Abstract
Our objective was to explore interest in genetic testing among Ashkenazi Jewish (AJ) Parkinson’s Disease (PD) cases and first-degree relatives, as genetic testing for LRRK2 G2019S is widely available. Approximately 18 % of AJ PD cases carry G2019S mutations; penetrance estimations vary between 24 and 100 % by age 80. A Genetic Attitude Questionnaire (GAQ) was administered at two New York sites to PD families unaware of LRRK2 G2019S mutation status. The association of G2019S, age, education, gender and family history of PD with desire for genetic testing (outcome) was modeled using logistic regression. One-hundred eleven PD cases and 77 relatives completed the GAQ. Both PD cases and relatives had excellent PD-specific genetic knowledge. Among PD, 32.6 % “definitely” and 41.1 % “probably” wanted testing, if offered “now.” Among relatives, 23.6 % “definitely” and 36.1 % “probably” wanted testing “now.” Desire for testing in relatives increased incrementally based on hypothetical risk of PD. The most important reasons for testing in probands and relatives were: if it influenced medication response, identifying no mutation, and early prevention and treatment. In logistic regression, older age was associated with less desire for testing in probands OR=0.921 95%CI 0.868–0.977, p=0.009. Both probands and relatives express interest in genetic testing, despite no link to current treatment or prevention.
Keywords: Genetic testing, Parkinson’s disease, LRRK2, Ashkenazi jewish, Genetic counseling
Introduction
Genetic susceptibility plays a role in the development of Parkinson’s disease (PD), however, how, and to what extent remain unclear. The most common mutation associated with PD in Caucasian populations is the G2019S mutation in the leucine-rich repeat kinase-2 (LRRK2) gene (Kachergus et al. 2005). Penetrance is variable, ranging from 24 to 100 % by age 80 (Goldwurm et al. 2011; Trinh et al. 2014) depending on ethnic group and methodology employed.
The goal of this study was to establish baseline knowledge regarding genetic testing, genetics of PD specifically, current diagnosis and treatment of PD, attitudes toward genetic testing for PD and reasons to pursue or decline genetic testing in PD cases and their asymptomatic family members participating in the Michael J Fox Foundation LRRK2 Ashkenazi Jewish (AJ) Consortium (Alcalay et al. 2013). We sought the reasons individuals are more, or less, likely to pursue testing and if those reasons corresponded to desire for genetic testing ’now’, given what is currently known about PD and the options for treatment.
The LRRK2 G2019S mutation is particularly common among AJ with PD. It was identified in 14.8 % of all AJ with PD and in 26 % of those with familial PD; resulting in approximately a 9 fold increased chance of developing PD in mutation carriers compared to age and sex matched controls (Giladi et al. 2011; Orr-Urtreger et al. 2007). In the Michael J Fox AJ Consortium, the largest systematically examined to date, 19.9 % of Ashkenazi PD cases had LRRK2 G2019S mutations, after excluding cases with glucocerebrosidase (GBA) mutations (Alcalay et al. 2013). Given that up to 30 % of AJ PD patients have either LRRK2 G2019S or mutations in GBA, genetic testing may be a consideration for AJ PD cases and their family members. As genetic testing becomes more accessible, not only through health care providers, but also through direct-to-consumer testing, it is important to understand what individuals know about the test, why they opt for testing, and what they believe can be concluded from the results. There have been few papers that explore patient’s and family member’s knowledge and attitudes toward genetic testing for PD. Studies have found that knowledge regarding genetics and PD is correlated with both higher level of education and Jewish ancestry (Falcone, Wood, Xie, Siderowf, and Van Deerlin 2011; Sakanaka et al. 2013; Tan and Jankovic 2006). In contrast, desire to obtain genetic testing results was associated with non-Jewish ancestry but not education, gender or family history of PD (Sakanaka et al. 2013). Therefore, the major research questions investigated in the present study were 1.) What is the understanding of what is currently known about PD and genetic testing? 2.) Is there a desire to obtain testing at this time in those already affected with PD or in their unaffected first-degree relatives? 3.) Is there an association between the desire or lack of desire for testing and reasons individuals deem important in the decision to pursue testing? 4.) Do the above questions vary by G2019S genotype?
