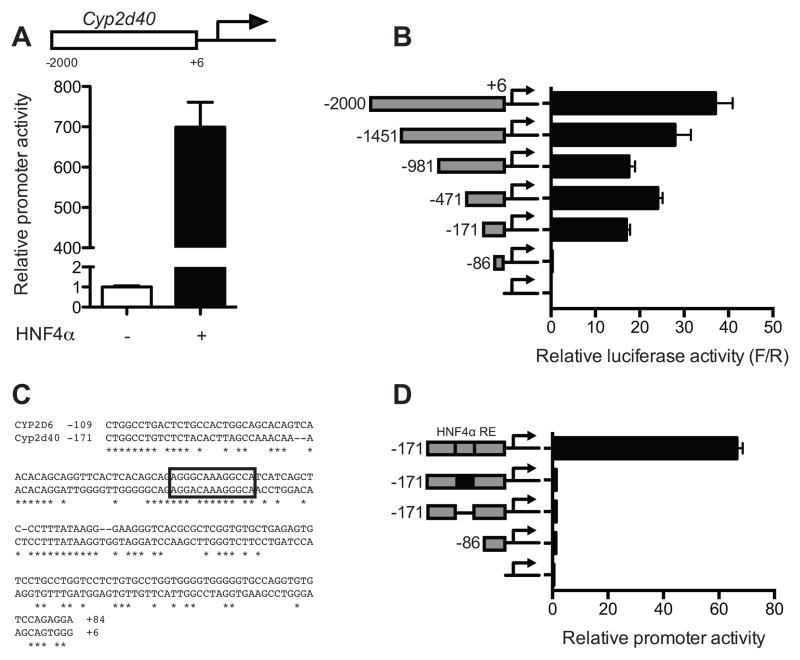
To further characterize the role of HNF4α in the regulation of Cyp2d40 expression, transient transfection and luciferase reporter assays were conducted. Transfection of HNF4α in HEK293T cells led to a dramatic increase in Cyp2d40 promoter activity (Fig. 3A), indicating that HNF4α transactivates Cyp2d40 promoter. To map the cis-element(s) responsible for HNF4α transactivation, a series of luciferase vectors harboring 5′-truncated upstream regulatory region of Cyp2d40 were constructed, and luciferase assays were performed. Ectopic HNF4α expression led to transactivation of Cyp2d40 promoter as short as −171 to +6 (Fig. 3B). However, the transactivation by HNF4α was completely abrogated when the region between −171 and −86 was deleted (Fig. 3B), indicating the presence of key cis-element(s) for HNF4α action within the region. In silico promoter analysis revealed a putative HNF4α binding site in this region, which is conserved between CYP2D6 and Cyp2d40 (Fig. 3C). To further validate the putative HNF4α binding site in Cyp2d40, mutation and deletion assays were performed. HNF4α transactivation of Cyp2d40 promoter was completely abolished when the putative binding site of HNF4α was deleted or mutated (Fig. 3D).
Figure 3. HNF4α transactivates Cyp2d40 promoter where a putative HNF4 binding site is critical for the activation.
(A) HEK293T cells were co-transfected with pGL3-Cyp2d40 with HNF4α expression plasmid (or empty vector as a control), and dual luciferase assays were performed (n=3, mean ± S.D.). (B) HEK293T cells were co-transfected with HNF4α expression plasmid and one of 5′-truncated Cyp2d40 promoter luciferase vectors, and dual luciferase assays were performed (n=3, mean ± S.D.). (C) A putative HNF4 binding site in Cyp2d40 upstream regulatory region (marked using a box) is aligned with the known HNF4α binding site in CYP2D6 [36]. (D) HEK293T cells were co-transfected with HNF4α expression plasmid, along with a luciferase construct where HNF4α response element (RE) of Cyp2d40 (marked using a white box) was mutated (marked using a black box) or deleted, and dual luciferase assays were performed (n=3, mean ± S.D.).
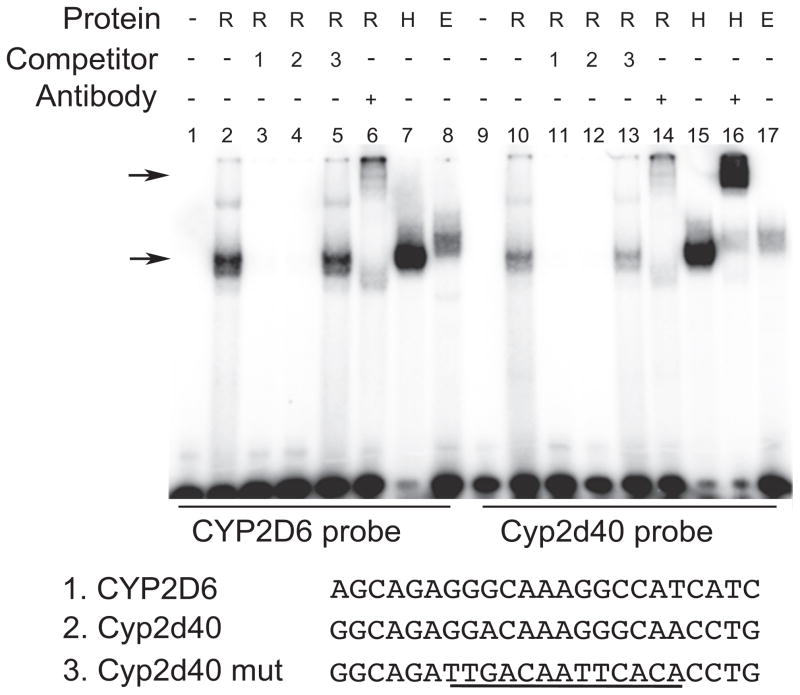
To verify direct interaction between HNF4α and the putative response element identified above, EMSA was performed. Recombinant HNF4α protein or nuclear extracts from HEK293T cells transfected with HNF4α expression vector (or empty vector as a control) were incubated with radiolabeled DNA probes harboring HNF4α response element of CYP2D6 (as positive control) or that of Cyp2d40, and the reaction mixtures were resolved on non-denaturing gels. A shifted band was observed when recombinant protein was incubated with either probe (Fig. 4, lane 2 and 10 for CYP2D6 and Cyp2d40 probe, respectively). Of note, a weaker signal was observed for Cyp2d40 than CYP2D6 (Fig. 4, lane 2 vs. 10), suggesting differences in binding affinity of HNF4α to the DNAs. Shifted bands at the similar location were obtained when nuclear extracts from HEK293T cells were used (Fig. 4, lane 7 and 15 for CYP2D6 and Cyp2d40 probe, respectively). The signal intensity of shifted bands for CYP2D6 and Cyp2d40 probes significantly decreased when unlabeled CYP2D6 or Cyp2d40 probe was added to the reaction mixture as a competitor (Fig. 4, lane 3, 4, 11, and 12). On the other hand, the probe harboring mutated sequences of HNF4α binding site failed to compete for the binding (Fig. 4, lane 5 and 13 for CYP2D6 and Cyp2d40, respectively). Addition of HNF4α antibody to the reaction mixture led to a super-shift of the band (Fig. 4, lane 6, 14 and 16). These EMSA results indicate that HNF4α directly binds to the putative binding site of Cyp2d40.
Figure 4. HNF4α physically interacts with the putative HNF4 binding site in Cyp2d40 promoter.
Recombinant HNF4α protein (R, 0.2 μg) or nuclear proteins (5 μg) prepared from HEK293T cells transfected with HNF4α expression vector (H) or empty control vector (E) were incubated with 32P-labeled DNA probes harboring putative HNF4α cis-element of Cyp2d40 or the known HNF4α binding sequence of CYP2D6, in the presence or absence of HNF4α antibody or indicated unlabeled DNA probes as competitors (in 50-fold excess). The mixture was resolved on non-denaturing gel. The lower arrow indicates the location of shifted bands by apparent HNF4α binding to DNA. The upper arrow indicates the super-shift complex. The probe sequences are shown below the gel image, with the mutated sequence underlined.