Methods
Participants
The Michael J Fox Ashkenazi Jewish Consortium was designed to focus on LRRK2 G2019S probands and their first-degree relatives. In the first stage, 553 PD probands were recruited from 3 sites, Beth Israel Medical Center (n=138), and Columbia University Medical Center (n=143) both in New York, NY, and Tel Aviv University, in Tel Aviv, Israel (n=272) from April 2010 to May 2012 (Alcalay et al. 2013). Institutional review boards at all sites approved the protocols. Probands were recruited if they reported at least two AJ grandparents, and were diagnosed with PD by a movement disorder specialist, based on the United Kingdom Parkinson’s Disease brain bank criteria (with the exception that reporting a family history of PD was not exclusionary as stipulated in the criteria) (Hughes, Daniel, Kilford, and Lees 1992). All NY participants were genotyped for the G2019S mutation but were not informed of the results.
An in-depth assessment was offered to probands who carry G2019S mutations and their family members and to a subset of non-carrier probands. The in-depth analysis consisted of detailed neurological, neuropsychological, psychiatric assessments, medical and family history, non-motor symptomatology, and a genetic attitudes questionnaire that we designed and is the focus of this report. Participants from Tel Aviv were not administered the genetic attitudes questionnaire, since results are offered to all individuals who undergo testing, and will not be further discussed. Probands from the NY sites found to carry G2019S mutations (n=83) and a random sample of non-carrier probands (n=69) were recruited for an in-depth analysis. We attempted to recruit first-degree relatives of all G2019S carriers for the in-depth analyses. Eight probands and five relatives knew their carrier status prior to the study. These 5 relatives came from 3 families (2 relatives from 2 families, 1 from a single family). The proband in each family was a known LRRK2 G2019S carrier.
Assessments
All probands completed a questionnaire regarding demographics and family history of PD. Details of this assessment have been reported elsewhere (Alcalay et al. 2013). Specific scores from the in-depth assessment included in these analyses were the Geriatric Depression Scale (GDS 15), Unified Parkinson’s Disease Rating Scale (UPDRS) Part III, and Montreal Cognitive Assessment (MoCA). Family history of PD was assessed using a reliable, valid interview (Marder et al. 2003).
We designed the Genetic Attitudes Questionnaire (GAQ) that was completed by each participant and reviewed by the interviewer for completeness. The GAQ consisted of four sections: A) Parkinson’s disease and your life (10 items), B) attitudes towards genetic testing (25 items), C) work and retirement (6 items), D) privacy and insurance (22 items). A range of question formats were employed including yes/no/ uncertain, 5-item response scale, and a 1–100 scale. For example, the 5-item response scale regarding desire for testing, response options included definitely want testing, probably want testing, definitely do not want testing probably do not want testing, and cannot decide about testing. All questions in the section on genetic knowledge have been previously published (Falcone et al. 2011). Specific questions about privacy and insurance were derived from the Prospective Huntington At Risk Observational Study (PHAROS) survey (Oster et al. 2008), and will be the subject of a future publication. The goal of the GAQ was to establish the degree of baseline knowledge regarding genetic testing, genetics of PD specifically, current diagnosis and treatment of PD, attitudes toward genetic testing for PD and reasons to pursue or decline genetic testing.
Statistical Analyses
Demographics, genotype, UPDRS, MoCA, GDS, and GAQ, were compared using chi-square tests, and Student’s t-tests as appropriate. Logistic regression models were constructed to test the association between demographic and clinical features in probands (predictors) and interest in genetic testing for PD (outcome) dichotomized as one group being definitely or probably desire testing and the second group included definitely or probably do not want testing and unsure about testing. Models were adjusted for G2019S mutation status, age, gender, disease duration, UPDRS III score, and education (categorized as with or without post-high-school education). Similar analyses were conducted in asymptomatic relatives adjusting for age, gender, G2019S status, and relative class (parent, sibling, child).