2.4. HNF4α recruitment to Cyp2d40 promoter increases at term pregnancy
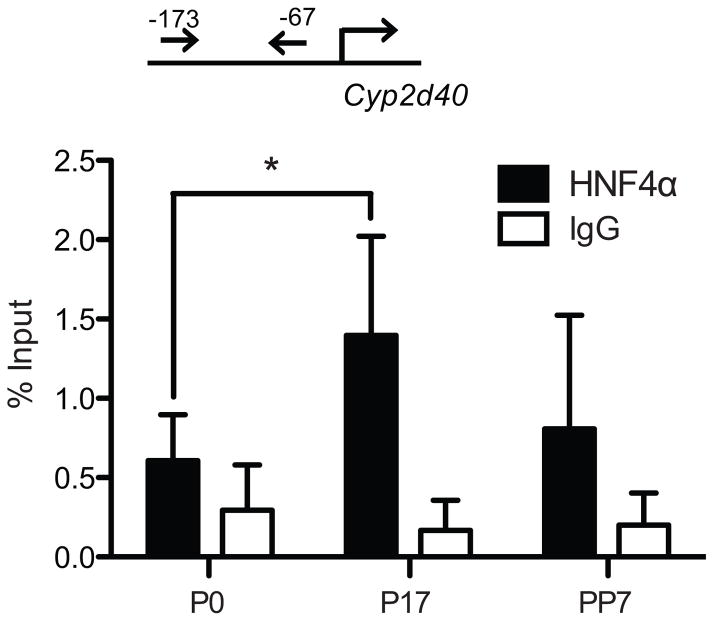
To examine the pregnancy-mediated changes in HNF4α transactivation of Cyp2d40 promoter in vivo, ChIP assays were performed using mouse liver tissues collected at different gestational time points. The amount of HNF4α-bound DNA was measured by qRT-PCR using a primer set that can detect the HNF4α response element of Cyp2d40. The results showed that HNF4α recruitment to Cyp2d40 was increased by 2.3-fold at term pregnancy as compared to pre-pregnancy (Fig. 5), suggesting that enhanced transactivation of Cyp2d40 promoter is in part responsible for Cyp2d40 induction during pregnancy.
Figure 5. HNF4α recruitment to Cyp2d40 promoter increases at term pregnancy.
Liver tissues were collected from Tg-CYP2D6 mice at pre-pregnancy (P0), 17 days of pregnancy (P17), and 7 days post-partum (PP7). ChIP assays were performed using HNF4α antibody (or IgG as a control), and the pulled-down DNA was quantified by qRT-PCR using a set of primers that bind −171/−67 of Cyp2d40 (n=7, mean ± S.D.; *, p<0.05).
2.5. HNF4α activity on CYP2D6 and Cyp2d40 promoter is differentially regulated during pregnancy
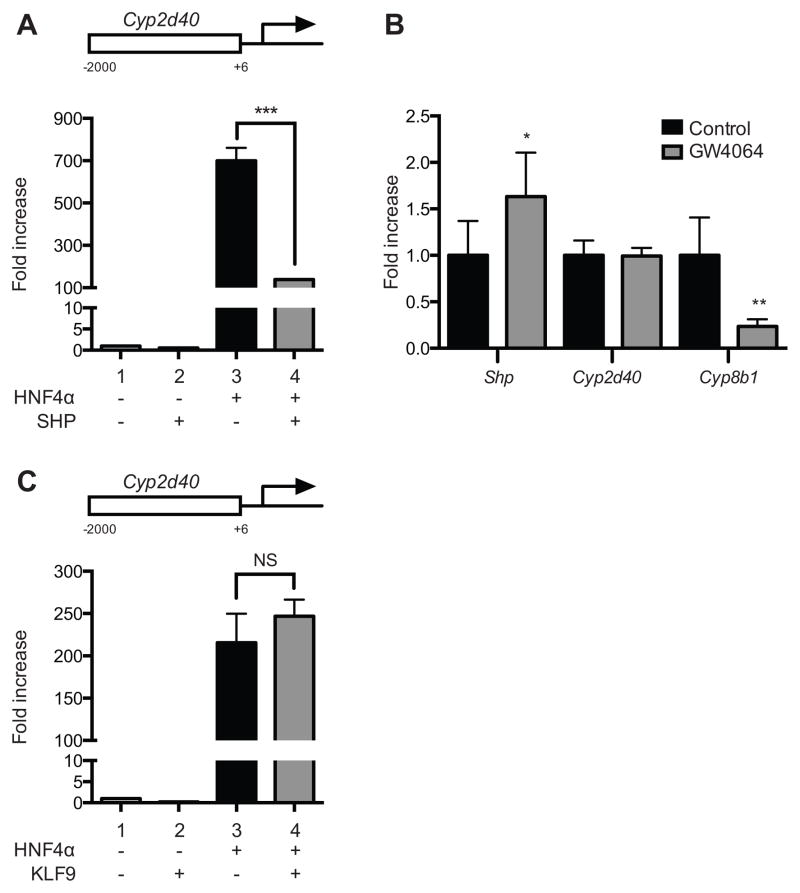
Previously, we have shown that SHP represses and KLF9 potentiates HNF4α transactivation of CYP2D6 promoter [13, 18]. To examine whether SHP and KLF9 modulate HNF4α transactivation of Cyp2d40 promoter (similarly to CYP2D6 promoter), we performed promoter reporter assays in HEK293T cells. HEK293T cells were co-transfected with pGL3-Cyp2d40 along with HNF4α and/or SHP expression vectors, and luciferase activity was measured. The results showed that SHP overexpression led to repressed HNF4α transactivation of Cyp2d40 promoter in HEK293T cells (Fig. 6A, lane 3 vs. 4). Considering that findings in HEK293T cells could result from super-physiological levels of SHP upon transient transfection, the role of SHP in Cyp2d40 regulation was further studied in vivo. To this end, mice were treated with GW4064, an inducer of SHP expression [21, 26], and mRNA expression levels of Cyp2d40 were measured. Cyp8b1 expression is known to be repressed when SHP is upregulated [27], and thus Cyp8b1 was included as a positive control. Unexpectedly, Cyp2d40 mRNA was not affected when SHP expression was induced in mice, whereas the expression of Cyp8b1 was significantly repressed in mice (Fig. 6B), suggesting that SHP does not modulate Cyp2d40 expression in in vivo systems. Results from promoter reporter assays for KLF9 showed that KLF9 did not affect HNF4α transactivation of Cyp2d40 promoter (Fig. 6C, lane 3 vs. 4), unlike the previously-shown potentiation of HNF4α transactivation of CYP2D6 promoter by KLF9 [18]. Together, these indicated that neither SHP nor KLF9 modulates Cyp2d40 expression, and the mechanism(s) underlying increased HNF4α transactivation of Cyp2d40 promoter likely differ from that of CYP2D6.
Figure 6. SHP and KLF9 do not modulate HNF4α transactivation of Cyp2d40.
(A) and (C), HEK293T cells were transfected with pGL3-Cyp2d40 with HNF4α and/or SHP expression vectors (A) or KLF9 expression plasmid (C), and dual luciferase assays were performed (n=3, mean ± S.D.). ***, p<0.001; NS, not significant. (B) C57BL/6J mice were injected with 15mg/kg GW4064 or olive oil as control for 5 days, and liver mRNA expression levels 6 hours after last injection were determined by qRT-PCR (n=3, mean ± S.D.; *, p<0.05, **, p<0.01 versus siRNA-Control).