Results
Demographics
We first compared those that did and did not complete the GAQ. One-hundred eleven probands (111/152 [73 %] of eligible probands) and 77 relatives (77/100 [77 %] of eligible asymptomatic first-degree relatives representing 45 families) completed the GAQ. There were no significant differences between those that did and did not complete GAQ (Appendix Table 1), except that probands who completed the GAQ had a lower GDS 15 score than non-responders (p=0.017), and relatives who completed the questionnaire were more likely to have post-high school education (p=0.013). Data presented from this point forward will only pertain to those completing the GAQ.
Demographic characteristics of G2019S carriers and non-carriers who completed the GAQ are shown in Table 1. Similar to the 553 cases that underwent screening at all three sites (Alcalay et al. 2013), probands who were G2019S carriers had longer disease duration (10.39 years [SD=6.73] compared to 7.92 years [SD 6.45] for non-carriers, p=0.054); a higher percent reporting a family history of PD (39.7 % of carriers vs. 21.2 % non-carriers, p=0.041); and carriers were more likely to believe that PD is hereditary (36.4 % of carriers vs. 12 % non-carriers, p=0.007). Among the relatives, there were no significant differences in demographic characteristics seen between G2019S carriers and non-carriers.
Table 1.
Demographics by Genotype for those completing GAQ
| Probands
|
Relatives
|
|||||
|---|---|---|---|---|---|---|
| G2019S Carriers (n=58) | G2019S Non-carriers (n=53) | Significance | G2019S Carriers (n=45) | G2019S Non-carriers (n=32) | Significance | |
| Percent Male | 60.3 | 73.6 | 0.162 | 35.8 | 47.7 | 0.301 |
| Age, years (SD) | 67.74 (10.05) | 68.97 (9.62) | 0.551 | 54.96 (17.32) | 51.52 (19.51) | 0.36 |
| Percent with post high school education | 96.4 | 88.7 | 0.157 | 84.6 | 86.4 | 1 |
| Number (Percent) with previous PD genetic testing outside of research | 7 (12.1 %) | 5 (9.4 %) | 0.600 | 3 (7.7 %) | 2 (6.5 %) | 0.521 |
| Number with genetic testing for another condition outside of research setting | 6 | 6 | 0.767 | 6 | 8 | 0.293 |
| Percent positive family history in 1st degree relative | 39.7 | 21.2 | 0.041 | 31.6 | 41.9 | 0.453 |
| Percent that believe PD is hereditary (Yes/No/Unsure) | 36.4/25.5/38.2 | 12/48/40 | 0.007 | 40.5/19/40.5 | 40/20/40 | 0.995 |
| Age-at-onset, years (SD) | 57.05 (12.20) | 60.94 (9.78) | 0.070 | N/A | N/A | N/A |
| PD duration, years (SD) | 10.39 (6.73) | 7.92 (6.45) | 0.054 | N/A | N/A | N/A |
| Mean UPDRS motor (SD) | 10.32 (7.49) | 13.31 (9.13) | 0.065 | N/A | N/A | N/A |
| MoCA (SD) | 24.96 (3.48) | 25.51 (3.58) | 0.444 | 26.86 (2.56) | 27.58 (2.24) | 0.214 |
Genetic Knowledge
The majority of probands and relatives are aware that scientists have identified genes associated with a higher risk of developing PD, and that a genetic test will not predict age of onset, PD severity, or if one will develop PD with certainty (Fig. 1). This did not change when stratified by genotype. In terms of knowledge regarding genetic testing for other diseases, among probands, a high percentage are aware that genetic testing exists for breast cancer (78.5 %) and Huntington’s Disease (61.9 %), but fewer individuals are aware of genetic testing for Cystic Fibrosis (51 %) and Gaucher Disease (47.2 %). Relatives had similar knowledge to probands (Fig. 1).
Fig. 1.
Performance of probands and relatives on genetic knowledge questions. The answer choices were either true or false; correct answer is given in parenthesis
Desire for Testing
Hypothetically, 32.6 % of the probands and 23.6 % of the asymptomatic relatives responded that they would definitely want to pursue genetic testing if offered “now,” while 41.1 % and 36.1 %, respectively, said they probably want to pursue testing “now.” On the 5-point scale (definite yes, probably yes, unsure, probably no, definitely no) there was no significant difference in responses by G2019S genotype (p=0.826 probands, p=0.07 relatives).