3. Discussion
Our previous study in Tg-CYP2D6 mice showed that CYP2D6 induction during pregnancy is potentially triggered by enhanced HNF4α transactivation of CYP2D6 promoter, attributable to decreased expression of transcriptional repressor (SHP) and increased expression of transcriptional activator (KLF9) during pregnancy [13, 18]. We hereby examined whether this regulatory mechanism is conserved for mouse endogenous Cyp2d genes.
Among the Cyp2d homologs, we found that Cyp2d40 expression is significantly up-regulated during pregnancy. This result is in part consistent with the previous report where expression of most Cyp2d genes examined in the study (i.e., Cyp2d11, 2d22, 2d26, and 2d40) was shown increased during pregnancy [28]. Interestingly, our results showed that expression of most Cyp2ds (except Cyp2d40) decreased during pregnancy. The discrepancy is potentially due to differences in mouse strains used in the studies (FVB vs. C57BL/6). While the directional changes in the expression of other mouse Cyp2ds remain to be verified, both our and the previous studies clearly demonstrated that mouse Cyp2d40 expression is increased during pregnancy similarly to human CYP2D6.
The finding of enhanced CYP2D6 and Cyp2d40 expression during pregnancy suggests that the physiological roles of these two enzymes may be conserved in mice and humans. CYP2D6 in the liver metabolizes and eliminates a variety of xenobiotics, and its enhanced activity during pregnancy may be an evolutionary consequence of providing protection against potentially harmful xenobiotics in developing fetus. CYP2D6 expressed in the brain, on the other hand, has been implicated in the synthesis of neurotransmitters [29, 30] although the roles of CYP2D6 in brain physiology remains to be verified. Whether pregnancy alters CYP2D6 expression in brain is currently unknown. CYP2D40 is highly homologous (i.e., 81%) to CYP2D6, and this suggests that CYP2D40 may play similar roles as CYP2D6 by participating in hepatic drug detoxification and modulating brain functions. Indeed, CYP2D proteins have been detected in mouse brain [1], and the contribution of different mouse CYP2D isoforms (especially CYP2D40) to the CYP2D proteins expressed in the brain is yet to be determined.
Our previous study has shown that CYP2D6 induction during pregnancy is due to enhanced transactivation of CYP2D6 promoter by HNF4α [13]. Results from this study showed that HNF4α also plays a key role in enhanced expression of Cyp2d40 during pregnancy; Cyp2d40 induction during pregnancy was abrogated in mice with decreased hepatic HNF4α expression, and HNF4α recruitment to Cyp2d40 promoter increased at term pregnancy. We further showed that Cyp2d40 is a direct target gene of HNF4α, and mapped a HNF4α response element to −117/−105 of Cyp2d40. Interestingly, the HNF4α binding site was found conserved in the promoters of the other mouse Cyp2d genes (except Cyp2d22) (data not shown). This suggests that the presence of HNF4α binding sequence in Cyp2d40 promoter alone does not explain the unique feature of Cyp2d40 regulation during pregnancy. HNF4α transactivation of its target gene promoters is likely modulated in a Cyp2d gene-specific manner during pregnancy, similarly to other HNF4α target genes whose expression is altered during pregnancy in a pattern different from that for CYP2D6 [13].
Enhanced HNF4α transactivation of CYP2D6 promoter is triggered in part by decreased SHP and increased KLF9 expression during pregnancy [13, 18]. However, results from this study suggest that neither SHP nor KLF9 plays a key role in modulating HNF4α transactivation of Cyp2d40 promoter. This is potentially due to differences between CYP2D6 and Cyp2d40 in the promoter sequences, resulting in altered interplay among SHP, KLF9, and HNF4α. Firstly, our EMSA results indicate that HNF4α binding to Cyp2d40 promoter may be of lower affinity than that to CYP2D6 promoter. In an in vivo system, this may lead to dampened SHP action on HNF4α transactivation of Cyp2d40 promoter. In accordance with this notion, although SHP significantly repressed Cyp2d40 promoter activity in HNF4α-overexpressing HEK293T cells, such finding was not recapitulated in mice. Secondly, whereas CYP2D6 harbors multiple binding sites for KLF9 that enhances HNF4α transactivation of the promoter [18], Cyp2d40 lacks such KLF9 binding sites in its promoter (based on in silico promoter analysis; data not shown). Our results from promoter reporter assays also correspond to the lack of KLF9 binding to Cyp2d40. The detailed molecular mechanisms underlying enhanced HNF4α transactivation of Cyp2d40 promoter during pregnancy remain unclear. The roles of other known regulators of HNF4α activity [31–35], such as PGC1α, in the regulation of HNF4α activity on Cyp2d40 promoter warrant further investigation.
Taken together, we have shown that mouse Cyp2d40 expression is enhanced in the liver during pregnancy, similarly to CYP2D6. The enhanced expression of CYP2D6 and Cyp2d40 during pregnancy is attributed to an increase in HNF4α transactivation of both promoters. These results suggest as-yet-unknown, evolutionarily conserved physiological functions of human CYP2D6 and mouse CYP2D40 during pregnancy.
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development [Grant HD065532].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Miaoran Ning, Email: mning2@uic.edu.
Kwi Hye Koh, Email: noble.koh@gmail.com.
Xian Pan, Email: xpan9@uic.edu.
Hyunyoung Jeong, Email: yjeong@uic.edu.
References
- 1.Miksys SL, Cheung C, Gonzalez FJ, Tyndale RF. Human CYP2D6 and mouse CYP2Ds: organ distribution in a humanized mouse model. Drug Metab Dispos. 2005;33:1495–502. doi: 10.1124/dmd.105.005488. [DOI] [PubMed] [Google Scholar]
- 2.Siegle I, Fritz P, Eckhardt K, Zanger UM, Eichelbaum M. Cellular localization and regional distribution of CYP2D6 mRNA and protein expression in human brain. Pharmacogenetics. 2001;11:237–45. doi: 10.1097/00008571-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Yu AM, Idle JR, Gonzalez FJ. Polymorphic cytochrome P450 2D6: humanized mouse model and endogenous substrates. Drug Metab Rev. 2004;36:243–77. doi: 10.1081/dmr-120034000. [DOI] [PubMed] [Google Scholar]
- 4.Zanger UM, Raimundo S, Eichelbaum M. Cytochrome P450 2D6: overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:23–37. doi: 10.1007/s00210-003-0832-2. [DOI] [PubMed] [Google Scholar]
- 5.Lu Y, Peng Q, Zeng Z, Wang J, Deng Y, Xie L, et al. CYP2D6 phenotypes and Parkinson’s disease risk: a meta-analysis. J Neurol Sci. 2014;336:161–8. doi: 10.1016/j.jns.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 6.Smith CA, Gough AC, Leigh PN, Summers BA, Harding AE, Maraganore DM, et al. Debrisoquine hydroxylase gene polymorphism and susceptibility to Parkinson’s disease. Lancet. 1992;339:1375–7. doi: 10.1016/0140-6736(92)91196-f. [DOI] [PubMed] [Google Scholar]