In univariate analyses, probands who did not want testing or who were unsure, were older (73.8 years; SD=9.44) than probands who definitely or probably wanted testing (69.9 years; SD=9.4), p =0.002. Disease duration was significantly longer in those who did not want testing (12.32; SD=8.39, vs. 8.5; SD=6.14), p=0.046; however UPDRS scores were not different. The percentage of G2019S carriers, or those who reported a family history of PD did not differ. Response did not differ by gender, or marital status (married compared to single).
In a multivariate model, including G2019S, gender, education, age, belief that PD was hereditary, and UPDRS score, only age was associated with definite or probable desire for testing (OR=0.921 95%CI 0.868–0.977, p=0.009), such that older age was associated with less interest in desire for testing now. Seventy asymptomatic relatives who did not know their genetic status were included in an analysis to predict interest in genetic testing. In a multivariate model, adjusting for relationship to the proband, gender, education, age, belief that PD is hereditary and genotype, there were no significant predictors of interest in testing. However, all interviewed asymptomatic parents of PD probands (6 from 4 families) expressed interest in testing. Thirty percent of siblings and 70.6 % of children were interested in testing.
Interest in genetic testing among relatives based on hypothetical risk of PD is shown in Fig. 2. The higher the likelihood that a test predicts the development of PD, the more likely an individual says he/she would want to be tested. There is equipoise at 25 % hypothetical risk, with approximately 25 % in each of the four groups.
Fig. 2.

Percent of asymptomatic relatives likely to choose testing based on hypothetical risk of PD
Reasons for Testing
Figures 3a and b show the relative importance of reasons in deciding to be tested for probands and relatives, respectively. The most important reasons cited by both groups were learning they do not carry a mutation, and being identified early for prevention or treatment. Additionally, 93 % of probands and 93 % of relatives reported they would definitely pursue testing if it determined response to medications. Reproductive decision-making was the least important reason for wanting testing for both probands and relatives.
Fig. 3.
a Probands’ reasons for testing b Relatives’ reasons for testing
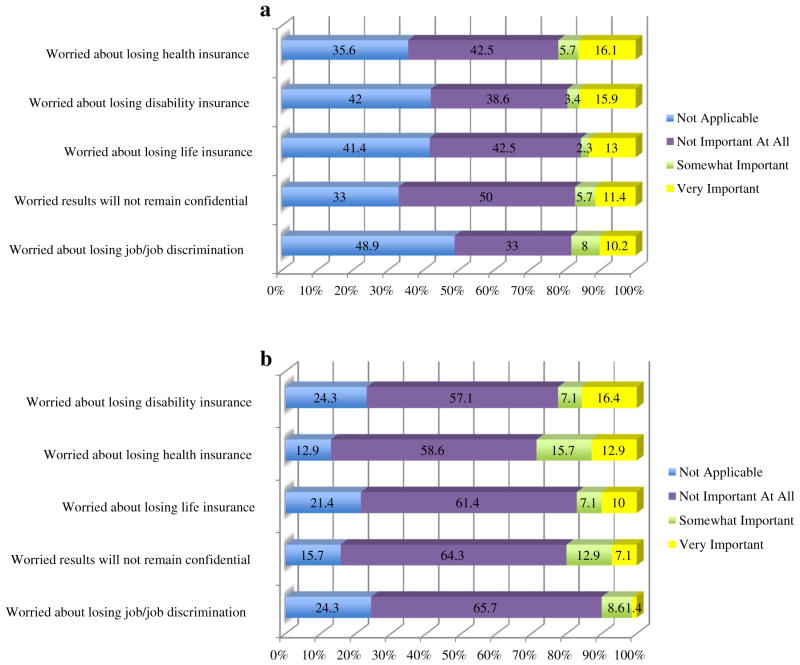
Figures 4a and b explore some negative consequences of being tested as related to insurance and privacy. The interview was administered prior to initiation of the Affordable Care Act. A total of 21.8 % of probands were somewhat or very concerned about losing health insurance, compared to 28.6 % of relatives. A total of 19.3 % of probands and 23.5 % of relatives were very or somewhat worried about losing disability insurance.
Fig. 4.
a Proband’s reasons for testing related to insurance and privacy b Relatives reasons for testing related to insurance and privacy
Discussion
Interest in genetic testing in AJ PD families depends on how likely a test would predict the development of PD, medication response, and the relationship to early detection. The majority of probands (73.7 %) and relatives (59.7 %) express interest in genetic testing now, despite the fact that there is no known link to treatment or prevention. We established that in this population there is a basic understanding of PD genetics, the importance of various reasons to consider testing, and that most affected individuals and unaffected first-degree relatives indicate that they would choose to be tested now.
Knowledge
Other studies have reported low scores on genetic knowledge questions in both PD patients and caregivers (Falcone et al. 2011; Sakanaka et al. 2013; Tan and Jankovic 2006). Though we did not calculate a genetic knowledge score, the majority of participants answered general PD genetics questions correctly. Sakanaka et al. reported 51.6 % of PD patients and 55.6 % of non-blood related caregivers answered correctly that “scientists have identified genes associated with a higher risk of developing PD,” with a positive correlation of those with Jewish ancestry responding correctly (Sakanaka et al. 2013, p. 116, Fig. 1). Our findings further support this increased knowledge in the Ashkenazi Jewish population, with 77.5 % of probands and 88.3 % of relatives answering correctly. However, other than for breast cancer, relatively few individuals were aware of genetic testing for other conditions. It is possible participants’ greater knowledge of PD genetics motivated them to participate in a PD genetics study. They may have also received information regarding PD genetics from their clinicians or through participation in research.
The high percentage knowing about genetic testing for breast cancer is expected given a high prevalence of BRCA1/2 mutations in the AJ population (Struewing et al. 1997). Particularly surprising though, is the low percentage aware of genetic testing for Gaucher Disease, associated with a GBA mutation in this population and linked to PD (Sidransky et al. 2009). GBA genetic testing is included in the recommended prenatal testing panel for the AJ population, though it was added several years after testing for Tay Sachs. Knowledge of both BRCA mutations and GBA homozygosity could lead to earlier detection and intervention for breast cancer and Gaucher Disease, respectively, as compared to PD, for which there is no current intervention based on genetic mutation status. Lack of an intervention has been cited as reducing interest in genetic testing in PD (Dahodwala et al. 2007).
As seen in other studies (Falcone et al. 2011; Sakanaka et al. 2013; Tan and Jankovic 2006), there is a desire to pursue genetic testing for PD with 73.7 % of probands answering they would definitely or probably desire testing now, and 59.7 % of relatives in this study responding similarly. However, when questioned how likely individuals were to pursue testing if a positive result meant they had a 25 % risk of developing PD, which is closest to the estimated lifetime penetrance of G2019S (Clark et al. 2006), there was equipoise.
Among PD probands, older individuals were less likely to definitely/probably want to pursue testing “now.” We found no single factor that predicted desire for testing in relatives including LRRK2 genotype, demographic features, or belief that PD is hereditary. This may reflect that each individual has different reasons for choosing to be tested or that individuals think they would want to be tested, but may not actually follow through when given the opportunity, especially following genetic counseling. Giladi et al. (2011) found that out of 46 Ashkenazi first-degree relatives, of PD patients known to be carriers of the G20919S mutation, only five wanted to pursue genetic testing following clinical counseling.
In a previous study (Falcone et al. 2011) the most important themes cited by PD patients to pursue testing were personal information/curiosity (30.6 %), to help research (26.5 %), and information for family (22.4 %). Similarly, we found in probands, personal information (i.e., finding out they do not carry the mutation and participation in research for early identification for treatment) were the most frequently cited reasons for wanting to be tested.
Practice Implications
Genetic counselors advising the Ashkenazi Jewish population regarding testing for G2019S, should be aware that penetrance of the G2019S mutation has been reported from approximately 24–100 %, (Goldwurm et al. 2011), but that penetrance among Ashkenazi Jews may be at the lower end of the spectrum (Clark et al. 2006). It is currently unknown whether the range of penetrance estimates is due to differing methodology, the inclusion of both clinic and community based samples or whether there are genetic modifiers in specific ethnic groups. The reduced penetrance could influence the decision to undergo testing. Currently, mutation status would not result in answers regarding age of onset of PD or treatment options. There are no reports of preimplantation genetic diagnosis being utilized for G2019S. As we learn more about the manifestations, progression and targeted interventions related G2019S carrier status; decisions based on knowledge of G2019S carrier status may be informative.