- 7.Kirchheiner J, Seeringer A, Godoy AL, Ohmle B, Maier C, Beschoner P, et al. CYP2D6 in the brain: genotype effects on resting brain perfusion. Mol Psychiatry. 2011;16:237, 333–41. doi: 10.1038/mp.2010.42. [DOI] [PubMed] [Google Scholar]
- 8.Corchero J, Granvil CP, Akiyama TE, Hayhurst GP, Pimprale S, Feigenbaum L, et al. The CYP2D6 humanized mouse: effect of the human CYP2D6 transgene and HNF4alpha on the disposition of debrisoquine in the mouse. Mol Pharmacol. 2001;60:1260–7. doi: 10.1124/mol.60.6.1260. [DOI] [PubMed] [Google Scholar]
- 9.Scheer N, Kapelyukh Y, McEwan J, Beuger V, Stanley LA, Rode A, et al. Modeling human cytochrome P450 2D6 metabolism and drug-drug interaction by a novel panel of knockout and humanized mouse lines. Mol Pharmacol. 2012;81:63–72. doi: 10.1124/mol.111.075192. [DOI] [PubMed] [Google Scholar]
- 10.Tracy TS, Venkataramanan R, Glover DD, Caritis SN. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A Activity) during pregnancy. Am J Obstet Gynecol. 2005;192:633–9. doi: 10.1016/j.ajog.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 11.Hogstedt S, Lindberg B, Peng DR, Regardh CG, Rane A. Pregnancy-induced increase in metoprolol metabolism. Clin Pharmacol Ther. 1985;37:688–92. doi: 10.1038/clpt.1985.114. [DOI] [PubMed] [Google Scholar]
- 12.Ververs FF, Voorbij HA, Zwarts P, Belitser SV, Egberts TC, Visser GH, et al. Effect of cytochrome P450 2D6 genotype on maternal paroxetine plasma concentrations during pregnancy. Clin Pharmacokinet. 2009;48:677–83. doi: 10.2165/11318050-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Koh KH, Pan X, Shen HW, Arnold SL, Yu AM, Gonzalez FJ, et al. Altered expression of small heterodimer partner governs cytochrome P450 (CYP) 2D6 induction during pregnancy in CYP2D6-humanized mice. J Biol Chem. 2014;289:3105–13. doi: 10.1074/jbc.M113.526798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang-Verslues WW, Sladek FM. HNF4alpha--role in drug metabolism and potential drug target? Curr Opin Pharmacol. 2010;10:698–705. doi: 10.1016/j.coph.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yusuf D, Butland SL, Swanson MI, Bolotin E, Ticoll A, Cheung WA, et al. The transcription factor encyclopedia. Genome Biol. 2012;13:R24. doi: 10.1186/gb-2012-13-3-r24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jover R, Bort R, Gomez-Lechon MJ, Castell JV. Cytochrome P450 regulation by hepatocyte nuclear factor 4 in human hepatocytes: a study using adenovirus-mediated antisense targeting. Hepatology. 2001;33:668–75. doi: 10.1053/jhep.2001.22176. [DOI] [PubMed] [Google Scholar]
- 17.Kamiyama Y, Matsubara T, Yoshinari K, Nagata K, Kamimura H, Yamazoe Y. Role of human hepatocyte nuclear factor 4alpha in the expression of drug-metabolizing enzymes and transporters in human hepatocytes assessed by use of small interfering RNA. Drug Metab Pharmacokinet. 2007;22:287–98. doi: 10.2133/dmpk.22.287. [DOI] [PubMed] [Google Scholar]
- 18.Koh KH, Pan X, Zhang W, McLachlan A, Urrutia R, Jeong H. Kruppel-like Factor 9 (KLF9) Promotes Cytochrome P450 (CYP) 2D6 Expression during Pregnancy in CYP2D6-humanized Mice. Mol Pharmacol. 2014 doi: 10.1124/mol.114.093666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seol W, Choi HS, Moore DD. An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors. Science. 1996;272:1336–9. doi: 10.1126/science.272.5266.1336. [DOI] [PubMed] [Google Scholar]