Study Strengths
The strengths of our study include that we were able to explore an educated population of the same ethnicity with a high prevalence of the G2019S mutation using a comprehensive survey, which highlighted various aspects of perceived knowledge and consequences of genetic testing. We had both symptomatic probands and asymptomatic at-risk family members. All individuals were genotyped, but the majority did not know their genotype. This allowed us to analyze if mutation status alone affected belief of PD being hereditary.
Study Limitations and Research Recommendations
Limitations of our study include generalizability outside of this population. The individuals (probands and relatives) knew that they were participating in a genetic study of PD, though they were not informed of results. Not everyone completed the GAQ. We did not perform test retest reliability on the GAQ, though several questions were derived from other studies. We did not include specific knowledge questions regarding LRRK2. Currently, to our knowledge, there is no information available regarding possible psychological stress following identification of G2019S mutation status for LRRK2. As mutation status was not revealed to individuals in our study, we could not answer this question. The focus of future research could include follow up of individuals who are informed about their mutation status. Our findings should be replicated in a larger sample of family members of PD pro-bands who carry LRRK2 mutations. Given the wide penetrance estimates, comparison of the GAQ in Ashkenazi Jewish families and other ethnic groups who carry LRRK2 mutations would be important. Attitudes toward genetic testing for PD mutations may change, given the health care legislation. In addition, were there a disease modifying therapy for PD or specifically for LRRK2 PD, attitudes and desire for testing might change.
Conclusion
In conclusion, our study explores the knowledge, attitudes, and desires toward the complexities of genetic testing in PD, in a high-risk population, and finds a high desire for genetic testing with no clear predictive factor other than age. As genetic screening for PD becomes more widely available, it will be imperative to ensure proper counseling regarding expectations of consequences of results. Further studies should be pursued in other populations and consider other PD associated genes to provide generalizability.
Acknowledgments
This study was supported by the Michael J Fox Foundation, NIH (R56NS036630, NS050487, NS060113, UL1 TR000040, and 10628097) and the Parkinson’s Disease Foundation.
Footnotes
Disclosure Manisha Gupte MD, Helen Mejia Santana MS, Anat Mirelman PhD, Deborah Raymond MS, Avi Orr-Urtreger MD PhD, Nir Giladi MD report nothing to disclose.
Dr. Alcalay receives research support from the NIH (K02NS080915), the Parkinson’s Disease Foundation, the Smart Foundation and the Michael J Fox foundation.
Dr. Saunders-Pullman serves on the Scientific Advisory Board of the Dystonia Medical Research Foundation. She receives research support from the NIH (K02 NS073836), the Michael J Fox Foundation for Parkinson’s Research, the Bachmann-Strauss Dystonia and Parkinson’s Foundation, and the Marcled Foundation.
Dr. Ozelius receives salary support from NIH [NS037409, NS075881, DC011805]. She is a current member of the scientific advisory boards of the National Spasmodic Dysphonia Association, the Benign Essential Blepharospasm Research Foundation and Tourette Syndrome Association, Inc. Dr. Ozelius receives royalty payments from Athena Diagnostics related to patents.
Dr. Clark receives research support from the NIH [NINDS #R01 NS060113 (principal investigator), NINDS #R01 NS073872 (Co-principal investigator), NIA #5P50AG008702 (Project 3, principal investigator), and NINDS #NS036630 (co-investigator) and 2P50NS038370-11 (Co-Investigator)], and the Parkinson’s Disease Foundation (principal investigator) and the Michael J Fox Foundation (co-investigator).
Dr. Bressman serves on the advisory boards of the Michael J. Fox Foundation, the Dystonia Medical Research Foundation, the Bachmann Strauss Dystonia and Parkinson’s Foundation, and the Board of We Move. She has consulted for Bristol Meyer Squibb. She has received research support from the Michael J. Fox Foundation, National Institutes of Health (NIH), and Dystonia Medical Research Foundation.
Dr. Marder receives research support from the NIH [#NS036630 (PI), 1UL1 RR024156-01 (Director PCIR), PO412196- G (Co-I),]. She received compensation for participating on the steering committee for U01NS052592 and from the Parkinson Disease Foundation, Huntington’s Disease Society of America, the Parkinson Study Group, CHDI, and the Michael J Fox Foundation.
Conflict of Interest Manisha Gupte, Roy N. Alcalay, Helen Mejia-Santana, Deborah Raymond, Rachel Saunders-Pullman, Ernest Roos, Martha Orbe-Reily, Ming-X Tang, Anat Mirelman, Laurie Ozelius, Avi Orr-Urtreger, Lorraine Clark, Nir Giladi, Susan Bressman and Karen Marder declare that they have no conflict of interest.
Human Studies and Informed Consent All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Contributor Information
Manisha Gupte, Department of Neurology, College of Physicians and Surgeons, Columbia University, 630 W 168th St, New York, NY 10032, USA.
Roy N. Alcalay, Department of Neurology, College of Physicians and Surgeons, Columbia University, 630 W 168th St, New York, NY 10032, USA. Taub Institute for Research on Alzheimer’s Disease and the Aging Brain, Columbia University, New York, NY, USA
Helen Mejia-Santana, Department of Neurology, College of Physicians and Surgeons, Columbia University, 630 W 168th St, New York, NY 10032, USA.
Deborah Raymond, The Alan and Barbara Mirken Department of Neurology, Beth Israel Medical Center, New York, NY, USA.
Rachel Saunders-Pullman, The Alan and Barbara Mirken Department of Neurology, Beth Israel Medical Center, New York, NY, USA. Department of Neurology, Albert Einstein College of Medicine, Bronx, New York, NY, USA.
Ernest Roos, Department of Neurology, College of Physicians and Surgeons, Columbia University, 630 W 168th St, New York, NY 10032, USA.
Martha Orbe-Reily, Department of Neurology, College of Physicians and Surgeons, Columbia University, 630 W 168th St, New York, NY 10032, USA.
Ming-X Tang, Department of Neurology, College of Physicians and Surgeons, Columbia University, 630 W 168th St, New York, NY 10032, USA.
Anat Mirelman, Movement Disorders Unit, Department of Neurology, Tel-Aviv Medical Center, Sackler School of Medicine, Tel-Aviv University, Tel-Avv, Israel.
Laurie Ozelius, Departments of Genetics and Genomic Sciences and Neurology, Mount Sinai School of Medicine, New York, NY, USA.
Avi Orr-Urtreger, Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel. Genetics Institute, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel.
Lorraine Clark, Taub Institute for Research on Alzheimer’s Disease and the Aging Brain, Columbia University, New York, NY, USA. Department of Pathology and Cell Biology, College of Physicians and Surgeons, Columbia University, New York, NY, USA. Center for Human Genetics, College of Physicians and Surgeons, Columbia University, New York, NY, USA.
Nir Giladi, Movement Disorders Unit, Department of Neurology, Tel-Aviv Medical Center, Sackler School of Medicine, Tel-Aviv University, Tel-Avv, Israel. Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel.
Susan Bressman, The Alan and Barbara Mirken Department of Neurology, Beth Israel Medical Center, New York, NY, USA. Department of Neurology, Albert Einstein College of Medicine, Bronx, New York, NY, USA.
Karen Marder, Email: ksm1@cumc.columbia.edu, Department of Neurology, College of Physicians and Surgeons, Columbia University, 630 W 168th St, New York, NY 10032, USA. Taub Institute for Research on Alzheimer’s Disease and the Aging Brain, Columbia University, New York, NY, USA.