- 20.Boulias K, Katrakili N, Bamberg K, Underhill P, Greenfield A, Talianidis I. Regulation of hepatic metabolic pathways by the orphan nuclear receptor SHP. Embo j. 2005;24:2624–33. doi: 10.1038/sj.emboj.7600728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–26. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 22.Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol. 2001;21:1393–403. doi: 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 24.Koh KH, Jurkovic S, Yang K, Choi SY, Jung JW, Kim KP, et al. Estradiol induces cytochrome P450 2B6 expression at high concentrations: implication in estrogen-mediated gene regulation in pregnancy. Biochem Pharmacol. 2012;84:93–103. doi: 10.1016/j.bcp.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong G, Itakura T, Kawajiri K, Skow L, Negishi M. Gene family of male-specific testosterone 16 alpha-hydroxylase (C-P-450(16 alpha)) in mice. Organization, differential regulation, and chromosome localization. J Biol Chem. 1989;264:2920–7. [PubMed] [Google Scholar]
- 26.Lee YK, Schmidt DR, Cummins CL, Choi M, Peng L, Zhang Y, et al. Liver receptor homolog-1 regulates bile acid homeostasis but is not essential for feedback regulation of bile acid synthesis. Mol Endocrinol. 2008;22:1345–56. doi: 10.1210/me.2007-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Chiang JY. Transcriptional regulation of the human sterol 12alpha-hydroxylase gene (CYP8B1): roles of heaptocyte nuclear factor 4alpha in mediating bile acid repression. J Biol Chem. 2001;276:41690–9. doi: 10.1074/jbc.M105117200. [DOI] [PubMed] [Google Scholar]
- 28.Topletz AR, Le HN, Lee N, Chapman JD, Kelly EJ, Wang J, et al. Hepatic Cyp2d and Cyp26a1 mRNAs and activities are increased during mouse pregnancy. Drug Metab Dispos. 2013;41:312–9. doi: 10.1124/dmd.112.049379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu AM, Idle JR, Byrd LG, Krausz KW, Kupfer A, Gonzalez FJ. Regeneration of serotonin from 5-methoxytryptamine by polymorphic human CYP2D6. Pharmacogenetics. 2003;13:173–81. doi: 10.1097/01.fpc.0000054066.98065.7b. [DOI] [PubMed] [Google Scholar]
- 30.Hiroi T, Imaoka S, Funae Y. Dopamine formation from tyramine by CYP2D6. Biochem Biophys Res Commun. 1998;249:838–43. doi: 10.1006/bbrc.1998.9232. [DOI] [PubMed] [Google Scholar]
- 31.Rha GB, Wu G, Shoelson SE, Chi YI. Multiple binding modes between HNF4alpha and the LXXLL motifs of PGC-1alpha lead to full activation. J Biol Chem. 2009;284:35165–76. doi: 10.1074/jbc.M109.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez-Jimenez CP, Gomez-Lechon MJ, Castell JV, Jover R. Underexpressed coactivators PGC1alpha and SRC1 impair hepatocyte nuclear factor 4 alpha function and promote dedifferentiation in human hepatoma cells. J Biol Chem. 2006;281:29840–9. doi: 10.1074/jbc.M604046200. [DOI] [PubMed] [Google Scholar]
- 33.Surapureddi S, Rana R, Reddy JK, Goldstein JA. Nuclear receptor coactivator 6 mediates the synergistic activation of human cytochrome P-450 2C9 by the constitutive androstane receptor and hepatic nuclear factor-4alpha. Mol Pharmacol. 2008;74:913–23. doi: 10.1124/mol.108.048983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nedumaran B, Hong S, Xie YB, Kim YH, Seo WY, Lee MW, et al. DAX-1 acts as a novel corepressor of orphan nuclear receptor HNF4alpha and negatively regulates gluconeogenic enzyme gene expression. J Biol Chem. 2009;284:27511–23. doi: 10.1074/jbc.M109.034660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie YB, Nedumaran B, Choi HS. Molecular characterization of SMILE as a novel corepressor of nuclear receptors. Nucleic Acids Res. 2009;37:4100–15. doi: 10.1093/nar/gkp333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cairns W, Smith CA, McLaren AW, Wolf CR. Characterization of the human cytochrome P4502D6 promoter. A potential role for antagonistic interactions between members of the nuclear receptor family. J Biol Chem. 1996;271:25269–76. doi: 10.1074/jbc.271.41.25269. [DOI] [PubMed] [Google Scholar]