References
- Alcalay RN, Mirelman A, Saunders-Pullman R, Tang MX, Mejia Santana H, Raymond D, Marder KS. Parkinson disease phenotype in Ashkenazi Jews with and without LRRK2 G2019S mutations. Movement Disorders. 2013;28(14):1966–1971. doi: 10.1002/mds.25647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LN, Wang Y, Karlins E, Saito L, Mejia-Santana H, Harris J, Marder K. Frequency of LRRK2 mutations in early-and late-onset Parkinson disease. Neurology. 2006;67(10):1786–1791. doi: 10.1212/01.wnl.0000244345.49809.36. 01.wnl.0000244345.49809.36 [pii] [DOI] [PubMed] [Google Scholar]
- Dahodwala N, Connolly J, Farmer J, Stern MB, Jennings D, Siderowf A. Interest in predictive testing for Parkinson’s disease: impact of neuroprotective therapy. Parkinsonism & Related Disorders. 2007;13(8):495–499. doi: 10.1016/j.parkreldis.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Falcone DC, Wood EM, Xie SX, Siderowf A, Van Deerlin VM. Genetic testing and Parkinson disease: assessment of patient knowledge, attitudes, and interest. Journal of Genetic Counseling. 2011;20(4):384–395. doi: 10.1007/s10897-011-9362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi N, Mirelman A, Thaler A, Bar-Shira A, Gurevich T, Orr-Urtreger A. Fighting the risk of developing Parkinson’s disease; clinical counseling for first degree relatives of patients with Parkinson’s disease. Journal of the Neurological Sciences. 2011;310(1–2):17–20. doi: 10.1016/j.jns.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Goldwurm S, Tunesi S, Tesei S, Zini M, Sironi F, Primignani P, Pezzoli G. Kin-cohort analysis of LRRK2-G2019S penetrance in Parkinson’s disease. Movement Disorders. 2011;26(11):2144–2145. doi: 10.1002/mds.23807. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. Journal of Neurology, Neurosurgery, and Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachergus J, Mata IF, Hulihan M, Taylor JP, Lincoln S, Aasly J, Toft M. Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across european populations. American Journal of Human Genetics. 2005;76(4):672–680. doi: 10.1086/429256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder K, Levy G, Louis ED, Mejia-Santana H, Cote L, Andrews H, Ottman R. Accuracy of family history data on Parkinson’s disease. Neurology. 2003;61(1):18–23. doi: 10.1212/01.wnl.0000074784.35961.c0. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A, Shifrin C, Rozovski U, Rosner S, Bercovich D, Gurevich T, Giladi N. he LRRK2 G2019S mutation in Ashkenazi Jews with Parkinson disease: is there a gender effect? Neurology. 2007;69(16):1595–1602. doi: 10.1212/01.wnl.0000277637.33328.d8. [DOI] [PubMed] [Google Scholar]
- Oster E, Dorsey ER, Bausch J, Shinaman A, Kayson E, Oakes D Huntington Study Group, Pharos Investigators. Fear of health insurance loss among individuals at risk for Huntington disease. American Journal of Medical Genetics Part A. 2008;146(16):2070–2077. doi: 10.1002/ajmg.a.32422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka K, Waters CH, Levy OA, Louis ED, Chung WK, Marder KS, Alcalay RN. Knowledge of and interest in genetic results among Parkinson disease patients and caregivers. Journal of Genetic Counseling. 2013 doi: 10.1007/s10897-013-9618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Ziegler SG. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. The New England Journal of Medicine. 2009;361(17):1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struewing JP, Hartge P, Wacholder S, Baker SM, Berlin M, McAdams M, Tucker MA. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. The New England Journal of Medicine. 1997;336(20):1401–1408. doi: 10.1056/NEJM199705153362001. [DOI] [PubMed] [Google Scholar]
- Tan EK, Jankovic J. Genetic testing in Parkinson disease: promises and pitfalls. Archives of Neurology. 2006;63(9):1232–1237. doi: 10.1001/archneur.63.9.1232. [DOI] [PubMed] [Google Scholar]
- Trinh J, Amouri R, Duda JE, Morley JF, Read M, Donald A, Farrer MJ. A comparative study of Parkinson’s disease and leucine-rich repeat kinase 2 p.G2019S parkinsonism. Neurobiol Aging. 2014;35(5):1125–1131. doi: 10.1016/j.neurobiolaging.2013.11.015. [DOI] [PubMed] [Google Scholar]